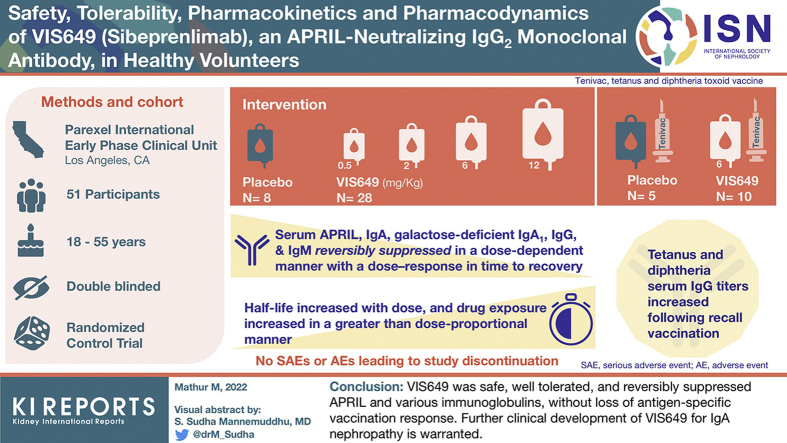
Abstract
Introduction
VIS649 (sibeprenlimab), a humanized IgG2 monoclonal antibody that inhibits APRIL, is being developed as a potential treatment for IgA nephropathy (IgAN). This phase 1, first-in-human, randomized, double-blind, single ascending dose study aimed to evaluate the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of VIS649 in healthy adults.
Methods
Participants were randomized to VIS649 (sequential i.v. dosing cohorts: 0.5, 2.0, 6.0, 12.0 mg/kg) or placebo; a further cohort received VIS649 6.0 mg/kg or placebo followed by a tetanus/diphtheria vaccine challenge.
Results
A total of 51 participants were randomized, dosed, and analyzed for safety (7 for each VIS649 dose; 8 for placebo; 10 for VIS649 + vaccine; 5 for placebo + vaccine). There were no serious adverse events (AEs) or AEs leading to study discontinuation. VIS649 had nonlinear PK: half-life increased with dose and drug exposure increased in a greater than dose-proportional manner. Serum APRIL, IgA, galactose-deficient (Gd) IgA1, IgG, and IgM were reversibly suppressed in a dose-dependent manner, with a dose–response in time to recovery. Tetanus and diphtheria serum IgG titers increased after recall vaccination.
Conclusion
VIS649 was safe, well tolerated, and reversibly suppressed APRIL and various immunoglobulins, without loss of antigen-specific vaccination response. Further clinical development of VIS649 for IgAN is warranted. Trial registration: ClinicalTrials.gov: NCT03719443.
Keywords: APRIL, clinical trial, galactose-deficient IgA, glomerulonephritis, IgA nephropathy, monoclonal antibody
Graphical abstract
The cytokine, a proliferation-inducing ligand (APRIL; also known as TNFSF13), is thought to play a key role in the pathogenesis of IgAN.1, 2, 3, 4, 5, 6 For example, increased APRIL levels are associated with the progression of IgAN,2 and genome-wide association studies have identified TNFSF13 as a risk locus for IgAN.7,8 APRIL acts through the following 2 receptors: the transmembrane activator and calcium-modulator and cytophilin ligand interactor and the B cell maturation antigen.9,10 It is hypothesized that APRIL induces B cell class switching to IgA production by the transmembrane activator and calcium-modulator and cytophilin ligand interactor signaling10 and promotes mature plasma cell survival by B cell maturation antigen signaling.11,12 APRIL may also enhance T-cell–independent immune responses,13 modulate IgA production in the mucosa,14,15 and play a direct role in Gd-IgA1 production by reprogramming the glycosylation machinery in autoantigen-reactive B cells.14 Thus, APRIL is a valid potential target for IgAN therapy.1,14
VIS649 (sibeprenlimab), a humanized IgG2 monoclonal antibody that binds to and blocks the biological actions of APRIL, is in clinical development as a potential treatment for IgAN.14 In preclinical primate studies, treatment with VIS649 resulted in a dose-dependent reduction of serum IgA levels by up to approximately 70%, and a surrogate mouse anti-APRIL monoclonal antibody reduced pathogenic immune complex formation/deposition, and thereby reduced kidney damage and loss of kidney function.14 The primary objective of the present first-in-human study was to evaluate the safety and tolerability of VIS649 in healthy volunteers. Secondary objectives included characterization of the PK and PD of VIS649. Exploratory objectives included investigating whether VIS649 suppression of APRIL influences antibody responses to tetanus and diphtheria toxoid vaccination.
Methods
Study Design and Participants
This was a phase 1, randomized, double-blind, placebo-controlled, single ascending dose study of VIS649 in healthy volunteers (ClinicalTrials.gov identifier: NCT03719443). The study was conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonisation and the Declaration of Helsinki. Participants were enrolled by investigators at Parexel International, Early Phase Clinical Unit (Los Angeles, CA), which was the location of the study. The protocol was approved by the site’s institutional review board, and all participants provided written informed consent to participate in the study before enrolling and after procedures and possible side effects were explained to them.
Participants (male or nonpregnant female) were eligible for the study if they were aged 18 to 55 years, had a body mass index of 18 to 32 kg/m2, were healthy as judged by the principal investigator based on medical evaluation and laboratory tests, and had serum IgG >750 mg/dl, serum IgM >55 mg/dl, and serum IgA >80 mg/dl. The study enrolled participants of Japanese descent and non-Japanese descent, as established by verbal confirmation (all 4 grandparents were born in Japan or all 4 grandparents were non-Japanese, respectively). The Japanese subgroup was included to satisfy regulatory requirements from the Japanese health authority. Participants were excluded if they had a history or presence of a serious medical condition, including a mental disorder; a history or presence of proteinuria, chronic kidney disease, or were considered to be immunosuppressed; had previously received an antibody or biological therapy within 30 days or 5 half-lives; had a history of severe allergic reaction or hypersensitivity reaction to tetanus/diphtheria toxoid-containing vaccine; had received a tetanus vaccine in the past 5 years; had known hypoglobulinemia disorder; had a pre-existing latent infection, infection requiring hospitalization or treatment with antivirals or antibiotics, or vaccination within 30 days; were concomitantly using systemic immunosuppressive or immunomodulatory medications; or had a positive urine drug, alcohol, or cotinine test.
