Graphical abstract
Keywords: Nanomaterials, Engineered nano-matrices, Enzyme immobilization, Bio-catalysis, Surface functionalization
Highlights
-
•
Explores Applications of Enzymatic catalysis.
-
•
Enzymes destabilize in harsh conditions which increase their bioprocess cost.
-
•
Enzyme immobilization enhances the biocatalytic performance of enzymes.
-
•
Nanomaterials are intriguing supporting matrices for enzyme immobilization.
-
•
Discussed enzyme immobilization on multifunctional magnetic nanomaterials (MNPs).
-
•
Biomedical applications and future prospects of enzyme coated MNPs are summed up.
Abstract
Background
Enzymes based bio-catalysis has wide range of applications in various chemical and biological processes. Thus, the process of enzymes immobilization on suitable support to obtain highly active and stable bio-catalysts has great potential in industrial applications. Particularly, surface-modified magnetic nanomaterials have garnered a special interest as versatile platforms for biomolecules/enzyme immobilization.
Aim of review
This review spotlights recent progress in the immobilization of various enzymes onto surface-coated multifunctional magnetic nanostructured materials and their derived nano-constructs for multiple applications. Conclusive remarks, technical challenges, and insightful opinions on this field of research which are helpful to expand the application prospects of these materials are also given with suitable examples.
Key scientific concepts of review
Nanostructured materials, including surface-coated magnetic nanoparticles have recently gained immense significance as suitable support materials for enzyme immobilization, due to their large surface area, unique functionalities, and high chemical and mechanical stability. Besides, magnetic nanoparticles are less expensive and offers great potential in industrial applications due to their easy recovery and separation form their enzyme conjugates with an external magnetic field. Magnetic nanoparticles based biocatalytic systems offer a wide-working temperature, pH range, increased storage and thermal stabilities. So far, several studies have documented the application of a variety of surface modification and functionalization techniques to circumvent the aggregation and oxidation of magnetic nanoparticles. Surface engineering of magnetic nanoparticles (MNPs) helps to improve the dispersion stability, enhance mechanical and physicochemical properties, upgrade the surface activity and also increases enzyme immobilization capabilities and biocompatibility of the materials. However, several challenges still need to be addressed, such as controlled synthesis of MNPs and clinical aspects of these materials require consistent research from multidisciplinary scientists to realize its practical applications.
Introduction
Magnetic nanoparticles (MNPs) have recently emerged as fascinating nanomaterials that have garnered extensive research attention among the scientific community and researchers owing to their broad-spectrum applications in numerous fields including nano-biomedicine [1], environmental protection [2], catalysis [3], electronic communication [4], magnetic fluids [5], data storage [6], etc. In addition to some special magnetic features, i.e., superparamagnetism, magnetic nanomaterials also exhibit exceptional physical attributes, stability, and biocompatibility [7]. Amongst various kinds of MNPs, including iron oxides (Fe3O4 and γ-Fe2O3), alloy-based (CoPt3 and FePt), pure metal (Fe and Co), and spinel-type ferromagnet (MgFe2O4, MnFe2O4, and CoFe2O4) MNPs, magnetite iron oxide (Fe3O4) nanoparticles are of paramount importance and gained popularity in biomedicine and biocatalysis due to facile fabrication, low toxicity, and acquiescence to surface functionalization [8], [9], [10], [11], [12]. Many literature reports have revealed the frequent use of magnetic Fe3O4 nanoparticles as a drug vehicle in cancer theranostics, protein purification, gene delivery, MRI agents, cell labeling, bioseparation, immunoassays, biosensors, and hyperthermia treatment [13], [14], [15], [16], [17].
Different attributes such as morphology, shape, size, and dispersibility of the Fe3O4 nanoparticles might have an influence on their application in different fields [18]. Thus, researchers have adopted multiple routes to develop MNPs for controlling their shape, size, and morphology with requisite and tailorable features. To date, a large number of fabrication approaches, including microemulsion, co-precipitation, thermal decomposition, hydrothermal, laser pyrolysis, electrochemical deposition, sonochemical and solvothermal methods, microwave-driven method, aerosol pyrolysis, chemical vapor deposition and biobased methods have been proposed for the development of magnetic Fe3O4 nanoparticles [7]. The biobased synthesis method has been emerged as a promising alternative for obtaining MNPs [19]. These methods are generally executed under the conditions of temperature and pressure close to those of the environment, and utilizing biomolecules extracted from plant extracts or biological entities (e.g., alkaloids, terpenoids, polyphenols, exopolysaccharides, carbohydrates, auxins, flavonoids) [20], [21]. The biobased procedures entail low energy requirements and safer reagents compared to traditional co-precipitation or reduction by agents with adverse impacts. Thus, this biosynthetic approach is recognized as one the most interesting options to advance trans-materialization and energy efficiency processes in the nanotechnology perspective [22], [23]. Table 1 depicts notable synthesis procedures along with their merits and demerits. In this report, we elaborate on recent progress in the surface coating strategies and immobilization of various enzymes on the surface-functionalized magnetic nanostructured materials and their derived nanocomposites.
Table 1.
Synthesis methods for magnetic nanoparticles.
| Name of synthesis method | Merits | Demerits |
|---|---|---|
| Chemical co-precipitation | Simple and efficient | poor crystallinity, size distribution, and aggregation Not appropriate to prepare highly pure, accurate stoichiometric phase |
| Microemulsion | Good homogeneous nature Precise control of particle size | time laborious, requirement of large amounts of solvent and poor yield |
| Hydrothermal reactions | Easy to control particle Shape and size | High pressure, prolonged reaction duration and high reaction temperature |
| Thermal decomposition | High yield and good control of size and shapes | Elevated reaction temperature |
| Sol-gel reactions | Good control of size and structure | Prolonged reaction time and cost expensive |
| Electrochemical method | Easy control of size | Reproducibility |
| Vapor phase method | Greater yield | High temperatures |
| Bio-based method | Nontoxic, cost effective, cheap materials and solvents, environmentally friendly, and synthesis at room temperature and atmosphere | Scale up limitations, Reproducibility, tedious purification, and poor understanding of the explicit mechanism. |
Surface modification/functionalization of MNPs for enzyme immobilization
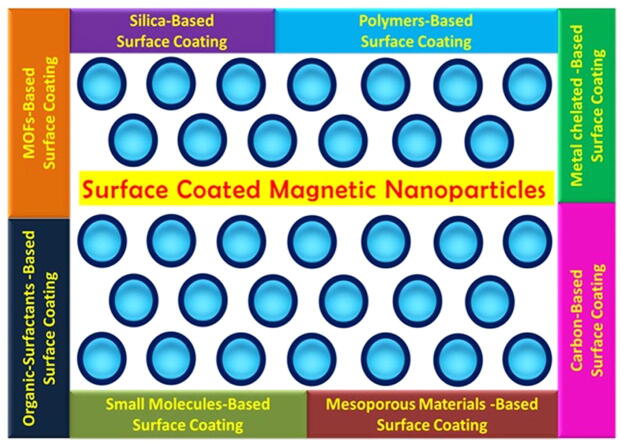
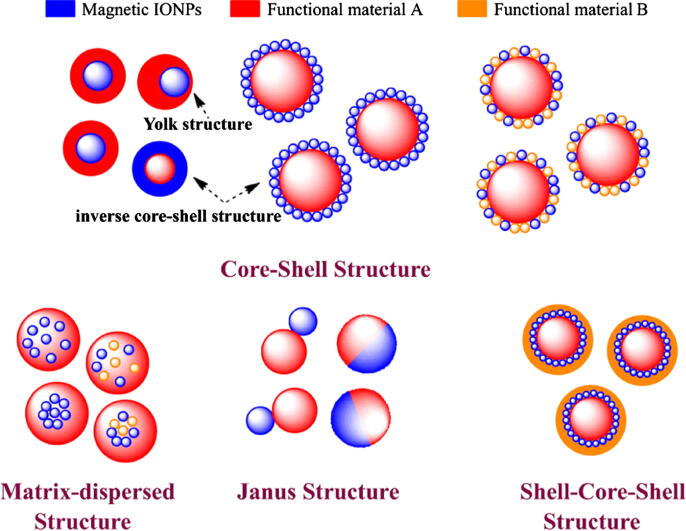
It is demonstrated that Fe3O4 nanoparticles are prone to aggregate due to their high surface energies, reactivity, and considerably high specific surface area. Moreover, the pristine form of Fe3O4 NPs exhibits high chemical activity and tend to oxidize in the air resulting in the loss of dispersibility and magnetic properties [24], [25]. Due to these factors, the efficiency of magnetic-driven separation is reduced, and consequently, limited the capability of bare Fe3O4 NPs for direct immobilization of biomolecules and enzymes. In this avenue, surface modification and functionalization is a necessary step to circumvent the aggregation and oxidation of these nanoparticles. Fig. 1 represents the surface functionalization strategies of MNPs to improve their properties for efficient enzyme immobilization. Four major purposes of surface engineering of MNPs are; 1) improve the dispersion stability of MNPs 2) modify the mechanical and physicochemical properties, 3) upgrade the surface activity of MNPs, and (4) enhance the biocompatibility of MNPs. Taking into account many strategies, researchers have made efforts to fabricate various kinds of magnetic iron oxide nanocomposites as shown in Fig. 2 [26].
Fig. 1.
Surface functionalization strategies of MNPs to improve their properties for enzyme immobilization.
Fig. 2.
Typical morphologies of magnetic composite nanomaterials. Reprinted from Ref. [26] with permission under the terms of the Creative Commons Attribution 3.0 license. IONPs—iron oxide nanoparticles.
Silica-coated MNPs for enzyme immobilization
Silica coating, in which silica shells are formed on the surface of magnetic cores, is a persuasive technique to functionalize Fe3O4 NPs. The formation of silica shells is likely to augment the chemical steadiness by providing protection to magnetic cores both from oxidation and aggregation. It can also increase the biocompatibility and hydrophilicity. Insertion of a vast number of silanol groups on the magnetic core’s surface by modification process affords the underlying foundation for additional functionalization with reagents for enzymes attachment. Fe3O4 NPs can be directly functionalized with silica shells by a well-characterized sol–gel procedure [27], in which tetraethoxysilane is hydrolyzed under alkaline environments to form silica shells on the surface of magnetic cores yielding core–shell Fe3O4@SiO2 NPs. The resultant core–shell Fe3O4@SiO2 NPs are first coated with amino silane [28], or epoxy silane coupling agents [29] to insert amine or epoxy moieties on the surfaces of carrier support, respectively, for effective attachment of enzymes. Afterward, enzymes can be integrated on the surface of the amino group bearing Fe3O4@SiO2 NPs based on Schiff base linkages by using bifunctional reagent glutaraldehyde. Whereas, epoxy-functionalized support matrices can be applied to direct enzyme immobilization via the reaction between epoxy groups of support material and amino groups of enzymes. With reference to amino-functionalized silica-coated magnetite nanoparticles, epoxy-functionalized Fe3O4@SiO2 NPs do not necessitate any additional linkage for enzyme immobilization. However, the immobilization procedure is time-consuming and ineffective due to the slow reactions between epoxy and amino groups, by virtue of steric hindrance associated with the dimensions of the macromolecule [30]. Amino and carboxyl group incorporated MNPs were prepared to gain an insight into whether the basic or acidic modification was more effective for the immobilization of L-asparaginase (ASNase) enzyme. The prepared nanoparticles were characterized by Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDAX), X-ray diffraction (XRD), and vibrating-sample magnetometer (VSM). In comparison to the free state of the enzyme, the nanocarriers-supported ASNases were more stable in a broad range of temperature and pH values under the optimum reaction conditions. Likewise, the nanobiocatalysts presented high stability at a raised temperature of 50 °C for 3 h. Free form of the enzyme showed only 30% of its original activity after preserving at 4 °C for 1 month, whereas Fe3O4/SiO2/COOH and Fe3O4/SiO2/NH2 ASNase preserved above 56.5% and 78.9% of their preliminary activities, respectively, under identical conditions. Both of the engineered ASNase revealed outstanding functioning stability after 17 consecutive batch cycles [31]. The MNPs surface was functionalized with aminopropyltriethoxysilane (APTES) and polyamidoamine (PAMAM) dendrimer to comprehend the covalent binding of cholesterol oxidase and esterase for the development of cholesterol biosensor [32].
Silica-encapsulated MNPs synthesized by microemulsion techniques were deemed as a good support matrix for immobilizing glucose oxidase (GOD). The binding of GOD onto the support was confirmed by the FTIR spectra. Immobilized bioconjugate preparation maintained over 95% and 90% of its primary activity after storage for 45 days, and 12 consecutive reaction cycles. Substantial improvements in thermal stability profiles were also recorded at high temperatures up to 80 °C. Moreover, the immobilized biocatalyst was less likely to be affected by alterations in pH values [33]. Correa et al. (2020) tested three different types of immobilization methods for covalent bonding of β-Glucuronidases on MNPs and three catalysts for Si particle deposition [34]. Among the nine different immobilized micron-sized biocatalytic preparations, only two showed insignificant activity. All the preparations had superior thermal, storage, and functional stability than the free enzyme. Different bioconjugates with MNPs and Si maintained 40% of their original activities at a high temperature of 80 °C after 6 h, while the free form of enzyme dropped over 90% of its activity within 10 min.
Polymer-modified MNPs for enzyme immobilization
Generally, ex-situ and in-situ modification are two methods, which are involved in the functionalization of MNPs with organic polymers [35], [36], [37], [38]. The in-situ modification method entails the inclusion of organic polymers in the precursor solution as a stabilizer to generate Fe3O4 NPs, whereas, in the ex-situ modification approach, the monomers are polymerized on the surface of Fe3O4 NPs. The generation of effective steric repulsion forces from polymer coatings weakens the magnetic and Van der Waals interactions of Fe3O4 NPs that subsequently prevent aggregation and augment their stability and dispersibility attributes. In the last few years, a plethora of polymers has been proposed as adequate coating materials to functionalize Fe3O4 NPs for efficient enzyme immobilization. Many of these polymers have been found suitable agents, such as starch, alginate, albumin, chitosan, polyvinyl alcohol (PVA), polyethyleneimine (PEI), polydopamine, polyethylene glycol (PEG), and different polyoxamines for MNPs development because of their desired biodegradable and biocompatible properties [36], [37], [38], [39], [40], [41]. Chitosan-modified MNPs are largely synthesized by the in-situ modification approach [42]. Dextran is among the choice coating polymers that are used for MNPs functionalization due to non-toxicity, biocompatibility, and degradability by dextranase [43], [44]. It is demonstrated that MNPs coating with dextran can ameliorate their attributes for colloidal stability, and drug delivery, and thus seemed a useful approach in fabricating MNPs [45], [46]. Indeed, dextran is a hydrophilic polymer that is composed of numerous glucose monomers linked together by α-1, 6-glycosidic bonds. This polymer is physically adsorbed onto MNPs by non-covalent linkages in alkaline solutions. The utilization of carboxymethyl dextran (CMD), a derivative of dextran, to fabricate MNPs provides hydroxyl as well as carboxyl functional moieties, which render easy chemical modification. CMD is endowed with excellent biocompatibility, biodegradability, and high-water solubility [47]. Vasić et al. (2020) inspected the influence of different concentrations of CMD on the properties of CMD-wrapped MNPs [48]. The surface morphology and functional groups of CMD-MNPs were monitored by SEM FTIR. The as-synthesized CMD-coated MNPs were then employed as support carriers for immobilizing alcohol dehydrogenase (ADH). Coating of CMD onto MNPs imparts preferable structural and magnetic properties and can thus be applied as a nanocarrier for enzyme immobilization. In contrast to the free form of ADH that dropped 70% of its original activity at 20 °C, and complete loss of its activity at 40 °C after 24 h. The nanoimmobilized biocatalyst retained more than 50%, and 75% of its remaining activity at 20 °C and 40 °C, respectively, under the same incubation period of 24 h.
