Introduction
Chronic interstitial nephritis in agricultural communities (CINAC), also named chronic kidney disease (CKD) of unknown origin or Mesoamerican nephropathy, is defined as a form of CKD affecting young farmers (mainly men). CINAC is not directly related to diabetes mellitus, glomerulonephritis, hypertension, or other known causes of CKD. Patients with CINAC live and work mainly in poor agricultural communities, often in hot tropical regions, and are occupationally exposed to potentially toxic agrochemicals through work, ingestion, water, and/or inhalation.1,2 There are 2 major hypotheses currently proposed: (i) repeated exposure to ambient heat with dehydration and (ii) exposure to nephrotoxic substances (e.g., agrochemicals, associated or not with metals).3 The disease is characterized by low or absent proteinuria and small kidneys with irregular contours in CKD stages 3 to 4. Renal biopsy results show tubulointerstitial lesions and moderate glomerulosclerosis associated with recently described sensitive pathognomonic lysosomal lesions in the proximal tubular cells (PTCs) indicative of a nephrotoxic tubulopathy.4,5
The epidemic dimension of CINAC was first observed in the 1990s in Sri Lanka and Central America. CINAC currently is an important cause of CKD-related deaths in an increasing number of countries.4,6,7 In Paraguay, as in most South American countries, cases of CINAC have not been described clinically and associated to histopathology by light microscopy (LM) and electron microscopy (EM). Here, we identified a farmer in Paraguay presenting the clinicoepidemiologic profile of CINAC in the absence of diabetes mellitus, glomerulonephritis, arterial hypertension, proteinuria, and polycystic kidney disease.
Case Presentation
On February 18, 2021, a 56-year-old male agricultural worker came to the outpatient clinic. He lived and worked in the Department of San Pedro, northeast from Asunción. Anamnesis by use of a questionnaire (see Supplementary S1) revealed that he worked in the cotton and corn fields since his 18 years of age. He was in charge of fumigation (application of pesticides in gaseous form) with a backsprayer without personal protection. During his career, he used several pesticides. In the first years, mainly paraquat and organophosphates. In the last decade, deltamethrin, methomyl, organophosphate, and glyphosate, the latter nearly exclusively over the last 4 years. The patient worked from 5:00 AM to 11:00 AM and, after a rest, from 3:00 PM to 5:00 PM. In summer, temperature ranged from 30 to 35 °C. In winter, from 20 to 24 °C, although some days up to 35 °C.
He came to the nephrology consultation for a routine medical checkup. The laboratory data showed hemoglobin at 12.2 g/dl and hematocrit at 37%. White blood cell and platelet counts were normal; blood glucose 79 mg/dl, urea 91 mg/dl, creatinine 2.5 mg/dl, estimated glomerular filtration rate 29 ml/min per 1.73 m2, sodium ion 136 mEq/l, and potassium ion 3.6 mEq/l. Urinalysis 1 + protein by dipstick, sediment with few white and red cells. Chronic deterioration of kidney function was confirmed by 2 increased serum creatinine values taken over a 6-month period.
His medical history is quiet, feeling well, without problems related to his agricultural work. He did not complain of headache or lower back pain, nor presented difficulties to urinate, nor took medication. Serology results revealed no hanta or leptospirosis infection. He was working until a few days before the consultation. Physical examination results revealed height at 171 cm, weight 71 kg, eutrophia, blood pressure 110/70 mm Hg, heart rate 76×/min, no fever, and normal cardiorespiratory system.
Ultrasonographic assessment of the kidneys revealed sizes of 10.5 cm (right) and 11 cm (left) with discrete irregular contour and loss of the corticomedullary demarcation. No renal cysts, ureteral dilation, nor a solid tumor was detected. Prostate had a normal size and no residue was observed. A renal biopsy specimen was analyzed by LM and EM.
