Abstract
Purpose
The coronavirus disease (COVID-19) outbreak has created a global health crisis. Secondary pulmonary bacterial infection is a COVID-19 complication, increasing morbidity and mortality. This study aimed to determine the pathogens, antibiotic susceptibility patterns, and risk factors for mortality in hospitalized COVID-19 patients.
Patients and Methods
This retrospective study used secondary data from patients’ electronic medical records at Hasan Sadikin General Hospital and Santo Borromeus Hospital between March 2020 and March 2021. Overall, 2230 hospitalized COVID-19 patients were screened, and 182 of them who were hospitalized ≥48 hours with a procalcitonin level of ≥0.25 ng/mL were enrolled. Culture examination was performed on sputum samples to determine pathogen and antibiotic susceptibilities. Univariate and multivariate analyses were used to determine mortality-related risk factors in hospitalized COVID-19 patients.
Results
The prevalence of secondary pulmonary bacterial infections in COVID-19 patients was 8.2%, with 161/182 pathogen growth from sputum samples. Mainly gram-negative bacteria (64.8%) were present, including Acinetobacter baumannii (31.9%), Klebsiella pneumoniae (19.8%), and Pseudomonas aeruginosa (8.8%). High rate of multidrug-resistant (MDR) pathogens was found among isolate (45.9%), ie carbapenem-resistance A.baumannii (CR-Ab) was 84.2%, extended-spectrum β-lactamase (ESBL) among K. pneumoniae was 61.1%. Secondary infection of MDR pathogens was associated with a higher risk of mortality (AOR 5.63, p = 0.001). Other associated factors were age ≥60 years, ventilator use, and female gender.
Conclusion
Gram-negative bacteria are the predominant pathogens causing secondary pulmonary bacterial infection in COVID-19 patients, implying nosocomial infection. High resistance to first-line antimicrobial drugs was observed in Gram-negative bacteria and Gram-positive bacteria. High rate of MDR pathogens was found among isolate and was associated with a significant risk of mortality.
Keywords: COVID-19, secondary pulmonary bacterial infection, antibiotic susceptibility, MDR pathogens, West Java, Indonesia
Introduction
The outbreak of coronavirus disease (COVID-19) has created a global health crisis and has become the largest pandemic in human history. From December 31, 2019, until mid-November 2021, a total of 257,904,476 COVID-19 cases have been reported, including 5,163,335 COVID-19-associated deaths.1 A high mortality rate was reported early in the pandemic, with nearly 36% of patients dying between January and February 2020 in China,2 and previous studies in Indonesia reported an overall mortality of 9.4%3 and 12%.4 Mortality has been associated with older age, pre-existing comorbidities, disease severity, admission to the intensive care unit (ICU), and ventilator use.5–8 In addition to the risk factors mentioned, secondary pulmonary bacterial infection has been identified as a common complication of viral respiratory infection, which increases morbidity and mortality.5,9
A similar pattern was observed in previous viral pandemics. The 1918 Spanish flu pandemic resulted in 50 million deaths worldwide, with the majority of deaths attributable to secondary bacterial pneumonia.9,10 During the 2009 H1N1 pandemic, secondary bacterial infections were detected in 18–34% of influenza cases, with incidence peaking at 1 week after influenza infection.11,12 Studies conducted in China,13 Hong Kong,14 and Mexico15 reported that bacterial secondary infections in COVID-19 patients were observed only in 6–12% of patients. One meta-analysis of COVID patients from 2019 to April 2020 also showed the overall proportion of bacterial infection in COVID patients was 6.9% (95% CI 4.3–9.5%), with a higher proportion being observed in critically ill patients.16
However, early in the pandemic, empiric treatment for bacterial secondary infections in hospitalized COVID-19 patients was common, especially with penicillin and macrolide agents, which were recommended as first-line treatments for community-acquired pneumonia.17–19 A meta-analysis showed that 71.9% of patients hospitalized with COVID-19 before mid-April 2020 received antibiotics.16 Improper administration of antibiotics will increase antibiotic resistance, which, in turn, will increase mortality in COVID-19 patients. Secondary bacterial pulmonary infections need to be recognized and treated effectively to reduce antibiotic resistance, which is increasing at an alarming rate.16,20 Previous studies in Brazil analyzed bacterial infections in patients with severe COVID, and already reported multi-drug resistance was present in 96% of A. baumannii and 57% of K. pneumoniae that were associated with longer ICU stay, mechanical ventilation use, and higher mortality.21 Currently, there are limited studies on secondary infections and antimicrobial use in COVID-19 patients in Indonesia. Therefore, this study aimed to determine the pathogens of secondary pulmonary bacterial infection, antibiotic susceptibility, and risk factors for mortality in COVID-19 patients admitted in two referral hospitals in West Java, Indonesia.
