Abstract
Oxygen-sensitive gallic acid decarboxylase from Pantoea (formerly Enterobacter) agglomerans T71 was purified from a cell extract after stabilization by reducing agents. This enzyme has a molecular mass of approximately 320 kDa and consists of six identical subunits. It is highly specific for gallic acid. Gallic acid decarboxylase is unique among similar decarboxylases in that it requires iron as a cofactor, as shown by plasma emission spectroscopy (which revealed an iron content of 0.8 mol per mol of enzyme subunit), spectrophotometric analysis (absorption shoulders at 398 and 472 nm), and inhibition of the enzyme activity by 2,2′-bipyridyl, o-phenanthroline, and EDTA. Another interesting feature of this strain is the fact that it contains a tannase, which is used together with the gallic acid decarboxylase in a two-enzyme resting cell bioconversion to synthesize valuable pyrogallol from readily available tannic acid.
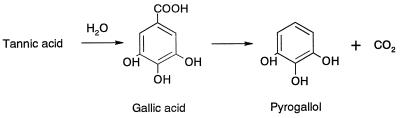
Gallic acid decarboxylases catalyze the second step in the degradation of the polyphenol tannic acid (13), the decarboxylation of gallic acid to pyrogallol (Fig. 1). These enzymes are very unstable due to their oxygen sensitivity, and none of them has been purified so far (6, 10–12, 19, 21, 26, 34). During a screening experiment we isolated a microorganism, identified as Pantoea (formerly Enterobacter) agglomerans, that exhibits a high level of gallic acid decarboxylase activity. We determined how to stabilize this enzyme as a prerequisite for the purification and characterization study described here.
FIG. 1.
Degradation of tannic acid.
P. agglomerans T71 contains both a gallic acid decarboxylase and a tannase; the latter enzyme initiates tannic acid degradation. By using the combined activities of tannase and gallic acid decarboxylase in a two-enzyme bioconversion, resting cells synthesized useful pyrogallol from tannic acid.
MATERIALS AND METHODS
Materials.
Tannic acid, gallic acid, and pyrogallol were obtained from Ishizu. DEAE-Sephacel, Superdex 16-60 Hi-load, a fast protein liquid chromatograph (FPLC), and the low-molecular-weight markers used for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) were obtained from Pharmacia. The molecular marker proteins used for gel filtration were purchased from Oriental Yeast. Unless otherwise stated, all other chemicals were obtained from Wako, Osaka, Japan.
Screening and culture conditions.
To prepare an enrichment culture, 3 g of different soil samples from areas surrounding Okayama, Japan, was added to 50 ml of medium containing (per liter) 10 g of tannic acid, 2 g of (NH4)2HPO4, 1 g of KH2PO4, 0.5 g of MgSO4 · 7 H2O, and 0.5 g of yeast extract (pH 6.0). The enrichment culture was refreshed four times at 7-day intervals by transferring 100 μl of the culture into fresh medium. Microorganisms were isolated on agar plates containing the medium described above except that 2 g of gallic acid per liter replaced tannic acid as the main source of carbon and energy. The isolates were cultivated on the medium containing tannic acid as the main source of carbon and energy, and pyrogallol formation was determined by high-performance liquid chromatography (HPLC) after 12 h of growth. The homogeneity of T71, which was selected because it was the strain that produced the most pyrogallol, was confirmed by growing the organism on agar plates containing 10 g of polypeptone (Daigo) per liter, 5 g of meat extract (Mikuni) per liter, and 5 g of NaCl per liter (pH 6.0) and by microscopic analysis.
In order to obtain large amounts of induced biomass, an overnight preculture grown on the medium containing 10 g of polypeptone per liter, 5 g of meat extract per liter, and 5 g of NaCl per liter (pH 6.0) was used to inoculate a 2-liter shaking flask containing 400 ml of medium. The medium used to induce gallic acid decarboxylase contained (per liter) 3 g of gallic acid, 5 g of glycerol, 5 g of polypeptone, 10 g of yeast extract, 1 g of KH2PO4, 0.5 g of MgSO4 · 7 H2O, and 0.01 g of FeSO4 · 7 H2O (pH 6.0). The medium used to induce tannase contained (per liter) 15 g of tannic acid, 5 g of sucrose, 2 g of (NH4)2HPO4, 1 g of KH2PO4, 0.5 g of MgSO4 · 7 H2O, and 0.5 g of yeast extract (pH 6.0). Cultivation was carried out at 28°C for 24 h with reciprocal shaking. Cells were harvested by centrifugation at 10,000 × g at 4°C and were washed twice with 50 mM potassium phosphate buffer (pH 6.0) (buffer A) containing 1 mM dithiothreitol and 50 mM Na2S2O3.
