Abstract
Introduction
Chemotherapeutics are known to have undesirable side effects (i.e. nausea, weight loss, hair loss, weakened immune system, etc.) due to the non-specificity of the drugs. Encapsulation of these chemotherapeutics inside nanoparticles significantly improves the bioavailability and half-life of drugs, while increasing their tumor penetration and localization. However, most, if not all, nanoparticles in clinics or research are synthetic, with no long-term studies on the effect of these nanoparticles in vivo. Herein, we developed a synergistic resveratrol nanoparticle system by using lecithin encapsulation. Lecithin, being a fully natural phospholipid derived from soybean, possesses inherent anti-tumor activity.
Methods
Lec(RSV) was successfully prepared using the nanoprecipitation method, and characterized by particle size and zeta potential analysis, and transmission electron microscopy (TEM). The in vitro cellular uptake and cytotoxic effects of Lec(RSV) were investigated in human breast cancer cell line BT474. Finally, the in vivo tumoral uptake of Lec(RSV) was carried out in the BT474 orthotopic model.
Results
Lec(RSV) showed a uniform distribution of ~120 nm, with prolonged stability. Lec(RSV) showed high cellular uptake and anti-cancer properties in vitro. Time-dependent uptake in the BT474 xenograft model indicated an increased tumoral uptake and apoptosis rate at 4 hours after tail vein injection of Lec(RSV).
Conclusion
Taken together, we successfully developed a fully natural Lec(RSV) that possesses potent anti-cancer activity in vitro, with good tumoral uptake in vivo. We hypothesize that Lec(RSV) could be a safe anti-cancer therapeutic that could be easily translated into clinical application.
Keywords: fully natural nanoparticle, lecithin, resveratrol, anti-tumor properties
Introduction
Conventional chemotherapy is a crucial component of cancer treatments for various cancer types, where the treatment strategy includes using toxic drugs to kill cancer cells.1,2 Although chemotherapy brings great breakthroughs in cancer treatment, it causes many undesirable side effects, such as nausea, weight loss, hair loss as well as a weakened immune system. Therefore, there is a constant lookout for natural ingredients demonstrating biological properties supporting chemotherapy, as well as having lower toxic effects.3,4 Resveratrol (RSV), a non-flavonoid polyphenol natural bioactive compound found in various plants,5 demonstrated cancer chemo-preventive properties in 1997.6,7 However, even though RSV was demonstrated to be a promising anti-cancer candidate in several in vitro cancer studies, it was restricted to clinical use due to its quick elimination from the body, and because it lacked therapeutically relevant levels in the bloodstream.8 Therefore, it is necessary to establish a proper carrier to deliver RSV so that it can take effect on site.
Lipid-based drug delivery vehicles, including liposomes and micelles, have been intensively studied and widely used for multifarious biomedical applications, such as cancer therapy.9–11 As a drug carrier, nanoparticles have some unique superiorities that protect the drug from degradation in circulation and reduce systemic toxic effects. Moreover, due to their small diameters, nanoparticles can penetrate through the fissures of intra-tumor vessels into the tumor and remain in the tumor because of the obstruction of tumor lymphatic drainage, a phenomenon known as the enhanced permeability and retention effect (EPR).12,13 Therefore, the ideal strategy for RSV to be applied as an effective clinical anti-cancer agent is to establish proper nanocarriers for delivery. Al-jubori et al successful created layer-by-layer nanoparticles of Tamoxifen and RSV for a dual drug delivery system and potential triple-negative breast cancer treatment.14
Currently, nanoparticle delivery systems usually include the use of poly(ethylene)-glycol (PEG). It is a synthetic polymer that is capable of encapsulating most small molecules into spherical nano-sized particles. However, previous studies mentioned that while the main concern was carcinogenic contaminants, PEG compounds themselves demonstrated some findings of genotoxicity15,16 besides resulting in irritation and systemic toxicity in serious cases.17 Contrary to PEG, Lec is naturally present in plant and animal tissue, with a combination of glycerophospholipids, which includes phosphatidylethanolamine, phosphatidylcholine, phosphatidic acid, and phosphatidylinositol. Due to its phosphatidylcholine content, lecithin is a reservoir of choline, which is also an essential nutrient.18
Herein, we combined two natural substances, Lec and RSV, and designed a nanoplatform based on the lipid structure of lecithin to encapsulate resveratrol. Lec(RSV) has a uniformed size distribution of ~120 nm, with a resveratrol encapsulation efficiency of 85.2%. This nanoparticle is stable at ambient temperature as well as at 4°C for up to twelve months. The release kinetics of the Lec(RSV) nanoparticle revealed a slow release of resveratrol from the Lec nanoparticle, with inherent anti-oxidant and anti-cancer properties; indicating the feasibility of using this Lec(RSV) system as an alternative, cost-effective, and low side-effect anti-cancer therapeutic.