Methods
Study Design and Participants
This retrospective study used secondary data extracted from the medical records of hospitalized patients with confirmed COVID-19 between March 2020 and March 2021. This study was carried out at two referral hospitals in Bandung: Hasan Sadikin Hospital, a public hospital with 944 inpatient services, and Santo Borromeus Hospital, a large private hospital with 394 inpatient services. Both hospitals included nearly 70 ICUs. Patients with confirmed COVID-19 were identified based on clinical signs and symptoms and the detection of severe acute respiratory syndrome coronavirus 2 in polymerase chain reaction assay.
We enrolled confirmed COVID-19 patients with secondary bacterial infection, defined as confirmed COVID-19 patients aged >18 years old and a procalcitonin (PCT) level of up to ≥0.25 ng/mL at 48 hours or more during hospitalization. Patients with other conditions, such as human immunodeficiency virus, acquired immunodeficiency syndrome, pregnancy, autoimmune diseases, malignancy, and urinary tract infection, were excluded from the study.
Data Collection
Data were extracted by two authors and cross-checked by another author. Data were extracted from medical records and kept anonymous. Data on the following variables were extracted: age, sex, body mass index, comorbidity, severity, oxygen support, laboratory findings, antibiotic history, and outcome. Pathogen and antibiotic susceptibility data from sputum cultures were also collected. Sputum culture data used in this study were the first sputum cultures obtained from the patient. Sputum samples in which epithelial cells were more than 10 cells per large visual field regardless of the number of leukocytes were not processed for culture.22 Bacterial and fungal identifications were performed in the microbiology laboratory of each hospital using the Vitek 2 Compact platform (BioMerieux, Marcy L’Etoile, France). Antimicrobial susceptibility testing for bacterial and fungi was performed using Minimum-Inhibitory Concentration (MIC) using the Vitek platform and reported according to the Clinical and Laboratory Standards Institute (CLSI) guideline 2020 (M100 and M60). The antimicrobial susceptibility (AST) results were interpreted as susceptible, intermediate, or resistant according to the CLSI breakpoint. We also used the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint, if the breakpoint of certain antimicrobial drugs was not available at CLSI. An intermediate test result was considered resistant. A cumulative AST report was generated using WHOnet software, according to the World Health Organization (WHO) Global Antimicrobial Resistance and Use Surveillance System (WHO GLASS) recommendation.23 Susceptibility results of cumulative AST were reported as the number of isolates tested and percentage.
All statistical analyses were performed using IBM SPSS, version 26.0 (SPSS, Chicago, Illinois, USA). Variables associated with mortality in the hospitalized patients were analyzed using univariate and multivariate logistic regression models, with p-values of <0.05 considered statistically significant.
Definition
Secondary bacterial infection was defined as a PCT level of up to ≥0.25 ng/mL at 48 hours or more during hospitalization. Hypertension was defined as a blood pressure of ≥140/90 mmHg.24 Obesity was defined according to the WHO's proposed classification of weight in adult Asians, that is, a body mass index of ≥25 kg/m2.25 Type 2 diabetes mellitus was defined as a fasting plasma glucose level of more than 126 mg/dL (7.0 mm/L) or HbA1C ≥6.5.26 Chronic kidney disease was defined as either kidney damage or a decreased glomerular filtration rate of <60 mL/min/1.73 m2 for at least 3 months.27 MDR pathogens are defined as pathogens that acquire non-susceptibility to at least one agent in three or more antimicrobial categories.28 Severe COVID-19 was defined as oxygen saturation below 90% at room air, or respiratory rate of >30 breaths/min, or partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio <300, and/or lung infiltrates >50% within 24–48 hours.29,30
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Hasan Sadikin Hospital and Santo Borromeus Hospital (ethics approval number LB 02.01/X.6.5/83/2021, May 12, 2021, and 014/KEPK/VI/2021, June 3, 2021). Informed consent was not required for this secondary use of medical data; thus, the need for written patient consent was waived by the ethics committee.
Results
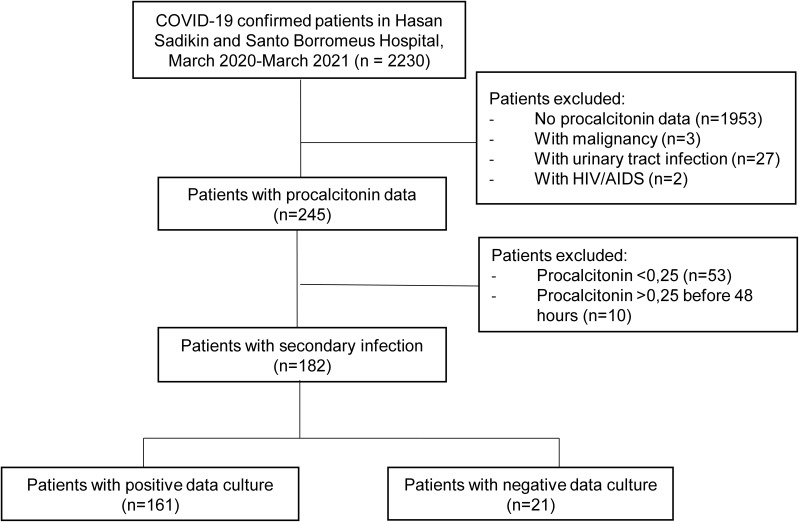
In this study, we screened 2230 confirmed COVID-19 patients and enrolled 182 confirmed COVID-19 patients with secondary infection (Figure 1). The prevalence of secondary pulmonary infections was 8.2% (182 of 2230 patients). The baseline patient characteristics are presented in Table 1 and Supplementary Table 1.