Enzyme assay.
Unless otherwise noted, decarboxylase activity was assayed at 30°C in 2 ml of buffer A containing 7.5 mM gallic acid and an appropriate amount of enzyme. The reaction was stopped after 10 min with 2 ml of acetonitrile, the preparation was centrifuged, and the gallic acid and pyrogallol contents were determined by HPLC. One unit of activity was defined as the amount of enzyme that catalyzed the formation of 1 μmol of pyrogallol per min. Tannase activity was tested under the same reaction conditions except that 1% (wt/vol) tannic acid replaced gallic acid as the substrate.
Resting cell bioconversions.
Experiments to determine resting cell conversion of tannic acid and gallic acid to pyrogallol were performed in 2 ml of buffer A containing 50 mM l-ascorbate, 1.0% (wt/vol) tannic acid or 300 mM gallic acid, and eightfold-concentrated resting cells. l-Ascorbate was used as the stabilizer instead of dithiothreitol and Na2S2O3 because of its lower price and therefore its higher relevance for application even if the stabilizing effect was reduced (76% of the enzyme activity remained after 3 h of dialysis, compared to 98% of the activity when 1 mM dithiothreitol and 50 mM Na2S2O3 were used). For tannic acid conversion, a 20:1 (vol/vol) mixture of tannic acid-induced resting cells and gallic acid induced resting cells and a combination of gallic acid-induced resting cells and 13.5 U of tannase from Aspergillus oryzae were used as biocatalysts. For conversion of gallic acid, gallic acid-induced cells were employed.
Enzyme purification.
All purification steps were performed at 4°C in buffer A containing 50 mM Na2S2O3 and 1 mM dithiothreitol unless otherwise specified. Centrifugation was carried out for 30 min at 20,000 × g. Gallic acid-induced cells from 1 liter of culture broth (4.2 g [dry weight]) were suspended in 40 ml of buffer A, disrupted for 20 min by ultrasonication (19 kHz; Insonator model 201M; Kubota), and centrifuged. The crude extract was fractionated with ammonium sulfate (30 to 50% saturation), and the 50% precipitate was dissolved in buffer A and loaded onto a DEAE-Sephacel column (2.0 by 2.5 cm) previously equilibrated with this buffer. The enzyme eluted at 40 mM KCl in a linear 0 to 200 mM KCl gradient in this buffer; the enzyme was fractionated with ammonium sulfate (40 to 50% saturation), centrifuged, and redissolved in buffer A. Then it was applied to an FPLC Superdex 16-60 Hi-load gel filtration column (1.6 by 60 cm) equilibrated with buffer A containing 0.2 M NaCl, and it eluted as a single, symmetrical peak with this buffer at a flow rate of 30 ml/h. The purified enzyme was dialyzed against buffer A containing 50% (vol/vol) glycerol, 50 mM Na2S2O3, and 1 mM dithiothreitol and stored at −20°C.
Enzyme characterization.
Enzyme stability was examined by dialyzing the crude extract against various reducing agents at different concentrations in buffer A. The pH stability and temperature stability were determined by incubating the enzyme for 3 h in 50 mM buffers at various pH values at 4°C and for 30 min in buffer A at various temperatures, respectively, and then performing activity tests under standard conditions. The Km was estimated from a Lineweaver-Burk plot. Metals and group-specific inhibitors were tested by incubating the enzyme for 10 min with each compound at a concentration of 1 mM at 30°C in the standard reaction mixture without gallic acid.
Analytical methods.