The study consisted of a ≤28-day screening period, an in-house stay of 2 to 3 days (admission to the study center on day −1 [baseline], randomization and dosing on day 1, and discharge on day 2), and a 16 to 24-week follow-up period with frequent outpatient visits. The study was conducted in sequential dose-escalating cohorts. The first 4 cohorts (0.5, 2.0, 6.0, and 12.0 mg/kg, respectively) each enrolled 9 participants who were randomized to VIS649 or placebo in a 7:2 ratio. Escalation to the next dosage level occurred after review of blinded safety data by a Safety Monitoring Committee composed of the principal investigator, an independent medical monitor, and the sponsor. In addition, a fifth cohort was finalized after the study had started (protocol amendment on March 12, 2019). Cohort 5 enrolled 15 adults randomized in a 2:1 ratio to receive VIS649 6.0 mg/kg or placebo on day 1, followed by a tetanus and diphtheria toxoid vaccine (TENIVAC, Sanofi Pasteur Limited) on day 28 (during the anticipated APRIL neutralization window) to evaluate the effect of APRIL suppression by VIS649 on recipients’ ability to generate a response to routine vaccine boost.
VIS649 (manufactured by the sponsor) doses were calculated on a per-participant weight basis and diluted in 100 ml of 0.9% sodium chloride; placebo (manufactured by Baxter Corporation, Deerfield, IL) consisted of 0.9% sodium chloride. VIS649 and placebo were administered i.v. using a volumetric pump through a 0.22-μm inline i.v. filter over the course of 1 hour, in the morning after a light meal. In cohorts 1 to 4, 2 participants (1 receiving VIS649 and 1 receiving placebo) were dosed at least 24 hours before the others, in case of unanticipated adverse reactions. Treatments were assigned based on a randomization code provided by Parexel International. Cohorts 1 to 4 were stratified to include 4 participants of Japanese descent and 5 of non-Japanese descent; no more than 1 Japanese participant per cohort was randomized to receive placebo. Treatment assignments were blinded to participants, investigators, and sponsor personnel, with the following 2 exceptions: (i) pharmacy staff who prepared the study drug and were not involved in any other study activities; and (ii) PK/PD analysts, who had access to unblinded data but did not interact with participants, and shared treatment-specific PD responses with the sponsor in a manner that did not unblind individual participant treatment assignments.
Concomitant medication use was not permitted in the period from 30 days (or 5 half-lives, if longer) preceding baseline until the end of the study, except for acetaminophen, ibuprofen, hormonal contraceptives, topical medications, vitamins, and dietary or herbal remedies. Medication for the treatment of AEs was permitted if agreed by the principal investigator and medical monitor.
The study could be prematurely terminated if the principal investigator, sponsor, or medical monitor became aware of a possible hazard to participants, or at any time at the sponsor’s discretion.
Assessments
The primary objective of the study was to evaluate the safety and tolerability of VIS649. Standard safety assessments including AEs, clinical laboratory tests, vital signs, electrocardiograms, and physical examinations were performed at regular intervals.
Secondary and exploratory objectives included to characterize the PK profile of VIS649, the effect of VIS649 on various PD parameters, and the immune response to tetanus/diphtheria toxoid vaccination after VIS649 administration. Blood samples for PK were collected on days 1 (predose and postdose), 2, and 3 and at week 1, 2, 4, 6, 8, 10, and 16 visits. Serum samples were analyzed for VIS649 concentrations by Syneos Health (Princeton, NJ) using a validated electrochemiluminescence immunoassay: VIS649 was captured on a Meso Scale Discovery (MSD) plate coated with mouse anti-VIS649 antibody; ruthenylated anti-VIS649 and MSD read buffer were added; then plates were read using an MSD electrochemiluminescence plate reader.
Blood samples to measure immunoglobulin levels were collected at baseline; day 3; at week 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, and 16 visits; and on additional visits at weeks 20 and 24 for participants in cohorts 3 and 4. Serum samples were analyzed for total IgA, IgG, and IgM by GenX Laboratory (Los Angeles, CA) using an AU480 Chemistry Analyzer (Beckman Coulter, Brea, CA). Serum Gd-IgA1 was analyzed using a validated solid-phase sandwich enzyme-linked immunosorbent assay with the #27600 Gd-IgA1 Assay Kit (KM55 anti-Gd-IgA1) (Immuno-Biological Laboratories Co., Ltd., Fujioka-Shi, Gunma, Japan) by Syneos Health. Serum APRIL was analyzed using a validated APRIL electrochemiluminescence immunoassay by Syneos Health: APRIL was captured on an MSD plate coated with anti-recombinant human APRIL; biotinylated anti-recombinant human APRIL, SULFO-TAG streptavidin (detection reagent) and MSD read buffer were added; then plates were read using an MSD electrochemiluminescence plate reader. Serum B cell activating factor (BAFF) was analyzed post hoc using human premixed Luminex assay kits (R&D Systems, Minneapolis, MN) and a MAGPIX (Luminex, Austin, TX), as per the manufacturer’s instructions.