Figure 1.
Flowchart of the study patient selection process.
Table 1.
Clinical Characteristics of COVID-19 Inpatients
| Characteristic | Total (n = 182) |
|---|---|
| Age, median (IQR), years | 60 (46–69) |
| Age group, n (%), years | |
| ≥60 | 92 (50.5) |
| <60 | 90 (49.5) |
| Sex, n (%) | |
| Male | 117 (64.3) |
| Female | 65 (35.7) |
| Comorbidities, n (%) | |
| Any | 140 (76.9) |
| Hypertension | 87 (47.8) |
| Obesity | 83 (45.6) |
| Type 2 DM | 64 (35.2) |
| Chronic kidney disease | 57 (31.3) |
| Cardiovascular diseases | 52 (28.6) |
| Stroke | 13 (7.1) |
| No comorbidities | 42 (23.1) |
| Time from illness onset to hospital admission, median (IQR), days | 3 (2–5) |
| Duration of hospitalization, median (IQR), days | 13 (9–18) |
| <13 days | 88 (48.4) |
| ≥13 days | 94 (51.6) |
| Laboratory findings, median (IQR) | |
| Procalcitonin | 1.27 (0.67–4.39) |
| Days of PCT measurement | 6 (5–8) |
| Oxygen support (%) | |
| Nasal cannula | 57 (33.7) |
| Non-rebreathing mask | 40 (23.7) |
| Ventilator | 30 (17.8) |
| High-flow nasal cannula | 20 (11.8) |
| Without oxygen support | 18 (10.7) |
| Simple mask | 4 (2.4) |
| Antibiotics history, n (%) | |
| No | 112 (61.5) |
| Yes | 70 (38.5) |
| Outcome, n (%) | |
| Survivor | 94 (51.6) |
| Nonsurvivor | 88 (48.4) |
Abbreviations: COVID-19, coronavirus disease; IQR, interquartile range; PCT, procalcitonin; DM, diabetes mellitus.
Median patient age was 60 years (range–46–69 years). Most of the patients were male (64.3%). Comorbidities were present in more than half of the patients (76.9%), with hypertension being the most prevalent (47.8%), followed by obesity (45.6%) and type 2 diabetes mellitus (35.2%). The increase in PCT levels mostly occurred on the sixth day of hospitalization, with the levels ranging from 0.67 ng/mL to 4.39 ng/mL (median: 1.27 ng/mL). Seventy COVID-19 patients (38.5%) received empirical antibiotics, while the remaining 112 patients (61.5%) did not. The most commonly used antibiotics were third-generation cephalosporins (38.5%), fluoroquinolones (37.9%) and carbapenems (18.1%). The length of hospital stays for COVID-19 patients with secondary pulmonary infections, ranged from 9 to 18 days, with a median of 13 days. The mortality rate of COVID-19 patients with secondary pulmonary infection was as high as 48.4%.
Bacterial growth had a predominance of gram-negative bacteria (64.8%), whereas the growth of gram-positive bacteria was 9.3%, and fungal growth was 8.7%. The three most common gram-negative bacteria were A. baumannii (31.86%), K. pneumoniae (19.78%), and P. aeruginosa (8.79%) (Table 2). MDR strains were found in 74 out of 182 (40.5%) pathogens of secondary pulmonary infection in this study. More than half of A. baumannii and coagulase-negative Staphylococcus were found to have MDR strains, followed by P. aeruginosa (43.7%). Ventilator-acquired pneumonia was particularly related to gram negative bacterial infections. Half of which were caused by infections of A. baumannii and 13.1% were caused by K. pneumoniae infections (Table 2).
Table 2.
