Abstract
Purpose
It is believed that vascular endothelial dysfunction is involved in the occurrence of cardiovascular disease (CVD), and diabetic peripheral neuropathy (DPN) is associated with flow-mediated dilation (FMD), however, the correlation is still unclear. Aims of the present study is to explore the relationship between DPN parameters and FMD, providing a new approach for the prevention of CVD.
Patients and Methods
A total of 272 patients with T2DM from the Department of Endocrinology of The First Hospital of Lanzhou University according to the grading criteria were selected. FMD was measured by a new vascular ultrasound system and patients were divided into FMD>7%, 4%≤FMD≤7%, and FMD<4% groups. The Toronto Clinical Scoring System (TCSS) was used to assess the severity of DPN. The nerve conduction studies (NCS) assessed large fibre neuropathy by nerve conduction velocity (CV), compound muscle action potential (CMAP) amplitude (Amp), and distal motor latency (DML). SPSS 25.0 was used for statistical analysis.
Results
TCSS evaluation revealed that the percentage of patients with severe nerve injury was significantly higher in FMD<4% (70%) compared to FMD>7% (2%). Among the TCSS indicators of all subjects, the proportion of temperature disturbance was the most (73%), and joint position disturbance was the least (0). TCSS scores were negatively correlated with FMD (r=−0.756, p<0.001). More interesting, in FMD<4% group, CV and Amp were positively correlated with FMD, while DML was negatively correlated (p<0.05). Linear regression analysis model showed that different systolic blood pressure (SBP), triglyceride (TG), TCSS and CV had statistically different effects on FMD.
Conclusion
High TCSS score and decreased CV of common peroneal and tibial nerves are risk factors of FMD injury, which provide potential value for timely prevention and treatment of cardiovascular diseases.
Keywords: type 2 diabetes, diabetic peripheral neuropathy, vascular endothelial function, cardiovascular disease, Toronto clinical scoring system
Introduction
Diabetic neuropathy is one of the common diabetic complications with serious consequences, with an incidence of more than 50%. The injury may involve central and peripheral nerves, with the latter being more common.1 Diabetic peripheral neuropathy (DPN), including autonomic and somatic neuropathy, is a common microvascular complication of diabetes.2 The diabetic distal symmetrical polyneuropathy (DSPN) is the most common one of diabetic somatic neuropathies.2 Clinical manifestations of DSPN are broad, this neuropathic pattern tends to occur after 50 years of age, mostly in patients with longstanding diabetes mellitus.3 There are many screening and diagnostic methods for DPN, among which neuroelectrophysiological examination is objective, sensitive and reliable, and is often regarded as the gold standard.4,5 Nerve conduction studies (NCS) are an essential tool in the evaluation of the peripheral nervous system.6 It determines the functional status of peripheral nerves by examining the muscular nerve conduction function of the affected limb.4 The Toronto Clinical Scoring System (TCSS) was proposed by Perkins et al7 in 2001 and suggested to be used for DPN screening and severity evaluation. Therefore, although the onset of DPN is hidden and the mechanism is complex, early diagnosis and treatment can be achieved through timely screening and electrophysiological examination.8
The effect of diabetic peripheral nerve injury on cardiovascular disease has little been reported, and that the results are inconsistent.9,10 It is well known that diabetes is one of the risk factors leading to the development of vascular dysfunction and atherosclerotic disease, and diabetes cardiovascular disease is the main cause of death in patients with diabetes.11 Although blood glucose, blood pressure, and lipid are key factors in diabetic cardiovascular disease, the results of a cohort study suggest that peripheral neuropathy is a predictor of cardiovascular disease in diabetic patients without cardiovascular disease at baseline, even after controlling for standard cardiovascular risk factors.12 In contrast, an epidemiological study shows that cardiovascular risk factors are predictors of the development of peripheral neuropathy in patients with type 2 diabetes.13 These studies show inconsistent causality, but they could still confirm an association between peripheral neuropathy and the risk of cardiovascular disease in patients with type 2 diabetes, moreover, the mechanism of the association remains largely unknown.
Vascular endothelium is a dynamic cellular interface that is essential for normal vascular function and if endothelial cells are dysfunctional, this promotes vascular smooth muscle proliferation, inflammatory cell infiltration, and platelet aggregation, which are key events in cardiovascular disease.14,15 Some studies have linked risk factors for cardiovascular disease to endothelial dysfunction, for example, endothelial dysfunction is considered to be an important factor in atherosclerosis, hypertension, and heart failure.16 Endothelial function assessment can directly measure the factors produced by endothelial cells, but these factors have a short half-life, and most of them require specialized laboratories, detection instruments, detection reagents, etc. So we use a noninvasive ultrasonic technique, which can indirectly reflect the vascular endothelial function, and it is using cuff pressure caused reflex hyperemia, increased blood flow to add the shear stress and stimulate the endothelial vascular active factor NO release,17 causing blood flow mediated endothelial dependent vasodilation (FMD), which can be used as a quantitative imaging indicator of vascular endothelial function. A recent study showed a negative association between FMD and the severity of coronary heart disease, regardless of age, sex, or cholesterol level.18 Several studies have confirmed the repeatability of FMD, which is one of the predictors of cardiovascular disease progression,19,20 so application of FMD serves as a bridge between diabetes peripheral neuropathy and cardiovascular disease. This present study explored the relation between type 2 diabetic neuropathy and vascular endothelial function, and provided a new idea for the prevention of diabetic cardiovascular disease.
Materials and Methods
Subjects
A total of 272 patients (148 males and 124 females) with type 2 diabetes were included in the Department of Endocrinology, First Hospital of Lanzhou University from December 2019 to December 2021, according to the classification and diagnostic criteria of diabetes by the World Health Organization (WHO) in 1999. All investigations were conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of the First Hospital of Lanzhou University. Exclusion: ①Neuropathy caused by other causes, such as cervical and lumbar lesions (nerve root compression, spinal stenosis), cerebral infarction, Guillain-Barre syndrome, etc.; Severe arteriovenous vascular lesions (venous embolism, lymphangitis, etc.); Neurotoxic effects caused by drugs especially chemotherapeutic drugs and nerve damage from metabolic toxins caused by renal dysfunction; ②Patients who took thiazolidinediones, DPP-IV inhibitors, SGLT-2 inhibitors, GLP-1 receptor agonists and neurotrophic drugs within 3 months; ③Have a history of coronary heart disease, severe secondary or malignant hypertension; ④Patients with significant bradycardia (heart rate<50bpm)21 and hypotension (Blood Pressure<90/60mmHg); ⑤Pregnant or lactating women.