Gallic acid and pyrogallol were analyzed with a Shimadzu model LC-6A HPLC equipped with a Spherisorb S5ODS column (4.6 by 150 mm); 10 mM KH2PO4-H3PO4 (pH 2.8)–acetonitrile (99:1, vol/vol) a flow rate of 1 ml/min was the eluent, and the eluate was monitored at 230 nm. Authentic gallic acid and pyrogallol were used for calibration. The retention times of gallic acid and pyrogallol were 10.5 and 5.9 min, respectively. SDS-PAGE was performed in 10% (wt/vol) polyacrylamide gels (29), and gradient PAGE (3 to 10% [wt/vol] polyacrylamide) was performed with an Ato model AE-6000 NPG-310L apparatus. The proteins in gels were stained with Coomassie blue R-250. The proteins were quantified by the method of Bradford (5) by using bovine serum albumin as the standard. Absorption spectra were recorded with a Shimadzu model UV-240 spectrophotometer. The N-terminal amino acid sequence of 1 μg of enzyme was analyzed by automated Edman degradation with a model 470A gas phase amino acid sequencer (Applied Biosystems).
For the metal analysis, all glassware was briefly boiled in 0.1 M HCl and exhaustively rinsed with bidistilled and deionized water before use. The iron content of a 10-μg/ml purified enzyme preparation was measured with an inductively coupled radiofrequency plasma spectrophotometer (model ICPV-1000; 27,120 MHz; Shimadzu) by using a cooling gas flow rate of 15 liters/min, a plasma gas flow rate of 1.2 liters/min, and a carrier gas flow rate of 1.0 liter/min. For qualitative analysis, spectra were scanned from 400 to 190 nm at a rate of 25 nm/min. Iron was quantified from the plasma emission spectrum at 259.9 nm by using calibration curves prepared with standard solutions. The iron dependence of the enzyme was examined by adding 0 to 100 mg of FeSO4 · 7 H2O per liter to a medium containing (per liter) 3 g of gallic acid, 5 g of glycerol, 2 g of (NH4)2HPO4, 1 g of KH2PO4, and 0.5 g of MgSO4 · 7 H2O (each compound was the purest grade commercially available) in deionized and bidistilled water (pH 6.0).
The molecular mass of native gallic acid decarboxylase was estimated by gradient PAGE and gel filtration on FPLC and HPLC with a TSK G-3000SW column (0.75 by 60 cm; Toyo Soda) by using buffer A containing 0.2 M NaCl at a flow rate of 0.7 ml/min as the eluent. The molecular mass was calculated from a linear regression curve obtained from the mobilities of the standard proteins glutamate dehydrogenase (290 kDa), lactate dehydrogenase (142 kDa), enolase (67 kDa), adenylate kinase (32 kDa), and cytochrome c (12.4 kDa).
RESULTS
Microorganism.
Strain T71, which utilized tannic acid and gallic acid as sole sources of carbon and energy, was isolated from a soil sample from an orchard near Okayama, Japan. Preliminary studies revealed that this organism is a motile, gram-negative, catalase-positive oxidase-negative strain which produces round, cream-colored colonies that are 2 mm in diameter and which exhibits fermentative growth on glucose. These characteristics suggest that strain T71 belongs to the soil- and plant-associated, facultatively anaerobic Enterobacter-Erwinia group. Additional biochemical tests revealed that strain T71 produces acid from a variety of sugars, lacks urease, arginine dihydrolase, lysine decarboxylase, cytochrome oxidase, and gelatinase activities, and does not produce H2S. On the basis of these and other results (particularly the lack of indole production and gas formation from d-glucose), strain T71 was shown to belong to the new genus Pantoea (formerly Enterobacter) (9) and was identified as P. agglomerans, which is consistent with the results of a recent biochemical characterization of this species (17). T71 has been deposited in the collection of the Fermentation Research Institute, Ministry of International Trade & Technology, Japan, as strain FERM P-16375.
Stability and activity of the enzyme.
Probably due to oxygen sensitivity, the purified gallic acid decarboxylase activity without stabilization was totally lost after incubation for 3 h at 4°C. The enzyme was stable in crude extract, and 82% of the activity remained after 3 days. However, dialysis of the crude extract against buffer A for 3 h resulted in a complete loss of activity. If reducing agents were added to the dialysis buffer, enzyme activity after 3 days was maintained best by 50 mM Na2S2O3 plus 1 mM dithiothreitol (about 77% of the enzyme activity was retained). Preparations containing other reducing agents, such as 50 mM Na2S2O3 alone, as well as 50 mM Na2S2O4, 50 mM Na2S2O5, and 50 mM ascorbate, retained about 50% of the enzyme activity. A preparation containing 10 mM dithiothreitol retained 17% of the enzyme activity, while no activity was observed with preparations containing 1 to 10 mM gallic acid, 1 to 10 mM pyrogallol, or 1 to 10 mM FeSO4 · 7H2O. The purified enzyme was stored at −20°C in buffer A containing glycerol and reducing agents with no loss of activity for 3 weeks. The enzyme was stable below at temperatures 50°C and at pH 6.0 to 10.0. The pH and temperature optima of the enzyme were determined to be 6.0 and 50°C, respectively.