Pathogens of Secondary Pulmonary Infection
| Pathogens | Total n = 182 (%) | MDR Pathogens n = 74 (%) | HAP n = 136 (%) | VAP n = 46 (%) |
|---|---|---|---|---|
| Acinetobacter baumannii | 58 (31.9) | 42 (72.4) | 35 (25.7) | 23 (50.0) |
| Klebsiella pneumoniae | 36 (19.8) | 14 (38.8) | 30 (22.1) | 6 (13.1) |
| Pseudomonas aeruginosa | 16 (8.8) | 7 (43.7) | 13 (9.5) | 3 (6.4) |
| Enterobacter cloacae | 6 (3.3) | 0 | 5 (3.6) | 1 (2.1) |
| Stenotrophomonas maltophilia | 2 (1.1) | 0 | 1 (0.7) | 1 (2.1) |
| Coagulase-negative Staphylococcus | 12 (6.6) | 10 (83.3) | 11 (8.1) | 1 (2.1) |
| Staphylococcus aureus | 5 (2.7) | 1 (20) | 3 (2.1) | 2 (4.2) |
| Enterococcus faecalis | 2 (1.1) | 0 | 1 (0.7) | 1 (2.1) |
| Others | 24 (13.2) | 0 | 18 (13.3) | 6 (13.1) |
| No growth | 21 (11.5) | - | 19 (13.9) | 2 (4.2) |
Notes: Others are Staphylococcus epidermidis, Staphylococcus hominis, Streptococcus sanguinis, Streptococcus gordonii, Citrobacter koseri, Candida albicans, Candida tropicalis, and Candida glabrata.
Abbreviations: MDR, multidrug resistance; HAP, hospital-acquired pneumonia; VAP, ventilator-acquired pneumonia.
High resistance to antimicrobial drugs (≥80%) that are used as the first-line treatment (fluoroquinolone, cephalosporin) for bacterial pneumonia was observed among gram-negative bacteria. This also indicates the MDR-organisms, ie extended-spectrum β-lactamase (ESBL) among K. pneumoniae was shown to be 61.1%, Carbapenem-resistance A. baumannii was found to be 84.2%, and Carbapenem-resistance P. aeruginosa was found to be 50% (Table 3). Meanwhile, the resistance of fluoroquinolone (levofloxacin, ciprofloxacin, moxifloxacin) among coagulase-negative Staphylococcus (CoNS) and Staphylococcus aureus were ranging between 33.3% and 100%. The methicillin-resistant coagulase-negative Staphylococci (MRCoNS) was observed in all isolates, while methicillin-resistant Staphylococcus aureus (MRSA) was observed to be as high as 33.3%. Among MRCoNS, vancomycin-resistance was observed to be as high as 14.1% (Table 4).
Table 3.
Antibiotic Susceptibility of Gram-Negative Bacteria
| Antibiotics | Acinetobacter baumannii | Klebsiella pneumonia | Pseudomonas aeruginosa | Enterobacter cloacae | Stenotrophomonas maltophilia | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | %S | n | %S | n | %S | n | %S | n | %S | |
| Amikacin | 58 | 57.9 | 36 | 72.2 | 16 | 62.5 | 6 | 100 | - | − |
| Ampicillin-sulbactam | 58 | 5.9 | 36 | 21.4 | - | - | 6 | 0 | - | − |
| Aztreonam | 58 | 21.7 | 36 | 38.9 | 16 | 56.3 | 6 | 66.7 | - | − |
| Cefazolin | 58 | 0 | 36 | 0 | 16 | 0 | 6 | 0 | - | − |
| Cefepime | 58 | 2.9 | 36 | 50 | 16 | 37.5 | 6 | 50 | - | − |
| Cefotaxime | 58 | 17.4 | 36 | 52 | 16 | 37.5 | 6 | 100 | - | − |
| Ceftazidime | 58 | 2.9 | 36 | 28.6 | 16 | 37.5 | 6 | 50 | - | − |
| Ceftriaxone | 58 | 7 | 36 | 38.9 | 16 | 37.5 | 6 | 83.3 | - | − |
| Ciprofloxacin | 58 | 14 | 36 | 38.9 | 16 | 50 | 6 | 83.3 | - | − |
| Ertapenem | 58 | 39.1 | 36 | 72.2 | 16 | 62.5 | 6 | 100 | - | − |
| Gentamicin | 58 | 17.5 | 36 | 58.3 | 16 | 62.5 | 6 | 83.3 | - | − |
| Levofloxacin | 58 | 30.4 | 36 | 50 | 16 | 62.5 | 6 | 100 | 2 | 50 |
| Meropenem | 58 | 15.8 | 36 | 72.2 | 16 | 50 | 6 | 100 | - | − |
| Moxifloxacin | 58 | 30.4 | 36 | 50 | 16 | 62.5 | 6 | 100 | - | − |
| Piperacillin-tazobactam | 58 | 2.9 | 36 | 42.9 | 16 | 37.5 | 6 | 50 | - | − |
| Tigecycline | 58 | 52.6 | 36 | 80.6 | 16 | 25 | 6 | 83.3 | - | − |
| Trimethoprim-sulfamethoxazole | 58 | 52.6 | 36 | 44.4 | 16 | 50 | 6 | 83.3 | 2 | 50 |
Abbreviations: %S, percentage susceptibility; n, number of isolate tested to certain antimicrobial drugs; −, not tested.
Table 4.