Data Collection
Clinical Characteristics
The patient’s age, gender, duration of diabetes, medication status, smoking and drinking history were collected from reported previously. The patient’s height, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR) were measured and the mean levels and standard deviations were recorded.
Laboratory Tests
Blood sample collections were performed in the morning after 12 hours of fasting. Using high-performance liquid chromatography, we determined glycated haemoglobin (HbA1c) levels. Fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), homocysteine (HCY) and 25-hydroxyvitamin D [25(OH) D] levels were performed by Siemens Advia 1800.
Determination of Vascular Endothelial Function
Patients who had undergone biochemical assessment were given brachial artery flow-mediated endothelial dependent vasodilation (FMD) in next morning by a novel vascular ultrasound system (UNEXEF 18VG, Nagoya, Japan). A fixed ultrasound physician was assigned to measure the brachial artery blood flow and unaware of the grouping of patients. Patients were asked to abstain from vitamin C, a high-fat diet, coffee, and to fast for at least 8 hours prior to the examination, such as coffee has a injurious effect on the endothelium by stimulating sympathetic nerves. Similarly, patients were asked to avoid strenuous exercise and rest quietly for at least 20 minutes before checking. The specific experimental conditions are as follows: The ambient temperature is kept at 20–24°C, the ambient humidity is 30–85%, the subject was in quiet state, supine position, and the cuff was tied to the subject’s forearm. The examination physician placed the electronic linear probe at the brachial artery of the subject’s forearm, and obtained the basic diameter of brachial artery lumen D0 when it was quiet. After obtaining the consent of the subject, the cuff was inflated, pressurized for about 2 minutes and 30 seconds, and then rapidly deflated to induce the dilation of the brachial artery. The maximum dilated diameter D1 of flow-mediated vasodilation within 2 minutes was recorded. The left and right brachial arteries were measured by the above procedure, and the average value of the two brachial arteries was taken as the record value of FMD. Calculation formula of FMD: FMD (%)= (D1-D0)/D0×100%.
Other Vascular Function Indicators
Endothelial dysfunction is an important cause of macrovascular and microvascular injury and is considered as the earliest change in the progression of arteriosclerosis.22,23 The ankle-brachial index (ABI) and brachial-ankle pulse wave velocity (baPWV) were used to evaluate arteriosclerosis. The ABI measurements were performed with the Omron Colin BP-203RPE III device (Omron Health Care). The systolic blood pressure (SBP) was measured in the ankle of both lower limbs and the brachial artery of both upper limbs. The SBP of each limb was collected twice with an interval of more than 1 minute. The value of the ABI for each limb was calculated dividing the greater SBP obtained in lower limb by the SBP of whichever was the higher in the upper limbs. The lowest value obtained was considered the ABI for that individual; abnormal threshold value is ≤0.9. baPWV measurement: After resting in supine position for 5 minutes, patients were asked to use an automatic arteriosclerosis meter (BP-203RPE II Colin Komaki, Japan) to measure atherosclerosis. Appropriate cuff was tied to the upper arm and lower limb, the mark of the cuff bag of the upper arm was aligned with the brachial artery, and the lower edge of the cuff was 2–3 cm away from the horizontal line of the fossa elbows. The mark of the cuff bag of the lower limb was located in the inner side of the lower limb, and the lower edge of the cuff was 1–2cm away from the medial malleolus. The sensor was placed in the precardiac area, and the arterial pulse waveform was recorded for 5 minutes, and the instrument automatically output baPWV value.
TCSS
The TCSS was applied to all patients in the current study by a professional clinician blinded to the grouping of patients. TCSS consists of nerve symptoms (lower limb pain, numbness, picotement, fatigue, walking imbalance, and upper limb symptoms), Sensory examinations (acmesthesia, temperature sensation, light touch, vibration sensation, joint position sensation) and nerve reflexes (ankle reflex and knee reflex). The total score of nerve symptoms was 6, 1 for the presence and 0 for the absence of symptoms. The total sensory score was 5, 0 for normal and 1 for abnormal, the total score of neural reflexes was 8, 0 for normal, 1 for weakened and 2 for disappeared. According to Perkins7 grading standards, a score of 0–5 indicates no nerve injury, 6–8 indicates mild nerve injury, 9–11 indicates moderate nerve injury, and 12–19 indicates severe nerve injury.
Peripheral Nerve Electrophysiological Examination
Nerve conduction studies (NCS) involves eliciting nerve action potentials at sites along a peripheral nerve and recording the response from another site along the nerve or from a muscle innervated by that nerve.24 Motor NCS are performed by stimulating a nerve trunk and recording the response from a muscle supplied by that nerve. The patients were placed in supine position and examined with KEYPOINT4 electromyogram-induced potentiometer by a clinician who specializes in electromyography. The ambient temperature was 25°C and the skin temperature was 33–35°C. Stimulation was initiated at 0 mA and slowly increased (usually up to 50mA) until a maximal response was obtained. The resultant biphasic waveform was the compound muscle action potential (CMAP). The upper extremity protocol involved stimulation of the median and ulnar nerves of both wrists and elbows, recorded from the musculi abductor pollicis brevis and abductor digiti minimi, respectively. In the lower extremity, peroneal and tibial stimulation was performed at both ankle and knee joints while recording from the extensor digitorum brevis (peroneal) and abductor hallucis (tibial) muscles. The main CMAP parameters that were used for analysis include the conduction velocity (CV), distal motor latency (DML), and amplitude (Amp).