Materials and Methods
Materials
Resveratrol (RSV, 99%) was acquired from Aladdin (Shanghai, China). Lecithin and Coumarin-6 were acquired from Sigma Aldrich, and Alamar Blue was purchased from ThermoFisher Scientific. Organic solvents (DMSO, DMF, 75% Ethanol) were purchased from Sigma Aldrich. The water was prepared with a Millipore (Bedford, MA, USA) Milli-Q® system, and all other chemicals were of analytical grade.
Synthesis of Lec(RSV)
Lec(RSV) was prepared using the nanoprecipitation method as described in our previous publication.19 Briefly, RSV (100 mg/mL) and Lec (100 mg/mL) were dissolved separately in DMSO. To create a working solution of Lec(RSV), 10 µL of Lec was added to 1 µL of RSV. 89 µL of DMSO was added to the mixture to make 100 µL of the solution. On a separate glass vial, 10 mL of Millipore grade water was added and stirred continuously at 12,000 rpm at ambient temperature. The mixture solution was added slowly into the vial via pipetting. The solutions were then transferred to Amicon Filter (Life Science Research) and centrifuged at 2800 rpm for 10 min. After each round of centrifugation, the remaining solutions were washed with 5 mL of water and centrifuged again at 4000 rpm for 10 min. This was repeated twice to eliminate all remaining DMSO. The final Lec(RSV) was diluted to a final concentration of 1 mL in PBS and stored at 4°C until further use.
Size Distribution and Zeta Potential
To determine the size and zeta potential of Lec and Lec(RSV), each formulation was transferred into transparent cuvettes for Dynamic Light Scattering (DLS) analysis. Their hydrodynamic diameter and zeta potentials were measured using the Zetasizer nano ZS90 (Malvern Instruments, UK).20 Data for each sample were obtained from three replicates, with five runs in each replicate.
Transmission Electron Microscopy (TEM) Analysis
Five µL aliquots of Lec(RSV) (10 mg/mL) were applied onto TEM-grade carbon-only mesh copper grids. Particles were left on the grid at ambient temperature for 5 min. Each grid was washed five times with distilled water. The specimens subsequently underwent negative staining using 2% uranyl acetate and were left at ambient temperature for 2 min. Grids were then washed thrice with distilled water and air-dried. The specimens were visualized using a TECNAI F20 electron microscope (Philips Electronic Instruments Corp., Mahwah, NJ).
Encapsulation Efficiency
Although the UV absorbance of RSV is 303 nm, lecithin has a wide UV absorbance of 200-500 nm, and a peak at 355 nm. This could lead to reading interference. Therefore, we have substituted a fluorescence molecule Coumarin-6 (Cou; ex: 420 nm) as it has a similar molecular weight to RSV. The synthesis of Lec(Cou) is the same as indicated in Synthesis of Lec(RSV). After purification, the samples were rehydrated to a volume of 1 mL in PBS. Then, 10 µL of samples of Lec(Cou) were taken and added to 90 µL of DMSO. Positive control would be 10 µL from the original stock of the working solution. The fluorescence intensity of coumarin was detected via a fluorospectrometer. To determine the encapsulation efficiency (EE) of coumarin-6, we calculated the amount of coumarin-6 in the Lec(Cou) as compared to the original amount of coumarin-6 used initially, with the following formula:
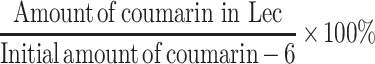 |
Stability of Lec and Lec(RSV)
To determine the stability of Lec and Lec(RSV), we synthesized Lec and Lec(RSV) as above mentioned at 10 mg/mL. The Lec and Lec(RSV) particles were then stored in PBS with 10% FBS to mimic the physiological condition. The vials were then kept in a 37°C chamber with constant stirring. At pre-determined time points (1, 2, 4, 8, 12, 24, and 48 hours), the size of Lec and Lec(RSV) particles was assessed by DLS and recorded.
In vitro Release Profile of RSV
To determine the release profile of encapsulated RSV, we used a reversed method to detect the released RSV in the outer PBS compartment. 2 mg/mL of RSV and Lec (RSV) were respectively added into 1 mL of PBS (containing 10% FBS, pH 7.4) respectively and then moved to a Float-a-Lyzer G2 dialysis device (MWCO 100 kD, Spectrum Lab) that was immersed in 1 mL of PBS (pH 7.4) at 37°C. At predetermined intervals (1, 2, 4, 8, 12, 24, 48, 72, 96 hours), the outer PBS solution was collected in an Eppendorf tube. At each time point, 1 mL of fresh PBS (pH 7.4) was replaced for the outer solution. This method was repeated until PBS solutions for all time points were collected. The amount of RSV in each Eppendorf tube was then determined by UV absorbance at 303 nm by a Synergy HT multi-mode microplate reader.
