Abstract
Background
Same-visit bidirectional endoscopy (esophagogastroduodenoscopy and colonoscopy) is widely performed under sedation. At present, the optimal sedation regimen remains unclear. This study aims to test the hypothesis that a low-dose esketamine added to propofol sedation reduces hemodynamic and respiratory adverse events in these procedures.
Methods
In this multicenter, randomized, double-blind, placebo-controlled trial, 660 adult patients scheduled for same-visit bidirectional endoscopy under sedation from 3 teaching hospitals in China will be recruited. Patients will be randomly allocated, in a 1:1 ratio, to an esketamine group or a normal saline group (n = 330 in each group), stratified by study center. All patients will receive intravenous propofol 0.5 mg/kg and sufentanil 0.1 μg/mL for induction of sedation, followed by intravenous esketamine 0.15 mg/kg or the same volume of normal saline. Propofol will be titrated to the target sedation levels during the procedures. The primary endpoint is a composite of desaturation (peripheral oxygen saturation < 90%) and hypotension (systolic blood pressure <80 mmHg or decrease >30% of baseline). Secondary endpoints include desaturation, hypotension, total dose of propofol, pain scores and fatigue scores on the 0–10 numerical rating scale, dizziness or headache, hallucination or nightmare, nausea or vomiting, endoscopist satisfaction, and patient satisfaction. All analyses will be intention-to-treat.
Discussion
We expect that a low-dose esketamine adjunct to propofol-based sedation will improve cardiorespiratory stability in patients undergoing same-visit bidirectional endoscopy, providing reference for clinical sedation practice during these procedures.
Trial Registration
Chinese Clinical Trial Registry (Identifier: ChiCTR-ChiCTR2200055938).
Keywords: esketamine, propofol, sedation, bidirectional endoscopy, desaturation, hypotension
Introduction
According to a recent nationwide survey, the overall number of gastrointestinal endoscopic procedures in China is 14 million per year.1 With the rapid growth rate of aging, this number is predicted to reach 51 million by 2030. Over the recent years, bidirectional endoscopy (esophagogastroduodenoscopy and colonoscopy) in a same visit has been increasingly implemented. Its benefits include reduction in medical costs and facilitation of healthcare decision-making.2 During the same-visit bidirectional endoscopy, the sequence of esophagogastroduodenoscopy followed by colonoscopy is the preferred sequence.3,4
Sedation is widely performed to facilitate the implementation of gastrointestinal endoscopy and to alleviate patients’ discomfort.5,6 In the United States and European countries, sedation is used in more than 90% of gastroscopies or colonoscopies.7,8 In China, the current sedation rate is approximately 50% and increasing rapidly.1 The frequently used technique is the propofol-based sedation combined with analgesics, but all these regimens are associated with complications such as oversedation, airway obstruction, respiratory depression, and hypotension.9,10 In addition, bidirectional endoscopy requires a prolonged duration of sedation than esophagogastroduodenoscopy or colonoscopy alone, which may increase the risk of adverse events. To date, the best sedation regimen for these procedures remains unknown. Esketamine, a novel N-methyl-D-aspartate receptor antagonist, has a 2-fold higher sedative potency and a lower risk of side effects than racemic ketamine.11,12 A recent study showed that the use of esketamine countered opioid-induced respiratory depression.13 Hence, esketamine can be an ideal adjuvant to propofol sedation in gastrointestinal endoscopic procedures.
In this multicenter randomized controlled study, we hypothesize that the use of a low-dose esketamine as an adjuvant to propofol-based sedation would lead to a decrease in the occurrence of desaturation and hypotension events in patients undergoing same-visit bidirectional endoscopy.
Methods
Study Design
This is an investigator-initiated, multicenter, randomized, double-blind, placebo-controlled trial. The study will be conducted at 3 teaching hospitals in eastern China. The leading study center is The First Affiliated Hospital of Soochow University, Suzhou, China. The two participating centers are Taicang First People’s Hospital, Taicang, China and The People’s Hospital of SND, Suzhou, China. The implementation of this study will be in accordance with the Declaration of Helsinki. This trial protocol follows the guidelines of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Supplementary File 1).14
Ethic Approval and Trial Registration
The study protocol was approved by the Medical Ethics Committee of The First Affiliated Hospital of Soochow University (Approval No. 2021–233) and then by the Ethics Committee of each participating center. The trial is registered at the Chinese Clinical Trial Registry (Identifier: ChiCTR2200055938) on January 26, 2022.