Statistical Analysis
SPSS 25.0 statistical software (IBMSPSS statistical software V25.0) was used for statistical analysis of data in this study. Normal distribution measurements are expressed as mean±standard deviation. Univariate ANOVA was used for inter-group comparison of quantitative data of multiple groups. If ANOVA found significant differences, s-N-K test was used for multiple comparisons to determine which group had differences. Chi-square test was used for qualitative data. Kruskal-Wallis H-test was used for the grade distribution data of multiple groups. Correlational and multiple linear regression analyses were used to analyze the influencing factors of vascular endothelial function in type 2 diabetes patients. P<0.05 was considered statistically significant.
Results
Clinical and Biochemical Features
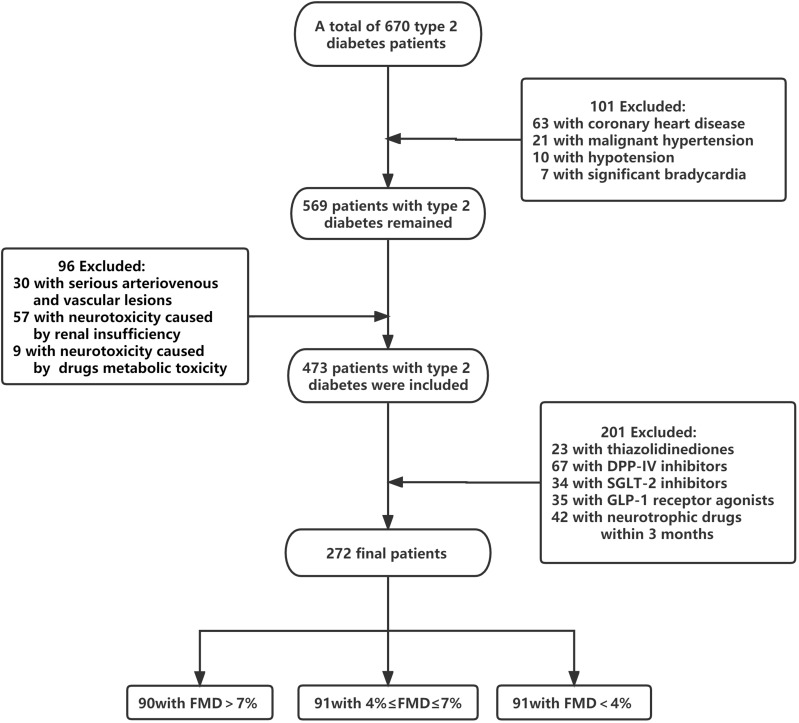
Figure 1 shows the flowchart. Patients were divided by FMD into three groups: FMD>7%=90 subjects (9.0±1.2%), 4%≤FMD≤7%=91 subjects (5.3±0.9%), and FMD<4%=91 subjects (2.4±0.9%). Table 1 lists the subjects’ indicators. There were no significant differences in age, sex, BMI, duration of disease, FPG, HbA1c, TC, HDL, LDL, TG, SBP, DBP, HR, 25(OH)D, drinking rate and smoking rate among the three groups (p>0.05). It can be seen that duration of diabetes and SBP in FMD<4% group were higher than those in the first two groups, but there were no significant differences.
Figure 1.
Flow diagram. The chart shows patient inclusion and exclusion in the study.
Table 1.
Comparison of Clinical and Biochemical Features of Patients with T2DM
| FMD>7% (n, 90) | 4%≤FMD≤7% (n, 91) | FMD<4% (n, 91) | p value | |
|---|---|---|---|---|
| Age (y) | 53.4±9.6 | 53.4±10.7 | 53.4±8.9 | 0.91 |
| Gender (male/female) | 51/39 | 43/48 | 54/37 | 0.37 |
| BMI (kg/m2) | 22.9±1.8 | 24.8±4.0 | 24.3±3.1 | 0.17 |
| Duration (y) | 9.1±6.5 | 9.3±7.1 | 10.3±7.6 | 0.64 |
| FPG (mmol/L) | 9.5±3.6 | 9.9±4.0 | 9.9±4.2 | 0.76 |
| HbA1c (%) | 8.4±2.1 | 8.5±1.8 | 8.4±1.8 | 0.99 |
| TC (mmol/L) | 3.8±1.0 | 4.2±1.3 | 3.8±1.0 | 0.48 |
| TG (mmol/L) | 1.6±0.8 | 1.6±2.1 | 1.6±1.1 | 0.10 |
| HDL (mmol/L) | 1.0±0.2 | 1.0±0.3 | 0.9±0.2 | 0.18 |
| LDL (mmol/L) | 2.5±0.8 | 2.7±0.9 | 2.5±0.8 | 0.70 |
| SBP (mmHg) | 121.5±24.1 | 129.5±17.9 | 131.7±18.7 | 0.36 |
| DBP (mmHg) | 76.2±8.7 | 80.0±11.3 | 76.7±8.3 | 0.43 |
| HR (bpm) | 83.6±11.6 | 88.6±11.4 | 83.1±12.5 | 0.22 |
| Smoking (%) | 20(22.2%) | 26(28.6%) | 23(25.3%) | 0.16 |
| Drinking (%) | 14(15.6%) | 18(19.8%) | 12(13.2%) | 0.16 |
| 25(OH)D(ng/mL) | 24.6±9.1 | 17.9±6.1 | 17.5±6.8 | 0.11 |
| HCY (µmol/L) | 13.1±3.4 | 13.4±3.6 | 16.8±13.5 | 0.23 |
| baPWV (cm/s) | 1627.1±377.3 | 1623.1±263.7 | 1630.1±595.2 | 0.57 |
| ABI | 1.2±0.1 | 1.2±0.1 | 1.2±0.1 | 0.87 |
Note: Univariate ANOVA and Chi-square test are used in this table.
Abbreviations: FMD, flow-mediated dilation; F, female; M, male; BMI, body mass index; FPG, fasting blood glucose; HbA1C, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL, High density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HCY, homocysteine; baPWV, brachial ankle pulse wave velocity; ABI, ankle-brachial index.