Cell Culture
Human breast cancer cells BT474 were acquired from ATCC and were cultured and utilized according to the protocols provided by the supplier. The cells were kept at 37°C in a humidified cell culture chamber with 5% CO2. Cells were suspended in RPMI-1640/F-12K medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin.
In vitro Cellular Uptake
To illustrate the in vitro uptake and internalization of Lec, Coumarin-6 (1µM) was added to Lec(RSV) during encapsulation. BT474 cells were grown to ~80% confluence on glass coverslips (12 ×12 mm; Fisher Scientific, Texas, USA). Prior to the addition of 200 µg/mL of Lec(RSV+Cou), the medium was substituted with a serum-free medium. After 1-hour incubation at 37°C, the cells were washed three times with PBS and fixed with 4% (w/v) paraformaldehyde (PFA). Coverslips with fixed cells were mounted on glass slides with Dako® mounting media and examined using an Olympus Fluoview 1000 confocal microscope (Olympus Imaging Co., Tokyo, Japan).
In vitro Anti-Cancer Properties of Lec(RSV)
To determine the cellular toxicity, BT474 cell lines were grown at 5000 cells/well at increasing concentrations in a 96-well plate.) Lec, RSV, and Lec(RSV) were respectively added at specific concentrations (10 ~ 40 µM) and incubated in a 5% CO2 cell incubator. After 24 hours, a 10 µL Alamar Blue assay was added and cells were incubated in dark for 2 hours. Fluorescence was detected with a Multimode Reader (Biotech), Ex/Em = 500/525 nm.
In vitro ROS Scavenging Properties of Lec(RSV)
The ROS Assay Kit was used to assess the ROS scavenging ability of Lec(RSV) on BT474. Briefly, the cells (1×105/well) were incubated in 6-well plates for 24 hours in a 5% CO2 incubator. After that, the media was substituted with fresh media containing RSV and different concentrations of Lec(RSV) for 24 hours. Then, 100 μL of 10 μmol/L DCFH-DA in PBS was added to each sample. Cells were incubated in a 5% CO2 incubator for 30 min and transferred onto 96-well black microplates. Fluorescence was detected with a Multimode Reader (Biotech), Ex/Em = 500/525 nm.
In vivo Tumoral Uptake of Lec(RSV)
All in vivo studies were carried out in accordance with the National Institutes of Health animal care guidelines and under strict pathogen-free conditions in the animal facility of Sun Yat-sen University. The animal protocol was authorized by the Institutional Animal Care and Use Committees on Animal Care (Sun Yat-sen University; SYSU-IACUC-2020-B0167). 1.2×107/0.2 mL BT-474 cells were administered into the breast tissue of Balb/C athymic nude mice to create the breast cancer model. When the tumor volumes grew up to 120 ~ 200 mm3, the mice were used to assess the targeting ability of the Lec(RSV). For in vivo analysis, we added FITC as a fluorescence molecule for imaging. Briefly, mice were separated into two groups (n = 3), one group with Lec(RSV+FITC) circulating in the body for 1 hour, and the other group for 4 hours. Then, we produced Lec(RSV+FITC) suspended in PBS, which was administered to the mice via tail vein injection (0.2 mL, equivalent to RSV 2 mg/kg). After 1 or 4 hours, the tumor tissues of the sacrificed mice were harvested and observed with fluorescence microscopy.
Statistical Analysis
The normal distribution of data from parametric results were confirmed by the Kruskal Wallis analysis of variance on ranks followed by the Tukey or the Dunn’s test when the data were not normally distributed or by the Student’s t-test as appropriate. Upon confirmation, the results were then analyzed by the t-test or one-way analysis of variance followed by the Tukey’s test, and are all presented as mean ± S.D. A p < 0.05 was required for statistical significance. All statistical analyses were performed with SPSS 25.0 (SPSS Inc., Chicago, IL).