Trial Status
At the time of manuscript submission, the recruitment of patients has not yet started. The recruitment process will begin in March 2022 and continue until December 2022 at three hospitals in Jiangsu province, China.
Study Patients
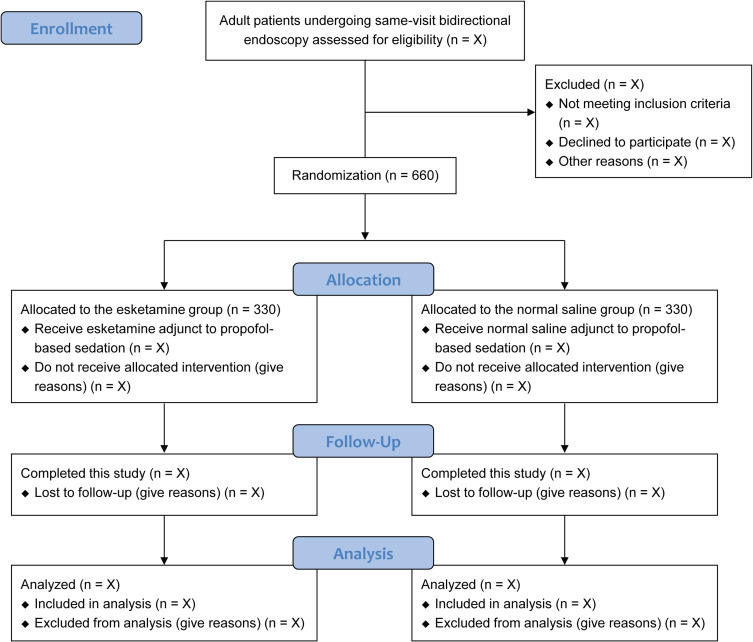
At each study center, an experienced investigator will screen the patients before the procedures and explain the details of this study to them. To be included in this study, patients should meet the eligibility criteria and provide their written informed consent. At any time during this study, patients can withdraw their consent without any consequence. A total of 660 eligible patients who are scheduled for same-visit bidirectional endoscopy under sedation will be randomly allocated to an esketamine group or a normal saline group (n = 330 in each group). The flow chart of this study is presented in Figure 1.
Figure 1.
Flow chart of patients in this study.
Eligibility Criteria
Patients are eligible for participation in this study if they are 18–70 years old, American Society of Anesthesiologists (ASA) physical status I or II, body mass index (BMI) 18–30 kg/m2, and scheduled for same-visit bidirectional endoscopy under sedation. The exclusion criteria are as follows: (1) severe cardiovascular or pulmonary diseases (uncontrolled hypertension [systolic blood pressure ≥ 180 mmHg], coronary heart disease, myocardial infarction, congestive heart failure, left ventricular ejection fraction <40%, tachyarrhythmia, second-degree or greater atrioventricular block, sick sinus syndrome, severe bradycardia [heart rate < 50 beats/min], chronic obstructive pulmonary disease, asthma, emphysema, bronchiectasis, or pulmonary fibrosis); (2) renal or liver dysfunction (Child Pugh grade C or need for renal replacement therapy); (3) neurocognitive disorders (Parkinson’s disease or Alzheimer’s disease) or psychiatric disease; (4) seizures or epilepsy; (5) hypersensitivity to study medications; (6) alcoholism or preoperative use of sedatives and analgesics; or (7) refusal for participation.
Randomization and Blinding
A biostatistician who is independent of data management and analyses generates the randomization list using an online tool (https://www.sealedenvelope.com/simple-randomiser/v1/lists). The randomization is performed with an allocation ratio of 1:1, permuted blocks of 2 and 4, and stratification by study center. The details of group allocation will be sealed in opaque envelopes until the end of the study. Based on the randomization list, an independent research nurse in each center will distribute the study medications (esketamine or normal saline placebo) in identical syringes labeled with study numbers only. It is impossible to distinguish the content in these syringes, because both esketamine and normal saline are clear and colorless. To avoid potential interference with blinding, esketamine or normal saline will be administered immediately after the induction of sedation. Patients, anesthesiologists, endoscopists, peri-procedure care providers, and outcome observers will all remain masked to the group assignment until the end of final analysis.