Purification and structure of gallic acid decarboxylase.
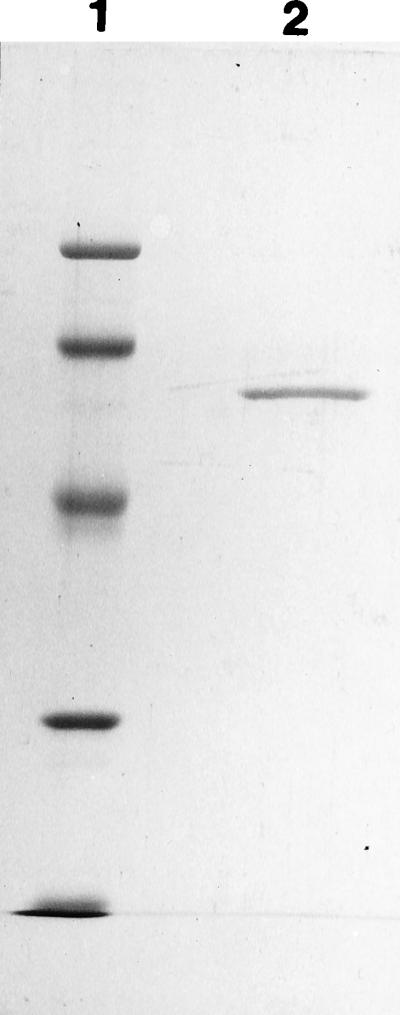
Gallic acid decarboxylase, induced only by its substrate, was purified and both the yield and enrichment were limited by the instability of the enzyme (Table 1). The SDS-PAGE results indicated that the purified enzyme was homogeneous and that the subunit molecular mass was 57 kDa (Fig. 2). The purity of the enzyme was confirmed by the fact that it eluted as a single symmetrical peak on HPLC and FPLC gels, which revealed that the native molecular mass was 320 kDa. Gradient PAGE revealed two bands, one at 165 kDa and one at 330 kDa, suggesting that the native enzyme is a homohexamer that might also appear as a trimer. The pure enzyme catalyzed the decarboxylation of gallic acid to stoichiometric amounts of pyrogallol with a Vmax of 150 U/mg and a Km of 0.96 mM.
TABLE 1.
Purification of gallic acid decarboxylase from P. agglomerans T71a
| Step | Amt of protein (mg) | Total activity (U) | Sp act (U/mg) | Fold purification | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 814 | 7,420 | 9.1 | 1.0 | 100 |
| (NH4)2SO4 (30–50%) | 305 | 6,120 | 20 | 2.2 | 82 |
| DEAE-Sephacel | 44.8 | 3,380 | 75 | 8.3 | 46 |
| (NH4)2SO4 (40–50%) | 17.3 | 1,810 | 105 | 11.5 | 24 |
| Superdex 16-60 Hi-load | 5.2 | 782 | 150 | 16.5 | 11 |
The original preparation contained 4.2 g (dry weight) of cells.
FIG. 2.
SDS-PAGE of purified gallic acid decarboxylase from P. agglomerans T71. Lane 1 contained low-molecular-weight marker proteins (molecular weights, 94,000, 67,000, 43,000, and 30,000); the 20.1- and 14.4 kDa markers at the bottom produced one band. Lane 2 contained 2 μg of purified enzyme.
N-terminal amino acid sequence.
The sequence of the 15 N-terminal amino acids was found to be SNTEN LPAND VYDLR. A database search, in which SWISS PROT and PIR were used, revealed no homology to similar enzymes. However, currently the sequences of only two similar aromatic acid decarboxylases are available (18, 27). The level of homology to the nucleotide-derived N terminus of a hypothetical 28-kDa protein of a red alga was 45%.
Effects of metals, inhibitors, and activators.