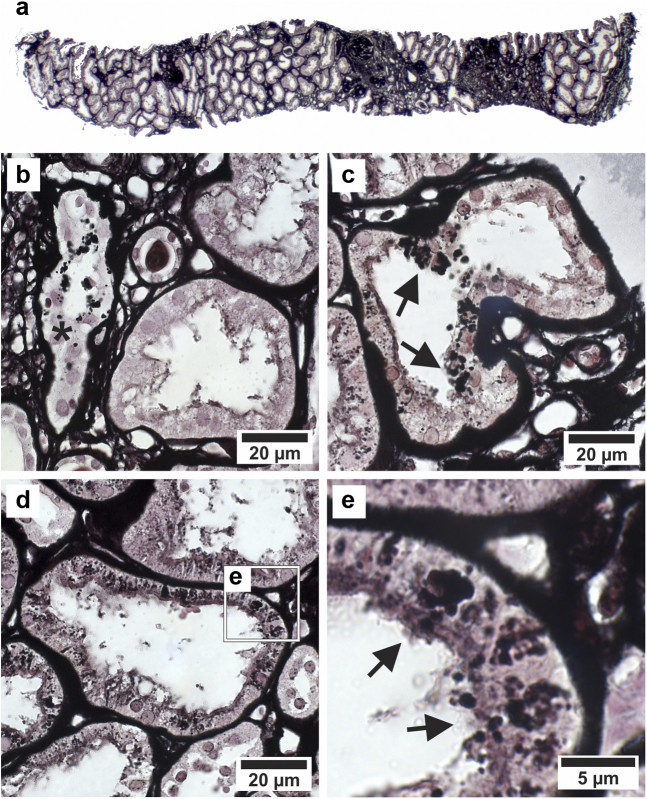
On LM (Figure 1a-e), there was an overall mild to severe degree of interstitial fibrosis with cellular infiltration. The biopsy specimen contained 6 glomeruli, 4 of them sclerotic. The proximal tubular epithelium was often atrophic with occasionally thickened basement membranes, suggesting a chronic tubular injury. Multiple PTCs showed argyrophilic granules in their cytoplasm. The distal tubular epithelium is not affected. In view of the combination of clinical and LM histologic findings, this patient was considered as suffering from CINAC, yet necessitating EM evaluation to confirm the presence (or not) of the lysosomal lesions.4
Figure 1.
Renal histology by LM of patient with CINAC from Paraguay. (a) Overview of part of the biopsy showing striped fibrosis. (b) Asterisk marks tubule with cells containing enlarged argyrophilic lysosomes and thickened basement membrane. (c) Atrophic proximal tubule with multiple epithelial cells containing enlarged argyrophilic lysosomes (arrows). (d) Atrophic proximal tubule with decreased epithelial height containing normal-sized to enlarged lysosomes. (e) Details of d with arrows pointing toward proximal epithelial cells containing enlarged, dysmorphic lysosomes. (a–e) Periodic acid–Schiff methenamine staining. The argyrophilic granules were previously demonstrated to be lysosomes by immunofluorescent staining for the lysosomal markers cathepsin B and LAMP1 in combination with Periodic acid–Schiff methenamine staining.4 CINAC, chronic interstitial nephritis in agricultural communities; LM, light microscopy.
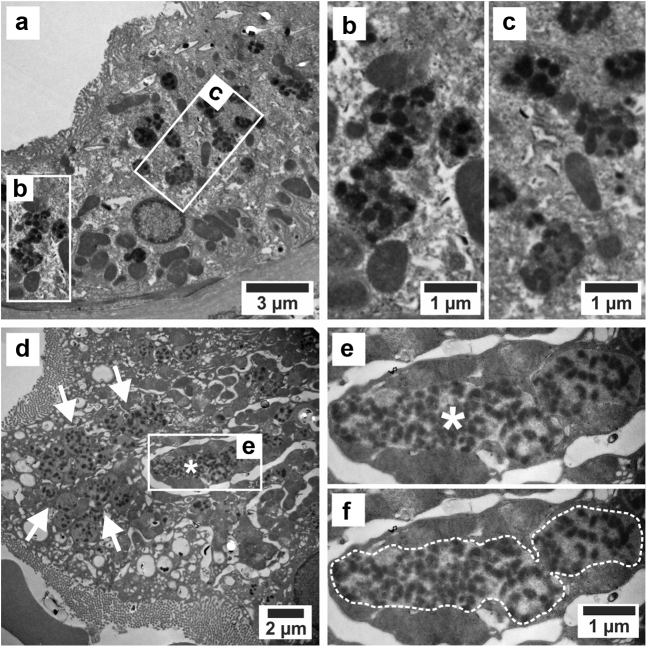
On EM (Figure 2a-f), PTCs contained enlarged dysmorphic granules with a mottled appearance at multiple sites; that is, both very large (up to several μm) as well as clusters of smaller granules with the same features, as previously reported in patients with CINAC.4
Figure 2.