Tetanus/diphtheria serology results were examined in cohort 5, and antitetanus toxoid and antidiphtheria quantitative enzyme-linked immunosorbent assays were performed by Q2 Solutions LLC (Valencia, CA) (IgG) and the University of Maryland Center for Vaccine Development and Global Health (Baltimore, MD) (IgM and IgA) at week 4 (prevaccination); day 31; and week 5, 6, 8, 12, and 16 visits. In enzyme-linked immunosorbent assays, tetanus and diphtheria antitoxoid IgG titers ≥0.1 IU/ml are considered to be protective.16, 17, 18
Statistical Analyses
No formal sample size calculation was performed; the target of 51 enrolled participants was chosen in consideration of limiting exposure to this new chemical entity while providing sufficient information to evaluate the safety and tolerability of VIS649 in a phase 1 setting.
Safety outcomes were summarized using descriptive statistics in the safety sample, defined as all randomized participants who received at least 1 dose of the study drug. PK outcomes were summarized using descriptive statistics in the PK sample, defined as all randomized participants with at least 1 quantifiable VIS649 concentration. Values below the lower limit of quantification (LLQ; 0.1 μg/ml for VIS649) were imputed as zero, with the exception of the VIS649 concentration–time figure, which used a log axis (imputed as the LLQ). PD outcomes were summarized using descriptive statistics in the PD sample, defined as the safety sample subset with at least 1 PD parameter assessment (IgA, IgG, IgM) post–study drug dosing. Values below the LLQ (0.5 μg/ml for Gd-IgA1, 50 pg/ml for APRIL, 0.1 IU/ml for diphtheria IgG) were imputed as the LLQ, and values above the upper limit of quantification (16.0 IU/ml for tetanus IgG, 2.00 IU/ml for diphtheria IgG) were imputed as the upper limit of quantification.
Statistical analyses were performed using SAS® Version 9.3 (SAS Institute Inc., Cary, NC) and Phoenix® WinNonlin® Version 8.0 or higher (Certara USA, Inc., Princeton, NJ).
Results
Participants
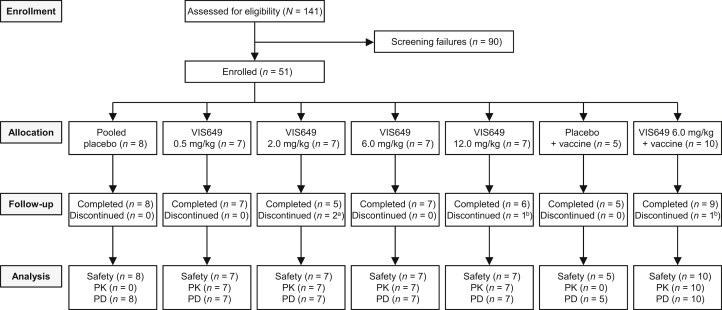
The study ran from October 9, 2018, to August 10, 2019. Overall, 51 participants were enrolled, randomized, and dosed with the study drug, of whom 47 (92.2%) completed the study (Figure 1). In cohort 5, 14 of 15 participants received the vaccine; 1 participant in the VIS649 + vaccine group was lost to follow-up and did not receive the vaccine.
Figure 1.
Participant disposition. a1 participant lost to follow-up, 1 participant withdrew. b1 participant lost to follow-up. PD, pharmacodynamics; PK, pharmacokinetics.
Baseline demographic characteristics were generally similar across the treatment groups (Table 1). Cohort 5 (participants who also received the vaccine) had a different ethnic mixture as there was no requirement for Japanese/non-Japanese descent in this cohort.
Table 1.
Baseline demographics (safety sample)
| Parameter | Pooled placebo (n = 8) | VIS649 0.5 mg/kg (n = 7) | VIS649 2.0 mg/kg (n = 7) | VIS649 6.0 mg/kg (n = 7) | VIS649 12.0 mg/kg (n = 7) | Placebo + vaccine (n = 5) | VIS649 6.0 mg/kg + vaccine (n = 10) |
|---|---|---|---|---|---|---|---|
| Age (yr), mean (SD) | 32.6 (9.2) | 29.4 (6.7) | 40.4 (11.7) | 41.0 (9.6) | 40.9 (11.9) | 33.4 (8.4) | 30.9 (7.3) |
| BMI (kg/m2), mean (SD) | 25.1 (4.3) | 25.4 (2.8) | 24.0 (3.6) | 24.9 (4.2) | 24.0 (2.9) | 25.5 (2.9) | 24.3 (2.0) |
| Female | 3 (37.5) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 4 (57.1) | 3 (60.0) | 4 (40.0) |
| Black or African American | 3 (37.5) | 2 (28.6) | 3 (42.9) | 1 (14.3) | 3 (42.9) | 3 (60.0) | 7 (70.0) |
| Asiana | 4 (50.0) | 3 (42.9) | 3 (42.9) | 3 (42.9) | 3 (42.9) | 0 | 0 |
| White | 1 (12.5) | 2 (28.6) | 1 (14.3) | 3 (42.9) | 1 (14.3) | 2 (40.0) | 3 (30.0) |
BMI, body mass index.
Data are presented as n (%), unless otherwise specified.
All Asian participants were of Japanese ethnicity.
Safety and Tolerability
VIS649 was well tolerated, with no serious AEs or AEs that led to study discontinuation. Treatment-emergent AEs (TEAEs) were experienced by 4 of 8 (50.0%) participants who received placebo, 11 of 28 (39.3%) who received VIS649 (all doses), 3 of 5 (60.0%) who received placebo + vaccine, and 4 of 10 (40.0%) who received VIS649 + vaccine (Table 2). Across all cohorts, there was a numerically greater incidence of upper respiratory tract infection among participants receiving placebo (2 of 13; 15.4%) compared with those receiving VIS649 (all doses; 2 of 38; 5.3%). The incidence of TEAEs was not dose dependent.
Table 2.