The enzyme was totally inhibited by oxidants, such as K2CrO4, (NH4)2S2O8, and H2O2, by thiol-specific p-chloromercuribenzoate, by Ag2SO4, by HgCl2, and by CuCl2; it was inhibited to lesser extents by KCN (37% inhibition), NaNO3 (25%), and cuprizione (31%). No significant effects on enzyme activity were observed with NaCl, BaCl2, CaCl2, MnCl2, MgSO4, PbCl2, ZnSO4, CoCl2, SnCl2, NiCl2, CdCl2, Al2(SO4)3, Na2MoO4, NaN3, NaF, iminodiacetic acid, iodoacetic acid, 5,5′-dithiobis(2-nitrobenzoate), and phenylmethylsulfonyl chloride. Cysteamine, semicarbazide, phenylhydrazine, and hydroxylamine, which are known to inhibit pyridoxal 5′-phosphate-dependent decarboxylases, also had no influence.
Iron cofactor.
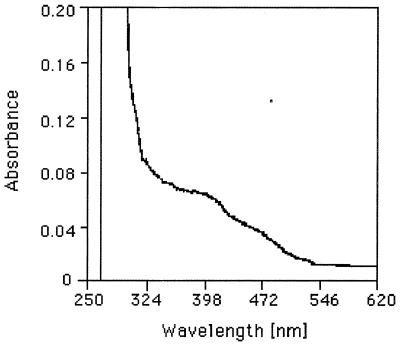
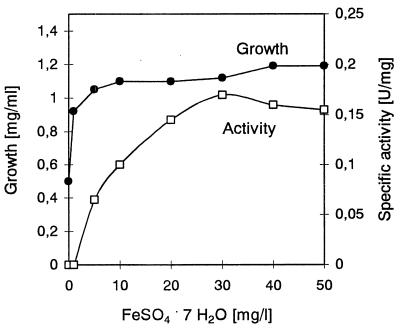
The purified enzyme produced absorption shoulders in the UV-visible spectrum at 398 and 472 nm (Fig. 3); the ratio of absorption at 398 nm (A398) to A280 was 0.064, and the A472/A280 ratio was 0.038. The addition of gallic acid slightly enhanced the A398 and A472. After Fe2+ was added to an iron-free synthetic medium, the specific activity increased; the highest levels of enzyme activity were observed at concentrations greater than 30 mg/liter FeSO4 · 7 H2O (Fig. 4). Plasma emission spectroscopy showed that the pure enzyme contained 0.82 mol of iron per mol of subunit. The enzyme was inhibited by Fe2+-chelating agents, such as 2,2′-bipyridyl (87% inhibition), o-phenanthroline (50%), and EDTA (28%), at a concentration of 1 mM. On the other hand, thiourea, N,N′-diethyldithiocarbamate, 8-hydroxyquinoline, and Fe3+-specific Tiron (4,5-dihydroxy-m-benzene disulfonate) had no effect, and FeCl2 and FeCl3 inhibited the enzyme slightly (29 and 24%, respectively).
FIG. 3.
Absorption spectrum of purified gallic acid decarboxylase from P. agglomerans T71. The spectrum was recorded by using 1 mg of purified enzyme per ml in buffer A.
FIG. 4.
Activation of gallic acid decarboxylase from P. agglomerans T71 by iron. Different amounts of Fe2+ were added to an iron-free synthetic medium. After 30 h of cultivation, cell growth (dry weight) and enzyme activity were determined.
Substrate specificity.
None of the structural analogs of gallic acid (namely, benzoate, 3- and 4-hydroxybenzoates, 2,3-, 2,4-, 2,5-, 2,6-, 3,4-, and 3,5-dihydroxybenzoates, 2,3,4-, 2,4,5-, and 2,4,6-trihydroxybenzoates, 4-hydroxy-3-methoxybenzoate, and 3- and 4-aminobenzoates) was decarboxylated when it was added at a concentration of 10 mM to 20 U of enzyme. Furthermore, the enzyme did not catalyze reverse carboxylation of 100 mM pyrogallol in a reaction mixture containing 1 M NaHCO3 in buffer A.
Resting cell bioconversion of tannic acid to pyrogallol.