Proportion of Peripheral Neuropathy Severity in Patients with T2DM
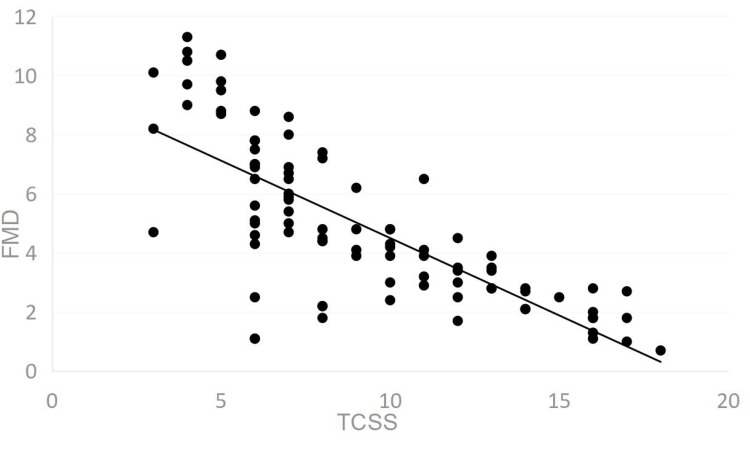
The proportion of severity of peripheral neuropathy in patients with T2DM obtained from TCSS is shown in Table 2. Percentage of patients with severe nerve injury was significantly higher in FMD<4% (70%) compared to FMD>7% (2%). Percentage of patients with severe nerve injury (70%) was significantly higher than those without (3%) in FMD<4% group, while in FMD>7% group, the proportion of patients with severe nerve injury (2%) was significantly lower than those without (62%). The severity of peripheral neuropathy can be considered different among the three groups (p<0.05). As shown in Figure 2 under section 3.4, FMD decreased gradually with the increase of TCSS, and the two were negatively correlated.
Table 2.
Proportion of Peripheral Neuropathy Severity in Three Groups
| FMD>7% (n, 90) | 4%≤FMD≤7% (n, 91) | FMD<4% (n, 91) | |
|---|---|---|---|
| No nerve injury (n; %) | 56(62%) | 30(33%) | 3(3%) |
| Mild nerve injury (n; %) | 22(25%) | 24(26%) | 9(10%) |
| Moderate nerve injury (n; %) | 10(11%) | 19(21%) | 16(17%) |
| Severe nerve injury (n; %) | 2(2%) | 18(20%) | 63(70%) |
Notes: Kruskal-Wallis H-test is used in this table X2, 11.114; p, 0.004; the severity of peripheral neuropathy can be considered different among the three groups.
Figure 2.
Scatter diagram of FMD and TCSS (0–19 points).
TCSS
Table 3 shows TCSS evaluation. The proportion of temperature disturbance (73%) was the most and joint position disturbance (0) was the least in the entire subject population. In FMD>7% group, temperature disturbance accounted for 55%, lower limb fatigue accounted for 50%, lower limb pain accounted for 44%; In 4%≤FMD≤7% group, temperature disturbance accounted for 71%, followed by vibration disturbance accounted for 63%, and fatigue accounted for 59%; In FMD<4% group, temperature disturbance accounted for 91%, followed by reflex disorder (ankle reflex disorder accounted for 90%, knee reflex disorder accounted for 88%), and vibration disturbance accounted for 78%. Neurological symptoms, sensory examination, and neural reflexes were considered to be different among the three groups using chi-square analysis.
Table 3.
Abnormal Ratio of TCSS Indicators of Three Groups
| Total (n, 272) | FMD>7% (n, 90) | 4%≤FMD≤7% (n, 91) | FMD<4% (n, 91) | X2 | p | |
|---|---|---|---|---|---|---|
| Nerve symptomsa | 44.249 | <0.001 | ||||
| Lower limb pain | 141(52%) | 40(44%) | 50(55%) | 51(56%) | ||
| Numbness | 60(22%) | 5(5%) | 10(11%) | 45(49%) | ||
| Picotement | 44(16%) | 5(5%) | 3(3%) | 36(40%) | ||
| Fatigue | 137(50%) | 45(50%) | 54(59%) | 68(75%) | ||
| Walking imbalance | 33(12%) | 5(5%) | 9(10%) | 19(21%) | ||
| Upper-limb symptoms | 35(13%) | 0 | 9(10%) | 26(29%) | ||
| Sensory examinationsb | 26.758 | 0.003 | ||||
| Acmesthesia | 25(9%) | 5(5%) | 7(8%) | 13(14%) | ||
| Temperature sensation | 198(73%) | 50(55%) | 65(71%) | 83(91%) | ||
| Light touch sensation | 54(20%) | 0 | 18(20%) | 36(40%) | ||
| Vibration sensation | 138(51%) | 10(11%) | 57(63%) | 71(78%) | ||
| Joint-position sensation | 0 | 0 | 0 | 0 | ||
| Nerve reflexesc | 76.631 | <0.001 | ||||
| Ankle reflex | 127(47%) | 9(10%) | 36(40%) | 82(90%) | ||
| Knee reflex | 119(44%) | 9(10%) | 30(33%) | 80(88%) |
Notes: Chi-square test is used in this table TCSS, Toronto clinical scoring system. a is for nerve symptoms; aX2, 44.249; p<0.001; b is for sensory examinations; bX2, 26.758; p, 0.003; c is for nerve reflexes; cX2, 76.631; p<0.001. Nerve symptoms; sensory examinations; and nerve reflexes can be considered different among the three groups.
Correlation Analysis Between TCSS and FMD
Pearson correlation analysis showed that TCSS was significantly negatively correlated with FMD (r=−0.756, p<0.001) in Table 4. Nerve symptoms (r=−0.328, p<0.001), sensory examinations (r=−0.081, p<0.05) and nerve reflexes (r=−0.714, p<0.001) were correlated with FMD, respectively.
Table 4.
Correlation Between Components of TCSS and FMD
| FMD (%) | ||
|---|---|---|
| r | p | |
| TCSS total points (0–19) | −0.756 | <0.001 |
| Nerve symptoms (0–6) | −0.328 | <0.001 |
| Sensory examination (0–5) | −0.081 | <0.05 |
| Nerve reflexes (0–8) | −0.714 | <0.001 |
Note: Pearson correlation analysis is used in this table.
Abbreviations: TCSS, Toronto clinical scoring system; FMD, flow-mediated dilation.