Antibiotic Susceptibility of Gram-Positive Bacteria
| Antibiotics | Coagulase-Negative Staphylococcus | Staphylococcus aureus | Enterococcus faecalis | |||
|---|---|---|---|---|---|---|
| n | %S | n | %S | n | %S | |
| Amoxicillin-clavulanic acid | 12 | 0 | 5 | 0 | - | − |
| Ampicillin | 12 | 0 | 5 | 0 | 2 | 100 |
| Clindamycin | 12 | 100 | 5 | 66.7 | - | − |
| Erythromycin | 12 | 0 | 5 | 66.7 | 2 | 0 |
| Levofloxacin | 12 | 0 | 5 | 66.7 | - | − |
| Linezolid | 12 | 57.2 | 5 | 66.7 | 2 | 100 |
| Moxifloxacin | 12 | 0 | 5 | 66.7 | - | − |
| Oxacillin | 12 | 0 | 5 | 66.7 | - | − |
| Penicillin | 12 | 0 | 5 | 0 | - | − |
| Piperacillin-tazobactam | 12 | 0 | 5 | 0 | - | − |
| Tetracycline | 12 | 51.5 | 5 | 66.7 | 2 | 50 |
| Tigecycline | 12 | 85.7 | 5 | 100 | 2 | 100 |
| Trimethoprim-sulfamethoxazole | 12 | 68.7 | 5 | 100 | 2 | 0 |
| Vancomycin | 12 | 85.9 | 5 | 100 | 2 | 100 |
Abbreviations: %S, percentage susceptibility; n, number of isolate tested to certain antimicrobial drugs; −, not tested.
Univariate logistic regression analysis revealed patients that had a secondary infection of MDR strains pathogen had a higher risk of mortality (odds ratio [OR] 4.94, 95% confidence interval [CI] 2.17–11.24, p < 0.001). Other factors related with higher risk of mortality were ventilator use during hospitalization (OR 4.34, 95% CI 1.58–11.95, p = 0.003), followed by older than 60 years (OR 3.12, 95% CI 1.50–6.51, p = 0.002) and patients with severe COVID-19 (OR 1.07, 95% CI 1.07–4.52, p = 0.03 (Table 5). Multivariate logistic regression analysis also demonstrated that the main risk factors associated with mortality were MDR strains pathogen infection, ventilator use during hospitalization, and patients older than 60 years (Table 6).
Table 5.
Univariate Analysis of the Association of Risk Factors with Mortality in COVID-19 Patients
| Variable | Survived (n = 64) | Died (n = 60) | p-value | Crude OR (95% CI OR) |
|---|---|---|---|---|
| Age (≥60 years) | 25 (39.1) | 40 (66.7) | 0.002* | 3.12 (1.50–6.51) |
| Sex (female) | 19 (29.7) | 28 (46.7) | 0.051 | 2.07 (0.99–4.34) |
| Comorbidity (any) | 47 (73.4) | 45 (75.0) | 0.876 | 1.09 (0.49–2.43) |
| Ventilator | 6 (9.8) | 18 (32.1) | 0.003* | 4.34 (1.58–11.95) |
| Severity (severe) | 27 (42.2) | 37 (61.7) | 0.030* | 2.21 (1.07–4.52) |
| MDR pathogens | 29 (45.3) | 45 (80.4) | <0.001* | 4.94 (2.17–11.24) |
Note: *Statistically significant.
Abbreviations: OR, odds ratio; CI, confidence interval; COVID-19, coronavirus disease.
Table 6.
Multivariate Analysis of the Association of Risk Factors with Mortality in COVID-19 Patients
| Variable | Initial Model | Final Model | ||
|---|---|---|---|---|
| AOR (95% CI AOR) | p-value | AOR (95% CI AOR) | p-value | |
| Age (≥60 years) | 4.20 (1.67–10.54) | 0.002* | 4.03 (1.63–9.98) | 0.003* |
| Sex (female) | 2.73 (1.06–7.02) | 0.037* | 2.59 (1.02–6.57) | 0.045* |
| Comorbidity (any) | 0.80 (0.30–2.12) | 0.651 | ||
| Ventilator | 3.08 (0.81–11.73) | 0.100 | 4.07 (1.20–13.80) | 0.024* |
| Severity (severe) | 1.77 (0.67–4.68) | 0.251 | ||
| MDR pathogens | 5.64 (2.09–15.20) | 0.001* | 5.63 (2.12–14.93) | 0.001* |
Note: *Statistically significant.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; COVID-19, coronavirus disease.