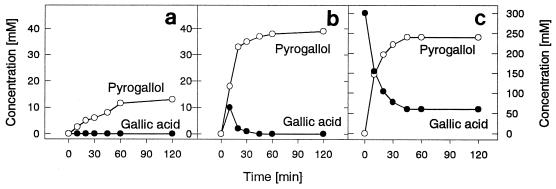
After induction by the substrates, the tannase activity was 85-fold lower (0.013 μmol of tannic acid per min per mg [dry weight] of cells) than the gallic acid decarboxylase activity (1.103 U/mg). Furthermore, tannase was not induced after growth on gallic acid, and the times required for maximal biomass yield (3.5 mg [dry weight] of cells per ml) and enzyme activity were 60 h on tannic acid-containing medium and 7 h on gallic acid-containing medium. Low gallic acid decarboxylase activity was observed after growth on tannase due to the formation of gallic acid. In a two-enzyme reaction mixture containing both tannic acid-induced resting cells and gallic acid-induced resting cells, 13 mM pyrogallol was formed from tannic acid (Fig. 5a). When 13.5 U of tannase from Aspergillus oryzae was added to gallic acid-induced cells, 39 mM pyrogallol was formed (Fig. 5b). In a one-step bioconversion in which gallic acid-induced resting cells were used, 240 mM pyrogallol (30.2 g of pyrogallol per liter) was formed from 300 mM gallic acid (Fig. 5c).
FIG. 5.
Bioconversion of tannic acid (a and b) and gallic acid (c) to pyrogallol by resting cells of P. agglomerans T71. The results obtained with a mixture of tannic acid-induced cells and gallic acid-induced cells (a), a combination of gallic acid-induced cells and A. oryzae tannase (b), and gallic acid-induced cells (c) are shown.
DISCUSSION
A number of gallic acid decarboxylases from mainly anaerobic sources have been described; however, these enzymes have not been purified due to their instability (6, 10–12, 19, 21, 26, 34). P. agglomerans T71 gallic acid decarboxylase was also found to be unstable; however, it was stabilized with reducing agents as a prerequisite for purification. The stabilizing effect of reducing agents suggests that the enzyme is sensitive to oxygen. The enzyme can be induced by gallic acid. The other gallic acid decarboxylases include both constitutive (10, 19, 21) and inducible (6, 12, 21, 26, 34) enzymes. The high substrate specificity of P. agglomerans T71 gallic acid decarboxylase is consistent with data obtained for Eubacterium oxidoreducens gallic acid decarboxylase (12), whereas most other gallic acid decarboxylases have a broader substrate spectrum (6, 21, 26, 34).
Nonoxidative, aromatic acid decarboxylases generally have no cofactor requirement (10, 12, 14, 15, 18, 19, 21, 22, 24, 26–28, 34); the only exception is a gallic acid decarboxylase from anaerobic Pelobacter acidigallici that requires Mg2+ (6). We found evidence that iron is involved in the catalysis of P. agglomerans T71 gallic acid decarboxylase. Since only 0.8 mol of iron per mol of enzyme subunit was detected, we assumed that a small amount of iron was lost during purification. Similar losses of iron during purification have been described for several oxygenases (3, 4). The oxygen sensitivity of this enzyme might be due to an oxidable iron-sulfur cluster like the clusters found previously in dioxygenases (30, 33). Electron spin resonance studies should help substantiate this hypothesis, characterize the cofactor role of iron, and determine whether Fe2+ or Fe3+ is catalytically active.
Gallic acid is the product of acidic or enzymatic hydrolysis of tannic acid, a readily available polyphenol in plants. Tannases are used in the processing of tea and other plant products (20). These enzymes have been found mainly in fungi (1, 2, 16, 25, 31, 32) and in some bacteria (7, 8). No gallic acid decarboxylase has been found in a variety of gram-negative bacteria that contain tannases (23). On the other hand, no tannases have been found in gallic acid decarboxylase-containing microorganisms (6, 10–12, 19, 21, 26, 34). P. agglomerans T71 is unique in that it contains both a tannase and a gallic acid decarboxylase. Pyrogallol, the product of tannase and gallic acid decarboxylase, has widespread industrial applications; it is used as a developer in photography, for staining leather, fur, and hair, as a precursor for dyes, and for determining oxygen concentrations in gas analyses. This compound is produced industrially from tannic acid by using an A. oryzae tannase and then autoclaving gallic acid in the presence of 6 N HCl. The acid step requires subsequent neutralization, which is accompanied by the formation of huge amounts of salt. From an economic and environmental view, a two-enzyme bioconversion under mild reaction conditions is advantageous. The first attempts described here resulted in pyrogallol yields of 13 to 39 mM, which were limited by the tannase activity. In order to make this process more economical for biotechnological applications, we are currently optimizing simultaneous induction of tannase and gallic acid decarboxylase. When gallic acid was used as the substrate, the pyrogallol yield was increased to 240 mM, which is in the range reported previously for microbial conversion by Citrobacter sp. (35). Both the one- and two-enzyme bioconversions described here are promising procedures for providing pyrogallol preparations in an environmentally acceptable manner.