Results and Discussion
The Synthesis of Lec(RSV)
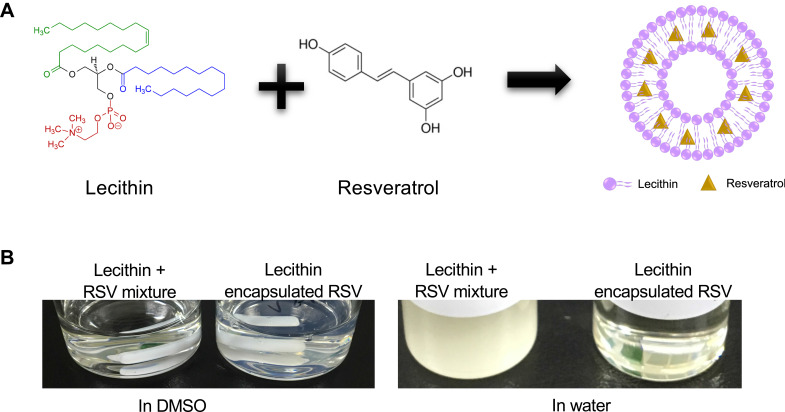
Our ultimate aim was to develop a fully natural nanoparticle with inherent anti-cancer properties. RSV is soluble in inorganic solvent but insoluble in water or buffer, which hinders its usability in the systemic delivery of RSV.21 Herein, we synthesized Lec(RSV) as a synergistic anti-cancer therapeutic platform (Figure 1A). As seen in Figure 1B, a simple mixture of both lecithin and RSV does not automatically encapsulate RSV and form nanoparticles. Therefore, we utilized the nanoprecipitation method, which was used in similar work in our group and others previously.19,22 Only through the precipitation method detailed above, a clear solution of Lec(RSV) could be observed (Figure 1B, right).
Figure 1.
The schematic diagram and feature of the synthesis of Lec(RSV). (A) Schematic representation of the utilization of lecithin and resveratrol to form Lec(RSV) nanoparticles, (B) nanoprecipitation method was used to achieve soluble Lec(RSV).
The Properties of Lec and Lec(RSV) NPs
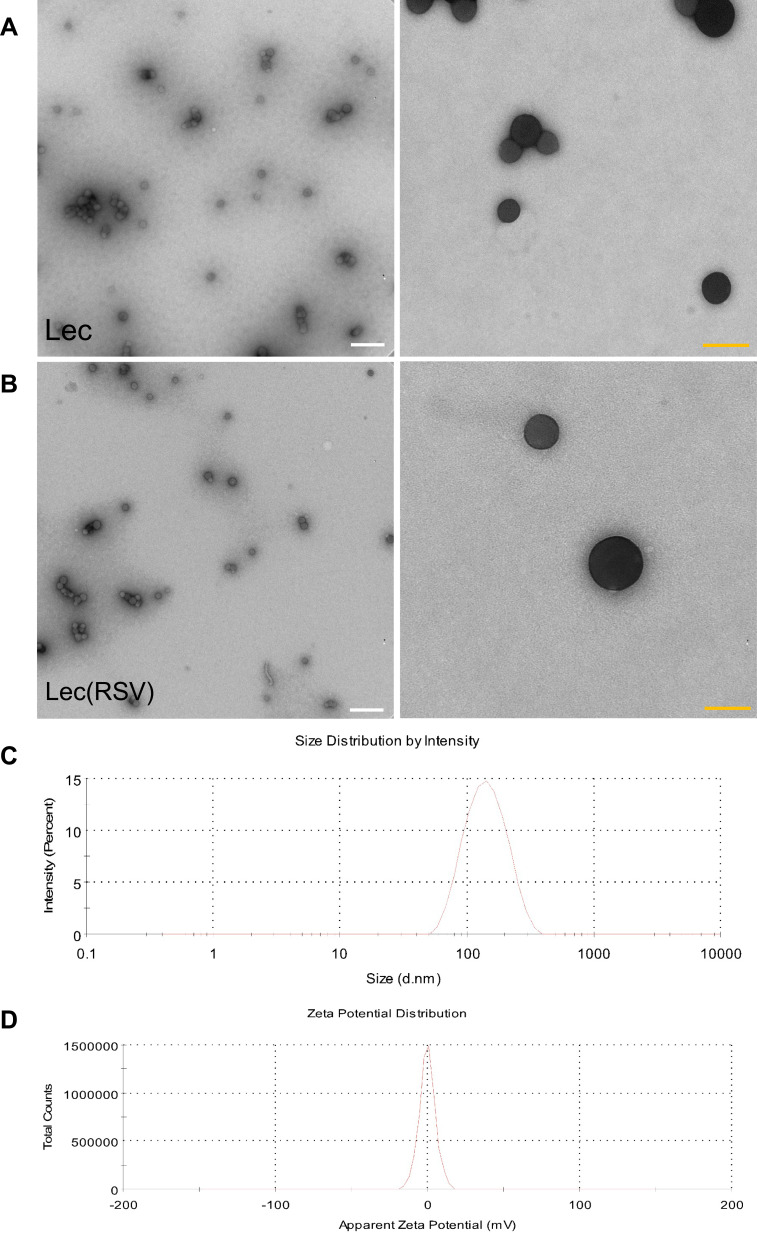
To determine the morphology and size of both Lec and Lec(RSV), TEM and DLS analyses were carried out. As seen in Figure 2A, TEM analysis revealed that both empty Lec NPs showed uniformed spherical morphology, similar to Lec(RSV) NPs in Figure 2B. This indicated the encapsulation of RSV does not alter the physical properties of Lec NPs. However, although both groups showed similar physical morphology in TEM, the DLS analysis revealed that the hydrodynamic size of Lec(RSV) was larger than empty Lec NPs (151.0 ± 22.93 nm vs 128.4 ± 19.88 nm) (Figure 2C, Table 1). This observation indicated that the encapsulation of RSV was successful23 as it did project a significant increase in the average size of the Lec(RSV) compared to the empty Lec control. In our research, the Lec(RSV) nanoparticles possessed an average size of 151 nm, which was approximately the ideal size to possess substantial loading capacity, stability, and favorable tumor penetration. The ideal size can be ascertained in regard to the disease site, therapeutic objectives, and other nanoparticle characteristics. The size of nanoparticles must be greater than 10 nm to evade the obstacle of renal filtration.24 A diameter larger than 200 nm will trigger the complement system and be rapidly eliminated from the bloodstream, with aggregation in the spleen and liver.25,26
Figure 2.