Comparison of CV, Amp and DML of Motor Nerve in Three Groups
NCS parameters include conduction velocity (CV), CMAP amplitude (Amp) and distal motor latency (DML) of median nerve, ulnar nerve, common peroneal nerve and tibial nerve as shown in Table 5. It can be seen that the CV and Amp of FMD<4% group were lower than those of the first two groups (p<0.05), and DML, on the other hand, was higher than the first two groups (p<0.05). However, there were no significant differences in CV, Amp and DML between FMD>7% and 4%≤FMD≤7% groups. According to nerve CV, the damage degree of common peroneal nerve and tibial nerve was more serious than that of median nerve and ulnar nerve at the same level of FMD. That is, when FMD>7%, CV of four nerves was within the normal range (upper extremity>50m/s, lower extremity>38m/s), when 4%≤FMD≤7%, median nerve and ulnar nerve CV remained within the normal range, while the common peroneal nerve and tibial nerve CV slowed down (37.38±7.50, 36.98±5.47).
Table 5.
Comparison of Peripheral Motor Nerve Measurements in Three Groups
| FMD>7% (n, 90) | 4%≤FMD≤7% (n, 91) | FMD<4% (n, 91) | |
|---|---|---|---|
| CV (m/s) | |||
| Median nerve | 51.18±6.56 | 50.23±5.32 | 48.75±5.43ab |
| Ulnar nerve | 50.61±4.85 | 50.16±5.23 | 45.05±5.05ab |
| Common peroneal nerve | 38.43±4.98 | 37.38±7.50 | 35.45±7.90ab |
| Tibial nerve | 38.04±3.51 | 36.98±5.47 | 34.3±5.55ab |
| Amp (mV) | |||
| Median nerve | 6.27±2.30 | 6.16±3.51 | 4.82±1.99ab |
| Ulnar nerve | 7.45±2.49 | 6.93±2.31 | 6.84±2.80ab |
| Common peroneal nerve | 1.19±1.39 | 0.91±1.17 | 0.80±0.87a |
| Tibial nerve | 5.25±2.84 | 5.16±2.50 | 4.40±3.45ab |
| DML (ms) | |||
| Median nerve | 3.08±0.72 | 3.50±0.72 | 4.29±3.07ab |
| Ulnar nerve | 2.53±0.97 | 2.56±0.81 | 2.67±0.52ab |
| Common peroneal nerve | 6.02±3.41 | 6.23±1.97 | 6.62±1.92ab |
| Tibial nerve | 4.43±1.05 | 4.89±2.08 | 5.64±2.27ab |
Notes: Univariate ANOVA and s-N-K test are used in this table Compared with FMD > 7% group; ap<0.05; Compared with 4%≤FMD≤7% group; bp<0.05.
Abbreviations: CV, conduction velocity; Amp, amplitude; DML, distal motor latency.
Correlation Analysis of Motor Nerve CV, Amp, DML and FMD
As shown in Table 6, correlation analysis showed that CV and Amp of median nerve, ulnar nerve, common peroneal nerve and tibial nerve were positively correlated with FMD, while DML was negatively correlated with FMD in FMD<4% group (p<0.05), however, there was no correlation in 4%≤FMD≤7% and FMD>7% group, respectively.
Table 6.
Correlation Analysis of Motor Nerve CV; Amp; DML and FMD
| FMD>7% (n, 90) | 4%≤FMD≤7% (n, 91) | FMD<4% (n, 91) | ||||
|---|---|---|---|---|---|---|
| CV (m/s) | r | p | r | p | r | p |
| Median nerve | 0.002 | 0.987 | 0.010 | 0.677 | 0.357 | 0.017 |
| Ulnar nerve | 0.194 | 0.297 | 0.190 | 0.267 | 0.472 | <0.001 |
| Common peroneal nerve | 0.040 | 0.779 | 0.121 | 0.665 | 0.650 | <0.001 |
| Tibial nerve | 0.059 | 0.681 | 0.098 | 0.543 | 0.798 | <0.001 |
| Amp (mV) | ||||||
| Median nerve | 0.005 | 0.972 | 0.111 | 0.767 | 0.340 | 0.028 |
| Ulnar nerve | 0.182 | 0.201 | 0.220 | 0.134 | 0.431 | 0.010 |
| Common peroneal nerve | 0.222 | 0.118 | 0.321 | 0.101 | 0.791 | <0.001 |
| Tibial nerve | 0.121 | 0.397 | 0.226 | 0.267 | 0.790 | <0.001 |
| DML (ms) | ||||||
| Median nerve | −0.274 | 0.072 | −0.254 | 0.102 | −0.359 | 0.017 |
| Ulnar nerve | −0.095 | 0.539 | −0.143 | 0.213 | −0.576 | 0.009 |
| Common peroneal nerve | −0.158 | 0.304 | −0.145 | 0.144 | −0.801 | <0.001 |
| Tibial nerve | −0.220 | 0.151 | −0.221 | 0.151 | −0.767 | <0.001 |
Note: Pearson correlation analysis is used in this table.
Abbreviations: CV, conduction velocity; Amp, amplitude; DML, distal motor latency.
Regression Analysis for Endothelial Function Injury in Type 2 Diabetes
Linear regression analysis was conducted in Model 1 with each baseline indicator as independent variable and vascular endothelial function indicator FMD as dependent variable in Table 7. Age, Duration, FPG, HbA1c, SBP and TG were forced to enter the model, and other indicators were entered by stepwise (p<0.05 entered, p>0.1 removed). The results of Model 1 show that SBP and TG levels are statistically and independently associated with FMD (β=−0.60 p=0.002, β=−0.57 p=0.017). TCSS was added to model 2 on the basis of model 1, and the results showed that high TCSS score is a risk factor of FMD (β=−0.51, p<0.001). Model 3 added the CV, Amp and DML of each nerve on the basis of model 1, and the results showed that decreased CV of common peroneal nerve and tibial nerve was a risk factor of FMD injury (β=0.13, p=0.014; β=0.27, p=0.003).
Table 7.