ACKNOWLEDGMENT
M.W. was supported by the Deutscher Akademischer Austauschdienst.
REFERENCES
- 1.Aoki K, Shinke R, Nishira H. Purification and some properties of yeast tannase. Agric Biol Chem. 1976;40:79–85. [Google Scholar]
- 2.Aoki K, Shinke R, Nishira H. Chemical properties and molecular weight of yeast tannase. Agric Biol Chem. 1976;40:297–302. [Google Scholar]
- 3.Bernhardt F-H, Meisch H-U. Reactivation studies on putidamonooxin—the monooxygenase of a 4-methoxybenzoate O-demethylase from Pseudomonas putida. Biochem Biophys Res Commun. 1980;93:1247–1253. doi: 10.1016/0006-291x(80)90623-3. [DOI] [PubMed] [Google Scholar]
- 4.Bill E, Bernhardt F-H, Trautwein A X, Winkler H. Mössbauer investigation of the cofactor iron of putidamonooxin. Eur J Biochem. 1985;147:177–182. doi: 10.1111/j.1432-1033.1985.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brune A, Schink B. Phloroglucinol pathway in the strictly anaerobic Pelobacter acidigallici: fermentation of trihydroxybenzenes to acetate via triacetic acid. Arch Microbiol. 1992;157:417–424. [Google Scholar]
- 7.Deschamps A M, Mahoudeau G, Conti M, Lebeault J-M. Bacteria degrading tannic acid and related compounds. J Ferment Technol. 1980;58:93–97. [Google Scholar]
- 8.Deschamps A M, Otuk G, Lebeault J-M. Production of tannase and degradation of chestnut tannin by bacteria. J Ferment Technol. 1983;61:55–59. [Google Scholar]
- 9.Evguenievahackenberg E, Selenskapobell S. Genome analysis of five soil bacterial isolates named formerly Enterobacter agglomerans. J Appl Bacteriol. 1995;79:49–60. [Google Scholar]
- 10.Grant D J W, Patel J C. The non-oxidative decarboxylation of p-hydroxybenzoic acid, gentisic acid, protocatechuic acid and gallic acid by Klebsiella aerogenes (Aerobacter aerogenes) Antonie Leeuwenhoek. 1969;35:325–343. doi: 10.1007/BF02219153. [DOI] [PubMed] [Google Scholar]
- 11.Gupta J K, Jebsen C, Keifel H. Sinapic acid degradation by yeast Rhodotorula glutinis. J Gen Microbiol. 1986;132:2793–2799. [Google Scholar]
- 12.Haddock J D, Ferry J G. Initial steps in the anaerobic degradation of 3,4,5-trihydroxybenzoate by Eubacterium oxidoreductans: characterization of mutants and role of 1,2,3,5-tetrahydroxybenzene. J Bacteriol. 1993;175:669–673. doi: 10.1128/jb.175.3.669-673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haslam E, Haworth R D, Jones K, Rogers H J. Gallotannins. Part I. Introduction: and the fractionation of tannase. J Chem Soc. 1961;1961:1829–1835. [Google Scholar]
- 14.He Z, Wiegel J. Purification and characterization of an oxygen-sensitive reversible 4-hydroxybenzoate decarboxylase from Clostridium hydroxybenzoicum. Eur J Biochem. 1995;229:77–82. doi: 10.1111/j.1432-1033.1995.tb20440.x. [DOI] [PubMed] [Google Scholar]
- 15.He Z, Wiegel J. Purification and characterization of an oxygen-sensitive, reversible 3,4-dihydroxybenzoate decarboxylase from Clostridium hydroxybenzoicum. J Bacteriol. 1996;178:3539–3543. doi: 10.1128/jb.178.12.3539-3543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iibuchi S, Minoda Y, Yamada K. Hydrolyzing pathway, substrate specificity and inhibition of tannin acyl hydrolase of Asp. oryzae No. 7. Agric Biol Chem. 1972;36:1553–1562. [Google Scholar]
- 17.Iimura K, Hosono A. Biochemical characteristics of Enterobacter agglomerans and related strains found in buckwheat seeds. Int J Food Microbiol. 1996;30:243–253. doi: 10.1016/0168-1605(96)00949-x. [DOI] [PubMed] [Google Scholar]