The size of empty Lec nanoparticle and Lec(RSV) nanoparticle. (A) TEM analysis of empty Lec nanoparticle. (B) TEM analysis of Lec(RSV) nanoparticles; Orange scale bar indicated 200 nm; while scale bar indicated 100 nm. (C) The plot of the average hydrodynamic size of Lec(RSV) nanoparticles; (D) the plot of zeta potential of Lec(RSV) nanoparticles.
Table 1.
The Average Size of Nanoparticles
| Run 1 | Run 2 | Run 3 | Average (nm) | SD | |
|---|---|---|---|---|---|
| Lecithin | 113.3 | 115.4 | 156.5 | 128.4 | 19.88 |
| Lec (RSV) | 133.5 | 136.1 | 183.4 | 151 | 22.93 |
The Zeta potential of Lec(RSV) NPs also indicated a neutral charge (Figure 2D). Neutral and negative charged particles are favored in clinical translation of NPs as they reduce the non-specific uptake of NPs compared to positive charged NPs, which could be easily uptaken by cells due to positive-negative charge interactions, which in turn leads to longer circulation lifetimes and less accumulation in major organs.27 Furthermore, nanoparticles with neutral and negative surface charges have been shown to reduce the adsorption of serum proteins, resulting in longer circulation half-lives.28
For the detection of encapsulation efficacy in vitro, we substituted RSV with a self-fluorescent molecule coumarin-6, with a similar molecular weight to determine the fluorescence intensity inside the Lec nanoparticles. The encapsulation efficacy of coumarin-6 was calculated to be around 85.15% (Table 2). An 85% encapsulation rate is a generally accepted efficiency and could be considered high.29
Table 2.
The Encapsulation Efficacy of RSV
| Coumarin Input | Lec (Coumarin) | |
|---|---|---|
| Run 1 | 2357 | 2031 |
| Run 2 | 2394 | 2075 |
| Run 3 | 2744 | 2277 |
| Average | 2498.33 | 2127.67 |
| Encapsulation efficiency (%) | 85.16 | |
The Stability of Lec and Lec(RSV) Nanoparticles
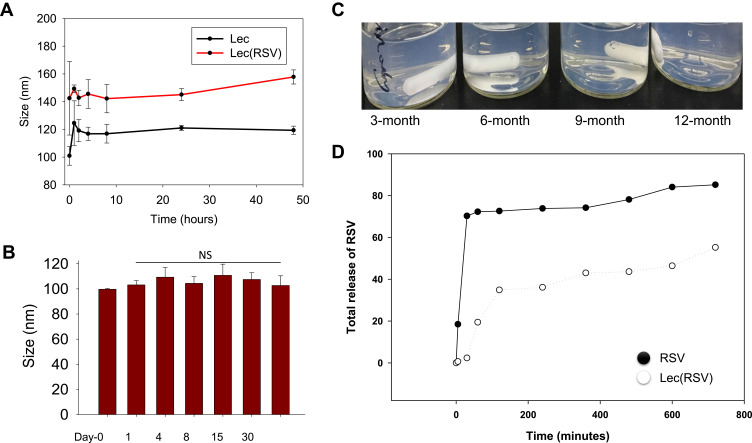
The stability of nanoparticles is crucial because systemic delivery of nanoparticles requires prolonged circulation of Lec(RSV) in vivo. Both the Lec and Lec(RSV) nanoparticles showed stability during a 48-hour period (Figure 3A). To determine the stability of Lec(RSV), we measured the size of Lec(RSV) at predetermined time points (Day-1, 4, 8, 15, 30, and 60). As seen in Figure 3B, the DLS measurement of Lec(RSV) revealed minimal changes through the 60-day period (~100 to 120 nm throughout), indicating good stability of Lec(RSV). Interestingly, long-term storage of Lec(RSV) in 4°C also indicated the excellent stability of this nanoplatform (Figure 3C) from the absence of aggregates even after 12 months of storage. Furthermore, RSV was slowly released after Lec encapsulation, revealing only ~55% cumulative release after 720 minutes (Figure 3D).