Francolini et al. (2020) prepared polymer-coated MNPs showing long alkyl chains, either hexadecyl (C16) or octyl (C8) to immobilize Candida rugosa lipase (CRL) [49]. Among the nanocarrier supports tested, the one displaying the longest alkyl chains deliver the most promising efficacies for immobilized enzyme. The nanoimmobilized biocatalytic system with the longest alkyl chains also presented superior tolerance to high temperature (ranging from 25 to 70 °C) than the soluble lipase. It also showed good recyclability in four successive cycles and conveniently recovered by a simple magnetic separation. Lipase enzyme from Thermomyces lanuginosus (TLL) was covalently attached to PEI-coated new heterobifunctional support, divinyl sulfone (DVS) superparamagnetic nanoparticles (SPMNs) and characterized. PEI is an organic polymer with the highest positive charge density, which provides colloidal stabilization to the protein molecules even at a high concentration of salt [50], [51], [52]. Moreover, as a smart polymer, PEI can respond to various external stimuli like pH, temperature, etc. [53]. Therefore, PEI can be utilized as a multipurpose agent to develop biocatalysts by simple adsorption or multipoint covalent coupling [52]. Its unique chemical structure can confer stabilization to multiple subunits of enzymes and provide stability against various organic solvents. Further, it is used for the co-immobilization of cofactors and enzymes, PEI-functionalized supports for enzyme immobilization might produce inter- and intra-molecular crosslinking, imparting a greater extent of homogeneity of activated amino groups [54]. Thermal inactivation profile at different pH values revealed that the immobilized TLL bioconjugates exhibited the most robust stability at an immobilization pH of 5.0. The nanobiocatalytic preparation obtained at pH 5.0 and blocked with ethanolamine (ETA) and ethylenediamine (EDA) for hydrolyzing racemic methyl mandelate. It achieved excellent enantioselectivity of 72% and 68%, respectively together with greater biocatalytic efficiencies in the reaction system at a neutral pH of 7.0. The engineered system had impressive operational stability retaining over 60% of conversion efficiency after seven repeated cycles.
Chitosan is a linear polysaccharide consisting of glucosamine with varying degrees of deacetylation. It can be utilized for the decoration of the magnetic nanocomposite’s surface. In addition to impart stability to nanocarrier supports, it provides functional groups (–NH2 and –OH groups), which are involved in chemical connection with biomolecules. Since the amine groups of chitosan have pKa value near 6.5, it possesses a coiled structure and can be more soluble in acidic solution. A set of desirable characteristics like bio-renewability, hydrophilicity, biodegradability, and biocompatibility [55], [56], the chitosan-induced chemical modifications do not alter the basic network of carrier support and even exert some advantageous activities such as adsorption, chelation, bacteriostatic properties activities [57], [58]. Due to its renewable nature (derived from shells of shellfish: krills, crabs, shrimps, and lobsters, and from the fishing industry waste), chitosan is potentially considered an inexpensive coating agent that additionally play a role in the development of competitive biocatalysts [59]. A novel type of magnetic nanobiocatalyst was designed by efficient immobilization of Trichoderma reesei cellulase onto chitosan modified Fe3O4/graphene oxide nanocomposite (Fe3O4/GO/CS). Using a covalent coupling method using glutaraldehyde as a cross-linker, cellulase was covalently attached to this nanocomposite. Transmission electron microscopy (TEM), SEM and FTIR confirmed the successful immobilization of cellulase with Fe3O4/GO/CS. With regard to the soluble enzyme, the nanobiocatalytic system showed highly enhanced bioactivity and retained over 75% of its actual activity. After the immobilization process, a substantial widening in pH, storage, and thermal stability were obtained. The immobilized cellulolytic enzyme was capable of maintaining a high degree of its original activity after repeatedly using for 8 cycles [60]. Covalent attachment of Lipase B from C. antarctica onto sebacoyl-modified chitosan-decorated MNPs constitutes a robust nanobiocatalyst to catalyze enzyme-assisted kinetic resolution of various racemic heteroarylethanols. It showed activity up to 10 repeated catalytic cycles under the optimized conditions (n-hexane, vinyl acetate, 45 °C) [61]. Manganese peroxidase isolated from Anthracophyllum discolor was immobilized to chitosan/magnetic Fe3O4 biocomposite to eliminate reactive orange 16 and methylene blue dye pollutants. The nanobioconjugate preparation retained its activity and demonstrated recycling ability in 5 consecutive reaction cycles [62]. Alnadari and coworkers (2020) investigated the comparative use of chitin, chitosan, and sodium alginate as biocompatible polymers to functionalize MNPs for immobilization of β-glucosidase from T. maritima (Tm-β-Glu) [63]. This exclusive technology entails a novel thermally resistant chitin-binding domain (Tt-ChBD), which was found desirable for larger-scale applications. Characterization indicated that chitin-coated MNPs exhibited the maximum enzyme loafing capability and galactooligosaccharides (GOS) biosynthesis from lactose among all the immobilization methods for Tm-β-Glu-Tt-ChBD, in comparison to the free form of the enzyme. Chitin represented the most robust binding capacity by combining target proteins with Tt-ChBD. Fascinatingly, magnetic separation enables the reusability of the nanobiocatalytic system in several successive batches for GOS synthesis without a substantial loss of enzyme activity. After the immobilization process, the immobilized enzyme showed operational stability under varying pH, temperature, storage, and thermal conditions.
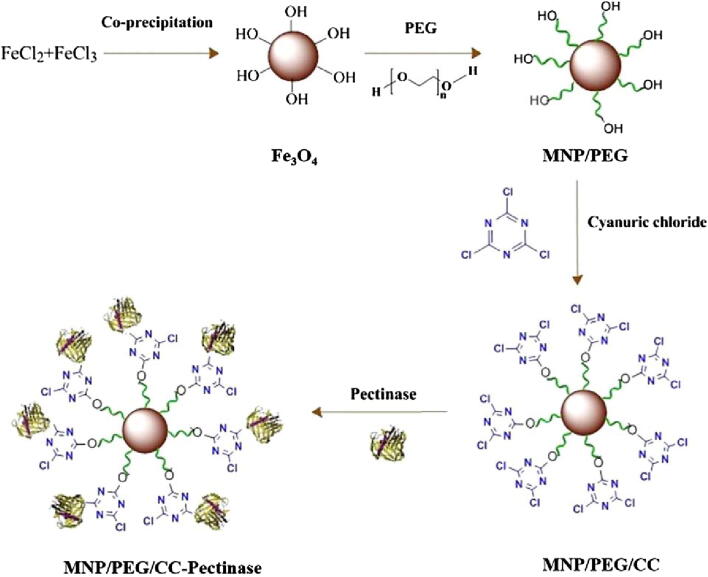
Polyethylene glycol (PEG) is a hydrophilic polymer of ethylene oxide that can improve the stability of MNPs. Inimitable attributes including water solubility, biocompatibility, flexibility, non-toxicity, and non-antigenic behavior render PEG a suitable coating material in various biotechnological applications. PEG-wrapped MNPs are found to be chemically stable and more dispersible than their pristine form of nanoparticles [64]. In a recent study, PEG-grafted MNPs were applied to covalent immobilization of pectinase via trichlorotriazine crosslinking. The MNPs-PEG were prepared under alkaline conditions by a chemical co-precipitation of FeCl3 and FeCl2 solutions using PEG as a coating agent. The prepared MNP-PEG nanosupport was then cross-linked with cyanuric chloride to introduce active groups on their surface facilitating the covalent attachment of pectinase enzyme. The resulted modified MNPs were allowed to react with pectinase solution yielding active MNP/PEG/CC-pectinase biocatalytic system (Fig. 3). In addition to high loading capacity, PEG-grafted MNPs immobilized enzyme presented improved satisfactory operational stability, improved catalytic efficiency, and easily recyclability in multiple cycles. pH and thermal stability profile revealed augmented enzyme performance even at extreme values than the free enzyme. Immobilized preparation was able to retain up to 94% and 55% of its actual activity after storage for 125 days at 25 °C, and 10 repeated catalytic runs, respectively. A prominent reduction in turbidity of pineapple juice (up to 59%) after treatment with the immobilized enzyme suggest its application in food-processing sectors [65].
Fig. 3.
Schematic representation of the synthesis of 1,3,5-triazine-functionalized PEG-coated Fe3O4 nanoparticles and immobilization of pectinase. Reprinted from Ref. [65] with permission from Elsevier. License Number: 5092320806715.
MNPs and carbon based nanocomposites for enzyme immobilization
MNPs and carbon based nanocomposites that integrates the useful magnetic attributes of the core (high saturation magnetization) and excellent physicochemical properties of carbonaceous materials have been well documented in the scientific literature [66], [67]. The integration of MNPs and carbonaceous materials including CNTs and graphene into nanocomposites has recently become a hot topic of research due to their new and/or enhanced functionalities that cannot be achieved by either component alone, and therefore holds great promise for a wide variety of applications in catalysis, optoeletronic materials, surface enhanced Raman Scattering, biomedical fields, and so on [68]. In comparison to iron oxides, the applications of the carbon-coated MNPs have been revealed in semi-heterogeneous catalysis, electrode supercapacitors, lithium-ion batteries, and blood and water purification [69], [70], [71]. Uniform coating of carbon on the surface of MNPs could protect from air oxidation and increase its stability, biocompatibility, and dispersible properties. For biocatalysis application, the aptitude of enzyme connection with the nanosupport by an adequate organic chemistry-based protocol is particularly alluring because it enables better enzyme reusability and negligible protein side-products in the final reaction mixture. Owing to high magnetic saturation, larger surface area, and tunable surface functionalities, carbon-coated MNPs have emerged as attractive choices of nanosupports for enzyme immobilization. Zlateski et al. have used chemically functionalized (diazonium chemistry) carbon-coated cobalt NPs, which is activated for bioconjugation (N,N-disuccinimidyl carbonate) and used for enzyme immobilization [72]. Three different kinds of enzymes including α-chymotrypsin, lipase B, and β-glucosidase were covalently attached to this magnetic nanosupport. After immobilization, the resultant enzyme–particle conjugates showed good stability and catalytic performance and could be recyclable from milliliter to liter volumes in short recycling durations. Magnetic carbon-coated NPs have been used for horseradish peroxidase (HRP) immobilization in combination with chitosan and cross-linking of glutaraldehyde and applied to constitute an enzyme-based novel amperometric electrode for H2O2 sensing [73].
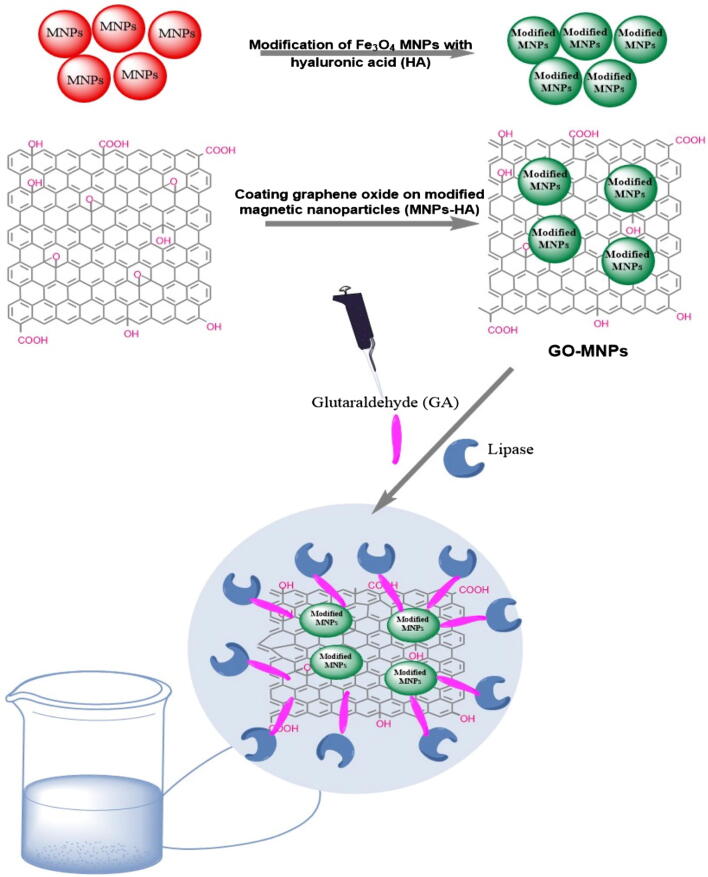
Carbon nanotubes (CNTs) are one-dimensional nanostructured materials, which are prepared by the coiling of one or more layers of graphite sheets around a central axis. Many studies have focused on functionalization (or coating) of CNTs (i.e., with magnetic or superparamagnetic nanoparticles) or filling their cavity with magnetic molecules to obtain versatile systems for biomedical applications [74]. CNTs have recently drawn much interest in the fabrication of many nanocomposites for enzyme immobilization due to high mechanical stability, porous structure, large surface area, metal–semiconductor feature, and exceptional adsorption capability [75], [76]. In a report, PAMAM dendrimer coated magnetic multi-walled carbon nanotubes (m-MWCNTs) were used for oriented immobilization of Rhizomucor miehei lipase (RML). Results revealed that the m-MWCNTs-PAMAM matrix immobilized lipase showed recovery activity as high as 2808% with a 27-fold higher esterification activity than the free form. Under the optimal conditions, the biodiesel conversion by immobilized enzyme reached 94% from waste vegetable oil in a tert-butanol solvent system. Furthermore, m-MWCNTs-PAMAM bound lipase was easily recoverable without loss of any significant reduction in conversion efficiency even after 10 repeated conversion runs [77]. Magnetic graphene nanocomposites have also been the subject of growing attention in recent years [78]. A set of fascinating properties, including easy surface amendment, magnetic response, simple fabrication, high enzyme loading, and noticeable reusability have rendered them useful in many application such as catalysis, sensors development, lithium batteries, dye and ion removal, microwave absorption, supercapacitor electrodes, etc. [79], [80], [81]. For the first time, hyaluronic acid-coated MNPs-functionalized graphene oxide composites (GO-MNPs) for the immobilization of lipase B from C. antarctica (Fig. 4). With reference to the free biocatalyst, the storage stability of lipase-GO-MNPs was substantially improved. GO-MNPs immobilized lipase showed activity at elevated temperatures retaining over 90% of its recovered activity at 60 °C, whereas the soluble enzyme could preserve only 45% of its activity [82]. Rouhani et al. (2020) immobilized laccase from T. versicolor onto magnetic-graphene nanocomposites via glutaraldehyde crosslinking for the green preparation of sulfa drugs [83]. For this, magnetic GO nanocomposite was first fabricated by in situ co-precipitation method and then functionalized with APTES followed by cross-linking with glutaraldehyde. The performance of the immobilized nanobiocatalyst was markedly increased than that to free laccase in terms of stability and activity under the optimal conditions. It retained about 70% of its relative activity after incubating at 55 °C for 2 h, while only 48% of activity was recorded by the free laccase under identical time duration. Furthermore, the nanobioconjugate preserved higher than 85% of its activity after 20 days and possessed satisfactory recycling efficiency exhibiting 85% of its initial activity after eight recurrent runs.
Fig. 4.
The diagram of the lipase-GO-MNPs-CLEAs assembly process. Reprinted from Ref. [82] with permission from Elsevier. License Number: 5092320991917. CLEAs-cross-linked enzyme aggregates.