- 18.Jones M E. Orotidylate decarboxylase of yeast and man. Curr Top Cell Regul. 1992;33:331–342. doi: 10.1016/b978-0-12-152833-1.50024-1. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz L R, Crawford R L, Hemling M E, Bryant M P. Metabolism of gallate and phloroglucinol in Eubacterium oxidoreductans via 3-hydroxy-5-oxohexanoate. J Bacteriol. 1987;169:1886–1890. doi: 10.1128/jb.169.5.1886-1890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekha P K, Lonsane B K. Production and application of tannin acyl hydrolase. State of the art. Adv Appl Microbiol. 1997;44:215–260. doi: 10.1016/s0065-2164(08)70463-5. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H, Otani C, Niimura T. Decarboxylation of gallate by cell-free extracts of Streptococcus faecalis and Klebsiella pneumoniae isolated from rat feces. J Food Hyg Soc Jpn. 1992;33:371–376. [Google Scholar]
- 22.Nakazawa T, Hayashi E. Phthalate and 4-hydroxyphthalate metabolism in Pseudomonas testosteroni: purification and properties of 4,5-dihydroxyphthalate decarboxylase. Appl Environ Microbiol. 1978;36:264–269. doi: 10.1128/aem.36.2.264-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemoto K, Osawa R, Hirota K, Ono T, Miyake Y. An investigation of gram-negative tannin-protein complex degrading bacteria in fecal flora of various mammals. J Vet Med Sci. 1995;57:921–926. doi: 10.1292/jvms.57.921. [DOI] [PubMed] [Google Scholar]
- 24.Pujar B G, Ribbons D W. Phthalate metabolism in Pseudomonas fluorescens PHK: purification and properties of 4,5-dihydroxyphthalate decarboxylase. Appl Environ Microbiol. 1985;49:374–376. doi: 10.1128/aem.49.2.374-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajakumar G S, Nandy S C. Isolation, purification, and some properties of Penicillium chrysogenum tannase. Appl Environ Microbiol. 1983;46:525–527. doi: 10.1128/aem.46.2.525-527.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samain E, Albagnac G, Dubourguier H-C. Initial steps of catabolism of trihydroxybenzenes in Pelobacter acidigallici. Arch Microbiol. 1986;144:242–244. [Google Scholar]
- 27.Santha R, Savithri H S, Rao A, Vaidyanathan C S. 2,3-Dihydroxybenzoic acid decarboxylase from Aspergillus niger. A novel decarboxylase. Eur J Biochem. 1995;230:104–110. [PubMed] [Google Scholar]
- 28.Santha R, Savithri H S, Rao A, Vaidyanathan C S. Identification of the active site-peptide of 2,3-dihydroxybenzoic acid decarboxylase from Aspergillus oryzae. Biochim Biophys Acta. 1996;1293:191–200. doi: 10.1016/0167-4838(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 29.Schägger H, van Jagow G. Tricin-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer D, Markus A, Seez M, Ruf H H, Lingens F. Purification and some properties of component B of the 4-chlorophenylacetate 3,4-diooxygenase from Pseudomonas species strain CBS3. J Biol Chem. 1987;262:9340–9346. [PubMed] [Google Scholar]
- 31.Weetal H H. Enzymatic gallic acid esterification. Biotechnol Bioeng. 1985;27:124–127. doi: 10.1002/bit.260270203. [DOI] [PubMed] [Google Scholar]
- 32.Yamada H, Adachi O, Watanabe M, Sato N. Studies on fungal tannase. Agric Biol Chem. 1968;32:1070–1078. [Google Scholar]
- 33.Yamaguchi M, Fujisawa H. Characterization of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem. 1978;255:5058–5063. [PubMed] [Google Scholar]
- 34.Yoshida H, Tani Y, Yamada H. Isolation and identification of a pyrogallol producing bacterium from soil. Agric Biol Chem. 1982;46:2539–2546. [Google Scholar]
- 35.Yoshida H, Yamada H. Microbial production of pyrogallol through decarboxylation of gallate. Agric Biol Chem. 1985;49:659–663. [Google Scholar]