EM of patient with CINAC from Paraguay. (a) Proximal epithelial cell containing enlarged dysmorphic single-membrane bound lysosomes with nonmembrane-bound, electron-dense, rounded/irregular aggregates dispersed throughout a light to medium-uniform, electron-dense matrix. (b and c) Details of a. (d) PTC containing a large dysmorphic lysosome (asterisk). Note the numerous associated smaller round, oval, and mildly dysmorphic lysosomes in the cytoplasm (arrows). (e) Higher magnification of the largest dysmorphic lysosome (asterisk) showing dispersed electron-dense aggregates in a medium to pale electron-dense matrix. (f) Same image as e with dotted delineation of the large lysosome. These granules were previously identified as lysosomes by performing TEM-EDX on PASM-stained sections.4 This technique allowed to confirm that the black silver and gold deposits of the PASM staining indeed were present in the argyrophilic granules, with the latter being positive for lysosomal markers on immunofluorescence.4 The biopsy sample was fixed in glutaraldehyde, postfixed in OsO4, and embedded in epoxy resin. Ultrathin sections were collected on carbon-coated formvar grids and stained with uranyl acetate and lead. EM, electron microscopy; OsO4, osmium tetroxide; PASM, Periodic acid–Schiff methenamine; TEM-EDX, transmission EM energy-dispersive X-ray spectroscopy.
During the follow-up of the patient since August 2021, he was advised to have a low protein diet and consume clean water. Control blood pressure was 120/80 mm Hg and serum creatinine was 2.1 mg/dl. Treatment with metformin (1000 mg/d) as renoprotector was started and justified by recent data on the pharmacodynamics and pharmacokinetics of metformin in patients with CKD with chronic administration, which established an optimal dosage of metformin in terms of safety and efficacy in this particular patient population at risk to accumulate metformin, hence lactic acidosis.8
Discussion
Ever since the discovery of CINAC in Sri Lanka and Central America, this kidney disease has been increasingly reported across the globe, mainly based on clinicoepidemiologic patient characteristics. The number of CINAC cases which are backed up by histopathology is scarce. One of the reasons thereof is that, in recent years, this diagnostic methodology is used less in view of the weak sensitivity/specificity of the classical histopathologic features (fibrosis, atrophy, mild infiltration). However, a recent sensitive histopathologic discovery in patients with CINAC (i.e., proximal tubular lysosomal lesions detectable on LM with additional features on EM) may reopen the gate for performing renal biopsies in clinical suspected cases.4 Given an updated set of diagnostic criteria (Table 1), we here report the first confirmed case of CINAC in South America (Paraguay). It involves an agricultural worker who complied to the clinicoepidemiologic CINAC criteria while also presenting the lysosomal lesions that have been described in CINAC biopsies across the globe.4 The findings in this case advocate for the diagnostic use of renal biopsies in suspected patients with CINAC.
Table 1.
Teaching points
| List of main characteristics defining CINAC | |
| Epidemiology | |
| 1 | Patient is male (less frequent female but possible). |
| 2 | Patient is mainly between 30 and 60 yrs of age, although younger and even children are not excluded. |
| 3 | The vast majority of currently described cases are living/working in an agricultural environment and <100 m altitude from sea level. |
| Clinical | |
| 4 | Patient complaints of nonspecific symptoms: fatigue, loss of appetite, headache, backache. |
| 5 | Chronically increased serum creatinine (>1.2 mg/100 ml). Confirmation by 2 consecutive measurements at least 3 months apart is required to determine chronicity. |
| 6 | No proteinuria (on dipstick maximally +); overt proteinuria is rare. |
| 7 | No clear-cut hypertension. Most cases have normal blood pressure. |
| 8 | Bilateral decrease in kidney length axis (<10 cm) on ultrasonography once patients reach CKD3. |
| Histopathology | |
| 9 | Features of chronic interstitial nephritis, proximal tubular atrophy, tubulointerstitial fibrosis, thickening and ruffling of tubular basement membranes, (mild) infiltration. |
| 10 | Light microscopy: nearly always several to multiple proximal tubular cross-sections presenting enlarged intracellular argyrophilic lysosomes, warranting electron microscopic analysis. |
| 11 | Electron microscopy: nearly always enlarged (>1.2 μm in largest diameter) dysmorphic lysosomes with electron-dense aggregates (or clusters of smaller such lysosomes) in proximal tubular epithelial cells |
CINAC, chronic interstitial nephritis in agricultural communities.