Multiple Linear Regression Analysis of Risk Factors for Endothelial Function Injury in T2DM
| Model1 (R2, 0.262) | Model2 (R2, 0.588) | Model3 (R2, 0.535) | ||||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Age | −0.71 | 0.395 | −0.70 | 0.395 | −0.71 | 0.395 |
| Duration | −0.68 | 0.121 | −0.67 | 0.122 | −0.69 | 0.125 |
| FPG | −0.54 | 0.111 | −0.53 | 0.110 | −0.53 | 0.110 |
| HbA1c | −0.55 | 0.159 | −0.55 | 0.156 | −0.56 | 0.159 |
| SBP | −0.60 | 0.002** | −0.60 | 0.002** | −0.60 | 0.002** |
| TG | −0.57 | 0.017* | −0.57 | 0.015* | −0.57 | 0.017* |
| Smoking | −0.015 | 0.056 | −0.015 | 0.056 | −0.014 | 0.055 |
| 25 (OH)D | 0.13 | 0.067 | 0.13 | 0.056 | 0.12 | 0.056 |
| TCSSa | −0.51 | <0.001** | ||||
| CV1b | 0.19 | 0.052 | ||||
| CV2b | 0.24 | 0.067 | ||||
| CV3b | 0.13 | 0.014* | ||||
| CV4b | 0.27 | 0.003** | ||||
| Amp3b | 0.37 | 0.070 | ||||
| Amp4b | 0.04 | 0.070 | ||||
| DML3b | −0.04 | 0.098 | ||||
| DML4b | −0.08 | 0.088 | ||||
Notes: *p<0.05; **p<0.01. Model1: Age; Duration; FPG; HbA1c; SBP and TG are forced to enter the model; and other indicators are entered by stepwise (p<0.05 entered; p>0.1 removed). Model2: Model1 +TCSS score (Age; Duration; FPG; HbA1c; SBP and TG are forced to enter the model; other indicators are entered stepwise; p<0.05 entered; p>0.1 removed) (a is removed from Model3). Model3: CV; Amp and DML of Model1 + median nerve1; ulnar nerve2; common peroneal nerve3 and tibial nerve4 (Age; Duration; FPG; HbA1c; SBP and TG were forced into the model; and other indicators were entered by stepwise. p<0.05 entered; p>0.1 removed) (b is removed from Model2).
Discussion
The endothelial is a complex structure that secretes special substances necessary to control blood vessel function and its dysfunction can lead to impaired vascular function, and then, impaired vascular function is also a risk factor for cardiovascular outcomes of diabetes, leading to the occurrence and development of atherosclerosis including coronary artery disease.25 As a non-invasive test, FMD has become widely used technique for measuring endothelial function.26 Peripheral endothelial function assessed by FMD is associated with coronary endothelial function,27 which can be explained by the fact that endothelial dysfunction is a systemic disease.28 Although FMD measures brachial arterial vascular function, the stimulation of FMD itself (reactive congestion) may be an important measure of peripheral microvascular function because reactive congestion is highly dependent on maximum forearm resistance.29,30 It has been widely documented that endothelial dysfunction has been associated with almost every risk factor for atherosclerosis and cardiovascular disease, such as hypertension, normal blood pressure with a family history of hypertension, smoking, dyslipidemia, diabetes and obesity.31
In addition to above causes of endothelial dysfunction, neuropathy can also cause vasodilation responses disorder. Previous studies have shown that the normal neurovascular response is conducted through the C-nociceptive nerve fibers. These fibers would stimulate adjacent C fibers, which secrete substance P, calcitonin gene-related peptide, and histamine, causing vasodilatation and increased blood flow to the injured tissues, thereby promoting wound healing, however, this impaired neurovascular reflex leads to a significant reduction in blood flow in diabetic neuropathy.32,33 A Caselli et al explored the role of C-nociceptive fibers in nerve axon reflex-related vasodilation through anesthesia block C-nociceptive fibers in diabetes divided the patients into diabetic neuropathy and non-neuropathy, and the results showed that the function of C-nociceptive fibers was the main factor affecting the vasodilation related to neuron-axon reflex, which provided evidence for the vascular dilation and injury caused by diabetic neuropathy.9 Nitric oxide (NO) is a potent vasodilator, which interacts with neural inputs and other local vasoactive mediators such as prostaglandin, endothelin, and endodermal hyperpolarizing factors co-determine overall vascular tone and blood flow.34 NO is synthesized by nitric oxide synthase, whose isoenzymes include neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS).35 nNOS is mainly expressed in specific neurons of the central nervous system, and nNOs-derived NO is also involved in vasodilation in the peripheral nervous system as an atypical neurotransmitter. eNOS is mainly expressed in vascular endothelial cells and also peripheral nerves, and its derived NO is a physiological vasodilator.35 Veves et al observed immunohistochemical staining of eNOS in skin biopsies of the dorsal feet of diabetic patients with peripheral nerve and vascular disease, diabetic patients with peripheral neuropathy and healthy subjects, and found that the intensity of eNOS staining decreased in both diabetic patients, and therefore is a possible mechanism of endothelial dysfunction in diabetic neuropathy.36 Candice Chapouly et al has confirmed that the loss of Desert Hedgehog (Dhh) signaling expression in the nerve was associated with increased endoneurial capillary permeability and decreased Claudin5 expression, which lead to the development of diabetic neuropathy.10 Thus, Dhh signaling plays a key role in maintaining blood nerve barrier integrity. Ando et al found that type 2 diabetic microfibrous neuropathy may be caused by impaired endothelial dysfunction.23 Above studies indicate that vascular dysfunction and peripheral neuropathy have a complex causal relationship.