Treatment-emergent adverse events (safety sample)
| Event | Pooled placebo (n = 8) | VIS649 0.5 mg/kg (n = 7) | VIS649 2.0 mg/kg (n = 7) | VIS649 6.0 mg/kg (n = 7) | VIS649 12.0 mg/kg (n = 7) | Placebo + vaccine (n = 5) | VIS649 6.0 mg/kg + vaccine (n = 10) |
|---|---|---|---|---|---|---|---|
| At least 1 TEAE | 4 (50.0) | 2 (28.6) | 3 (42.9) | 3 (42.9) | 3 (42.9) | 3 (60.0) | 4 (40.0) |
| TEAEs occurring in >1 participants who received VIS649 (across VIS649 groups) | |||||||
| URTI | 1 (12.5) | 0 | 1 (14.3) | 1 (14.3) | 0 | 1 (20.0) | 0 |
| Diarrhea | 1 (12.5) | 0 | 1 (14.3) | 0 | 1 (14.3) | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 1 (14.3) | 1 (14.3) | 0 | 0 |
TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection.
Data are presented as n (%).
Most TEAEs were mild. There were 2 moderate TEAEs that were reported: back pain by 1 participant who received 6.0 mg/kg (considered not related to the study drug) and vomiting and migraine by 1 participant who received 6.0 mg/kg (considered unlikely to be related to the study drug). A participant in the 2.0 mg/kg group experienced a severe TEAE of vasovagal syncope after phlebotomy on day 29 after dosing (considered unlikely to be related to the study drug). The severity of TEAEs was not dose dependent, and all TEAEs had resolved or were resolving by the end of the study.
No meaningful differences were noted between the Japanese and non-Japanese participants with regard to TEAEs.
There was no clinically relevant effect of treatment on laboratory tests, vital signs, electrocardiogram parameters, or physical examinations.
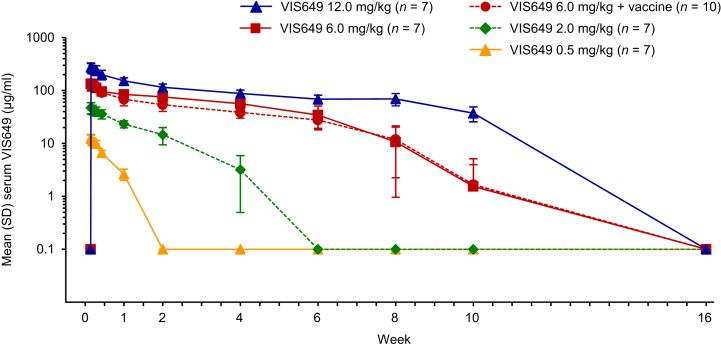
Pharmacokinetics
Concentration–time profiles indicated that VIS649 PK were nonlinear after a single dose (Figure 2). VIS649 exposure increased in a greater than dose-proportional manner. At the higher tested doses (6.0 and 12.0 mg/kg), serum VIS649 concentrations were biexponential, with a rapid distribution phase followed by an (initially) slower elimination phase. Across all doses, the rate of VIS649 elimination increased at concentrations below approximately 50 μg/ml.
Figure 2.
Mean serum VIS649 concentration over time after a single i.v. dose (pharmacokinetics sample). Values below LLQ (0.1 μg/ml) were imputed as the LLQ. LLQ, lower limit of quantification.
Maximum serum concentration (Cmax) increased in an approximately dose-proportional manner (Table 3). Other PK parameters indicated nonlinear PK: half-life (t½) increased with dose, drug exposure (area under the curve) increased in a greater than dose-proportional manner, and total clearance decreased with dose. Apparent volume of distribution was not dose dependent.
Table 3.
Mean pharmacokinetic parameters of VIS649 after a single i.v. dose (pharmacokinetics sample)
| Parameter | VIS649 0.5 mg/kg (n = 7) | VIS649 2.0 mg/kg (n = 7) | VIS649 6.0 mg/kg (n = 7) | VIS649 6.0 mg/kg + vaccine (n = 10) | VIS649 12.0 mg/kg (n = 7) |
|---|---|---|---|---|---|
| Cmax (μg/ml) | 12.6 | 48.6 | 136 | 131 | 283 |
| AUC0–W16 (h.μg/ml) | 1120 | 11,700 | 74,500 | 57,900 | 176,000 |
| AUC0–∞ (h.μg/ml) | 1120 | 11,700 | 74,400 | 57,900 | 187,000 |
| t½ (h) | 60.7 | 175 | 231 | 342 | 670 |
| CL (ml/h) | 31.5 | 11.7 | 6.13 | 8.72 | 4.51 |
| Vd (ml) | 2750 | 2850 | 2180 | 3910 | 4120 |
AUC0–W16/0–∞, area under the concentration–time curve from predose (time 0) to week 16/infinity; CL, apparent clearance; Cmax, maximum serum concentration; t½, terminal elimination half-life; Vd, apparent volume of distribution.
Administration of the vaccine had no noteworthy effects on VIS649 PK (Table 3), and there were no significant PK differences between Japanese and non-Japanese participants (data not shown).