Study Interventions
All patients will undergo bidirectional endoscopy in the esophagogastroduodenoscopy-colonoscopy sequence. Throughout the procedures, heart rate and peripheral oxygen saturation (SpO2) will be continuously monitored, and noninvasive blood pressure will be measured at 3-min intervals. All patients will receive supplemental oxygen at a flow of 3 L/min through a nasal cannula. A standardized intravenous sedation will be provided by the same anesthesiologist in each study center. Intravenous sufentanil 0.1 μg/kg and propofol 0.5 mg/kg will be administered for induction of sedation. Thereafter, the esketamine group will receive intravenous esketamine 0.15 mg/kg, while the normal saline group will receive the same volume of normal saline. Propofol will be titrated with additional doses of 0.2–0.3 mg/kg to reach the target sedation levels or to treat discomfort responses (such as grimaces, gag, moan, or body movement). The level of sedation will be assessed using the Modified Observer’s Alertness/Sedation scale (MOAA/S) every 30 seconds (Table 1).15,16 At the beginning of esophagogastroduodenoscopy, the target sedation level will be MOAA/S score = 1 (responding only after trapezius squeeze stimulus). Subsequently and during the colonoscopy, the target sedation level will be MOAA/S score = 2 (responding only after mild prodding or shaking). Following the procedures, patients will be transferred to a recovery room. Patients are ready for discharge when they are fully awake and a modified Aldrete score of 10 is reached.4 The schedule of patient enrollment, study interventions, and endpoint measurements will conform to the SPIRIT statement (Table 2).
Table 1.
Modified Observer’s Alertness/Sedation Scale (MOAA/S)
| MOAA/S Score | Responsiveness | ASA Continuum of Sedation |
|---|---|---|
| 5 | Prompt response to name spoken in a normal tone | Minimal sedation |
| 4 | Lethargic response to name spoken in a normal tone | Moderate sedation |
| 3 | Response only to name called loudly or repeatedly | Moderate sedation |
| 2 | Response only to mild prodding or shaking | Moderate sedation |
| 1 | Response only to painful stimulus (trapezius squeeze) | Deep sedation |
| 0 | No response to painful stimulus (trapezius squeeze) | General anesthesia |
Notes: Adapted from Kashiwagi K, Hosoe N, Takahashi K et al. Prospective, randomized, placebo-controlled trial evaluating the efficacy and safety of propofol sedation by anesthesiologists and gastroenterologist-led teams using computer-assisted personalized sedation during upper and lower gastrointestinal endoscopy. Digestive Endoscopy. 2016; 28: 657–64. © 2016 Japan Gastroenterological Endoscopy Society16.
Table 2.
Schedule of Patient Enrollment, Study Interventions, and Endpoint Measurements
| Study Period | ||||||
|---|---|---|---|---|---|---|
| Enrollment | Allocation | Post-Allocation | Close-Out | |||
| Timepoint | Anesthesia Clinic Visit | Prior to Sedation | During Sedation | Emergence from Sedation | 15 Min in Recovery Room | Hospital Discharge |
| Patient enrollment | ||||||
| Inclusion criteria | × | |||||
| Exclusion criteria | × | |||||
| Written informed consent | × | |||||
| Demographic data | × | |||||
| Baseline characteristics | × | |||||
| Randomization | × | |||||
| Allocation | × | |||||
| Study interventions | ||||||
| Esketamine | × | |||||
| Normal saline placebo | × | |||||
| Endpoint measurements | ||||||
| Desaturation events | × | |||||
| Hypotension events | × | |||||
| Total dose of propofol | × | |||||
| NRS pain scores | × | × | ||||
| NRS fatigue scores | × | × | ||||
| Dizziness | × | × | × | |||
| Hallucination or nightmare | × | × | × | |||
| Nausea and vomiting | × | × | × | |||
| Endoscopist satisfaction | × | |||||
| Patient satisfaction | × | |||||
Abbreviation: NRS, numerical rating scale.
Primary Endpoint
The primary endpoint of this trial is a composite of desaturation and hypotension events during the procedures. Desaturation is defined as SpO2 <90%. Hypotension is defined as systolic blood pressure (SBP) <80 mmHg or decrease in SBP >30% of baseline. The baseline blood pressure values will be obtained when patients are comfortably seated in a pre-procedure waiting area.