Figure 3.
The stability of Lec and Lec(RSV) nanoparticles. (A) The size of Lec and Lec(RSV) nanoparticles during a 48-hour period; (B) the size of Lec(RSV) nanoparticles at predetermined time points (day-1, 4, 8, 15, 30, and 60); (C) the appearance of Lec(RSV) after long-term storage of 12 months; (D) the release of RSV of Lec(RSV) nanoparticles after encapsulation.
The in vitro Uptake Ability and Cytotoxicity of Lec and Lec(RSV) Nanoparticles
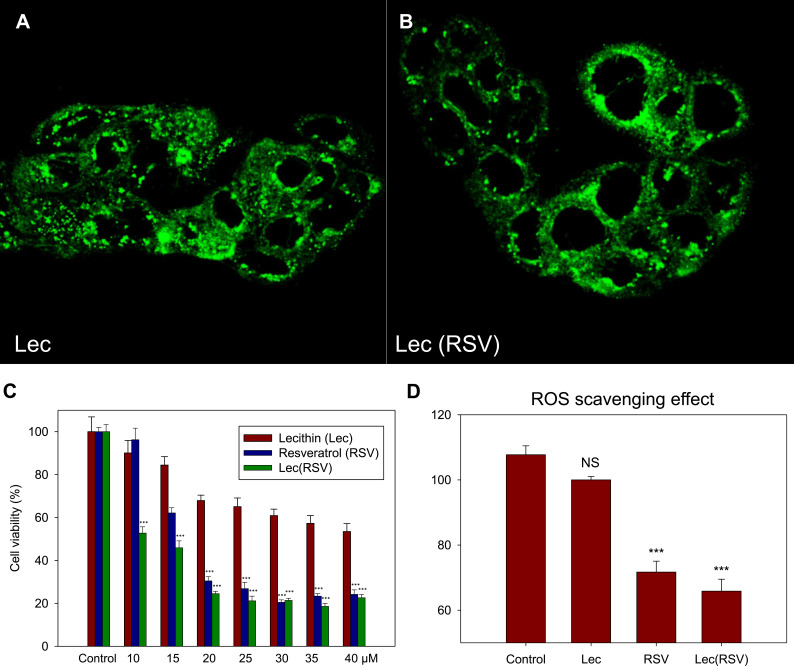
We then confirmed the in vitro uptake ability of Lec(RSV). To visualize this, we encapsulated FITC into both Lec and Lec(RSV) and treated these nanoparticles respectively to BT474 breast cancer cells. After 4 hours of co-incubation, both Lec and Lec(RSV) showed significant uptake into the cell cytoplasm (Figure 4A and B). Next, we carried out in vitro cytotoxicity assay to observe the cancer cell killing ability of RSV. As seen in Figure 4C, RSV treatment indicated high toxicity to the cells (IC50 ~18 µM). As an antioxidant, Lec also possesses an intrinsic cancer-killing ability, although not as significant as RSV (IC50 > 40 µM). Interestingly, Lec(RSV) showed significant and synergistic effects on the cancer cell killing ability compared to either Lec or RSV treatment alone (IC50 ~11 µM). A similar phenomenon can be observed in ROS scavenging assay, where RSV showed a significant ROS scavenging effect compared to control or Lec (p < 0.001), and the encapsulation of RSV did not interfere with the biological activity of RSV (Figure 4D).
Figure 4.
The in vitro uptake ability and cytotoxicity of Lec and Lec(RSV) nanoparticles. (A and B) The BT474 breast cancer cells uptake of Lec and Lec(RSV) encapsulated FITC; (C) in vitro cytotoxicity assay to observe the cancer cell killing ability of Lec, RSV, and Lec(RSV), respectively; (D) ROS scavenging effects of Lec, RSV and Lec(RSV). ***Indicated significance value of p < 0.001; compared to control.