So far, the mechanism of interaction between peripheral nerve injury and vascular endothelial disorder is still unclear.37 The relationship between DPN and FMD has been studied in recent years. By using laser Doppler imaging scanner to evaluate the response to iontophoresis of 1% acetylcholine chloride (Ach) and 1% sodium nitroprusside (NaNP), Arora et al compared these two responses in forearm and dorsolateral feet of healthy subjects, patients without and with diabetic peripheral neuropathy, and the results showed that endothelium-dependent and independent vasodilation was reduced in the group with peripheral neuropathy compared with the other two groups, and the reduction was more pronounced in feet than in forearm.38 In present study, TCSS was used to measure accurately the indicators of peripheral neuropathy, which has the advantage of being simple, comprehensive (including symptoms and signs), and can preliminarily evaluate the severity of DPN. Our study revealed incidence of defining variables in TCSS: lower limb pain developed in 52% of patients, lower limb fatigue in 50%, temperature disturbance in 73%, hypoesthesia of acupuncture pain in 25%, vibration disturbance in 51%, ankle reflexes reduced or absent in 47%, and knee reflexes reduced or absent results in 44%. In other words, hyposensitivity to temperature is the most common sign of peripheral neuropathy in patients with type 2 diabetes. Furthermore, we observed the incidence of parameters in TCSS for different FMD levels. It was found that small nerve fibers were mainly involved in FMD>7%; however, for FMD<4% group, the involvement of large nerve fibers increased. The correlation analysis showed that TCSS score was negatively correlated with FMD (r=−0.756, p<0.001), and neurological symptoms (r=−0.328, p<0.001), sensory examinations (r=−0.081, p<0.05), nerve reflexes (r=−0.714, p<0.001) were also negatively correlated with FMD, respectively. Arora et al’s research is consistent with us. More interesting, our paper applies TCSS that can evaluate the function of myelinated small or unmyelinated nerve fibers and small nerve fibers are the first to be damaged in the onset of diabetes, that is, Aδ myelinated small nerve fibers are first involved, and then C unmyelinated nerve fibers are gradually involved, and then Aβ myelinated large nerve fibers are developed. In the early stage of diabetes, sensory nerve damage is a significant clinical feature, which mainly caused by nerve demyelination and axonal degeneration. Therefore, TCSS can be used to detect DPN earlier and accurately.
Electrophysiology can be thought as an extension of the clinical neurologic examination.4 Because the measures are objective and the results do not depend on the patient’s subjective response, these are currently the most reliable methods of measuring the peripheral neuromuscular system.4 In the current study, NCS was used to objectively assess peripheral neuromuscular function. It detects large myelinated Aβ nerve fibers and records the presence, nature, distribution, and severity of peripheral nerve injury. In demyelinating polyneuropathies, there is a reduction of nerve conduction velocities with a relative preservation of nerve potential amplitudes, contrasted with the situation in axonal neuropathies where losing of amplitudes and relative preservation of conduction velocities are observed.4 In this study, we observed CV, Amp and DML of median nerve, ulnar nerve, common peroneal nerve, tibial nerve and their relationship with FMD. It can be seen that the CV and Amp of FMD<4% group were lower than those of the first two groups (p<0.05), and DML, on the other hand, was higher than the first two groups (p<0.05). However, there were no significant differences in CV, Amp and DML between FMD>7% and 4%≤FMD≤7% groups. Correlation analysis showed that CV and Amp of median nerve, ulnar nerve, common peroneal nerve and tibial nerve were positively correlated with FMD, while DML was negatively correlated with FMD in FMD<4% group (p<0.05), suggesting that the damage of large nerve fibers may be associated with severe vascular endothelial dysfunction. A study showed that after adjusting for age, sex, smoking status and body mass index, patients with diabetes have higher mortality risk than those without diabetes, moreover, with concurrent peripheral neuropathy can lead to an increased hazard ratio, however, the results did not reveal the effect of specific indicators of peripheral neuropathy on mortality.39 For the first time, we used multiple regression analysis to find that high TCSS score and decreased CV of common peroneal and tibial nerves are risk factors of FMD injury.
There is no doubt that the smaller the FMD, the more serious the endothelial function damage. In present study, 7% as the cut-off point value for endothelial dysfunction was based on previous studies and guidelines,31,40 and we also confirmed that when FMD>7%, the majority of patients did not have neuropathy and nerve CV, Amp and DML were not correlated with FMD. The data were collected and analyzed and for the first time we found that when FMD<4%, the majority of patients had neuropathy, and there was a significant correlation between nerve conduction indexes and FMD. When FMD was between 4 and 7%, patients have mild or severe peripheral nerve damage, but there was no significant correlation between nerve conduction indicators and FMD.
The advantage of this study is that TCSS is negatively correlated with FMD, and the simple and comprehensive advantages of TCSS can be used to quickly assess the vascular endothelial function of patients. Furthermore, the relationship between motor NCS and FMD was further studied, which provided a more detailed guidance for cardiovascular disease prevention in patients with peripheral neuropathy in type 2 diabetes. There are still deficiencies that this study is a retrospective study with a limited number of cases, requiring further large-scale prospective studies and the causal relationship between the two is difficult to explain clearly, and basic research is needed to further clarify its internal mechanism.
Conclusion
In conclusions, peripheral neuropathy of type 2 diabetes may be correlated with FMD, and damage to large nerve fibers may be associated with more severe vascular endothelial disorders. High TCSS score and decreased CV of common peroneal and tibial nerves are risk factors of FMD injury, which provide potential value for timely prevention and treatment of cardiovascular diseases.
Acknowledgments
The authors gratefully acknowledge the participation and cooperation of all patients in this study as well as the Department of Endocrinology of the First Hospital of Lanzhou University.
Funding Statement
This work was supported by Gansu Natural Science Foundation (No. 21JR1RA080); The First Hospital of Lanzhou University Research Fund (No. ldyyyn2019-39) and TCM Special Project of Gansu Province in 2021.