Small molecules and surfactants coating for enzyme immobilization
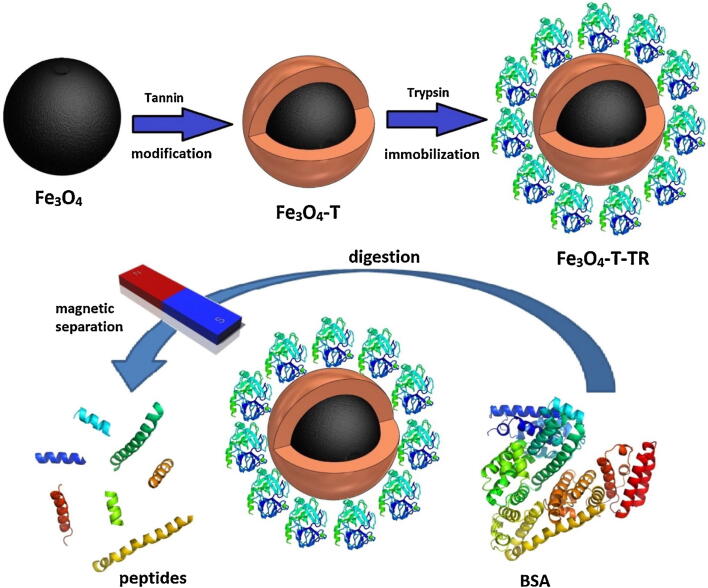
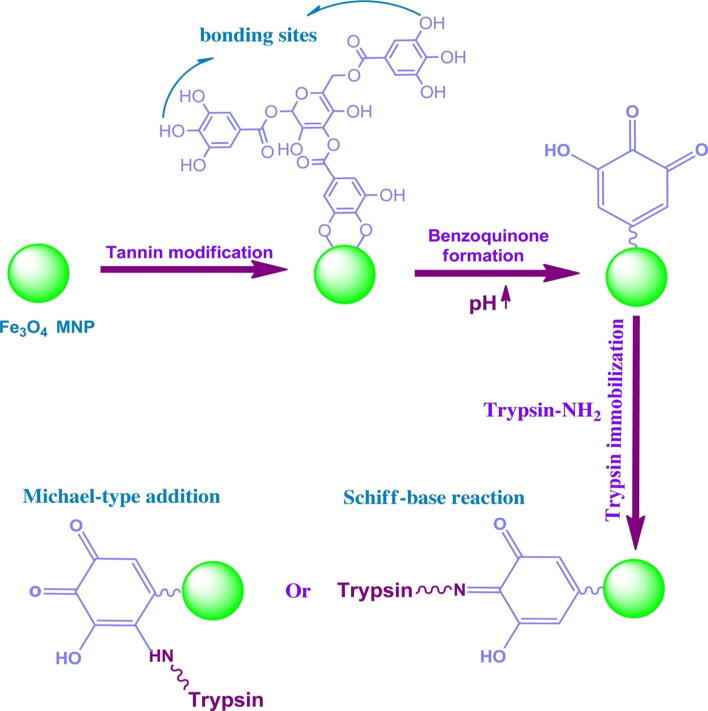
A number of small organic molecules such as gallic acid, citric acid, amino acids, tannic acid (TA), cyclodextrin, vitamins, lauric acid, and dopamine are often employed for the modification of MNPs surface following various strategies to enhance the biocompatibility, and stability of these MNPs [84], [85], [86], [87]. One of the approaches is the direct inclusion of small organic molecules during fabrication. Tannic acid is a hydrophilic polyphenolic compound with wide applications in the production of resin, leather, and as polymer flocculants or coagulants for water remediation [88]. Tannic acid can be used in combination with Fe(III) for the modification of MNPs [89], [90]. During the use of tannic acid as a cross-linker, three galloyl groups from the tannic acid molecule react with each of Fe III ion forming an octahedral complex [91]. The tannin-metal complex formation is significantly influenced by the initial pH value. A tannin-metal mono-complex is generated at pH below 2, whereas a pH of 3–6 yields a bis-complex, and a stable tris-complex is formed at a pH of above 7. At ambient temperature, the intermingling of TA and Fe III with each other in water results in film formation. It is demonstrated that protein molecules with open and random coil-like conformations display a high affinity towards polyphenols compared with tightly folded structures [92]. Tannic acid-coated MNPs were applied as a support material to the immobilization of β-agarase, which exhibited greater pH and thermal resistance as well as appreciable recycling ability compared with the free counterpart. In addition, the immobilized β-agarase-TA-MNPs system was applied to prepare neoagaro-oligosaccharides with varying degrees of polymerization and antioxidant activities [93]. Atacan and coworkers adopted a solvothermal method to prepare MNPs and modified with tannic acid by a novel binding process for covalent immobilization of trypsin (Fig. 5) [94]. The trypsin was attached to tannin-modified MNPs by generating Michael-type addition or Schiff-base reaction among the quinone moieties present on the tannin NPS, which are produced by of pH-driven oxidation of pyrogallol groups of tannin, and the amino groups of the trypsin (Fig. 6). Thus, efficient immobilization was demonstrated by carrying out a novel process. Finally, the trypsin bound to tannin-coated MNPs was applied to successful enzymatic digestion of bovine serum albumin (BSA), where it showed a satisfactory digestion performance for BSA and egg white proteins.
Fig. 5.
The illustration of modification and immobilization process on magnetic iron oxide nanoparticles for efficient BSA digestion. Reprinted from Ref. [94] with permission from Elsevier. License Number: 5092321139781.
Fig. 6.
The chemical structure of pH-catalyzed oxidation of pyrogallol groups of tannin and subsequent binding reactions with the amines on trypsin. Reprinted from Ref. [94] with permission from Elsevier. License Number: 5092321139781.
As a natural molecule, gum arabic (GA) has demonstrated the ability to improve the colloidal stability of magnetic nanomaterials because of non-specific adsorption [95]. Coating with GA increases colloidal stability as well as incorporates reactive functionalities that aid in the coupling of biomolecules or enzymes. Mahmood et al. used GA as a coating agent to circumvent MNPs agglomeration and augment their biocompatibility making it an attractive carrier for CRL immobilization [96]. GA-coated MNPs (GA-MNPs) were fabricated by chemical co-precipitation technique and functionalized with glutaraldehyde for effective attachment of lipase enzyme onto this magnetic support. Before immobilization, the lipase surface was covered with different surfactants for stabilization of enzyme in its open form. The resulted surfactant-coated immobilized lipase bio-system was applied to produce ethyl isovalerate, a flavor ester. Among the different surfactants tested, non-ionic surfactants showed better contribution with the retention of 80% esterification yield in 48 h than that to corresponding anionic/cationic surfactants. Such improvement in activity might be ascribed to the interfacial activation of immobilized non-ionic surfactant-coated lipase conjugate. Moreover, surfactant-coated forms of the magnetic nanobiocatalyst preserved good catalytic activity after seven consecutive reuse cycles. In another report, the surface of MNPs was coated with gallic acid biomolecule. The phenolic moieties in gallic acid are attractive to nucleophiles, such as primary amines, making them capable of immobilizing enzyme molecules [97]. Furthermore, gallic acid presents the benefits of low cost, abundant availability, and biocompatibility. Trypsin (EC 3.4.21.4) was efficiently immobilized on the surface of easily fabricated gallic acid-coated MNPs, which offers a decent support matrix due to high accessible surface area and facile separation properties. As compared to the free enzyme, the immobilized form of trypsin presented high stability and retained high enzyme relative activity in alkaline pH conditions (pH range of 6 to 10.5) and a temperature range of 45 to 55 °C. It also showed appreciable storage stability retaining over 90% of activity after four months at 4 °C, while 60% of activity was recorded using the free enzyme under comparable conditions. After 8 continuous reuse times, the activity of the immobilized enzyme was found to 54.5% of its primary activity. It was capable of hydrolyzing BSA that manifests its application in the field of diagnostics, pharmaceuticals, food, and waste treatments [98].
Metal-chelated MNPs for enzyme immobilization
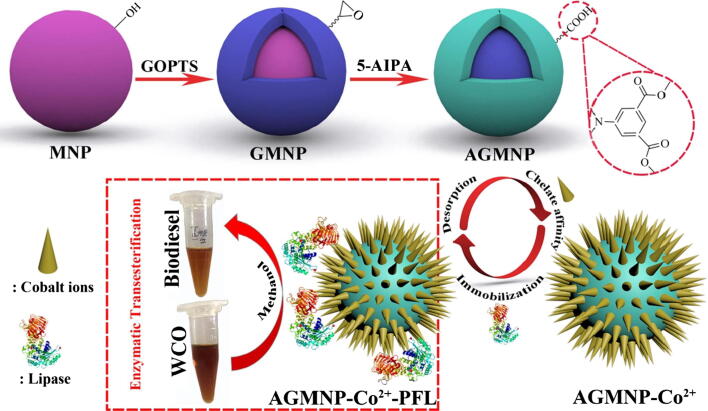
Surface functionalization of MNPs with metal elements may furnish an inert layer, which explicitly displays a core-satellite, core–shell, or dumbbell structural morphology. In addition, the functionalization of MNPs by metallic coatings results in improved compatibility and stability [99]. Loading of metal ions directly on the surface of support and chelation with enzyme molecules often display retention of high enzyme capacity and biocatalytic performance following the immobilization process [100]. Moreover, the metal-chelated affinity immobilization process is facile, fairly mild, and easy to perform, and the support could be recycled after desorption of the used enzyme. Hence, the metal-coated MNPs, integrating the benefits of paramagnetic property and immobilized metal ion affinity interaction, might have an incredible perspective for enzyme immobilization. Chen et al. (2014) synthesized agarose-coupled novel MNPs using by co-precipitation method under alkaline conditions [101]. MNPs were first coated with iminodiacetate using an epichlorohydrin agent followed by chelation with metal ions. The morphology and chemical properties of prepared support was analyzed by SEM, XRD, VSM, and FTIR. Among different metals ascertained, the Co2+-chelated agarose MNPs exhibited the highest β-glucosidase loading ability of 1.81 mg/g MNPs and attained the maximum recovered activity (117% per protein gram) for β-glucosidase immobilization. Immobilized bioconjugate displayed high operational and thermal stability and preserved over 90% of its preliminary activity after repeatedly using for 15 runs. A novel metal-chelating ligand, 5-aminoisophthalic acid (5-AIPA), was effectively coated onto MNPs, which were pre-decorated with (3-Glycidoxypropyl) trimethoxy silane (GOPTS) for Co2+-chelated affinity immobilization of lipase from P. fluorescens [102]. Covalent coating of support surface with GOPTS was initially used to link MNPs beads containing hydroxy to create reactive epoxy groups for additional functionalization. Afterward, the condensation of GOPTS-coated MNPs with 5-AIPA caused the epoxy group to react with the amine group of aromatic carboxylic acid. Finally, Co2+-chelated AGMNPs were applied to immobilize lipase from Pseudomonas fluorescens, and adsorption–desorption of lipase enzyme achieved cyclic use of this support matrix (Fig. 7). Under the optimal environment, the resultant immobilized lipase possessed 95% conversion efficiency to synthesize biodiesel from waste cooking oil. It also retained higher than 80% of biodiesel yield after 10 repeated conversion cycles that demonstrate excellent operational performance. The designed support was readily regeneratable after the desorption of the inactivated enzyme and can be re-chelated with the Co(II) ions [102].
Fig. 7.
Schematic diagram of Co2+-chelated MNP preparation for use in reversible immobilization of lipase. Reprinted from Ref. [102] with permission from Elsevier. License Number: 5092321359009.
Wang et al. [87] prepared Ni2+-functionalized silica-coated MNPs (SiMNPs) using isocyanatopropyltriethoxysilane as a metal-chelating ligand to immobilize prolidase from Escherichia coli [103]. To this end, water-soluble nanocrystallites of iron oxide were first produced by the co-precipitation synthetic method, which was subsequently wrapped with a silica shell by reacting with tetraethoxysilane (TEOS) in aqueous ammonia. Further coupling of silica shell iron oxide nanoparticles with the chelating agent 3-(triethoxysilyl)propyl isocyanate-nitrilotriacetic acid (ICPTES-NTA) and NiCl2 results in the formation of fully-armed Ni2+-functionalized silica-wrapped MNPs (NiNTASiMNPs). The resultant synthesized NiNTASiMNPs were employed as an affinity probe to adsorb His6-EcPepQ. Characterization of native and NiNTASiMNPs-bound enzyme revealed that His6-EcPepQ@NiNTASiMNPs enzyme showed greatly improved activity at elevated temperature of 70 °C and a wider pH range of 5.5 to 10 than that to free counter form. It also displayed enhanced stability when stored for 2 months and reusable for over 20 cycles by retaining 80% of its original activity. Moreover, the immobilized magnetic nanobiocatalyst was applied to degrade organophosphorus compounds, including diethyl p-nitrophenyl phosphate (ethyl paraoxon) and dimethyl p-nitrophenyl phosphate (methyl paraoxon). Results demonstrated dimethyl p-nitrophenyl phosphate as a preferred substrate for degradation by immobilized prolidase.
Mesoporous material-modified MNPs for enzyme immobilization
Mesoporous materials have been recognized as prodigious carriers for enzyme immobilization due to a set of desired features including designable pore size, huge surface area, non-toxicity, greater pore volume, and thermo-chemical strength [104]. Enzymes can be attached on the surface or trapped within the pores of mesoporous matrices by covalent coupling, cross-linking, or simple physical adsorption. A large number of various organosilanes are utilized to insert cyano, amino, epoxy, or sulfhydryl functionalities on the surface of mesoporous materials for efficient enzyme attachment [105]. The introduction of these functional groups generates many reactive sites to bind biomolecules and thus enhance the loading capacities. Furthermore, the penetration of organic groups into the mesoporous channel reduces the pore volume or size, resulting in the prevention of enzyme leaching. Likewise, Muñoz-Pina and coworkers (2018) also examined the polyphenol oxidase (PPO) immobilizing ability of many thiol-modified mesoporous silica materials with diverse geometrical shapes, structures, and pore sizes. UVM-7 is a silica-based mesoporous material that comprises of both mesopores and textural pores. It also has shown potential for enzyme immobilization such as polyphenol oxidase in both real and model systems [106]. More recently, the same group fabricated five different nanostructured materials to inspect their capability to increase the activity of the PPO enzyme. All these materials were based on a mesoporous silica material (UVM-7 support) and introduced with different functionalities (i.e. amine, alkane, isocyanate, pyridine, and carboxylic acid) to evaluate PPO immobilization capacity. Except for the carboxylic acid-modified nanomaterial, all other functionalized nanosupports offered high enzyme loading abilities and the immobilization rate increases with functionalization. Interestingly, amine-carrying support material captured not only the PPO enzyme but also sequestrated the resultant oxidation products. This nanomaterial was corroborated by reacting with fresh apple juice in which no browning occur even after exposure to 1.5 h in the presence of oxygen [106]. Furthermore, the modification of MNPs with mesoporous materials including silica helps in efficient encapsulation/adsorption of enzymes, due to various reasons including the presence of hollow spheres with mesoporous walls, ability of forming unique core/shell system etc [107]. For instance, khorshidi et al., reported the successfull immobilization of cross-linked cellulase aggregates (CLEA) on the amine-functionalized Fe3O4@silica core–shell magnetic nanoparticles (MNPs) [108]. In another study, Wang et al., have exploited the mesoporous properties of silica nanoparticles to fabricate wormhole framework structured mesoporous silica-magnetite nanocomposites [109]. The magnetic nanocomposites were prepared by using tetraethyl orthosilicate as the silica source and amine terminated Jeffamine surfactants as template. The nanocomposites were modified by chelating with copper to further enhance the adsorption capacity. The Cu2+ chelated magnetic nanocomposite showed higher adsorption capacity of 98.1 mg g−1 -particles and activity recovery of 92.5% for laccase via metal affinity adsorption. More recently, core − shell structured magnetic mesoporous silica microspheres with ultra-large mesopores were developed using a controllable solvent evaporation induced solution-phase interface co-assembly approach [110]. For this purpose. large-molecular-weight amphiphilic block copolymers poly(ethylene oxide)-blockpoly(methyl methacrylate) (PEO-b-PMMA) and small surfactant cetyltrimethylammonium bromide were employed as co-templates, which co-assembled with silica source in tetrahydrofuran/water solutions. The as-prepared composite was used to immobilize trypsin, which demonstrated a high loading capacity of 80 μg/mg. In another study, mesoporous yolk–shell nanoparticles with a movable Fe3O4 core inside the hollow capsules, with two different morphologies were fabricated using a template-assistant selectively etching method [111]. The composites were applied as carriers for Candida rugosa lipase (CRL) immobilization.