Discussion
Viral infections in the respiratory tract increase susceptibility to secondary bacterial infections through dysregulation of the host immune system, reduced pulmonary mucociliary clearance, and increased bacterial adhesion to the epithelial cells.31,32 This study found that the prevalence of secondary pulmonary bacterial infection in COVID-19 patients lower than that in the previous Spanish flu and H1N1 pandemic.9,11,12 The prevalence of secondary pulmonary bacterial infection in COVID-19 patients in this study was 8.2%, which is in concordance with the findings of previous studies that reported a prevalence of secondary infections ranging from 6% to 14% in COVID-19 patients.14,15,33
Empirical antibiotic therapy was widely used in the early pandemic, mainly because of concerns arising from previous pandemic experiences with high rates of mortality caused by secondary pulmonary bacterial infection.14,34 Some studies reported high empiric antibiotic therapy administered to more than 50% of hospitalized COVID-19 patients worldwide.5,33,35 This study found that most patients (70.3%) did not receive empiric antibiotics because the use of complete blood count, PCT, and evaluation of clinical signs of infection as guidance was quickly implemented by the special task team in both hospitals so that antibiotics were not given promptly from the beginning or were stopped immediately in less than 24 h of administration. The mortality rate of COVID-19 patients with secondary pulmonary infection in this study was as high as 48.4%, similar to the findings of other studies conducted in India and China.5,13,36 Along with the development of knowledge about COVID-19, the management of patients at the beginning of the pandemic may have been different, resulting in a higher mortality rate.
The growth of the pathogens in this study showed predominance of gram-negative bacteria, with the most common species being A. baumannii, K. pneumoniae, and P. aeruginosa. These pathogens are closely associated with nosocomial infection. These findings are similar to those in some studies that showed that the most common pathogens in the group of gram-negative bacteria were A. baumannii and K. pneumoniae.13,36,37 A study showed different results, with the most common pathogens being Mycoplasma sp., Haemophilus influenzae, and Pseudomonas aeruginosa.16 One of the reasons for the differences in the bacterial patterns in these studies may be related to the differences in bacterial colonization in each hospital and the differences in patients’ baseline characteristics. Other microorganisms found in this study were Staphylococcus epidermidis (2 patients), Staphylococcus hominis (2 patients), Streptococcus sanguinis (2 patients), Streptococcus gordonii (1 patient), Citrobacter koseri (1 patient), and fungal organisms (16 patients). A previous study conducted in India reported fungal infections as much as 9%.36 In this study, we found a similar prevalence of 8.7% fungal infections in hospitalized patients. The fungal organisms found in this study were Candida albicans (10 patients), Candida tropicalis (4 patients), and Candida glabrata (2 patients).
Antibiotic resistance has become a worldwide concern because it causes fatal therapeutic failure, particularly in critically ill patients. The growth rate of MDR bacteria in the samples from the two hospitals was 74 samples (45.9%). Some studies found that more than 50% of MDR and extensively drug-resistant (XDR) strains were found in A. baumannii and K. pneumoniae isolated from COVID-19 patients admitted in the ICU,36,38,39 with a significantly higher proportion after the pandemic than that before the pandemic.40 The increasing spread of MDR strains of nosocomial pathogens, especially A. baumannii, is likely due to the increase in required interventions for COVID-19 patients, antibiotics overuse, and aggravated by the decline in hygiene protocols in hospitals.21,41
High resistance to antimicrobial drugs that are used as the first-line treatment for bacterial pneumonia was observed in this study, especially among gram-negative bacteria. Some studies showed similar results about increased resistance toward antimicrobial targeting of gram-negative bacteria, especially MDR pathogens.42–46 High rate of MDR pathogens was found among isolate, extended-spectrum β-lactamase (ESBL) among K. pneumoniae was shown to be 61.1%, Carbapenem-resistance A. baumannii was found to be 84.2%, and Carbapenem-resistance P. aeruginosa was found to be 50%. One study also reported similar results that A. baumannii was not susceptible to almost all of the antibiotics tested, with the highest resistance to ceftazidime (96%), followed by meropenem (94%), fluoroquinolones (93.5%), imipenem (92%), and piperacillin/tazobactam (91%).36 K. pneumoniae showed no susceptibility to third-generation cephalosporins, ceftriaxone (91.7%), fluoroquinolones (82%), β-lactamases, piperacillin/tazobactam (79.2%), and cefoperazone-sulbactam (76.4%).36 A high rate of MDR pathogens was observed since most of the patients in this study had severe or critically severe COVID-19. Patients required intensive care, mechanical ventilation, steroid therapy, and more exposure to broad-spectrum antibiotics.