Pharmacodynamics
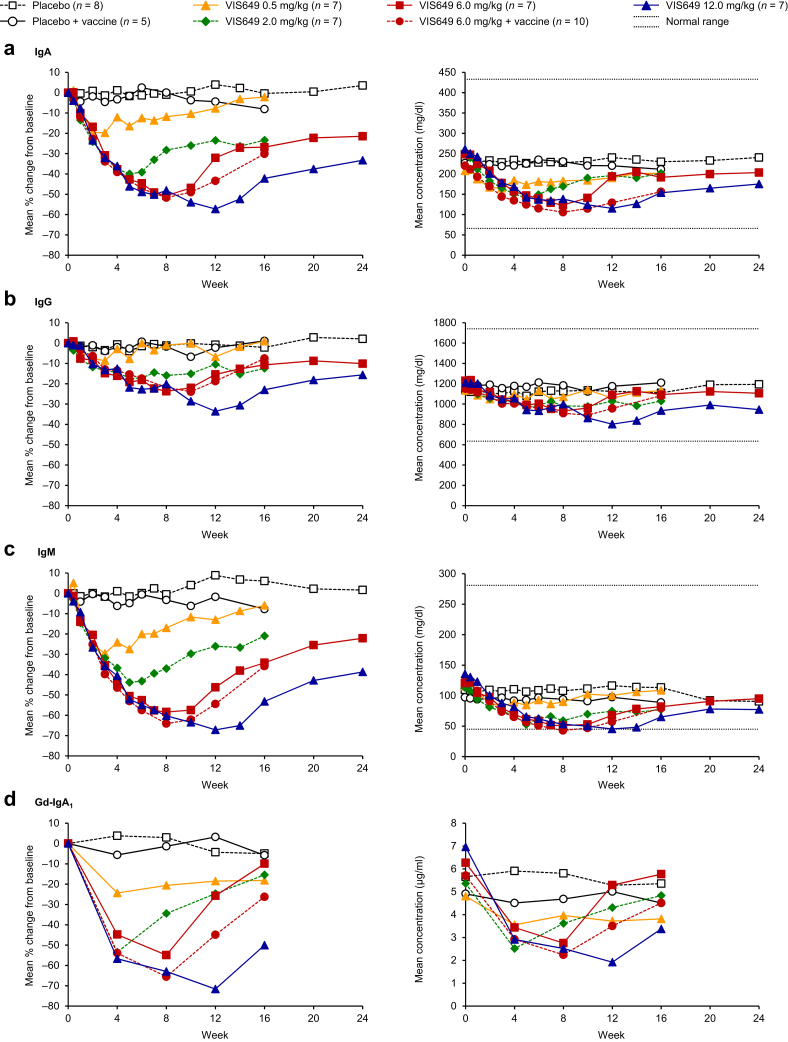
Serum immunoglobulins (IgA, IgG, IgM, and Gd-IgA1) were suppressed in a dose-dependent manner after VIS649 administration (Figure 3a-d and Supplementary Tables S1–S4). Maximum mean percentage reductions from baseline were observed at the week 12 time point with the 12.0 mg/kg dose: IgA, −57.2%; IgG, −33.6%; IgM, −67.2%; and Gd-IgA1, −71.6%. These reductions seemed to be reversible, with a dose response in time to recovery.
Figure 3.
Mean percentage change from baseline and absolute serum concentration for (a) IgA, (b) IgG, (c) IgM, and (d) Gd-IgA1, by treatment (pharmacodynamics sample). Normal ranges: IgA, 66–433 mg/dl; IgG, 635–1741 mg/dl; IgM, 45–281 mg/dl. Lower limit of quantification for Gd-IgA1, 0.5 μg/ml. Gd, galactose-deficient.
No mean or median values fell below the normal ranges for IgA or IgG; for IgM only, several mean and/or median values fell slightly below the normal range (i.e., <45 mg/dl) between weeks 5 and 14 in the VIS649 groups, but all values recovered and no AEs related to low IgM levels were recorded.
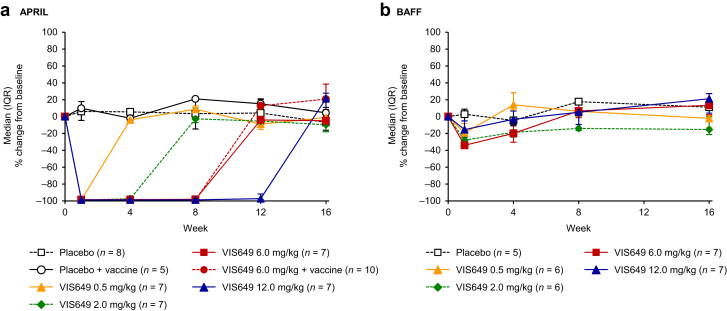
Mean serum-free (non-VIS649 bound) APRIL levels decreased from baseline to at or below the LLQ (50 pg/ml) for all VIS649 doses at week 1 and had a dose response in time to recovery. Recovery to predose levels was observed by week 4 for the 0.5 mg/kg group, week 8 for the 2.0 mg/kg group, week 12 for the 6.0 mg/kg group, and week 16 for the 12.0 mg/kg group (Figure 4a and Supplementary Table S5).
Figure 4.
Median (IQR) percentage change from baseline in serum (a) APRIL concentration and (b) BAFF concentration, by treatment (pharmacodynamics sample). BAFF, B cell activating factor; IQR, interquartile range.
Serum BAFF results revealed minimal change from baseline, with median changes <35% at all time points for all doses studied (Figure 4b).
No notable depletions in circulating lymphocyte populations (B cell, T cell, or natural killer cell types) were observed after VIS649 dosing at any of the dose levels or with placebo (Table 4).
Table 4.
Summary of lymphocyte subsets after a single i.v. dose of VIS649 (pharmacodynamics sample)
| Parameter | Visit | Pooled placebo (n = 8) | VIS649 0.5 mg/kg (n = 7) | VIS649 2.0 mg/kg (n = 7) | VIS649 6.0 mg/kg (n = 7) | VIS649 12.0 mg/kg (n = 7) |
|---|---|---|---|---|---|---|
| CD19 (B cells) (cells/μl) | Baseline | 205.4 (128.3) | 194.6 (23.7) | 225.7 (89.7) | 262.6 (80.0) | 175.9 (49.4) |
| Change at week 16 | −21.1 (97.3)a | 25.1 (56.1) | −5.4 (26.5)b | 84.6 (81.8) | 23.7 (24.6) | |
| CD20+ (B cells) (cells/μl) | Baseline | 209.4 (154.1) | 212.1 (47.6)b | 221.4 (95.7) | 273.2 (66.2)c | 173.0 (66.4) |
| Change at week 16 | −19.8 (134.8)a | 12.2 (73.0)b | −5.6 (50.4)b | 133.1 (139.8)c | 16.1 (16.3) | |
| CD4 (T cells) (cells/μl) | Baseline | 659.8 (178.0) | 633.6 (156.9) | 803.7 (145.8) | 579.3 (288.7) | 716.1 (166.1) |
| Change at week 16 | −14.9 (175.5)a | 14.0 (161.1) | −75.4 (80.7)b | 121.6 (149.2) | 135.6 (204.7) | |
| CD8 (T cells) (cells/μl) | Baseline | 432.3 (145.1) | 463.7 (165.3) | 395.0 (103.2) | 332.4 (108.1) | 362.6 (199.7) |
| Change at week 16 | −14.0 (113.7)a | −14.9 (135.7) | −49.0 (68.1)b | 86.0 (94.3) | 94.3 (96.8) | |
| CD16 + CD56 (NK cells) (cells/μl) | Baseline | 175.3 (156.1) | 147.3 (38.3) | 181.9 (88.4) | 113.4 (40.8) | 134.0 (48.3) |
| Change at week 16 | 0.4 (46.2)a | 13.9 (49.9) | 20.2 (91.4)b | 34.9 (51.4) | 15.7 (41.1) |
CD, cluster of differentiation; NK, natural killer.