The in vivo Tumoral Uptake of Lec(RSV) Nanoparticle
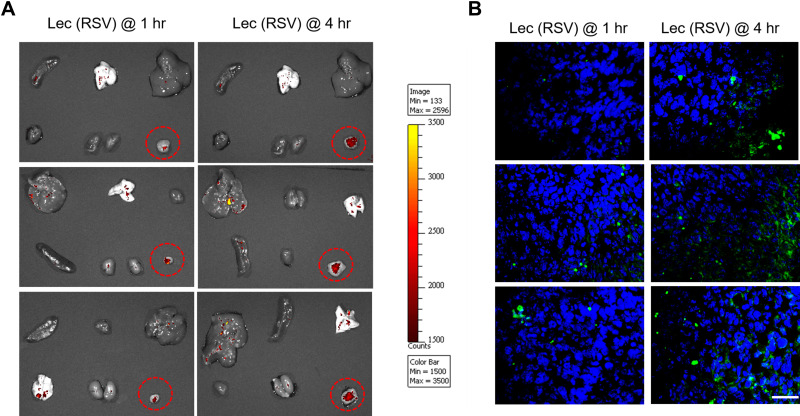
Finally, we carried out the in vivo tumoral uptake experiment in the BT474 orthotopic model. We treated one group of female Balb/C nude mice (n = 3) with Lec(RSV + FITC) NPs for 1 hour and another group (n = 3) for 4 hours. As seen in Figure 5, the accumulation of Lec(RSV) was significantly increased after 4 hours compared to the 1-hour group, indicating the reliability of Lec(RSV) to be used as a drug delivery system for the treatment of cancer. The TUNEL assay analysis revealed an increased level of apoptosis in the 4 hr treatment group compared to the 1 hr group, indicating the successful uptake of RSV into tumor cells, exerting its tumor cell killing effect in vivo.
Figure 5.
The in vivo tumoral uptake of Lec(RSV) nanoparticle. (A) BT474 orthotopic model was used to carry out in vivo tumoral uptake experiment. We treated one group of female Balb/C nude mice (n = 3) with Lec(RSV + FITC) NPs for 1 hour and another group (n = 3) for 4 hours. The red circles indicate the accumulation effects of the nanoparticles in the tumor at 1 hour and 4 hours after injection. It shows that the tumoral uptake of Lec(RSV) was significantly increased in the tumor after 4 hours compared to the 1-hour group, indicating the reliability of Lec(RSV) to be used as a drug delivery system for the treatment of cancer. (B) TUNEL assay analysis of the tumor section in (A). At 4 hours, the tumors treated with Lec(RSV) indicated higher apoptosis level as compared to the tumors treated only for 1 hour, indicated prolonged effect of tumor killing effect of Lec(RSV). Scale bar indicated 50 µm.
Conclusion
Herein, we developed a fully natural Lec(RSV) nanoparticle system. Lec(RSV) showed long-lasting stability, high cellular uptake in BT474 breast cancer cells, and significant retention in the BT474 breast cancer cells’ orthotopic model with minimal toxicity. Taken together, Lec(RSV) could be potentially used as an alternative chemotherapeutic and could be easily translated into clinical application as an anti-cancer therapeutic.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (82050410363, 82072930), the Hundred Talents Program for Young Scholars of Sun Yat-sen University (1320318003), 111 project (No. B20062), Guangzhou Science and Technology Bureau (201704020131), the Guangdong Province Outstanding Youth Award (2021B1515020066), and The Three Million for Three Years Project of SYSMH (132090023).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gilman A. The initial clinical trial of nitrogen mustard. Am J Surg. 1963;105:574–578. doi: 10.1016/0002-9610(63)90232-0 [DOI] [PubMed] [Google Scholar]
- 2.Qin SY, Zhang A-Q, Cheng S-X, et al. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234–247. doi: 10.1016/j.biomaterials.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 3.Aung TN, Qu Z, Kortschak R, et al. Understanding the effectiveness of natural compound mixtures in cancer through Their molecular mode of action. Int J Mol Sci. 2017;18(3):656. doi: 10.3390/ijms18030656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Liang S, Tan CH, et al. Nanocarriers in the enhancement of therapeutic efficacy of natural drugs. BIO Integration. 2021;2:40–49. doi: 10.15212/bioi-2020-0040 [DOI] [Google Scholar]
- 5.Wu PS, Li YS, Kuo YC, et al. Preparation and evaluation of novel transfersomes combined with the natural antioxidant resveratrol. Molecules. 2019;24(3):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218 [DOI] [PubMed] [Google Scholar]
- 7.Vesely DL. New anticancer agents: hormones made within the heart. Anticancer Res. 2012;32(7):2515–2521. [PubMed] [Google Scholar]