Abbreviations
FMD, flow-mediated dilation; F, female; M, male; BMI, body mass index; FPG, fasting blood glucose; HbA1C, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HCY, homocysteine; baPWV, brachial ankle pulse wave velocity; ABI, ankle-brachial index; TCSS, Toronto clinical scoring system; CMAP, compound muscle action potential; CV, conduction velocity; Amp, amplitude; DML, distal motor latency.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. doi: 10.1038/s41572-019-0092-1 [DOI] [PubMed] [Google Scholar]
- 2.Lai YR, Huang CC, Chiu WC, et al. The role of blink reflex R1 latency as an electrophysiological marker in diabetic distal symmetrical polyneuropathy. Clin Neurophysiol. 2020;131(1):34–39. doi: 10.1016/j.clinph.2019.09.022 [DOI] [PubMed] [Google Scholar]
- 3.Elkholy SH, Hosny HM, Shalaby NM, El-Hadidy RA, Abd El-Rahim NT. Blink reflex in type 2 diabetes mellitus. J Clin Neurophysiol. 2014;31(6):552–555. doi: 10.1097/WNP.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 4.Perkins B, Bril V. Electrophysiologic testing in diabetic neuropathy. Handb Clin Neurol. 2014;126:235–248. doi: 10.1016/B978-0-444-53480-4.00018-7 [DOI] [PubMed] [Google Scholar]
- 5.Dupuis JE, Li J, Callaghan BC, Reynolds EL, London ZN. Bilateral nerve conduction studies in the evaluation of distal symmetric polyneuropathy. Muscle Nerve. 2019;60(3):305–307. doi: 10.1002/mus.26616 [DOI] [PubMed] [Google Scholar]
- 6.Tavee J. Nerve conduction studies: basic concepts. Handb Clin Neurol. 2019;160:217–224. doi: 10.1016/B978-0-444-64032-1.00014-X [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Olaleye D, Zinman B. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250–256. doi: 10.2337/diacare.24.2.250 [DOI] [PubMed] [Google Scholar]
- 8.Gholami F, Nikookheslat S, Salekzamani Y, Boule N, Jafari A. Effect of aerobic training on nerve conduction in men with type 2 diabetes and peripheral neuropathy: a randomized controlled trial. Neurophysiol Clin. 2018;48(4):195–202. doi: 10.1016/j.neucli.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Caselli A, Rich J, Hanane T, Uccioli L, Veves A. Role of C-nociceptive fibers in the nerve axon reflex-related vasodilation in diabetes. Neurology. 2003;60(2):297–300. doi: 10.1212/01.wnl.0000040250.31755.f9 [DOI] [PubMed] [Google Scholar]
- 10.Chapouly C, Yao Q, Vandierdonck S, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res. 2016;109(2):217–227. doi: 10.1093/cvr/cvv263 [DOI] [PubMed] [Google Scholar]
- 11.Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21(4):21. doi: 10.1007/s11886-019-1107-y [DOI] [PubMed] [Google Scholar]
- 12.Brownrigg JR, de Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart. 2014;100(23):1837–1843. doi: 10.1136/heartjnl-2014-305657 [DOI] [PubMed] [Google Scholar]
- 13.Yang CP, Lin CC, Li CI, et al. Cardiovascular risk factors increase the risks of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: the Taiwan Diabetes Study. Medicine. 2015;94(42):e1783. doi: 10.1097/MD.0000000000001783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73(4):595–601. doi: 10.1253/circj.cj-08-1169 [DOI] [PubMed] [Google Scholar]
- 15.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology; clinical consequences; and medical therapy: part I. Eur Heart J. 2013;34(31):2436–2443. doi: 10.1093/eurheartj/eht149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. J Hypertens. 2005;23(2):233–246. doi: 10.1097/00004872-200502000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129(1):111–118. doi: 10.1016/s0021-9150(96)06018-2 [DOI] [PubMed] [Google Scholar]
- 19.O’Brien MW, Johns JA, Petterson JL, Mekary S, Kimmerly DS. The impact of age and sex on popliteal artery endothelial-dependent vasodilator and vasoconstrictor function. Exp Gerontol. 2021;145:111221. doi: 10.1016/j.exger.2020.111221 [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15(7):736–746. doi: 10.1093/ehjci/jet256 [DOI] [PubMed] [Google Scholar]
- 21.Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2019;140(8):e382–e482. doi: 10.1161/CIR.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA, Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando A, Miyamoto M, Saito N, et al. Small fibre neuropathy is associated with impaired vascular endothelial function in patients with type 2 diabetes. Front Endocrinol. 2021;12:653277. doi: 10.3389/fendo.2021.653277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Bryan R, Kincaid J. Nerve conduction studies: basic concepts and patterns of abnormalities. Neurol Clin. 2021;39(4):897–917. doi: 10.1016/j.ncl.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 25.Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ J. 2003;67(7):572–575. doi: 10.1253/circj.67.572 [DOI] [PubMed] [Google Scholar]
- 26.Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060–1071. doi: 10.1161/HYPERTENSIONAHA.118.11554 [DOI] [PubMed] [Google Scholar]
- 27.Takase B, Uehata A, Akima T, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82(12):1535–1539. doi: 10.1016/s0002-9149(98)00702-4 [DOI] [PubMed] [Google Scholar]
- 28.Anderson TJ, Gerhard MD, Meredith IT, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75(6):71B–74B. doi: 10.1016/0002-9149(95)80017-m [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44(2):134–139. doi: 10.1161/01.HYP.0000137305.77635.68 [DOI] [PubMed] [Google Scholar]
- 30.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–1474. doi: 10.1016/0735-1097(94)90141-4 [DOI] [PubMed] [Google Scholar]
- 32.Hile C, Veves A. Diabetic neuropathy and microcirculation. Curr Diab Rep. 2003;3(6):446–451. doi: 10.1007/s11892-003-0006-0 [DOI] [PubMed] [Google Scholar]
- 33.Hamdy O, Abou-Elenin K, LoGerfo FW, Horton ES, Veves A. Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001;24(3):344–349. doi: 10.2337/diacare.24.2.344 [DOI] [PubMed] [Google Scholar]
- 34.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1 [DOI] [PubMed] [Google Scholar]
- 35.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837;837a-837d. doi: 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veves A, Akbari CM, Primavera J, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy; vascular disease; and foot ulceration. Diabetes. 1998;47(3):457–463. doi: 10.2337/diabetes.47.3.457 [DOI] [PubMed] [Google Scholar]
- 37.Margariti A. Peripheral neuropathy may be a potential risk of cardiovascular disease in diabetes mellitus. Heart. 2014;100(23):1823–1824. doi: 10.1136/heartjnl-2014-306258 [DOI] [PubMed] [Google Scholar]
- 38.Arora S, Smakowski P, Frykberg RG, et al. Differences in foot and forearm skin microcirculation in diabetic patients with and without neuropathy. Diabetes Care. 1998;21(8):1339–1344. doi: 10.2337/diacare.21.8.1339 [DOI] [PubMed] [Google Scholar]
- 39.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus; fasting glucose; and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]