MOF-modified MNPs for enzyme immobilization
Amongst nanostructured materials, MOFs are considered an inspiring group of support materials for enzymes encapsulation owing to their exceptionally larger surface area, pore size uniformity, tunable surface and porosity, easy recovery, functional and structural versatility, and high thermal and chemical stability [112], [113]. Surface adsorption, co-precipitation, and pore encapsulation are three notable immobilization strategies that can be used to embed enzymes onto MOFs. Co-precipitation strategy can protect enzyme molecules from thermal, biological, and chemical degradation by embedding enzyme molecules within MOFs, and resultantly, generate immobilized enzyme with improved stability [114]. The use of MNPs-MOF nanocomposites produced by integrating features of both Fe3O4 NPs and MOFs have emerged as novel platforms for immobilizing a diverse array of enzymes [115], [116]. Generally, MNPs-MOF nanocomposites are produced by the reaction between metal ions and organic ligands using carboxylated Fe3O4 NPs. Wang et al. (2016) introduced an efficient, facile, and environmentally-responsive approach for the preparation of magnetic MOF nanocomposite [117], Fe3O4@MIL-100(Fe). For this, carboxyl-modified Fe3O4 nanorods were incorporated with three-dimensional MIL-100(Fe) nanocrystals. The as-prepared composite microsphere showed the substantial surface area and strong magnetic properties, which make them attractive contenders for enzyme immobilization. The CRL was immobilized onto Fe3O4@MOF core–shell microspheres by covalent attachment (strategy I) and metal-ion affinity interfaces (strategy II). The immobilized nanobiocatalytic system reserved 65% of its preliminary activity at 65 °C for the hydrolysis of olive oil over 6 h. It retained over 60% of remaining activity after 10 reiterated catalytic runs and presented a significant improvement in biocatalytic activities at broader temperature and pH ranges than that to the free enzyme. High enzyme loading capacity was ascribed to large pore size and surface area combined with the occurrence of free carboxyl groups and unsaturated metal sites in MOFs.
A novel magnetically active Ni-based MOF composite was prepared for efficient separation and immobilization of enzymes [118]. For this, a facile one-pot hydrothermal method was adopted to develop Ni-based MOF nanorods (Fe3O4/Ni-BTC) with a good magnetic response by the entrapment of citric acid-coated MNPs on Ni-BTC. Characterization revealed that these nanocomposites were fabricated in the form of nanorods, which contained MNPs on their surface. A variety of different interactions played a key role in enzyme immobilization, such as hydrogen bonding, electrostatic attraction, hydrophobic forces, and affinity between histidine tags and Ni2+. Based on the mechanistic understanding of these nanocomposites, a new approach was proposed for the immobilization of S-adenosylmethionine synthetase (SAMS), which showed high stability against extreme pHs and elevated temperature, and showed noteworthy repeatability profile after immobilization process [118].
Laccase from white-rot fungi was immobilized, for the first time, onto amino-functionalized magnetic MOF, Fe3O4-NH2@MIL-101(Cr) [119]. Immobilized laccase prepared by the covalent bonding and adsorption method revealed high immobilization yield, largely recovered activity, and better endurance to low pH and elevated temperature regimes. It also showed excellent storage stability holding over 85% of its initial bioactivity after storage of 28 days. At an extreme temperature of 85 °C, Fe3O4-NH2@MIL-101(Cr) bound biocatalyst presented about 50% of the remaining activity even after heating for 6 h. The tolerance of immobilized laccase was greatly improved in organic solvents like methanol. Finally, Fe3O4-NH2@MIL-101(Cr)-based biocatalytic system led to the rapid removal of 2,4-dichlorophenol, reaching the removal efficiency to 87%. After the reaction, laccase can be easily recovered by the mean of a magnet from the complex reaction solution. All these features make MOF-based MNPs nanocomposite a novel nanoplatform for immobilizing an array of enzymes with high catalytic activity and great biotechnological potential [119]. Table 2 portrays a current summary of the use of surface-engineered magnetic nanoparticles as novel paradigms for enzyme immobilization.
Table 2.
Some examples of surface-coated magnetic nanoparticles as support materials for enzyme immobilization and their applications.
| Magnetic nanocarrier | Enzyme | Functional reagent | Improved properties | Application | References |
|---|---|---|---|---|---|
| MNPs | Pseudomonas fluorescens lipase | Co2+ | Immobilized lipase possessed 95% conversion efficiency to synthesize biodiesel from waste cooking oil. Excellent operational performance retained higher than 80% of biodiesel yield after 10 repeated conversion cycles. |
Biodiesel production | [102] |
| Fe3O4-NH2@MIL-101(Cr) | Laccase from white rot fungi | MIL-101 | High recovered activity, and better endurance to low pH and elevated temperature regimes. Excellent storage stability retaining over 85% of its original bioactivity after storage of 28 days. At an extreme temperature of 85 °C, Fe3O4-NH2@MIL-101(Cr) bound biocatalyst presented about 50% of the remaining activity even after heating for 6 h. Rapid removal of 2,4-dichlorophenol, reaching the removal efficiency to 87%. |
Removal of phenolic compounds | [119] |
| Agarose-coupled novel MNPs | β-glucosidase from sweet almond | Co2+ | Immobilized bioconjugate displayed high operational and thermal stability, and preserved over 90% of its preliminary activity after repeatedly using for 15 runs. |
Production of aromatic compounds Ethanol from cellulosic agricultural residues |
[101] |
| MNPs-functionalized graphene oxide composites | Lipase B from Candida antarctica | Hyaluronic acid | As compared to the free enzyme, the storage stability of lipase-GO-MNPs was substantially improved. GO-MNPs immobilized lipase showed activity at elevated temperatures retaining over 90% of its recovered activity at 60 °C, whereas the free enzyme retained only 45% of its activity under the same temperature conditions. |
Biodiesel production, pharmaceuticals and cosmetic industry | [82] |
| MNPs | Porcine pancreatic lipase and penicillin G acylase | Cellulose | Improved catalytic activity and stability of immobilized enzymes. Easy separation of immobilized enzymes from the reaction system. |
Enzyme immobilization | [120] |
| MNPs | β-agarase | Tannic acid | Immobilized β-agarase, exhibited greater pH and thermal resistance as well as appreciable recycling ability compared with the free counterpart. The immobilized β-agarase-TA-MNPs system was applied to prepare neoagaro-oligosaccharides with varying degrees of polymerization and antioxidant activities |
Preparation of bioactive neoagaro-oligosaccharide | [93] |
| Trichlorotriazine-functionalized MNPs | Pectinase | Polyethylene glycol | Immobilized enzyme presented improved satisfactory operational stability, improved catalytic efficiency, and easily recyclability in multiple cycles. Augmented pH and thermal stability profile than the free enzyme. Retained up to 94% and 55% of its actual activity after storage for 125 days at 25 °C, and 10 repeated catalytic runs, respectively. A prominent reduction in turbidity of pineapple juice (up to 59%) after treatment with the immobilized enzyme. |
Fruit juice clarification | [65] |
| Fe3O4@MIL-100(Fe) | Candida rugosa lipase | MIL-100(Fe) | Immobilized nanobiocatalytic system retained more than 65% of its original activity at 65 °C for the hydrolysis of olive oil in 6 h. It retained over 60% of residual activity still after 10 repeated catalytic runs. Presented a significant improvement in biocatalytic activities at broader temperature and pH ranges than that to the free enzyme. |
Transesterification and synthesis of esters | [117] |
| MNPs | Cholesterol oxidase | Silica | In contrast to the soluble enzyme, the covalent immobilization of biocatalyst was able to retain about 50% of its activity. | Development of biosensing components | [121] |
| MNPs | Glucose oxidase | Silica | Immobilized bioconjugate preparation maintained over 95% and 90% of its original activity after storage for 45 days, and 12 consecutive reaction cycles. Substantial improvements in thermal stability profiles were also recorded at high temperatures up to 80 °C. Moreover, the immobilized biocatalyst was less likely to be affected by alterations in pH values |
Biomedical applications | [33] |
| MNPs | Phospholipase D | Silica | Increased tolerance of immobilized enzyme to high temperature. Catalytic activity of the immobilized biocatalyst retained to be 40% after eight recycles. | Synthesis of functional phosphatidylserine |
[122] |
| MNPs film | Horseradish peroxidase from horseradish cv. Balady |
Polymethyl methacrylate | Excellent reusability retaining 78.5% of its initial activity after 10 repeated cycles. High stability of the immobilized HRP against metal ions, a high urea concentration, isopropanol, and Triton X-100. Efficient removal of phenol in the presence of hydrogen peroxide. |
Removal of wastewater aromatic pollutants | [123] |
| Fe3O4–graphene nanocomposite | Trametes Versicolor laccase | APTES | Stability and activity of the immobilized nanobiocatalyst was markedly increased than that to free laccase. Retained about 70% of its relative activity after incubating at 55 °C for 2 h, while only 48% of activity was recorded by the free laccase under identical time duration. Nanobioconjugate preserved higher than 85% of its activity after 20 days of storage and possessed satisfactory recycling efficiency exhibiting 85% of its original activity after eight repeated cycles. |
Green preparation of sulfa drugs | [83] |
| Biomimetic silica-MNPs hybrid nanocomposite | β-glucuronidase from Patella vulgata limpets | silica | Superior storage, thermal, and operational stability of the enzyme immobilized in the composite material. Different bioconjugates with MNPs and Si maintained 40% of their original activities at a high temperature of 80 °C after 6 h, while the free form of enzyme dropped over 90% of its activity within 10 min. |
Pharmaceutical and food industry | [34] |
| Fe3O4/Ni-BTC | S-adenosylmethionine synthetase from Thermus thermophilus HB27 | Citric acid | Iimmobilized enzyme was more stable against temperature variation (by nearly 8-fold in an 80 °C water bath after 2 h) and extreme pH (by nearly 1.3-fold at pH 3). Excellent reusability after immobilization with high efficiency and stability. |
Biosynthesis of S-adenosylmethionine | [118] |
| Amino-functionalized MNPs |
Alkaline protease from Bacillus licheniformis | APTES | Excellent operational stability retaining 50.1% of its initial activity after 10 cycles. Efficient catalytic hydrolysis of oat bran into oat polypeptides. |
Preparation of oat polypeptides |
[124] |
| Ni2+-functionalized MNPs | Prolidase from Escherichia coli | Silica | Improved activity at elevated temperature of 70 °C and a wider pH range of 5.5 to 10 than that to free counter form. Enhanced stability at storage for 2 months and reusable for over 20 cycles by retaining 80% of its original activity. Degradation efficiency for organophosphorus compounds. |
Hydrolysis of organophosphorus compounds | [103] |
| MNPs | Candida rugosa lipase | Alkyl silane | Increased catalytic activities of lipases after immobilization. Good stability and recycling ability retained 65% of its initial activity after seven repeated cycles. |
Enzyme immobilization | [125] |
| NPs | Horseradish peroxidase from horseradish cv. Balady |
Carbon | Enzyme-based novel amperometric electrode | H2O2 sensing |
[73] |
| MNPs | Lipase from Thermomyces lanuginosus | Polydopamine | A broader pH and temperature adaptability as compared to the free enzyme. Improved pH, thermal, and solvent tolerance stabilities compared to the free enzyme. |
Biodiesel production, organic synthesis, and environmental protection |
[126] |
| MNPs | Cellulase from Aspergillus fumigatus |
– | Immobilized enzyme retained 56.87% of its maximal activity after 6 h of incubation at 60 °C. Efficient hydrolysis of pre-treated rice straw with saccharification efficiency of 52.67%. Reutilization for up to four saccharification cycles with retention of 50.34% activity. |
Enzymatic saccharification of rice straw | [127] |
| Magnetic carbon nanotubes | Glucoamylase from Aspergillus niger |
Poly(amidoamine) | superior stability and reusability, without compromising the substrate specificity of free glucoamylase |
Starch processing and glucose production |
[128] |
| Metallic nanomagnets | α-chymotrypsin, lipase B, and β-glucosidase | Carbon | Immobilized bioconjugate preparations showed good stability and catalytic performance and could be recyclable from milliliter to liter volumes in short recycling durations. | Analytical immunoprecipitation and cell separation | [72] |
| MNPs with long alkyl chains | Candida rugosa lipase | poly- N,N diethylaminoethyl-acrylamide |
Nanoimmobilized biocatalytic system with the longest alkyl chains presented superior tolerance to high temperature (ranging from 25 to 70 °C) than that to the free form of lipase. It also showed good recyclability in four successive cycles and conveniently recovered by a simple magnetic separation. |
Biodiesel production, food processing , cosmetic and pharmaceutical industry | [49] |
| Divinyl sulfone superparamagnetic nanoparticles |
Lipase from Thermomyces lanuginosus | Polyethyleneimine | Good enantioselectivities with high catalytic activities in the reaction medium at pH 7.0. Excellent operational stability in the esterification reaction obtaining up to 61 % conversion after the seventh reaction cycle. |
Biodiesel production, food processing , cosmetic and pharmaceutical industry |
[129] |
| Superparamagnetic nanoparticles (Fe3O4) | Lipase from Thermomyces lanuginosus | Polyethylenimine, APTES, and Glutaraldehyde | The SPMN (superparamagnetic nanoparticle) @APTES covalent preparation had around 450 min of half-life time at pH 7.0 and 70 °C while that of the free enzyme was 46 min. The conversion attained was 50% and the enantiomeric excess of the product was 99%. |
Recovery of the biocatalyst | [130] |
| MNPs | Alcohol dehydrogenase | Carboxymethyl dextran | In contrast to the free form of ADH that dropped 70% of its original activity at 20 °C, and complete loss of its activity at 40 °C after 24 h. Nanoimmobilized biocatalyst retained more than 50%, and 75% of its remaining activity at 20 °C and 40 °C, respectively, under the same incubation period of 24 h. |
Chemical industries | [48] |
| Fe3O4/SiO2/NH2 | L-asparaginase | APTES, and Glutaraldehyde | ASNases were more stable in a wide range of pH and temperature values under the optimum reaction conditions. High stability at an elevated temperature of 50 °C for 3 h. Free form of enzyme showed only 30% of its original activity after preserving at 4 °C for 1 month, whereas Fe3O4/SiO2/NH2 ASNase preserved above 78.9% of its preliminary activities. Outstanding functioning stability after 17 consecutive batch cycles. |
Anti-leukemia chemotherapy | [31] |
| Fe3O4/SiO2/COOH | L-asparaginase | APTES, and Glutaraldehyde | High stability in a wide range of pH and temperature values. Preservation of 56.5% of its initial activity. Outstanding operational stability in several consecutive cycles. |
Anti-leukemia chemotherapy | [31] |
| Magnetic graphene nanocomposite | Trichoderma reesei cellulase | Chitosan | With regard to the soluble enzyme, the nanobiocatalytic system showed highly enhanced bioactivity and retained over 75% of its actual activity. After the immobilization process, a substantial widening in pH, storage, and thermal stability were obtained. The immobilized cellulolytic enzyme was capable of maintaining a high degree of its original activity after repeatedly using for 8 cycles. |
Saccharification of microcrystalline cellulose |
[60] |
| Sebacoyl-modified MNPs | Lipase B from Candida antarctica | Chitosan | High activity up to 10 repeated catalytic cycles under the optimized conditions (n-hexane, vinyl acetate, 45 °C). | Enzymatic Kinetic Resolution of Racemic Heteroarylethanols |
[61] |
| MNPs | β-glucosidase from Thermotoga maritima | Chitin, chitosan, and sodium alginate | Marked reusability of the nanobiocatalytic system in several successive batches for GOS synthesis without a substantial loss of enzyme activity. Immobilized enzyme showed operational stability under varying pH, temperature, storage, and thermal conditions. |
Galacto-oligosaccharide production | [63] |
| Iron oxide magnetic nanocomposite | Manganese peroxidase from Anthracophyllum discolor | Chitosan | The nanobioconjugate preparation retained its activity and demonstrated recycling ability in 5 consecutive reaction cycles. | Decolorization of textile wastewater |
[62] |
| Fe3O4@SiO2_EDTA-TMS | Laccase | EDTA-Cu (II) | Good operational stability of the immobilized enzyme presenting 73% of its initial activity after five sequential reactive cycles. Successfully applied to the degradation of Indigo Carmine dye |
Biocatalysis and biosensors | [131] |
| MNPs | Tyrosine | Tannic acid | Enzymatic digestion of bovine serum albumin | Protein digestion | [94] |
| MNPs | Tyrosine | Gallic acid | Immobilized trypsin presented high stability and retained high enzyme relative activity in alkaline pH conditions (pH range of 6 to 10.5) and a temperature range of 45 to 55 °C. It also showed appreciable storage stability retaining over 90% of its original activity after storage for 4 months at 4 °C. After 8 continuous reuse times, the activity of the immobilized enzyme was found to 54.5% of its primary activity. |
Diagnostics, pharmaceuticals, food, and waste treatments | [98] |
| MNPs | Candida rugosa lipase | Gallic acid | Improved esterification activity. Surfactant-coated forms of the magnetic nanobiocatalyst preserved good catalytic activity after seven consecutive reuse cycles. |
Production of multicycle ethyl isovalerate | [96] |
| Fe3O4@silica yolk-shell nanospheres |
Catalase from bovine liver | TMOS, APTES | Enhanced recycling efficiency and high resistance to heat, proteolytic agent, and denaturants. | Enzyme shielding | [132] |
| Fe3+-TA@ Fe3O4/SiO2-catalase |
Catalase from bovine liver | TMOS, APTES | Improved stability and efficient recycling ability | Shielding effect to protect enzymes from thermal, biological, and chemical degradation |
[133] |
| Fe3O4@mSiO2 | Nitrile hydratase | Glutaraldehyde | Improved pH, thermal, mechanical and storage stability |
Catalysis production of nicotinamide |
[134] |
| CA-Fe3O4 NPs | Lipase | Citric acid | Excellent long-term storage stability and increased activity at high temperature and pH | Enzyme immobilization | [4] |
MNPs—Magnetic nanoparticles; TMOS— Tetramethyl orthosilicate.