A. baumannii is one of the most important pathogens associated with nosocomial infections and remains a major concern owing to the rapid development of resistance to various antimicrobials and the ability to survive in the environment for a very long time.47 The majority of A. baumannii strains were isolated from the respiratory tract of hospitalized patients, and it is the second most common gram-negative bacteria found in ICUs; the associated mortality rates are as high as 30%–75%.44–46 The high MDR isolate finding rate of A. baumannii and P. aeruginosa indicates poor environmental conditions associated with the patient, such as poor hand hygiene, poor infection control practices, and lack of adherence to hygiene protocols.48 Hand hygiene practices are severely affected during the COVID-19 pandemic because all health workers have been using gloves as part of their personal protective equipment. Continuous and inappropriate use of gloves can cause cross-contamination and affect the rate of nosocomial infection.49
This study showed that MDR strains pathogen infection, ventilator use during hospitalization, and older age were associated with a higher risk of mortality during hospitalization. In concordance with another study in Italy that analyzed patients with acute respiratory distress syndrome (ARDS) admitted to the ICU, mortality related to carbapenemase-producing Klebsiella pneumonia was reported as high as 28.6%.50 Previous studies also reported that gram-negative bacteria were associated with longer hospital stay, longer ICU stay, mechanical ventilator use, and higher mortality.13,21
This study also showed that older age was associated with a higher risk of mortality, as shown in previous studies, because older adults usually have a more complicated condition that prolongs hospitalization duration.5,51–54 It makes them more vulnerable to contracting secondary bacterial or nosocomial infections. Critical patients with respiratory failure or acute respiratory distress syndrome require higher levels of oxygen therapy, such as use of ventilators, which are associated with a higher risk of mortality and increased risk of secondary bacterial infection.2,5,52,55
This study has some limitations. The true rate of secondary pulmonary bacterial infection is likely to be higher than that reported in this study because of the retrospective nature of the study, and PCT testing was not routinely and uniformly performed at the same time. Another limitation was the isolation of anaerobic, atypical, and fastidious microorganisms that were not recorded in this study. Clinical symptoms are important in making decisions regarding the possibility of infection, initiation, and choice of antibiotics; hence, it is possible that PCT and bacterial microbiological examinations were not performed in patients with relatively mild symptoms, especially in patients who had been administered empiric antibiotics, which may produce inaccurate findings.
Conclusion
This study reveals that gram-negative bacteria are pathogens causing secondary lung infections in hospitalized COVID-19 patients, with A. baumannii as the most common pathogen, implying a nosocomial infection. High resistance to first-line antimicrobial drugs (fluoroquinolone) was observed in Gram-negative bacteria and Gram-positive bacteria. High rate of MDR pathogens was found among isolate and was associated with a significant risk of mortality.
Acknowledgments
We would like to express our gratitude to all the health workers in both hospitals for their service and sacrifice during the COVID-19 pandemic. We acknowledge the helpful input from Evan Susandi in this study. Financial support for the study was provided by Universitas Padjadjaran (grant No. 1735/UN6.3.1/LT/2020).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.CDC. COVID-19 case surveillance public use data; 2021.
- 2.Tian R, Wu W, Wang C, et al. Clinical characteristics and survival analysis in critical and non-critical patients with COVID-19 in Wuhan, China: a single-center retrospective case control study. Sci Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-74465-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozaliyani A, Savitri AI, Setianingrum F, et al. Factors associated with death in COVID-19 patients in Jakarta, Indonesia: an epidemiological study. Acta Med Indones. 2020;52(3):246–254. [PubMed] [Google Scholar]
- 4.Surendra H, Elyazar IR, Djaafara BA, et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: a hospital-based retrospective cohort study. Lancet Reg Health West Pac. 2021;9:100108. doi: 10.1016/j.lanwpc.2021.100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang J, Wang Q, Zhang H, et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan, China. Am J Med. 2021;134(1):e6–e14. doi: 10.1016/j.amjmed.2020.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598 [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38(1):213–265. doi: 10.1172/JCI103791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205(3):458–465. doi: 10.1093/infdis/jir749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Wang J, Yang Y, et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9(1):1–7. doi: 10.1186/s13756-020-00819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng LS-K, Chau SK-Y, Tso EY-K, et al. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: experience from a single hospital cluster. Ther Adv Infect Dis. 2020;7:2049936120978095. doi: 10.1177/2049936120978095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Guerra BA, Gonzalez-Lara MF, De-leon-cividanes NA, et al. Antimicrobial resistance patterns and antibiotic use during hospital conversion in the COVID-19 pandemic. Antibiotics. 2021;10(2):182. doi: 10.3390/antibiotics10020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn VM, Gandhi TN, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–e541. doi: 10.1093/cid/ciaa1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieringer TD, Furukawa D, Graber CJ, et al. Inpatient antibiotic utilization in the veterans’ health administration during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;42(6):751–753. doi: 10.1017/ice.2020.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buehrle DJ, Decker BK, Wagener MM, et al. Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob Agents Chemother. 2020;64(11):e01011–20. doi: 10.1128/AAC.01011-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staub MB, Beaulieu RM, Graves J, Nelson GE. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect Control Hosp Epidemiol. 2021;42(7):810–816. doi: 10.1017/ice.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa R, Lamas C, Simvoulidis LFN, et al. Secondary infections in a cohort of patients with COVID-19 admitted to an intensive care unit: impact of gram-negative bacterial resistance. Rev Inst Med Trop Sao Paulo. 2022;64. doi: 10.1590/s1678-9946202264006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray P, Washington J. Microscopic and baceriologic analysis of expectorated sputum; 1975: 339–344. [PubMed]
- 23.World Health Organization. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016–2017; 2017.
- 24.Whelton PK, Williams B. The 2018 European Society of Cardiology/European Society of hypertension and 2017 American College of Cardiology/American heart association blood pressure guidelines: more Similar s. JAMA. 2018;320(17):1749–1750. doi: 10.1001/jama.2018.16755 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment; 2000.