Data are presented as mean (SD).
n = 7.
n = 5.
n = 6.
There were no notable PD differences between Japanese and non-Japanese participants (data not shown).
Vaccine Response
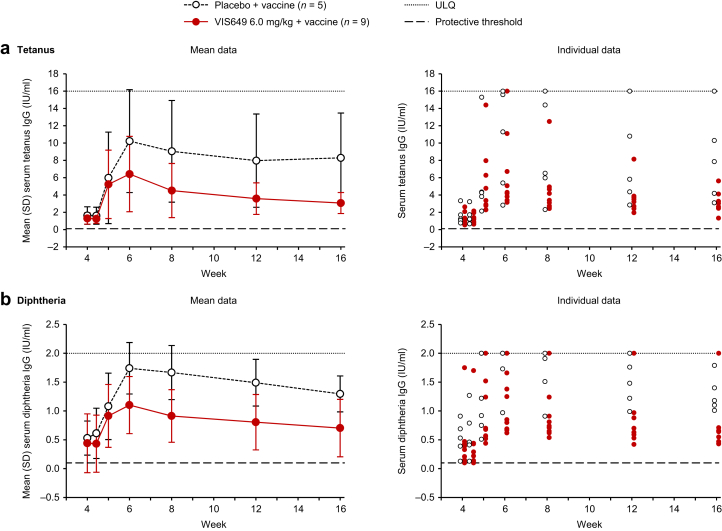
Both groups (placebo and VIS649 6.0 mg/kg) had increased tetanus toxoid IgG titers after immunization, with a maximum mean increase at week 6 (Figure 5a). At week 6 relative to prevaccination (week 4) values, a mean 7.9-fold increase in antibody titers was observed in the placebo group and a mean 6.4-fold increase was observed in the VIS649 group. Tetanus toxoid IgG titers declined from week 6 onward. Antibody levels in the VIS649 group were lower compared with the placebo group (consistent with the reduction in total IgG associated with VIS649 administration), but remained above the protective threshold of 0.1 IU/ml for all participants throughout the study (Figure 5a).
Figure 5.
(a) Tetanus and (b) diphtheria IgG titer levels (vaccinated safety sample). Vaccine administered at the week 4 visit (week 4 titers were prevaccination). Lower limit of quantification for diphtheria IgG, 0.1 IU/ml. ULQ for tetanus IgG, 16.0 IU/ml; for diphtheria IgG, 2.00 IU/ml. ULQ, upper limit of quantification.
Diphtheria IgG titers were higher in the placebo group than in the VIS649 group before immunization (week 4), which translated into higher titers in the placebo group after immunization (Figure 5b). Nevertheless, titer fold-change responses were similar between groups after immunization, with a mean 5.5-fold increase in concentration for placebo recipients and a mean 5.1-fold increase for VIS649 recipients at the week 6 visit. Diphtheria IgG titers declined from week 6 onward, with lower values in the VIS649 group compared with the placebo group (consistent with the reduction in total IgG associated with VIS649 administration). All participants maintained titers >0.1 IU/ml throughout the study, except for 1 participant in the VIS649 group who had titers of ≤0.10 IU/ml (the LLQ) at week 4 (prevaccination) and day 31 only (Figure 5b).
There was no evidence of tetanus- or diphtheria-specific IgM responses in either the placebo or VIS649 groups, consistent with the recall nature of the vaccination (Supplementary Figure S1a and b).
In a post hoc analysis, pre-existing serum tetanus/diphtheria antitoxoid IgA titers fell between day 1 and week 4 in the VIS649 group (consistent with the overall suppression of total serum IgA), were boosted after vaccination in both groups (with peak recall response >6-fold for tetanus and >4-fold for diphtheria relative to prevaccination values [i.e., week 4]), and declined faster in VIS649 recipients thereafter (Supplementary Figure S2a and b).