Biomedical applications of surface coated MNPs.
Detection, diagnostics and therapy
MNPs have often been widely used in several biomedical applications such as, biosensing, medical diagnostics, drug delivery, gene transfer etc. [135]. Among various extraordinary physicochemical properties of MNPs including fluorescence, photoacoustic effects, hyperthermia, magnetism, and photothermal properties [136], [137]. Particularly, enzyme-like catalytic properties of MNPs, such as peroxidase-like activity of iron oxide NPs, have received immense interest in the field of biomedicine [138]. Due to these enzymatic properties, in some cases modified MNPs are referred as nanoenzymes (enzyme mimetic MNPs), which are artificial enzymes with highly effective enzyme-like properties [139], [140]. Recently, these materials have gained considerable interests as they are easy to prepare, controllable in size and adjustable in function [141]. Besides, they offer stability and multifunctionality when compare to conventional enzymes and thus offer versatile applications in the field of biomedicine [138]. Enzyme mimetic MNPs demonstrate high stability even under harsh conditions including high temperatures, high acidic and basic environments. For example, Fe3O4 NPs stabilized with peroxidase-like casein showed excellent stability in wide range of pH between 1 and 12, and temperatures from 4 to 90 °C [142]. The as-prepared casein-MNPs were applied to catalyze the oxidation of a peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2 to the oxidized colored product which provides a colorimetric detection of H2O2. Contrarily, HRP enzyme did not exhibit any activity after treatment at lower pH (less than 5) and rapidly lost its activity as the temperature increased to 40 °C. Due to the smaller size of NPs and high surface area enzyme mimetic MNPs also offer excellent biocatalytic activity which can be further tuned by controlling the size and morphology of nanoparticles. This phenomenon has been successfully demonstrated by Gao et al., who has developed a novel immunoassay involving antibody-modified magnetite nanoparticles for the successful capturing, and separation of wastes in the treatment of wastewater. The small sized enzyme mimetic MNPs have exhibited highest catalytic activity in the order 30 nm greater than 150 nm greater than 300 nm [143]. In addition, the enzymatic activity of enzyme mimetic MNPs also dependent on the shape of NPs. Different shapes of iron oxide nanostructures like octahedral, spheres, and triangular plates have showed different peroxidase-like activities [144]. Apart from enzyme-like activity, iron oxide based enzyme mimetic MNPs also possess excellent superparamagnetism properties which can be exploited for multipurpose performances. For examples, the enzyme like activities of MNPs can be utilized to replicate peroxidase and catalase activities at acidic and neutral pH, respectively. Chen et al., have demonstrated the process of controlling the ability of free radicals under intracellular microenvironment through pH-dependent peroxidase-like and catalase-like activities of iron oxide NPs [145]. They have investigated the interaction of nanoenzyme with H2O2 within cells, the results revealed a concentration-dependent cytotoxicity on human glioma U251 cells, and they could dramatically enhance H2O2-induced cell damage. This pH controlled enzyme like activity can be effectively utilized under special circumstances like tumors or biofilms. Similarly, the enzyme mimetic MNPs offers great opportunity in other biomedical applications. Mostly, the peroxidase like activity of these materials have been utilized to enhance the signal detection during colorimetric reactions and generate free radicals to kill bacteria and cells or interfere ROS level. One of the primary applications of enzyme mimetic MNPs is the replacement of horseradish peroxidase (HRP) in enzyme-linked immunosorbent assay (ELISA) and other HRP-related molecular detection. Such as the development of MNPs based immunosorbent assay by Gao et al., using chitosan-modified magnetite nanoparticles to replace enzymes in conventional ELISA configurations [35].
Besides, these enzyme mimetic MNPs based novel immunoassays have also been used to detect various antigens or pathogens including human chorionic gonadotropin (HCG), IgG and epidermal growth factor receptor (EGFR) [138]. Apart from detections, enzyme mimetic MNPs have also been used for tumor diagnosis and therapy. Such as, magneto-ferritin nanoparticles (M−HFn) which targeted and visualized affected tissues (tumor) without using any targeting ligands or contrast agents [146]. In addition, these materials have also been used for the direct elimination of tumors. Particularly, iron oxide NPs have been very useful in this regard, they facilitate the generation of toxic radicals by catalyzing which affect tumor viability. However, in many cases the amount of H2O2 is not sufficient to initiate toxicity [147]. To enhance this, H2O2 is directly injected into the body or combine with an enzyme to generate H2O2 using in vivo substance as substrate. Indeed, the later process is more suitable compare to the direct injection of H2O2, which may cause unwanted damage to local tissues.
Bioelectronics
In this field, advance functional bio-devices such as biosensors, biofuel cells etc., are created by integrating biomolecules including enzymes with electronic systems. Recent advancements in the field of nanobiotechnology have enabled to create highly sensitive biomedical devices with advance functionalities. Biofuel cells involve biological materials, including proteins, microorganisms, enzymes etc., as catalysts to convert chemical energy into electrical power. The enzyme based biofuel cells typically consist of isolated enzymes as catalysts, anode, cathode and electrolyte materials [148]. So far, various oxidoreductase enzymes such as glucose dehydrogenase, aldehyde dehydrogenase, glucose oxidase etc., have been used in different types of enzymatic biofuel cells which have been applied as a power source for portable or implantable electronics, including pacemaker etc. For example, a ternary conducting nanocomposite, involving Fe3O4 MNPs, carbon nanotubes (CNT), gold nanoparticles (Au) and a conducting polymer polypyrrole (PPy), was developed and applied as the electrode support for the immobilization of glucose oxidase (GOD) [149]. The resulting composite improved the bioelectrocatalysis of the enzyme towards oxidation of glucose. In another study, magnetic carbon-encapsulated iron nanoparticles (CEINs) immobilized with laccase (Lc) and 1,4-naphthoquinone (NQ) and fructose dehydrogenase (FDH) were used for the fabrication of bioelectrodes in a biobattery and a biofuel cell [150]. In the device, the glassy carbon bioanode was coated with carbon-encapsulated iron nanoparticles, 1,4-naphthoquinone, fructose dehydrogenase, and Nafion, while the cathode was modified with carbon-encapsulated magnetic nanoparticles and laccase in the Nafion layer. A maximum power of 78 µW/cm2 at the voltage of 0.33 V and under 20 kΩ resistance, and the open-circuit voltage was 0.49 V was used. These enzymes worked effectively in the biofuel cell, and laccase also effectively worked in the biobattery.
Another application of enzyme coated MNPs are the fabrication of biosensors involving sensitive biological entities like antibodies, cell receptors, enzymes, transducers and, detectors associated with signal processing and electronics [32]. Commonly used MNPs and enzymes-based biosensors are glucose sensor for blood-sugar tests. Particularly, the process of enzyme immobilization is crucial to improve the sensitivity of the biosensors, which has been considerably progressed due to the advancement in the field of nanobiocatalysis [151]. Recently, Pakapongpan et al., have successfully immobilized glucose oxidase (GOD) on reduced graphene oxide (RGO), which is covalently conjugated to magnetic nanoparticles (Fe3O4 NPs) to obtain highly selective and stable glucose biosensor [152]. The proposed biosensor showed fast amperometric response (3 s) to glucose with a wide linear range from 0.05 to 1 mM, a low detection limit of 0.1 μM at a signal to noise ratio of 3 (S/N = 3) and good sensitivity (5.9 μA/mM). Similarly, a biosensor based on nanomagnet-silica core–shell conjugated to organophosphorous hydrolase (OPH) enzyme was designed for detection of paraoxon [153]. In another study, a novel core–shell Fe3O4@poly(dopamine) MNPs hybrid was fabricated using an in situ self-polymerization method [38]. The as-prepared nanohybrid was used as a solid support for the covalent immobilization of horseradish peroxidase (HRP), and the resulting biofunctionalized MNPS were employed to fabricate an amperometric biosensor for H2O2. The enzyme biosensor showed a high sensitivity of 442.14 mA M−1 cm−2, a low limit of detection of 182 nM, a wide linear range from 6.0 × 10−7 to 8.0 × 10−4 M and high stability for 1 month.
Proteomics
This field involve proteins analyses which maybe play a significant role in the field of biomarkers, drug treatment, medical diagnostics etc. Recently, this field has progressed due to the advancement of technologies in mass spectrometry analysis, protein quantification and bioinformatics data analysis. Particularly, Mass spectrometry plays a crucial role in large-scale protein analysis, but the downstream proteome analysis is typically effected by the cumbersome sample preparation methods [154]. This is typically simplified by the process of protein digestion in-solution using proteolytic enzymes such as trypsin. Trypsin (Try) is typically immobilized on a solid support including MNPs to make it more friendly for the mass spectrometric analysis [155], [156]. A method of combining trypsin-immobilized MNPs and microwave-assisted protein digestion is reported to study the proteins of human lens tissue, which were identified by liquid chromatography and mass spectrometry [157]. But, unprotected MNPs are very unstable and can be readily oxidize in atmospheric conditions [158]. Therefore, MNPs are often functionalized to protect them against oxidation and to facilitate enzyme immobilization using various stabilizing agents including polymers or other compounds containing amino (–NH2), hydroxide (–OH), carboxylic acid (–COOH) and phosphate groups [159], [160]. For example, polyaniline-coated nano-Fe3O4/carbon nanotube composite exhibited enhanced digestion efficiency of Try due to the high surface area-to-volume ratio of nanoparticles, which increased interaction between Try and the substrates (proteins) [161]. In another study, MNPs were stabilized with polyvinyl alcohol and activated with glutaraldehyde for trypsin immobilization [162]. Pristine (without MNPs) and immobilized trypsin on MNPs showed optimum activity at pH 6.0, 30 °C and pH 7.0, 40 °C, respectively, while trypsin immobilized MNPs was more stable than the free enzyme at 40 °C. Indeed, a recent study has demonstrated that trypsin immobilized on MNPs at the nano-scale performs better than the commercially available macroparticles counterpart [154].
A quantitative proteomics method based on liquid chromatography coupled to mass spectrometry has been widely used in allergen analysis, but it often requires long period of digestion which limits its application [163]. In this context, Qi et al., have applied a novel MNPs based hybrid material i.e., Try immobilized on hairy polymer-chain hybrid MNPs to shorten the digestion time and enhance the digestion efficiency [164]. Rapid digestion method based on as-prepared nanohybrid was used to detect milk allergens in baked food by ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Due to this technique, immobilized Try was digested in a short period of time (15 min), with higher or equal sequence coverage compared to conventional free trypsin, which required 12–16 h for digestion. Furthermore, Try immobilized MNPs have also been used for continuous hydrolysis of casein, which is the main protein in milk, and known to produce several bioactive peptides after hydrolysis [98]. Atacan et al., covalently immobilized Try on tannic acid (TA) coated Fe3O4 MNPs to investigate the digestion of casein from bovine milk [165]. Digestion efficiency of casein was investigated using liquid chromatography–mass spectrometry (LC–MS/MS) technique, which confirmed the efficient digestion of casein by immobilized Try compared to free Try due to prevention of autohydrolysis.
Conclusive remarks, challenges, and future directions
This review examines the prospects of surface-coated/functionalized magnetic nanostructured materials and their derived nanocomposites as exciting support candidates for the immobilization of various enzymes. It was observed that the surface coating/functionalization by inorganic materials (carbon, silicon groups, metal and metal oxides) and organic molecules (surfactants, small molecules, polymers, MOFs) can enhance the stability, and biocompatibility of MNPs, which consequently magnifies the application of these nanostructures for effective immobilization of enzymes. Although extensive research progress has been dedicated to the surface engineering of magnetic nanoparticles, a series of challenges still need to be addressed. For instance, a precise control over the size distribution and surface-coated shape of MNPs must be considered in future studies. Moreover, it is also important to address the issue of long-term stability of functionalized MNPs. Considering the future perspective, most of the applications, in particular, clinical aspects are still in the hypothetical phase necessitating consistent research investigations from multidisciplinary areas to realize its practical applications. Therefore, in addition to optimize the fabrication routes to synthesize magnetic nanostructures with better properties, the development of efficient, environmentally-friendlier, and stable surface-modification are also important. We envision that with sustained development, fabrication, and surface engineering strategies, numerous possibilities will arise to construct more and more multifunctional nanomaterials in the future to design immobilized biocatalytic systems for expanding the real-time application scope. Moreover, enzyme coated MNPs using the concepts of nanobiocatalysis also offer great potential in the field of biomedical applications including bioelectronics, bioconversion, and proteomics.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Muhammad Bilal: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. Hafiz M.N. Iqbal: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Syed Farooq Adil: Methodology, Writing – original draft, Writing – review & editing. Mohammed Rafi Shaik:Methodology, Writing – original draft, Writing – review & editing. Abdelatty Abdelgawad: Data curation. Mohammad Rafe Hatshan: Funding acquisition, Data curation. Mujeeb Khan: Data curation, Methodology, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Research Group no. RG-1436-032. Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico is thankfully acknowledged for partially supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M.N. Iqbal (CVU: 735340).
Biographies

Dr. Muhammad Bilal is an Associate Professor in the School of Life Science and Food Engineering, Huaiyin Institute of Technology, Huaian, China. He accomplished his Ph.D. from Shanghai Jiao Tong University with a specialization in Bioengineering. He has published more than 400 scientific contributions in the form of Research, Reviews, Book Chapters and Editorial type scientific articles in various areas of Science & Engineering. He has an H-index = 50 along with more than 9000 citations. Dr. Bilal has a collaborative network with national and international institutes/universities across the globe. His research interests include environmental biotechnology/engineering, nanotechnology, bio-catalysis, enzyme engineering, immobilization, chemical modifications and industrial applications of microbial enzymes, bioremediation of hazardous and emerging pollutants, liquid and solid waste management – valorization of agro-industrial wastes and biomaterials .