Secondary Endpoints
The secondary endpoints include (1) the incidence of desaturation, (2) the incidence of hypotension, (3) total dose of propofol, (4) pain scores at emergence from sedation and 15 min later assessed using the numerical rating scale (NRS, 0–10; 0 = no pain, 10 = the most severe pain), (5) fatigue scores at emergence from sedation and 15 min later assessed using the NRS (0–10; 0 = no fatigue, 10 = the most severe fatigue), (6) the incidence of dizziness or headache, (7) the incidence of hallucination or nightmare, (8) the incidence of nausea or vomiting, (9) endoscopist satisfaction, and (10) patient satisfaction. Satisfaction will be evaluated using a 5-point Likert scale (1 = very dissatisfied, 5 = highly satisfied).17
Data Collection and Management
Demographic data include age, sex, ethnicity, weight, height, and BMI. Baseline characteristics include smoking status, history of medications, comorbidities, ASA physical status, and baseline heart rate, blood pressure, and SpO2. Other peri-procedure data include induction dose of propofol, infusion dose of propofol, duration of endoscopy, time to emergence from sedation, and time to discharge. All raw data will be recorded in the Case Report Forms (CRFs) by an independent investigator in each study center. All medical records without personally identifiable information will be entered into an electronic database and monitored by an independent Data Monitoring Committee (DMC). After all patients have completed this trial, the primary investigator and data administrator will check and confirm the integrity and accuracy of data. Thereafter, all data will be locked. The administrator will import the data into a designated form and send it to a statistician for final statistical analyses.
Sample Size Calculation
Before implementing this trial, we prospectively investigated 32 patients receiving sufentanil in combination with propofol sedation for same-visit bidirectional endoscopy between May 2021 and July 2021. Our results showed that 7 patients (21.9%) developed desaturation and 5 patients (15.6%) experienced hypotension (unpublished data). These incidences are in line with the recent literature.17,18 We use a conservative composite incidence of 30% for the power analysis of this multicenter trial. It is hypothesized that the addition of a low-dose esketamine would reduce the composite incidence of desaturation and hypotension to 20% (ie, an absolute reduction rate of 10%). Based on this assumption, 294 patients will be required in each group with a power of 80% at an α level of 0.05. Considering a potential dropout rate of 10%, we decide to recruit a total of 660 patients, with 330 in each arm. The sample size is calculated using the PASS software (version 11.0.7, NCSS, LCC, Kaysville, UT, USA).
Statistical Analysis
Continuous variables will be tested for normal distribution using the Shapiro–Wilk test and presented as means ± standard deviations if normally distributed and medians (interquartile ranges) if not. The groups will be compared using the independent t-test and Mann–Whitney rank-sum test, as appropriate. Categorical variables will be presented as numbers (percentages) and analyzed using the chi-squared test or Fisher's exact test, as appropriate.
Demographic data and baseline characteristics will be presented using descriptive statistics only. For the primary and secondary endpoints, the therapeutic effect between the two study groups will be assessed using the odds ratio (OR) or mean difference (MD) with 95% confidence intervals (CI). Considering multiple comparison corrections for the secondary endpoints, the Benjamini–Hochberg approach will be applied to control for false discovery. In addition, these endpoints will be analyzed using the multivariate logistic regression model or the generalized linear model adjusting for baseline covariates (age, BMI, smoking status, history of hypertension, and history of diabetes) and study center. The P values before and after adjustment will be presented. In addition, subgroup analyses based on the primary endpoint will be carried out to further investigate whether the study interventions would produce different effects in the following subgroups: age (<60 years vs ≥60 years), BMI (<25 kg/m2 vs ≥25 kg/m2), current smoker (yes vs no), history of hypertension (yes vs no), and history of diabetes (yes vs no). The interaction analysis across the subgroups will be performed using a logistic regression model.
All analyses will be performed on the intention-to-treat basis. We expect that protocol violation would be uncommon in this study. We have no plans for an interim analysis or imputation of missing data. All statistical tests will be two-sided and a P value less than 0.05 indicates a statistically significant difference. Statistical analyses will be performed using the GraphPad Prism software (version 9.00; GraphPad, San Diego, CA) and R software (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria).