There are arguments pointing to heat stress as the major etiologic factor in development of CINAC.3 Alternatively, other researchers propose nephrotoxins as the most likely inducers of this renal disease.3,9 In this case report, no objective retrospective data could be gathered on whether and to what extent the patient might have experienced repetitive heat stress/dehydration during his career. However, given the working hours, it appears that workers spontaneously avoided the hottest times of the day. In contrast, qualitative information on his historical and unprotected exposure to several agrochemicals is documented. It is worthwhile to mention that the lysosomal lesion in PTCs of patients with CINAC is identical to the lesions that are (nearly exclusively) observed in toxin-induced nephropathies,4 thereby suggesting that the aberrant lysosomal phenotype is the reflection of exposure to currently not yet identified nephrotoxin(s). Whether the lysosomal lesion is mechanistically involved in renal dysfunction remains to be determined as well as the toxin(s) and conditions that might induce it. In addition, it cannot be excluded that heat stress or dehydration, when present among the patient’s antecedents, may have contributed to the development and progression of this nephropathy.
Overall, independent of the nomenclatures CINAC, Mesoamerican nephropathy, and CKD of unknown origin from different regions around the world, the presentation of the same histopathologic lesions, epidemiologic and climate profiles, exposure to agrochemicals, and clinical manifestations, indicate that this form of CKD is a globally spread renal disease entity. Further epidemiologic and in particular mechanistic and toxicologic studies are needed to identify what kind of toxins are the initiators of this progressive chronic interstitial nephritis already identified in many countries and most likely present in a lot of others. It is hoped that this case report may generate additional investigative interest of many nephrologists in South America confronted with analogue CKD cases.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained informed consent from the patient presented for this publication.
Acknowledgments
The following professional and laboratories contributed by providing biopsy specimens and technical support: Miguel Calvo (renal biopsy), José Bellasai (preparation of renal specimens), and Mayer Lab (specimen storage). Parts of this work was funded by the Research Foundation Flanders (PhD Fellowship Gerd Schreurs 1S52218N and Research Grant G0C0119N). Benjamin Vervaet is funded by SPRINT (European Horizon 2020 grant; project 862568).
Footnotes
S1. Patient questionnaire.
Contributor Information
Francisco Santa-Cruz, Email: fsantas@gmail.com.
Marc E. De Broe, Email: marc.debroe@uantwerpen.be.
Supplementary Material
S1. Patient questionnaire.
References
- 1.Ordunez P., Nieto F.J., Martinez R., et al. Chronic kidney disease mortality trends in selected Central America countries, 1997–2013: clues to an epidemic of chronic interstitial nephritis of agricultural communities. J Epidemiol Community Health. 2018;72:280–286. doi: 10.1136/jech-2017-210023. [DOI] [PubMed] [Google Scholar]
- 2.Jayasumana C., Orantes C., Herrera R., et al. Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational, and environmental determinants. Nephrol Dial Transplant. 2017;32:234–241. doi: 10.1093/ndt/gfw346. [DOI] [PubMed] [Google Scholar]
- 3.Johnson R.J., Wesseling C., Newman L.S. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 4.Vervaet B.A., Nast C.C., Jayasumana C., et al. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97:350–369. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Wijkström J., González-Quiroz M., Hernandez M., et al. Renal morphology, clinical findings, and progression rate in mesoamerican nephropathy. Am J Kidney Dis. 2017;69:626–636. doi: 10.1053/j.ajkd.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Herrera Valdés R., Almaguer López M., Orantes Navarro C.M., et al. Chronic interstitial nephritis of nontraditional causes in Salvadoran agricultural communities. Clin Nephrol. 2020;93:60–67. doi: 10.5414/CNP92S110. [DOI] [PubMed] [Google Scholar]
- 7.Torres C., Aragón A., González M., et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Lalau J.D., Kajbaf F., Bennis Y., Hurtel-Lemaire A.S., Belpaire F., De Broe M.E. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41:547–553. doi: 10.2337/dc17-2231. [DOI] [PubMed] [Google Scholar]
- 9.De Broe M.E., Vervaet B.A. Is an environmental nephrotoxin the primary cause of CKDu (mesoamerican nephropathy)? ProKidney360. 2020;1:591–595. doi: 10.34067/KID.0003172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.