Dr. Hafiz M. N. Iqbal is a full-time Professor at the School of Engineering and Sciences, Tecnologico de Monterrey, Mexico. He completed his Ph.D. in Biomedical Sciences with a specialization in Applied Biotechnology and Materials Science at the University of Westminster, London, UK. Dr. Iqbal’s research group is engaged in Environmental Engineering, Bioengineering, Biomedical Engineering, Materials Science, Enzyme Engineering, Bio-catalysis, Bioremediation, Algal Biotechnology, and Applied Biotechnology related research activities. Dr. Iqbal has an H-index = 51, along with more than 8500 citations. Dr. Iqbal had guest-edited several special issues and served as an Editorial Board member for several peer-reviewed journals. He has published more than 350 scientific contributions in the form of Research, Reviews, Book Chapters, and Editorials, at several platforms in various journals of national/international repute with high impact factor.

Dr. Syed Farooq Adil is an Associate Professor at the chemistry department, King Saud University, Riyadh, KSA. He as completed his Bachelor’s and Master’s degree from the Osmania University, Hyderabad. He carried out his doctoral research work at the Indian Institute of Chemical Technology, and his Ph.D. degree was awarded by the Jawaharlal Nehru Technological University, Hyderabad. His research is focused on nanomaterials, material sciences, graphene-based nanocomposites and other energy storage materials, which are prepared using conventional methods and employing green synthesis and their applications as catalytic and biological agents. He has published more than 100 publications, several patents and few books. He also worked as a visiting associate at California Institute of Technology (CALTECH), Pasadena, CA, USA. He is also associated with the Journal of Saudi Chemical Society as an assistant editor. He is also an editorial board member of various journals.

Dr. Mohammed Rafi Shaik is an Associate Professor in the Department of Chemistry at King Saud University, Riyadh, KSA. He completed his Ph.D. in Chemistry (2014) with a specialization in polymer nanocomposites from King Saud University, KSA. He has an H-index = 19 along with more than 1000 citations. He has guest-edited a couple of special issues and serves as a scientific reviewer in numerous peer-reviewed journals. He is a member of The Saudi Chemical Society, KSA. His research interests involve design, synthesis, structural and physical characterization as well as potential applications of functional materials including inorganic nanomaterials, graphene/inorganic nanocomposites, for catalysis, biomedical and energy storage applications. He has over 100 publications and published a book and a book chapter.

Mr. Abdelatty Abdelgawad working as a Researcher in the Industrial Engineering Department at King Saud University, Riyadh, KSA. He received his Pre-Masters in Public Education (2010) from Benisuef University, Egypt. His research interests involve materials design, synthesis, structural and physical characterization as well as potential applications of functional materials including nanomaterials for energy storage and industrial applications.

Dr. Mohammad Rafe Hatshan is an Assistant Professor at the chemistry department, college of science, King Saud University, Riyadh, Saudi Arabia. He is also working as a Director of Central Laboratory, College of Science, King Saud University. He has completed his Bachelor’s degree from King Saud University and Master’s degree from Wollongong University, Australia. He received his Ph.D. (2019) from Western Michigan University, USA. His research is focused on nanomaterials, material sciences, graphene-based nanocomposites and other energy storage materials, which are prepared using conventional methods and employing green synthesis and their applications as catalytic and biological agents. He has published more than 25 scientific publications contributions in the form of Research, Reviews, Book Chapters, and Editorials, at several platforms in various journals of national/international repute with high impact factor.

Dr. Mujeeb Khan is an Associate Professor in the Department of Chemistry at King Saud University, Riyadh, KSA. He received his MSc degree in Materials Chemistry and PhD (2008) from Johannes Gutenberg University of Mainz in Germany. Later he worked as a Post-doctoral fellow at Max Planck Institute for Polymer Research Mainz, Germany. His research interests involve design, synthesis, structural and physical characterization as well as potential applications of functional materials including inorganic nanomaterials, graphene/inorganic nanocomposites, for catalysis, biomedical and energy storage applications. He has over 100 publications and published a book, US patent and few book chapters. He is also working as an Associate Editor in the Arabian Journal of Chemistry.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Muhammad Bilal, Email: bilaluaf@hotmail.com.
Mohammed Rafi Shaik, Email: mrshaik@ksu.edu.sa.
Mujeeb Khan, Email: kmujeeb@ksu.edu.sa.
Reference
- 1.Reddy L.H., Arias J.L., Nicolas J., Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 2.Tang S.C., Lo I.M. Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res. 2013;47:2613–2632. doi: 10.1016/j.watres.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Rossi L.M., Costa N.J., Silva F.P., Wojcieszak R. Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem. 2014;16:2906–2933. [Google Scholar]
- 4.Li X.-B., Gao Y.-J., Wang Y., Zhan F., Zhang X.-Y., Kong Q.-Y., et al. Self-assembled framework enhances electronic communication of ultrasmall-sized nanoparticles for exceptional solar hydrogen evolution. J Am Chem Soc. 2017;139(13):4789–4796. doi: 10.1021/jacs.6b12976. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S., Dutz S., Häfeli U.O., Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166(1-2):8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H.-W., Liu Y., Sun S.-H. Synthesis and assembly of magnetic nanoparticles for information and energy storage applications. Front Phys China. 2010;5(4):347–356. [Google Scholar]
- 7.Liu S., Yu B., Wang S., Shen Y., Cong H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv Colloid Interface Sci. 2020;281:102165. doi: 10.1016/j.cis.2020.102165. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Qiao S.Z., Hu Q.H., Lu G.Q. Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small. 2011;7:425–443. doi: 10.1002/smll.201001402. [DOI] [PubMed] [Google Scholar]
- 9.Liu G., Gao J., Ai H., Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9(9-10):1533–1545. doi: 10.1002/smll.201201531. [DOI] [PubMed] [Google Scholar]
- 10.Del Arco J., Alcántara A.R., Fernández-Lafuente R., Fernández-Lucas J. Magnetic micro-macro biocatalysts applied to industrial bioprocesses. Bioresour Technol. 2021;322:124547. doi: 10.1016/j.biortech.2020.124547. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y., Wang Z., Liu W., Zhang H., Zuo W., Tang H., et al. Size-and shape-dependent peroxidase-like catalytic activity of MnFe2O4 nanoparticles and their applications in highly efficient colorimetric detection of target cancer cells. Dalton Trans. 2015;44(28):12871–12877. doi: 10.1039/c5dt01585e. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho T.C., Malafatti J.O.D., Paris E.C., Tardioli P.W., Farinas C.S. Hydroxyapatite-CoFe2O4 magnetic nanoparticle composites for industrial enzyme immobilization, use, and recovery. ACS Appl Nano Mater. 2020;3(12):12334–12345. [Google Scholar]
- 13.Baghayeri M., Nazarzadeh Zare E., Mansour Lakouraj M. A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly (p-phenylenediamine)@Fe3O4 nanocomposite. Biosens Bioelectron. 2014;55:259–265. doi: 10.1016/j.bios.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Peterson R.D., Chen W., Cunningham B.T., Andrade J.E. Enhanced sandwich immunoassay using antibody-functionalized magnetic iron-oxide nanoparticles for extraction and detection of soluble transferrin receptor on a photonic crystal biosensor. Biosens Bioelectron. 2015;74:815–822. doi: 10.1016/j.bios.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Lee N., Yoo D., Ling D., Cho M.H., Hyeon T., Cheon J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115(19):10637–10689. doi: 10.1021/acs.chemrev.5b00112. [DOI] [PubMed] [Google Scholar]
- 16.Blanco-Andujar C., Walter A., Cotin G., Bordeianu C., Mertz D., Felder-Flesch D., et al. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine. 2016;11(14):1889–1910. doi: 10.2217/nnm-2016-5001. [DOI] [PubMed] [Google Scholar]
- 17.Estelrich J., Escribano E., Queralt J., Busquets M. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci. 2015;16(12):8070–8101. doi: 10.3390/ijms16048070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A.G. Roca, L. Gutiérrez, H. Gavilán, M.E.F. Brollo, S. Veintemillas-Verdaguer, M. del Puerto Morales, Design strategies for shape-controlled magnetic iron oxide nanoparticles, Adv Drug Deliv Rev 138 (2019) 68-104. [DOI] [PubMed]
- 19.M. Jacinto, V. Silva, D. Valladão, R. Souto, Biosynthesis of magnetic iron oxide nanoparticles: a review, Biotechnology Letters 43 (2021) 1-12. [DOI] [PubMed]
- 20.Bishnoi Shahana, Kumar Aarti, Selvaraj Raja. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater Res Bull. 2018;97:121–127. [Google Scholar]
- 21.Majidi Sima, Zeinali Sehrig Fatemeh, Farkhani Samad Mussa, Soleymani Goloujeh Mehdi, Akbarzadeh Abolfazl. Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed B. 2016;44(2):722–734. doi: 10.3109/21691401.2014.982802. [DOI] [PubMed] [Google Scholar]
- 22.Mahdavi Mahnaz, Namvar Farideh, Ahmad Mansor, Mohamad Rosfarizan. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18(5):5954–5964. doi: 10.3390/molecules18055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miri A., Najafzadeh H., Darroudi M., Miri M.J., Kouhbanani M.A.J., Sarani M. Iron oxide nanoparticles: biosynthesis, magnetic behavior, cytotoxic effect. ChemOpen. 2021;10:327. doi: 10.1002/open.202000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W., He Q., Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397. doi: 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Jiakun, Sun Jingjing, Wang Yuejun, Sheng Jun, Wang Fang, Sun Mi. Application of iron magnetic nanoparticles in protein immobilization. Molecules. 2014;19(8):11465–11486. doi: 10.3390/molecules190811465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W., Wu Z., Yu T., Jiang C., Kim W.-S. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications, Sci Technol. Adv Mater. 2015 doi: 10.1088/1468-6996/16/2/023501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Yonghui, Qi Dawei, Deng Chunhui, Zhang Xiangmin, Zhao Dongyuan. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J Am Chem Soc. 2008;130(1):28–29. doi: 10.1021/ja0777584. [DOI] [PubMed] [Google Scholar]
- 28.Chang Qing, Tang Heqing. Immobilization of horseradish peroxidase on NH2-modified magnetic Fe3O4/SiO2 particles and its application in removal of 2, 4-dichlorophenol. Molecules. 2014;19(10):15768–15782. doi: 10.3390/molecules191015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tural Bilsen, Şimşek İlke, Tural Servet, Çelebi Bülent, Demir Ayhan S. Carboligation reactivity of benzaldehyde lyase (BAL, EC 4.1.2.38) covalently attached to magnetic nanoparticles. Tetrahedron Asymmetry. 2013;24(5-6):260–268. [Google Scholar]
- 30.Liu Dong-Mei, Chen Juan, Shi Yan-Ping. Advances on methods and easy separated support materials for enzymes immobilization, Trends. Anal Chem. 2018;102:332–342. [Google Scholar]
- 31.Noma S.A.A., Ulu A., Koytepe S., Ateş B. Preparation and characterization of amino and carboxyl functionalized core-shell Fe3O4/SiO2 for L-asparaginase immobilization: A comparison study. Biocatal Biotransform. 2020:1–13. [Google Scholar]
- 32.Doaga R., McCormac T., Dempsey E. Functionalized magnetic nanomaterials for electrochemical biosensing of cholesterol and cholesteryl palmitate. Microchim Acta. 2020;187:1–10. doi: 10.1007/s00604-020-4203-1. [DOI] [PubMed] [Google Scholar]
- 33.Ashtari Khadijeh, Khajeh Khosro, Fasihi Javad, Ashtari Parviz, Ramazani Ali, Vali Hojatollah. Silica-encapsulated magnetic nanoparticles: enzyme immobilization and cytotoxic study. Int J Biol Macromol. 2012;50(4):1063–1069. doi: 10.1016/j.ijbiomac.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Correa S., Ripoll M., Jackson E., Grazú V., Betancor L. Stabilization of b-Glucuronidase by Immobilization in Magnetic-Silica Hybrid Supports. Catalysts. 2020;10:669. [Google Scholar]
- 35.Gao Lizeng, Wu Jiamin, Lyle Sarah, Zehr Keith, Cao Liangliang, Gao Di. Magnetite nanoparticle-linked immunosorbent assay. J Phys Chem C. 2008;112(44):17357–17361. [Google Scholar]
- 36.Wang Y., Xu F., Zhang L., Wei X. One-pot solvothermal synthesis of Fe3O4–PEI composite and its further modification with Au nanoparticles. J Nanopart Res. 2013;15:1338. [Google Scholar]
- 37.Li Gui-yin, Jiang Yu-ren, Huang Ke-long, Ding Ping, Chen Jie. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J Alloy Compd. 2008;466(1-2):451–456. [Google Scholar]
- 38.Martín M., Salazar P., Villalonga R., Campuzano S., Pingarrón J.M., González-Mora J.L. Preparation of core–shell Fe3O4@poly (dopamine) magnetic nanoparticles for biosensor construction. J Mater Chem B. 2014;2:739–746. doi: 10.1039/c3tb21171a. [DOI] [PubMed] [Google Scholar]
- 39.Bar-Shir Amnon, Avram Liat, Yariv-Shoushan Shani, Anaby Debbie, Cohen Smadar, Segev-Amzaleg Niva, et al. Alginate-coated magnetic nanoparticles for noninvasive MRI of extracellular calcium. NMR Biomed. 2014;27(7):774–783. doi: 10.1002/nbm.3117. [DOI] [PubMed] [Google Scholar]
- 40.Silva A.H., Lima E., Jr, Mansilla M.V., Zysler R.D., Troiani H., Pisciotti M.L.M., et al. Superparamagnetic iron-oxide nanoparticles mPEG350–and mPEG2000-coated: cell uptake and biocompatibility evaluation. Nanomed Nanotechnol. 2016;12:909–919. doi: 10.1016/j.nano.2015.12.371. [DOI] [PubMed] [Google Scholar]
- 41.Ashrafi-Shahri S.M., Ravari F., Seifzadeh D. Smart organic/inorganic sol-gel nanocomposite containing functionalized mesoporous silica for corrosion protection. Prog Org Coat. 2019;133:44–54. [Google Scholar]
- 42.Liu Yong, Jia Shaoyi, Wu Qian, Ran Jingyu, Zhang Wei, Wu Songhai. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal Commun. 2011;12(8):717–720. [Google Scholar]
- 43.Berry C.C., Wells S., Charles S., Curtis A.S. Dextran and albumin derivatised iron oxide nanoparticles: influence on fibroblasts in vitro. Biomaterials. 2003;24:4551–4557. doi: 10.1016/s0142-9612(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 44.Gamarra L.F., Brito G.E.S., Pontuschka W.M., Amaro E., Parma A.H.C., Goya G.F. Biocompatible superparamagnetic iron oxide nanoparticles used for contrast agents: a structural and magnetic study. J Magn Magn Mater. 2005;289:439–441. [Google Scholar]
- 45.Remya N.S., Syama S., Sabareeswaran A., Mohanan P.V. Toxicity, toxicokinetics and biodistribution of dextran stabilized Iron oxide Nanoparticles for biomedical applications. Int J Pharm. 2016;511(1):586–598. doi: 10.1016/j.ijpharm.2016.06.119. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee A., Bandopadhyay R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int J Biol Macromol. 2016;87:295–301. doi: 10.1016/j.ijbiomac.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 47.Shin J.M., Song S.H., Rao N.V., Lee E.S., Ko H., Park J.H. A carboxymethyl dextran-based polymeric conjugate as the antigen carrier for cancer immunotherapy. Biomater Res. 2018;22:1–7. doi: 10.1186/s40824-018-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasić Katja, Knez Željko, Konstantinova Elizaveta A., Kokorin Alexander I., Gyergyek Sašo, Leitgeb Maja. Structural and magnetic characteristics of carboxymethyl dextran coated magnetic nanoparticles: From characterization to immobilization application. React Funct Polym. 2020;148:104481. doi: 10.1016/j.reactfunctpolym.2020.104481. [DOI] [Google Scholar]
- 49.Francolini I., Taresco V., Martinelli A., Piozzi A. Enhanced performance of Candida rugosa lipase immobilized onto alkyl chain modified-magnetic nanocomposites. Enzyme Microb Technol. 2020;132 doi: 10.1016/j.enzmictec.2019.109439. [DOI] [PubMed] [Google Scholar]
- 50.Mateo Cesar, Abian Olga, Fernandez-Lafuente Roberto, Guisan Jose M. Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support–polyethylenimine composites. Biotechnol Bioeng. 2000;68(1):98–105. doi: 10.1002/(sici)1097-0290(20000405)68:1<98::aid-bit12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Cai Hongdong, An Xiao, Cui Jun, Li Jingchao, Wen Shihui, Li Kangan, et al. Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coated iron oxide nanoparticles for biomedical applications. ACS Appl Mater Interfaces. 2013;5(5):1722–1731. doi: 10.1021/am302883m. [DOI] [PubMed] [Google Scholar]
- 52.Virgen-Ortíz J.J., dos Santos J.C., Berenguer-Murcia Á., Barbosa O., Rodrigues R.C., Fernandez-Lafuente R. Polyethylenimine: a very useful ionic polymer in the design of immobilized enzyme biocatalysts. J Mater Chem B. 2017;5:7461–7490. doi: 10.1039/c7tb01639e. [DOI] [PubMed] [Google Scholar]
- 53.Lin Guimei, Zhang Hong, Huang Leaf. Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm. 2015;12(2):314–321. doi: 10.1021/mp500656v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.dos Santos J.C., Rueda N., Barbosa O., del Carmen Millán-Linares M., Pedroche J., del Mar Yuste M., et al. Bovine trypsin immobilization on agarose activated with divinylsulfone: improved activity and stability via multipoint covalent attachment. J Mol Catal B Enzym. 2015;117:38–44. [Google Scholar]
- 55.Krajewska Barbara. Application of chitin-and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol. 2004;35(2-3):126–139. [Google Scholar]
- 56.Shukla Sudheesh K., Mishra Ajay K., Arotiba Omotayo A., Mamba Bhekie B. Chitosan-based nanomaterials: A state-of-the-art review. Int J Biol Macromol. 2013;59:46–58. doi: 10.1016/j.ijbiomac.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 57.Zamora-Mora V., Fernández-Gutiérrez M., González-Gómez Á., Sanz B., San Román J., Goya G.F., et al. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: From preparation to in vitro studies. Carbohydr Polym. 2017;157:361–370. doi: 10.1016/j.carbpol.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 58.Kalantari Katayoon, Afifi Amalina M., Jahangirian Hossein, Webster Thomas J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr Polym. 2019;207:588–600. doi: 10.1016/j.carbpol.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Cipolatti Eliane P., Silva María José A., Klein Manuela, Feddern Vivian, Feltes Maria Manuela C., Oliveira J. Vladimir, et al. Current status and trends in enzymatic nanoimmobilization. J Mol Catal B Enzym. 2014;99:56–67. [Google Scholar]
- 60.Asar Mohd Faisal, Ahmad Nafees, Husain Qayyum. Chitosan modified Fe3O4/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: its application in the saccharification of microcrystalline cellulose. Prep Biochem Biotech. 2020;50(5):460–467. doi: 10.1080/10826068.2019.1706562. [DOI] [PubMed] [Google Scholar]
- 61.Spelmezan C.G., Bencze L.C., Katona G., Irimie F.D., Paizs C., Toșa M.I. Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules. 2020;25:350. doi: 10.3390/molecules25020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.S.M. Siddeeg, M.A. Tahoon, W. Mnif, F. Ben Rebah, Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase for decolorization of textile wastewater, Processes 8 (2020) 5.