Discussion
This multicenter randomized controlled trial will include a total of 660 adult patients to assess the effects of low-dose esketamine versus normal saline placebo on the incidence of desaturation and hypotension during same-visit bidirectional endoscopy. In addition, we will compare the total dose of propofol, post-procedure pain and fatigue, the occurrence of dizziness or headache, hallucination or nightmare, nausea or vomiting, endoscopist satisfaction, and patient satisfaction between the two groups. The implementation of this trial and reporting of results will be based on the Consolidated Standards of Reporting Trials guidelines.19
Propofol is the most popular sedation drug for gastrointestinal endoscopic procedures. Compared with the traditional sedation with benzodiazepines such as midazolam or diazepam, sedation with propofol for digestive endoscopy leads to a shorter time to sedation, a faster recovery profile, and improved patient satisfaction.10 Despite these benefits, propofol use for sedation can cause cardiorespiratory complications. Studies showed that the incidence of these complications ranged from 20% to 60%, depending on the type of procedures and choice of sedation regimens.10,17,18,20,21 Forster and colleagues found that intravenous lidocaine infusion reduced propofol requirements by 50% (a mean dose of 58 mg vs 121 mg) and improved pain and fatigue after colonoscopy.20 Only a small number of patients were included in that study, and they did not measure the plasma concentration of lidocaine. Eberl and colleagues showed that sedation with dexmedetomidine led to prolonged hemodynamic depression and less satisfaction in patients undergoing endoscopic esophageal procedures, when compared with propofol sedation.22
Ketamine or esketamine can be used for sedation, alone or as an adjuvant to propofol. Yin and colleagues showed that, in elderly patients undergoing gastrointestinal endoscopy, the combination of propofol and ketamine better maintained hemodynamic and respiratory stability, when compared with propofol plus sufentanil, propofol plus dexmedetomidine, or propofol alone.23 As for esketamine, a recent study suggested that patients receiving esketamine for sedation during gastroscopy had reduced incidences of adverse events (such as dizziness, nausea, vomiting, and headache) and a shorter time to recovery than patients receiving racemic ketamine.24 Another randomized study found that a low-dose esketamine versus alfentanil reduced the total dose of propofol during endoscopic retrograde cholangiopancreatography, without affecting recovery, satisfaction, or adverse events.17 The role of esketamine in endoscopic procedures is not fully understood. To our knowledge, this will be the first multicenter randomized controlled study to assess whether a low-dose esketamine adjunct to propofol-based sedation would reduce the incidence of cardiorespiratory complications during same-visit bidirectional endoscopy. For our patients, intravenous propofol 0.5 mg/kg in combination with sufentanil 0.1 μg/mL will be used for sedation induction, followed by propofol titration to the target sedation levels. This strategy will minimize the risk of oversedation and associated complications.
This study has several limitations. First, a composite of desaturation and hypotension is designed as the primary endpoint. When using a single outcome as the study endpoint, a larger sample size is needed based on sample size calculation. Second, although a higher dose of esketamine may produce more potent sedative effects and further decrease the consumption of propofol, we select a low-dose at 0.15 mg/kg. This is because all our patients will receive a low-dose of sufentanil as an analgesic concurrently, and we intend to minimize the associated risk of psychotomimetic side effects. Last, we will exclude patients who are older than 70 years, because we believe that the optimal sedation regimen for the elderly or very elderly patients should be determined based on the results of this study.
In conclusion, this multicenter randomized controlled trial will evaluate the effects of a low-dose esketamine as an adjuvant to propofol-based sedation on the incidence of desaturation and hypotension in patients undergoing same-visit bidirectional endoscopy. Our findings, whether positive or negative, will provide reference for clinical practice of sedation during these procedures.
Acknowledgments
We thank Shuang-Jie Wu, Zhou-Lin Lu, Qi-Ming Zhang, and Li Zhou for their collaboration in the Ethics Committee and Data Monitoring Committee at The First Affiliated Hospital of Soochow University.
Funding Statement
This study is supported by the Science and Technology Development Plan Clinical Trial Project (SLT201909), 333 High-level Talent Training Project in Jiangsu Province (BRA2020089), and Six Talent Peaks Project in Jiangsu Province (WSN-022).