- 8.Francioso A, Mastromarino P, Masci A, et al. Chemistry, stability and bioavailability of resveratrol. Med Chem. 2014;10(3):237–245. doi: 10.2174/15734064113096660053 [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya S, Ahmmed SM, Saha BP, et al. Soya phospholipid complex of mangiferin enhances its hepatoprotectivity by improving its bioavailability and pharmacokinetics. J Sci Food Agric. 2014;94(7):1380–1388. doi: 10.1002/jsfa.6422 [DOI] [PubMed] [Google Scholar]
- 10.Chay SY, Tan WK, Saari N. Preparation and characterisation of nanoliposomes containing winged bean seeds bioactive peptides. J Microencapsul. 2015;32(5):488–495. doi: 10.3109/02652048.2015.1057250 [DOI] [PubMed] [Google Scholar]
- 11.Jantscheff P, Esser N, Graeser R, et al. Liposomal gemcitabine (GemLip)-efficient drug against hormone-refractory Du145 and PC-3 prostate cancer xenografts. Prostate. 2009;69(11):1151–1163. doi: 10.1002/pros.20964 [DOI] [PubMed] [Google Scholar]
- 12.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(2):219–233. doi: 10.1002/wnan.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/S0168-3659(99)00248-5 [DOI] [PubMed] [Google Scholar]
- 14.Al-Jubori AA, Sulaiman GM, Tawfeeq AT, et al. Layer-by-layer nanoparticles of tamoxifen and resveratrol for dual drug delivery system and potential triple-negative breast cancer treatment. Pharmaceutics. 2021;13(7). doi: 10.3390/pharmaceutics13071098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wangenheim J, Bolcsfoldi G. Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis. 1988;3(3):193–205. doi: 10.1093/mutage/3.3.193 [DOI] [PubMed] [Google Scholar]
- 16.Biondi O, Motta S, Mosesso P. Low molecular weight polyethylene glycol induces chromosome aberrations in Chinese hamster cells cultured in vitro. Mutagenesis. 2002;17(3):261–264. doi: 10.1093/mutage/17.3.261 [DOI] [PubMed] [Google Scholar]
- 17.Lanigan RS. Cosmetic ingredient review expert, final report on the safety assessment of PPG-11 and PPG-15 stearyl ethers. Int J Toxicol. 2001;20(4):53–59. [DOI] [PubMed] [Google Scholar]
- 18.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67(11):615–623. doi: 10.1111/j.1753-4887.2009.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Wu J, Liu Y, et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11(3):2618–2627. doi: 10.1021/acsnano.6b07195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo M, Marek L, Liang Y, et al. Transforming tea catechins into potent anticancer compound: analysis of three boronated-PEG delivery system. Micromachines. 2021;13(1):45. doi: 10.3390/mi13010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson K, Mock C, Liang D. Pre-formulation studies of resveratrol. Drug Dev Ind Pharm. 2015;41(9):1464–1469. doi: 10.3109/03639045.2014.958753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Bao X, Kolesnik I, et al. Enhancing the in vivo stability of polycation gene carriers by using PEGylated hyaluronic acid as a shielding system. BIO Integration. 2022. doi: 10.15212/bioi-2021-0033 [DOI] [Google Scholar]
- 23.Kumari A, Singla R, Guliani A, et al. Nanoencapsulation for drug delivery. EXCLI J. 2014;13:265–286. [PMC free article] [PubMed] [Google Scholar]
- 24.de Barros AB, Tsourkas A, Saboury B, et al. Emerging role of radiolabeled nanoparticles as an effective diagnostic technique. EJNMMI Res. 2012;2(1):39. doi: 10.1186/2191-219X-2-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni SA, Feng SS. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30(10):2512–2522. doi: 10.1007/s11095-012-0958-3 [DOI] [PubMed] [Google Scholar]
- 26.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17(8):2950–2962. doi: 10.1016/j.bmc.2009.02.043 [DOI] [PubMed] [Google Scholar]
- 27.Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13):3435–3446. doi: 10.1016/j.biomaterials.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon-Vazquez R, Tsapis N, Lorscheider M, et al. Improving dexamethasone drug loading and efficacy in treating arthritis through a lipophilic prodrug entrapped into PLGA-PEG nanoparticles. Drug Deliv Transl Res. 2022;12(5):1270–1284. doi: 10.1007/s13346-021-01112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]