- 63.Alnadari F., Xue Y., Zhou L., Hamed Y.S., Taha M., Foda M.F. Immobilization of β-Glucosidase from Thermatoga maritima on Chitin-functionalized Magnetic Nanoparticle via a Novel Thermostable Chitin-binding Domain. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-019-57165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.García-Jimeno Sonia, Estelrich Joan. Ferrofluid based on polyethylene glycol-coated iron oxide nanoparticles: Characterization and properties. Colloids Surf A. 2013;420:74–81. [Google Scholar]
- 65.Kharazmi Sara, Taheri-Kafrani Asghar, Soozanipour Asieh. Efficient immobilization of pectinase on trichlorotriazine-functionalized polyethylene glycol-grafted magnetic nanoparticles: A stable and robust nanobiocatalyst for fruit juice clarification. Food Chem. 2020;325:126890. doi: 10.1016/j.foodchem.2020.126890. [DOI] [PubMed] [Google Scholar]
- 66.Mehra Neelesh Kumar, Jain Keerti, Jain Narendra Kumar. Pharmaceutical and biomedical applications of surface engineered carbon nanotubes. Drug Discov Today. 2015;20(6):750–759. doi: 10.1016/j.drudis.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y., Jing X., Chen Y. Material chemistry of graphene oxide-based nanocomposites for theranostic nanomedicine. J Mater Chem B. 2017;5:6451–6470. doi: 10.1039/c7tb00680b. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Yi, Chen Biao, Zhang Liming, Huang Jie, Chen Fenghua, Yang Zupei, et al. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide. Nanoscale. 2011;3(4):1446. doi: 10.1039/c0nr00776e. [DOI] [PubMed] [Google Scholar]
- 69.Herrmann I.K., Grass R.N., Stark W.J. High-strength metal nanomagnets for diagnostics and medicine: carbon shells allow long-term stability and reliable linker chemistry. Nanomedicine. 2009;4:787–798. doi: 10.2217/nnm.09.55. [DOI] [PubMed] [Google Scholar]
- 70.Schätz A., Grass R.N., Kainz Q., Stark W.J., Reiser O. Cu (II)− azabis (oxazoline) complexes immobilized on magnetic Co/C nanoparticles: Kinetic resolution of 1, 2-diphenylethane-1, 2-diol under batch and continuous-flow conditions. Chem Mater. 2010;22:305–310. [Google Scholar]
- 71.Lim You Sing, Lai Chin Wei, Abd Hamid Sharifah Bee. Porous 3D carbon decorated Fe3O4 nanocomposite electrode for highly symmetrical supercapacitor performance. RSC Adv. 2017;7(37):23030–23040. [Google Scholar]
- 72.Zlateski Vladimir, Fuhrer Roland, Koehler Fabian M., Wharry Scott, Zeltner Martin, Stark Wendelin J., et al. Efficient magnetic recycling of covalently attached enzymes on carbon-coated metallic nanomagnets. Bioconjug Chem. 2014;25(4):677–684. doi: 10.1021/bc400476y. [DOI] [PubMed] [Google Scholar]
- 73.Lai Guo-Song, Zhang Hai-Li, Han De-Yan. Amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase by carbon-coated iron nanoparticles in combination with chitosan and cross-linking of glutaraldehyde. Microchim Acta. 2009;165(1-2):159–165. [Google Scholar]
- 74.Masotti Andrea, Caporali Andrea. Preparation of magnetic carbon nanotubes (Mag-CNTs) for biomedical and biotechnological applications. Int J Mol Sci. 2013;14(12):24619–24642. doi: 10.3390/ijms141224619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan Dahui, Gao Zan, Yang Wanlu, Wang Jun, Yuan Yao, Wang Bin, et al. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater Sci Eng, B. 2013;178(10):736–743. [Google Scholar]
- 76.Wu Yang, Wei Yang, Wang Jiaping, Jiang Kaili, Fan Shoushan. Conformal Fe3O4 sheath on aligned carbon nanotube scaffolds as high-performance anodes for lithium ion batteries. Nano Lett. 2013;13(2):818–823. doi: 10.1021/nl3046409. [DOI] [PubMed] [Google Scholar]
- 77.Fan Yanli, Wu Guiying, Su Feng, Li Kai, Xu Li, Han Xiaotao, et al. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel. 2016;178:172–178. [Google Scholar]
- 78.Alegret Nuria, Criado Alejandro, Prato Maurizio. Recent advances of graphene-based hybrids with magnetic nanoparticles for biomedical applications. Curr Med Chem. 2017;24(5):529–536. doi: 10.2174/0929867323666161216144218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie Guoqiang, Xi Pinxian, Liu Hongyan, Chen Fengjuan, Huang Liang, Shi Yanjun, et al. A facile chemical method to produce superparamagnetic graphene oxide–Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. J Mater Chem. 2012;22(3):1033–1039. [Google Scholar]
- 80.Teymourian Hazhir, Salimi Abdollah, Khezrian Somayeh. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens Bioelectron. 2013;49:1–8. doi: 10.1016/j.bios.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Chaohui, Wu Hui, Wang Mingliang, Huang Chusen, Yang Dapeng, Jia Nengqin. Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Mater Sci Eng, C. 2017;78:817–825. doi: 10.1016/j.msec.2017.04.139. [DOI] [PubMed] [Google Scholar]
- 82.V. Atiroğlu, Lipase immobilization on synthesized hyaluronic acid-coated magnetic nanoparticle-functionalized graphene oxide composites as new biocatalysts: Improved reusability, stability, and activity, Int J Biol Macromol 145 (2020) 456-465. [DOI] [PubMed]
- 83.Rouhani S., Azizi S., Kibechu R.W., Mamba B.B., Msagati T.A. Laccase Immobilized Fe3O4-Graphene Oxide Nanobiocatalyst Improves Stability and Immobilization Efficiency in the Green Preparation of Sulfa Drugs. Catalysts. 2020;10:459. [Google Scholar]
- 84.Mamani J.B., Costa-Filho A.J., Cornejo D.R., Vieira E.D., Gamarra L.F. Synthesis and characterization of magnetite nanoparticles coated with lauric acid. Mater Charact. 2013;81:28–36. [Google Scholar]
- 85.Jin Yinjia, Liu Fei, Shan Chao, Tong Meiping, Hou Yanglong. Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res. 2014;50:124–134. doi: 10.1016/j.watres.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 86.V. Sreeja, K. Jayaprabha, P. Joy, Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI, Appl Nanosci 5 (2015) 435-441.
- 87.Mumtaz Shazia, Wang Li-Sheng, Hussain Syed Zajif, Abdullah Muhammad, Huma Zille, Iqbal Zafar, et al. Dopamine coated Fe3O4 nanoparticles as enzyme mimics for the sensitive detection of bacteria. Chem Commun. 2017;53(91):12306–12308. doi: 10.1039/c7cc07149c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Özacar Mahmut, Şengil İ. Ayhan, Türkmenler Harun. Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin. Chem Eng J. 2008;143(1-3):32–42. [Google Scholar]
- 89.Tang Wen, Ma Tonghao, Zhou Lina, Wang Gaoya, Wang Xiaoli, Ying Hanjie, et al. Polyamine-induced tannic acid co-deposition on magnetic nanoparticles for enzyme immobilization and efficient biodiesel production catalysed by an immobilized enzyme under an alternating magnetic field, Catalysis. Science & Technology. 2019;9(21):6015–6026. [Google Scholar]
- 90.Moradi Samira, Khodaiyan Faramarz, Hadi Razavi Seyed. Green construction of recyclable amino-tannic acid modified magnetic nanoparticles: Application for β-glucosidase immobilization. Int J Biol Macromol. 2020;154:1366–1374. doi: 10.1016/j.ijbiomac.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 91.Wang Jiahong, Zheng Changle, Ding Shaolan, Ma Hongrui, Ji Yanfen. Behaviors and mechanisms of tannic acid adsorption on an amino-functionalized magnetic nanoadsorbent. Desalination. 2011;273(2-3):285–291. [Google Scholar]
- 92.Altun S., Çakıroğlu B., Özacar M., Özacar M. A facile and effective immobilization of glucose oxidase on tannic acid modified CoFe2O4 magnetic nanoparticles. Colloids Surf B. 2015;136:963–970. doi: 10.1016/j.colsurfb.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 93.Xiao Qiong, Liu Chunli, Ni Hui, Zhu Yanbing, Jiang Zedong, Xiao Anfeng. β-Agarase immobilized on tannic acid-modified Fe3O4 nanoparticles for efficient preparation of bioactive neoagaro-oligosaccharide. Food Chem. 2019;272:586–595. doi: 10.1016/j.foodchem.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 94.Atacan K., Çakıroğlu B., Özacar M. Efficient protein digestion using immobilized trypsin onto tannin modified Fe3O4 magnetic nanoparticles. Colloids Surf B. 2017;156:9–18. doi: 10.1016/j.colsurfb.2017.04.055. [DOI] [PubMed] [Google Scholar]
- 95.Bandyopadhyaya Rajdip, Nativ-Roth Einat, Regev Oren, Yerushalmi-Rozen Rachel. Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett. 2002;2(1):25–28. [Google Scholar]
- 96.Mahmood Iram, Ahmad Ishfaq, Chen Guo, Huizhou Liu. A surfactant-coated lipase immobilized in magnetic nanoparticles for multicycle ethyl isovalerate enzymatic production. Biochem Eng J. 2013;73:72–79. [Google Scholar]
- 97.Dai T., Yan X., Li Q., Li T., Liu C., McClements D.J., et al. Characterization of binding interaction between rice glutelin and gallic acid: Multi-spectroscopic analyses and computational docking simulation. Food Res Int. 2017;102:274–281. doi: 10.1016/j.foodres.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 98.Atacan Keziban, Çakıroğlu Bekir, Özacar Mahmut. Improvement of the stability and activity of immobilized trypsin on modified Fe3O4 magnetic nanoparticles for hydrolysis of bovine serum albumin and its application in the bovine milk. Food Chem. 2016;212:460–468. doi: 10.1016/j.foodchem.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Xu Chenjie, Sun Shouheng. New forms of superparamagnetic nanoparticles for biomedical applications. Adv Drug Deliv Rev. 2013;65(5):732–743. doi: 10.1016/j.addr.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Osman Bilgen, Kara Ali, Demirbel Emel, Kök Senay, Beşirli Necati. Adsorption equilibrium, kinetics and thermodynamics of α-amylase on poly (DVB-VIM)-Cu+2 magnetic metal-chelate affinity sorbent. Appl Biochem Biotechnol. 2012;168(2):279–294. doi: 10.1007/s12010-012-9771-z. [DOI] [PubMed] [Google Scholar]
- 101.Chen Tingting, Yang Wenjuan, Guo Yuling, Yuan Renjun, Xu Li, Yan Yunjun. Enhancing catalytic performance of β-glucosidase via immobilization on metal ions chelated magnetic nanoparticles. Enzyme Microb Technol. 2014;63:50–57. doi: 10.1016/j.enzmictec.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 102.Wang J., Li K., He Y., Wang Y., Han X., Yan Y. Enhanced performance of lipase immobilized onto Co2+-chelated magnetic nanoparticles and its application in biodiesel production. Fuel. 2019;255 [Google Scholar]
- 103.Wang T.-F., Lo H.-F., Chi M.-C., Lai K.-L., Lin M.-G., Lin L.-L. Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds. Int J Mol Sci. 2019;20:3625. doi: 10.3390/ijms20153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Zhou, Hartmann Martin. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev. 2013;42(9):3894. doi: 10.1039/c3cs60059a. [DOI] [PubMed] [Google Scholar]
- 105.Yiu Humphrey H.P, Wright Paul A, Botting Nigel P. Enzyme immobilisation using SBA-15 mesoporous molecular sieves with functionalised surfaces. J Mol Catal B Enzym. 2001;15(1-3):81–92. [Google Scholar]
- 106.Muñoz-Pina S., Ros-Lis J.V., Argüelles Á., Martínez-Máñez R., Andrés A. Influence of the functionalisation of mesoporous silica material UVM-7 on polyphenol oxidase enzyme capture and enzymatic browning. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125741. [DOI] [PubMed] [Google Scholar]
- 107.Sadasivan Sajanikumari, Sukhorukov Gleb B. Fabrication of hollow multifunctional spheres containing MCM-41 nanoparticles and magnetite nanoparticles using layer-by-layer method. J Colloid Interface Sci. 2006;304(2):437–441. doi: 10.1016/j.jcis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 108.Jafari Khorshidi Kaveh, Lenjannezhadian Hengameh, Jamalan Mostafa, Zeinali Majid. Preparation and characterization of nanomagnetic cross-linked cellulase aggregates for cellulose bioconversion. J Chem Technol Biotechnol. 2016;91(2):539–546. [Google Scholar]
- 109.Wang F., Guo C., Yang L.-R., Liu C.-Z. Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour Technol. 2010;101:8931–8935. doi: 10.1016/j.biortech.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Yu, Yue Qin, Zagho Moustafa M., Zhang Jiajie, Elzatahry Ahmed A., Jiang Yongjian, et al. Core-Shell magnetic Mesoporous silica microspheres with large Mesopores for enzyme immobilization in biocatalysis. ACS Appl Mater Interfaces. 2019;11(10):10356–10363. doi: 10.1021/acsami.8b18721. [DOI] [PubMed] [Google Scholar]
- 111.Ali Zafar, Tian Lei, Zhang Baoliang, Ali Nisar, Khan Muhammad, Zhang Qiuyu. Synthesis of fibrous and non-fibrous mesoporous silica magnetic yolk–shell microspheres as recyclable supports for immobilization of Candida rugosa lipase. Enzyme Microb Technol. 2017;103:42–52. doi: 10.1016/j.enzmictec.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 112.Stock N., Biswas S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev. 2012;112:933–969. doi: 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- 113.Talin A. Alec, Centrone Andrea, Ford Alexandra C., Foster Michael E., Stavila Vitalie, Haney Paul, et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science. 2014;343(6166):66–69. doi: 10.1126/science.1246738. [DOI] [PubMed] [Google Scholar]