Discussion
Patients with IgAN have increased levels of APRIL in plasma/serum and tonsils (thought to be a source of pathogenic IgA), which correlates with the severity of clinical presentation, including proteinuria, glomerular filtration rate, and risk of kidney failure.2, 3, 4, 5 Blocking the binding of APRIL to its receptors and thereby preventing downstream events (the production of Gd-IgA1 and the persistence of IgA-producing plasma cells) represents an attractive treatment target for IgAN, with the potential to prevent kidney damage and loss of function.14
In this study, a single dose of VIS649 (up to 12.0 mg/kg) suppressed serum-free APRIL to at or below the LLQ at the first postdose time point (week 1). Serum IgA and Gd-IgA1 decreased in parallel with serum APRIL and recovered in a dose-dependent manner after reappearance of free APRIL in serum in the following weeks. Serum IgM followed a similar pattern, reflecting a possible role for APRIL in IgM+ B cell proliferation and IgM secretion.13,19
Whereas serum-free APRIL was suppressed, serum BAFF—which shares receptors with APRIL9—was minimally affected. This indicates that VIS649 selectively inhibits APRIL, with intact BAFF signaling, thereby potentially avoiding the lymphocyte depletion and significant immunoglobulin suppression that is associated with dual APRIL/BAFF inhibition (e.g., with atacicept).20, 21, 22, 23 Treatment with the anti-CD20 monoclonal antibody, rituximab, is associated with an increase in BAFF levels,24, 25, 26, 27 which may partially explain the lack of efficacy of rituximab in patients with IgAN.28
VIS649 treatment did not interfere with participants’ ability to mount a substantial antigen-specific serum IgG or IgA booster response to tetanus and diphtheria toxoid vaccination (with fold increases in IgG titers similar to those of placebo recipients), despite an overall reduction in the respective immunoglobulins. There was no evidence of tetanus- or diphtheria-specific IgM responses in either the placebo or VIS649 groups 4 weeks postvaccination, consistent with recall response. These data indicate that qualitative antibody responses to routine vaccine antigens are preserved during APRIL suppression. Overall, VIS649 seems to have the ability to suppress pathogenic Gd-IgA1 (and other immunoglobulins) while allowing for a booster immune response to tetanus and diphtheria vaccines.
With regard to PK, VIS649 elimination became more rapid at concentrations below approximately 50 μg/ml. VIS649 t½ increased with dose, area under the curve increased in a greater than dose-proportional manner, and clearance decreased with dose, indicating nonlinear PK in this single-dose range. This concentration-dependent nonlinear PK may reflect target-mediated drug disposition, in which VIS649’s picomolar affinity and high specificity binding to APRIL affects the disposition of drug in the body.14,29,30 Regardless of this nonlinearity, VIS649 suppressed APRIL and serum immunoglobulins at all studied doses.
There were no safety concerns with a single dose of VIS649 in healthy participants. Notably, there was no increased risk of respiratory tract infections or other infections versus placebo, and no indication of infusion reactions, delayed allergic reactions, or i.v. infusion site reactions.
Limitations of this study include the small number of participants, meaning that outcome differences according to race cannot be determined with confidence. Because this was a single-dose study, the safety and tolerability of VIS649 will need to be investigated and confirmed in a larger study with repeated doses. Safety and efficacy are being investigated in an ongoing multiple-dose, phase 2 proof-of-concept trial (NCT04287985).
Conclusion
Through its anti-APRIL effects, VIS649 specifically targets a key underlying disease mechanism in IgAN (i.e., production of Gd-IgA1), and, together with its therapeutically relevant PK/PD parameters and favorable safety profile, these data support the further clinical development of VIS649 as a potential targeted treatment for IgAN. The results of this study in healthy volunteers will be used to inform the design and dose selection of subsequent studies in patients with IgAN.
Disclosure
MM, JY, SS, and DO are full-time employees of Visterra, Inc. JB has received research grants from Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Roche, Travere Therapeutics, and Visterra, Inc.; has acted as a consultant for Alnylam, Astellas, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Roche, Travere Therapeutics, UCB, and Visterra, Inc.; has provided expert scientific advice to Alnylam, Astellas, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Roche, Travere Therapeutics, UCB, and Visterra, Inc.; is a member of Kidney Health Initiative; has lectured, chaired, or participated in symposia/panel discussions for Calliditas, Omeros, and Travere Therapeutics; and has evaluated research grant proposals for Kidney Research UK and MRC. YS has acted as a consultant for Chinook Therapeutic, Chugai Pharmaceutical, Daiichi Sankyo, Kyowa Kirin, Mitsubishi Tanabe Pharma, Morpho Sys, Novartis Pharma, Retrophin, and Visterra, Inc.; has received grants or has grants pending from Chinook Therapeutic, Daiichi Sankyo, Japan Agency for Medical Research and Development, Japan Society for the Promotion of Science, Kyowa Kirin, Ministry of Health, Labour and Welfare in Japan, Moderna, Retrophin, and Visterra, Inc.; has participated in speakers bureaus for Asahi Kasei Pharma, Astellas Pharma Inc., Bayer Pharma AG, Chugai Pharmaceutical, Daiichi Sankyo, Kissei Pharmaceutical, Kyowa Kirin, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme K.K., Novartis AG, Novartis Pharma, Ono Pharmaceutical, and Sumitomo Dainippon Pharma. FE is a full-time employee of Certara USA, Inc., and received funding from Visterra to perform analyses presented in this study. MFP is a full-time employee of the University of Maryland and received funding from Visterra to conduct the tetanus and diphtheria IgM and IgA serologic assays presented in this study.
Acknowledgments
This work was supported by Visterra, Inc. (Waltham, MA), a member of the Otsuka family of companies. Authors affiliated with the sponsors were involved in the design of the study, the analysis and interpretation of data, the writing and reviewing of this article, and the decision to submit the article for publication. Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge—a Prime Global Agency (Cambridge, UK), and funded by Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ). This work was previously presented at the European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Congress; June 5–8, 2021; Berlin, Germany and virtual.
Data Sharing Statement
To submit inquiries related to Otsuka or Visterra clinical research, or to request access to individual participant data (IPD) associated with any Otsuka or Visterra clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
Author Contributions
MM, FE, and MFP contributed to the acquisition and interpretation of data. JB and YS contributed to the interpretation of data. JY, SS, and DO contributed to the design of the study and the acquisition and interpretation of data. All authors participated in the drafting or the critical review of the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Footnotes
Table S1. Mean (SD) serum IgA concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S2. Mean (SD) serum IgG concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S3. Mean (SD) serum IgM concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S4. Mean (SD) serum Gd-IgA1 concentration (μg/ml), by treatment (pharmacodynamics sample).