Data Sharing Statement
The data that support the findings of this study will be available from the corresponding author on reasonable request.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.Zhou S, Zhu Z, Dai W, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127:56–64. [DOI] [PubMed] [Google Scholar]
- 2.Urquhart J, Eisen G, Faigel DO, Mattek N, Holub J, Lieberman DA. A closer look at same-day bidirectional endoscopy. Gastrointest Endosc. 2009;69:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Yang J, Li J, et al. Comparison of procedural sequences in same-day painless bidirectional endoscopy: single-center, prospective, randomized study. Digestive Endoscopy. 2017;29:330–337. [DOI] [PubMed] [Google Scholar]
- 4.Laoveeravat P, Thavaraputta S, Suchartlikitwong S, et al. Optimal sequences of same-visit bidirectional endoscopy: systematic review and meta-analysis. Digestive Endoscopy. 2020;32:706–714. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003-2009. JAMA. 2012;307:1178–1184. [DOI] [PubMed] [Google Scholar]
- 6.Early DS, Lightdale JR, Vargo JJ, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018;87:327–337. [DOI] [PubMed] [Google Scholar]
- 7.Riphaus A, Geist F, Wehrmann T. Endoscopic sedation and monitoring practice in Germany: re-evaluation from the first nationwide survey 3 years after the implementation of an evidence and consent based national guideline. Z Gastroenterol. 2013;51:1082–1088. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LB, Wecsler JS, Gaetano JN, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967–974. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich-Rust M, Welte M, Welte C, et al. Capnographic monitoring of propofol-based sedation during colonoscopy. Endoscopy. 2014;46:236–244. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Zhu Z, Zheng Y. Effect and safety of propofol for sedation during colonoscopy: a meta-analysis. J Clin Anesth. 2018;51:10–18. [DOI] [PubMed] [Google Scholar]
- 11.Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: a Critical Review. CNS Drugs. 2018;32:411–420. [DOI] [PubMed] [Google Scholar]
- 12.Kamp J, Jonkman K, van Velzen M, et al. Pharmacokinetics of ketamine and its major metabolites norketamine, hydroxynorketamine, and dehydronorketamine: a model-based analysis. Br J Anaesth. 2020;125:750–761. [DOI] [PubMed] [Google Scholar]
- 13.Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120:1117–1127. [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barends CRM, Driesens MK, Struys M, Visser A, Absalom AR. Intranasal dexmedetomidine in elderly subjects with or without beta blockade: a randomised double-blind single-ascending-dose cohort study. Br J Anaesth. 2020;1:87. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwagi K, Hosoe N, Takahashi K, et al. Prospective, randomized, placebo-controlled trial evaluating the efficacy and safety of propofol sedation by anesthesiologists and gastroenterologist-led teams using computer-assisted personalized sedation during upper and lower gastrointestinal endoscopy. Digestive Endoscopy. 2016;28:657–664. [DOI] [PubMed] [Google Scholar]
- 17.Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37:394–401. [DOI] [PubMed] [Google Scholar]
- 18.Chiang MH, Wu SC, You CH, et al. Target-controlled infusion vs. manually controlled infusion of propofol with alfentanil for bidirectional endoscopy: a randomized controlled trial. Endoscopy. 2013;45:907–914. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster C, Vanhaudenhuyse A, Gast P, et al. Intravenous infusion of lidocaine significantly reduces propofol dose for colonoscopy: a randomised placebo-controlled study. Br J Anaesth. 2018;121:1059–1064. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SW, Choi GJ, Lee OH, et al. Comparison of propofol monotherapy and propofol combination therapy for sedation during gastrointestinal endoscopy: a systematic review and meta-analysis. Digestive Endoscopy. 2018;30:580–591. [DOI] [PubMed] [Google Scholar]
- 22.Eberl S, Preckel B, Bergman JJ, van Dieren S, Hollmann MW. Satisfaction and safety using dexmedetomidine or propofol sedation during endoscopic oesophageal procedures: a randomised controlled trial. Eur J Anaesthesiol. 2016;33:631–637. [DOI] [PubMed] [Google Scholar]
- 23.Yin S, Hong J, Sha T, et al. Efficacy and Tolerability of Sufentanil, Dexmedetomidine, or Ketamine Added to Propofol-based Sedation for Gastrointestinal Endoscopy in Elderly Patients: a Prospective, Randomized, Controlled Trial. Clin Ther. 2019;41:1864–77.e0. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Huang J, Yang S, et al. Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: a Randomized, Open-Label Clinical Study. Drug Des Devel Ther. 2019;13:4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]