- 114.Liang K., Ricco R., Doherty C.M., Styles M.J., Bell S., Kirby N., et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun. 2015;6:1–8. doi: 10.1038/ncomms8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ke Fei, Qiu Ling-Guang, Yuan Yu-Peng, Jiang Xia, Zhu Jun-Fa. Fe3O4@ MOF core–shell magnetic microspheres with a designable metal–organic framework shell. J Mater Chem. 2012;22(19):9497. doi: 10.1039/c2jm31167d. [DOI] [Google Scholar]
- 116.Pang Fei, He Mingyuan, Ge Jianping. Controlled synthesis of Fe3O4/ZIF-8 nanoparticles for magnetically separable nanocatalysts, Chemistry–A. European Journal. 2015;21(18):6879–6887. doi: 10.1002/chem.201405921. [DOI] [PubMed] [Google Scholar]
- 117.Wang Jianzhi, Zhao Guanghui, Yu Faquan. Facile preparation of Fe3O4@ MOF core-shell microspheres for lipase immobilization. J Taiwan Inst Chem Eng. 2016;69:139–145. [Google Scholar]
- 118.He Jie, Sun Shanshan, Zhou Zhao, Yuan Qipeng, Liu Yanhui, Liang Hao. Thermostable enzyme-immobilized magnetic responsive Ni-based metal–organic framework nanorods as recyclable biocatalysts for efficient biosynthesis of S-adenosylmethionine. Dalton Trans. 2019;48(6):2077–2085. doi: 10.1039/c8dt04857f. [DOI] [PubMed] [Google Scholar]
- 119.Wu Enhui, Li Yuexian, Huang Qing, Yang Zhenkai, Wei Anyu, Hu Qi. Laccase immobilization on amino-functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere. 2019;233:327–335. doi: 10.1016/j.chemosphere.2019.05.150. [DOI] [PubMed] [Google Scholar]
- 120.Suo H., Xu L., Xue Y., Qiu X., Huang H., Hu Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr Polym. 2020;234 doi: 10.1016/j.carbpol.2020.115914. [DOI] [PubMed] [Google Scholar]
- 121.Šulek Franja, Drofenik Miha, Habulin Maja, Knez Željko. Surface functionalization of silica-coated magnetic nanoparticles for covalent attachment of cholesterol oxidase. J Magn Magn Mater. 2010;322(2):179–185. [Google Scholar]
- 122.Han Q., Zhang H., Sun J., Liu Z., Huang W.-C., Xue C., et al. Immobilization of phospholipase D on silica-coated magnetic nanoparticles for the synthesis of functional phosphatidylserine. Catalysts. 2019;9:361. [Google Scholar]
- 123.Abdulaal W.H., Almulaiky Y.Q., El-Shishtawy R.M. Encapsulation of HRP Enzyme onto a Magnetic Fe3O4 Np–PMMA Film via Casting with Sustainable Biocatalytic Activity. Catalysts. 2020;10:181. [Google Scholar]
- 124.Hu Huawen, Xin John H., Hu Hong, Wang Xiaowen, Kong Yeeyee. Metal-free graphene-based catalyst—Insight into the catalytic activity: A short review. Appl Catal A. 2015;492:1–9. [Google Scholar]
- 125.Wang J., Meng G., Tao K., Feng M., Zhao X., Li Z., et al. Immobilization of lipases on alkyl silane modified magnetic nanoparticles: effect of alkyl chain length on enzyme activity. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0043478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bi Y., Wang Z., Zhang R., Diao Y., Tian Y., Jin Z. Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles. Processes. 2020;8:629. [Google Scholar]
- 127.Kaur Prabhpreet, Taggar Monica Sachdeva, Kalia Anu. Characterization of magnetic nanoparticle–immobilized cellulases for enzymatic saccharification of rice straw. Biomass Convers Biorefin. 2021;11(3):955–969. [Google Scholar]
- 128.Zhao Guanghui, Li Yanfeng, Wang Jianzhi, Zhu Hao. Reversible immobilization of glucoamylase onto magnetic carbon nanotubes functionalized with dendrimer. Appl Microbiol Biotechnol. 2011;91(3):591–601. doi: 10.1007/s00253-011-3299-y. [DOI] [PubMed] [Google Scholar]
- 129.Bezerra Rayanne M., Monteiro Rodolpho R.C., Neto Davino M. Andrade, da Silva Francisco F.M., de Paula Regina C.M., de Lemos Telma L.G., et al. A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone. Application in the immobilization of lipase from Thermomyces lanuginosus. Enzyme Microb Technol. 2020;138:109560. doi: 10.1016/j.enzmictec.2020.109560. [DOI] [PubMed] [Google Scholar]
- 130.Bezerra Rayanne M., Neto Davino M. Andrade, Galvão Wesley S., Rios Nathalia S., Carvalho Ana Caroline L. de M., Correa Marcio A., et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem Eng J. 2017;125:104–115. [Google Scholar]
- 131.Fernandes Raquel A., Daniel-da-Silva Ana Luísa, Tavares Ana P.M., Xavier Ana M.R.B. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem Eng Sci. 2017;158:599–605. [Google Scholar]
- 132.Cui Jiandong, Sun Baoting, Lin Tao, Feng Yuxiao, Jia Shiru. Enzyme shielding by mesoporous organosilica shell on Fe3O4@ silica yolk-shell nanospheres. Int J Biol Macromol. 2018;117:673–682. doi: 10.1016/j.ijbiomac.2018.05.227. [DOI] [PubMed] [Google Scholar]
- 133.Cui Jiandong, Ren Sizhu, Lin Tao, Feng Yuxiao, Jia Shiru. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@ silica core shell nanosphere. Chem Eng J. 2018;343:629–637. [Google Scholar]
- 134.Gao J., Yu H., Zhou L., He Y., Ma L., Jiang Y. Formation of cross-linked nitrile hydratase aggregates in the pores of tannic-acid-templated magnetic mesoporous silica: characterization and catalytic application. Biochem Eng J. 2017;117:92–101. [Google Scholar]
- 135.Cardoso V.F., Francesko A., Ribeiro C., Bañobre-López M., Martins P., Lanceros-Mendez S. Advances in magnetic nanoparticles for biomedical applications. Adv Healthc Mater. 2018;7:1700845. doi: 10.1002/adhm.201700845. [DOI] [PubMed] [Google Scholar]
- 136.Reddy K.R., Reddy P.A., Reddy C.V., Shetti N.P., Babu B., Ravindranadh K., et al. In: Methods in Microbiology. Gurtler V., Ball A.S., Soni S., editors. Elsevier; London: 2019. Functionalized magnetic nanoparticles/biopolymer hybrids: synthesis methods, properties and biomedical applications; pp. 227–254. [Google Scholar]
- 137.Li Xiaoming, Wei Jianrong, Aifantis Katerina E., Fan Yubo, Feng Qingling, Cui Fu-Zhai, et al. Current investigations into magnetic nanoparticles for biomedical applications. J Biomed Mater Res A. 2016;104(5):1285–1296. doi: 10.1002/jbm.a.35654. [DOI] [PubMed] [Google Scholar]
- 138.Gao L., Fan K., Yan X. In: Nanozymology. Yan X., editor. Springer; Singapore: 2020. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetics for Biomedical Application; pp. 105–140. [Google Scholar]
- 139.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 140.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem Soc Rev. 2019;48:1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 141.Yu Zhengze, Lou Ruxin, Pan Wei, Li Na, Tang Bo. Nanoenzymes in disease diagnosis and therapy. Chem Commun. 2020;56(99):15513–15524. doi: 10.1039/d0cc05427e. [DOI] [PubMed] [Google Scholar]
- 142.Liu Yan, Yuan Min, Qiao Longjiao, Guo Rong. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens Bioelectron. 2014;52:391–396. doi: 10.1016/j.bios.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 143.Gao Lizeng, Zhuang Jie, Nie Leng, Zhang Jinbin, Zhang Yu, Gu Ning, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 144.Liu Shanhu, Lu Feng, Xing Ruimin, Zhu Jun‐Jie. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity, Chemistry–A. European Journal. 2011;17(2):620–625. doi: 10.1002/chem.201001789. [DOI] [PubMed] [Google Scholar]
- 145.Chen Zhongwen, Yin Jun-Jie, Zhou Yu-Ting, Zhang Yu, Song Lina, Song Mengjie, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6(5):4001–4012. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 146.Fan Kelong, Cao Changqian, Pan Yongxin, Lu Di, Yang Dongling, Feng Jing, et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nanotechnol. 2012;7(7):459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 147.Zhang D., Zhao Y.-X., Gao Y.-J., Gao F.-P., Fan Y.-S., Li X.-J., et al. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J Mater Chem B. 2013;1:5100–5107. doi: 10.1039/c3tb20907e. [DOI] [PubMed] [Google Scholar]
- 148.Verma Madan L., Puri Munish, Barrow Colin J. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit Rev Biotechnol. 2016;36(1):108–119. doi: 10.3109/07388551.2014.928811. [DOI] [PubMed] [Google Scholar]
- 149.Perveen Ruma, Nasar Abu, Inamuddin, Kanchi Suvardhan, Kashmery Heba Abbas. Development of a ternerry condunting composite (PPy/Au/CNT@ Fe3O4) immobilized FRT/GOD bioanode for glucose/oxygen biofuel cell applications. Int J Hydrogen Energy. 2021;46(4):3259–3269. [Google Scholar]
- 150.Chomicz R., Bystrzejewski M., Stolarczyk K. Carbon-Encapsulated Iron Nanoparticles as a Magnetic Modifier of Bioanode and Biocathode in a Biofuel Cell and Biobattery. Catalysts. 2021;11:705. [Google Scholar]
- 151.Garcia Josep, Zhang Yue, Taylor Hannah, Cespedes Oscar, Webb Michael E., Zhou Dejian. Multilayer enzyme-coupled magnetic nanoparticles as efficient, reusable biocatalysts and biosensors. Nanoscale. 2011;3(9):3721. doi: 10.1039/c1nr10411j. [DOI] [PubMed] [Google Scholar]
- 152.Pakapongpan Saithip, Poo-arporn Rungtiva P. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater Sci Eng, C. 2017;76:398–405. doi: 10.1016/j.msec.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 153.Khaksarinejad Reza, Mohsenifar Afshin, Rahmani-Cherati Tavoos, Karami Rezvan, Tabatabaei Meisam. An organophosphorus hydrolase-based biosensor for direct detection of paraoxon using silica-coated magnetic nanoparticles. Appl Biochem Biotechnol. 2015;176(2):359–371. doi: 10.1007/s12010-015-1579-1. [DOI] [PubMed] [Google Scholar]
- 154.Martins Gonçalo, Fernández-Lodeiro Javier, Djafari Jamila, Lodeiro Carlos, Capelo J.L., Santos Hugo M. Label-free protein quantification after ultrafast digestion of complex proteomes using ultrasonic energy and immobilized-trypsin magnetic nanoparticles. Talanta. 2019;196:262–270. doi: 10.1016/j.talanta.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 155.Husain Q. Magnetic nanoparticles as a tool for the immobilization/stabilization of hydrolases and their applications: An overview. Biointerface Res Appl Chem. 2016;6 [Google Scholar]
- 156.Zhai Rui, Yuan Yifeng, Jiao Fenglong, Hao Feiran, Fang Xiang, Zhang Yangjun, et al. Facile synthesis of magnetic metal organic frameworks for highly efficient proteolytic digestion used in mass spectrometry-based proteomics. Anal Chim Acta. 2017;994:19–28. doi: 10.1016/j.aca.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 157.Miao Aizhu, Dai Ying, Ji Yinghong, Jiang Yongxiang, Lu Yi. Liquid-chromatographic and mass-spectrometric identification of lens proteins using microwave-assisted digestion with trypsin-immobilized magnetic nanoparticles. Biochem Biophys Res Commun. 2009;380(3):603–608. doi: 10.1016/j.bbrc.2009.01.132. [DOI] [PubMed] [Google Scholar]
- 158.Aslani E., Abri A., Pazhang M. Immobilization of trypsin onto Fe3O4@ SiO2–NH2 and study of its activity and stability. Colloids Surf B. 2018;170:553–562. doi: 10.1016/j.colsurfb.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 159.Sahin Selmihan, Ozmen Ismail. Determination of optimum conditions for glucose-6-phosphate dehydrogenase immobilization on chitosan-coated magnetic nanoparticles and its characterization. J Mol Catal B Enzym. 2016;133:S25–S33. [Google Scholar]
- 160.Atacan K., Özacar M. Characterization and immobilization of trypsin on tannic acid modified Fe3O4 nanoparticles. Colloids Surf B. 2015;128:227–236. doi: 10.1016/j.colsurfb.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 161.Wang Sheng, Bao Huimin, Yang Pengyuan, Chen Gang. Immobilization of trypsin in polyaniline-coated nano-Fe3O4/carbon nanotube composite for protein digestion. Anal Chim Acta. 2008;612(2):182–189. doi: 10.1016/j.aca.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 162.Sahin S., Ozmen I. Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J Pharm Biomed Anal. 2020;184 doi: 10.1016/j.jpba.2020.113195. [DOI] [PubMed] [Google Scholar]
- 163.Chen Qi, Zhang Jingshun, Ke Xing, Lai Shiyun, Tao Baohua, Yang Jinchuan, et al. Quantification of bovine β-casein allergen in baked foodstuffs based on ultra-performance liquid chromatography with tandem mass spectrometry. Food Addit Contam. 2015;32(1):25–34. doi: 10.1080/19440049.2014.990994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qi Kailun, Liu Tong, Yang Yunjia, Zhang Jing, Yin Jie, Ding Xiaojing, et al. A rapid immobilized trypsin digestion combined with liquid chromatography–Tandem mass spectrometry for the detection of milk allergens in baked food. Food Control. 2019;102:179–187. [Google Scholar]
- 165.Atacan Keziban, Çakıroğlu Bekir, Özacar Mahmut. Covalent immobilization of trypsin onto modified magnetite nanoparticles and its application for casein digestion. Int J Biol Macromol. 2017;97:148–155. doi: 10.1016/j.ijbiomac.2017.01.023. [DOI] [PubMed] [Google Scholar]