Table S5. Mean (SD) serum APRIL concentration (pg/ml), by treatment (pharmacodynamics sample).
Figure S1. (A) Tetanus and (B) diphtheria IgM titer levels (vaccinated safety sample).
Figure S2. (A) Tetanus and (B) diphtheria IgA titer levels (vaccinated safety sample).
Supplementary Material
Table S1. Mean (SD) serum IgA concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S2. Mean (SD) serum IgG concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S3. Mean (SD) serum IgM concentration (mg/dl), by treatment (pharmacodynamics sample).
Table S4. Mean (SD) serum Gd-IgA1 concentration (μg/ml), by treatment (pharmacodynamics sample).
Table S5. Mean (SD) serum APRIL concentration (pg/ml), by treatment (pharmacodynamics sample).
Figure S1. (a) Tetanus and (b) diphtheria IgM titer levels (vaccinated safety sample).
Figure S2. (a) Tetanus and (b) diphtheria IgA titer levels (vaccinated safety sample).
References
- 1.Kim Y.G., Alvarez M., Suzuki H., et al. Pathogenic role of a proliferation-inducing ligand (APRIL) in murine IgA nephropathy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han S.S., Yang S.H., Choi M., et al. The role of TNF superfamily member 13 in the progression of IgA nephropathy. J Am Soc Nephrol. 2016;27:3430–3439. doi: 10.1681/ASN.2015060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y.L., Zhu L., Shi S.F., Liu L.J., Lv J.C., Zhang H. Increased APRIL expression induces IgA1 aberrant glycosylation in IgA nephropathy. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto M., Manfroi B., Suzuki H., et al. Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J Am Soc Nephrol. 2017;28:1227–1238. doi: 10.1681/ASN.2016050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahara M., Nagato T., Nozaki Y., et al. A proliferation-inducing ligand (APRIL) induced hyper-production of IgA from tonsillar mononuclear cells in patients with IgA nephropathy. Cell Immunol. 2019;341:103925. doi: 10.1016/j.cellimm.2019.103925. [DOI] [PubMed] [Google Scholar]
- 6.Makita Y., Suzuki H., Kano T., et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 2020;97:340–349. doi: 10.1016/j.kint.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiryluk K., Li Y., Scolari F., et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Z., Feng S.Z., Xu R.C., et al. Association of TNFSF13 polymorphisms with IgA nephropathy in a Chinese Han population. J Gene Med. 2017;19 doi: 10.1002/jgm.2966. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F., Schneider P., Rennert P., Browning J. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 10.Castigli E., Scott S., Dedeoglu F., et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor B.P., Raman V.S., Erickson L.D., et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–97. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery D.T., Kalled S.L., Ellyard J.I., et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells [published correction appears in J Clin Invest. 2004;113:1069] J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein J.V., López-Fraga M., Elustondo F.A., et al. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myette J.R., Kano T., Suzuki H., et al. A proliferation inducing ligand (APRIL) targeted antibody is a safe and effective treatment of murine IgA nephropathy. Kidney Int. 2019;96:104–116. doi: 10.1016/j.kint.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Chorny A., Puga I., Cerutti A. Innate signaling networks in mucosal IgA class switching. Adv Immunol. 2010;107:31–69. doi: 10.1016/B978-0-12-381300-8.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J.L., Tiwari T., Moro P., et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2018;67:1–44. doi: 10.15585/mmwr.rr6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) The immunological basis for immunization series. module 3: tetanus. World Health Organization. https://apps.who.int/iris/handle/10665/275340 Published 2018. Accessed January 29, 2021.
- 18.Begg N. Manual for the Management and Control of Diphtheria in the European Region. WHO Regional Office for Europe. https://apps.who.int/iris/handle/10665/108107 Published 1994. Accessed January 29, 2021.
- 19.Soleto I., Morel E., Martín D., Granja A.G., Tafalla C. Regulation of IgM+ B cell activities by rainbow trout APRIL reveals specific effects of this cytokine in lower vertebrates. Front Immunol. 2018;9:1880. doi: 10.3389/fimmu.2018.01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dall’Era M., Chakravarty E., Wallace D., et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 21.Ginzler E.M., Wax S., Rajeswaran A., et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14:R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenberg D., Gordon C., Licu D., Copt S., Rossi C.P., Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial) [published correction appears in Ann Rheum Dis. 2016;75:946] Ann Rheum Dis. 2015;74:2006–2015. doi: 10.1136/annrheumdis-2013-205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaegi C., Steiner U.C., Wuest B., Crowley C., Boyman O. Systematic review of safety and efficacy of atacicept in treating immune-mediated disorders. Front Immunol. 2020;11:433. doi: 10.3389/fimmu.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie F., Miceli-Richard C., Ittah M., Sellam J., Gottenberg J.E., Mariette X. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann Rheum Dis. 2007;66:700–703. doi: 10.1136/ard.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard R.P.E., Abdulahad W.H., Vissink A., et al. Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjögren’s syndrome: data from a placebo-controlled clinical trial. Ann Rheum Dis. 2013;72:146–148. doi: 10.1136/annrheumdis-2012-202071. [DOI] [PubMed] [Google Scholar]
- 26.Perumal J.S., Kister I., Howard J., Herbert J. Disease exacerbation after rituximab induction in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;2:e61. doi: 10.1212/NXI.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hébert V., Maho-Vaillant M., Golinski M.L., et al. Modifications of the BAFF/BAFF-receptor axis in patients with pemphigus treated with rituximab versus standard corticosteroid regimen. Front Immunol. 2021;12:666022. doi: 10.3389/fimmu.2021.666022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafayette R.A., Canetta P.A., Rovin B.H., et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol. 2017;28:1306–1313. doi: 10.1681/ASN.2016060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An G. Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J Clin Pharmacol. 2020;60:149–163. doi: 10.1002/jcph.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56:248–252. doi: 10.1038/clpt.1994.134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.