- 26.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Supplement_1):S62–S67. doi: 10.2337/dc09-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 28.Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 29.Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis. 2020;71(15):740–747. doi: 10.1093/cid/ciaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. COVID-19 clinical management: living guidance; 2021.
- 31.Lee KH, Gordon A, Foxman B. The role of respiratory viruses in the etiology of bacterial pneumonia: an ecological perspective. Evol Med Public Health. 2016;2016(1):95–109. doi: 10.1093/emph/eow007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paget C, Trottein F. Mechanisms of bacterial superinfection post-influenza: a role for unconventional T cells. Front Immunol. 2019;10:336. doi: 10.3389/fimmu.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karataş M, Duman MY, Tünger A, Çilli F, Aydemir Ş, Ozenci V. Secondary bacterial infections and antimicrobial resistance in COVID-19: comparative evaluation of pre-pandemic and pandemic-era, A retrospective single center study. Ann Clin Microbiol Antimicrob. 2021;20. doi: 10.1186/s12941-021-00454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strathdee SA, Davies SC, Marcelin JR. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet. 2020;396(10257):1050–1053. doi: 10.1016/S0140-6736(20)32063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijay S, Bansal N, Rao BK, et al. Secondary infections in hospitalized COVID-19 patients: Indian experience. Infect Drug Resist. 2021;14:1893. doi: 10.2147/IDR.S299774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Santis V, Corona A, Vitale D, et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: results of a prospective observational multicenter study. Infection. 2022;50(1):139–148. doi: 10.1007/s15010-021-01661-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurana S, Singh P, Sharad N, et al. Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: implication on antimicrobial resistance. Indian J Med Microbiol. 2021;39(2):147–153. doi: 10.1016/j.ijmmb.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40(3):495–502. doi: 10.1007/s10096-020-04142-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur j Clin Microbiol Infect Dis. 2017;36(11):1999–2006. doi: 10.1007/s10096-016-2703-z [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez GV, Master RN, Clark RB, et al. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998–2010. Emerg Infect Dis. 2013;19(1):133. doi: 10.3201/eid1901.120310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald LC. Trends in antimicrobial resistance in health care–associated pathogens and effect on treatment. Clin Infect Dis. 2006;42(Supplement_2):S65–S71. doi: 10.1086/499404 [DOI] [PubMed] [Google Scholar]
- 44.Yu Y-S, Yang Q, Xu X-W, Kong H-S, Xu G-Y, Zhong B-Y. Typing and characterization of carbapenem-resistant Acinetobacter calcoaceticus–baumannii complex in a Chinese hospital. J Med Microbiol. 2004;53(7):653–656. doi: 10.1099/jmm.0.05513-0 [DOI] [PubMed] [Google Scholar]
- 45.Hsueh P-R, Liu Y-C, Yang D, et al. Multicenter surveillance of antimicrobial resistance of major bacterial pathogens in intensive care units in 2000 in Taiwan. Microb Drug Resist. 2001;7(4):373–382. doi: 10.1089/10766290152773383 [DOI] [PubMed] [Google Scholar]
- 46.Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 47.Abbas M, Nunes TR, Martischang R, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10(1):1–13. doi: 10.1186/s13756-020-00875-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almasaudi SB. Acinetobacter spp. as nosocomial pathogens: epidemiology and resistance features. Saudi J Biol Sci. 2018;25(3):586–596. doi: 10.1016/j.sjbs.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindberg M, Skytt B, Lindberg M. Continued wearing of gloves: a risk behaviour in patient care. Infect Prev Pract. 2020;2(4):100091. doi: 10.1016/j.infpip.2020.100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montrucchio G, Corcione S, Sales G, Curtoni A, De Rosa F, Brazzi L. Carbapenem-resistant Klebsiella pneumoniae in ICU-admitted COVID-19 patients: keep an eye on the ball. J Glob Antimicrob Resist. 2020;23:398–400. doi: 10.1016/j.jgar.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Yu L, Fu Y, et al. Tigecycline in combination with other antibiotics against clinical isolates of carbapenem-resistant Klebsiella pneumoniae in vitro. Ann Palliat Med. 2019;8(5):622–631. doi: 10.21037/apm.2019.09.11 [DOI] [PubMed] [Google Scholar]
- 52.Ali MM, Malik MR, Ahmed AY, et al. Survival analysis of all critically ill patients with COVID-19 admitted to the main hospital in Mogadishu, Somalia, 30 March–12 June 2020: which interventions are proving effective in fragile states? Int J Infect Dis. 2022;114:202–209. doi: 10.1016/j.ijid.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sousa G, Garces T, Cestari V, Florêncio R, Moreira T, Pereira M. Mortality and survival of COVID-19. Epidemiol Infect. 2020;148. doi: 10.1017/S0950268820001405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Smet R, Mellaerts B, Vandewinckele H, et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932. e1. doi: 10.1016/j.jamda.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]