Abstract
Viscum coloratum (Kom.) Nakai is a well-known medicinal hemiparasite widely distributed in Asia. The synthesis and accumulation of its metabolites are affected by both environmental factors and the host plants, while the latter of which is usually overlooked. The purpose of this study was to comprehensively evaluate the effects of host and habitat on the metabolites in V. coloratum through multiple chemical and biological approaches. The metabolite profile of V. coloratum harvested from three different host plants in two habitats were determined by multiple chemical methods including high-performance liquid chromatography-ultraviolet (HPLC-UV), gas chromatography-flame ionization detector (GC-FID) and ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-QTOF/MS). The differences in antioxidant efficacy of V. coloratum were determined based on multiple in vitro models. The multivariate statistical analysis and data fusion strategy were applied to analyze the differences in metabolite profile and antioxidant activity of V. coloratum. Results indicated that the metabolite profile obtained by various chemical approaches was simultaneously affected by host and environment factors, and the environment plays a key role. Meanwhile, three main differential metabolites between two environment groups were identified. The results of antioxidant assay indicated that the environment has greater effects on the biological activity of V. coloratum than the host. Therefore, we conclude that the integration of various chemical and biological approaches combined with multivariate statistical and data fusion analysis, which can determine the influences of host plant and habitat on the metabolites, is a powerful strategy to control the quality of semi-parasitic herbal medicine.
Keywords: Viscum coloratum, Host, Environment, Plant metabolomics, Multivariate statistical analysis, Biological activity
Graphical abstract
Highlights
-
•
The effects of environment and host on the metabolites and antioxidant activity of V. coloratum were investigated
-
•
Metabolite of V. coloratum were simultaneously affected by host and environment, and the environment plays a key role.
-
•
The identification of three differential metabolites between two origin groups was achieved.
-
•
The integration of chemical and biological approaches is a powerful strategy to control the quality of herbal medicine.
1. Introduction
Viscum coloratum (Kom.) Nakai (mistletoe) is a well-known medicinal hemiparasite and is widely distributed in Asia. It has been used as a traditional Chinese medicine (TCM) for a long history in China. According to the basic theory of TCM, V. coloratum is used to treat ailments such as arthralgia, soreness of the waist and knees, and threatened abortion. In many pharmacological studies, V. coloratum has been confirmed to have various pharmacological activities, such as treating cardiovascular system diseases [[1], [2], [3]], anti-aging, anti-oxidation [4], and anti-tumor activities [[5], [6], [7]], and immune regulation [8].
Hemiparasites are capable of photosynthesis. Therefore, like other plants, the synthesis and accumulation of its metabolites are affected by environmental factors. For example, the podophyllotoxin content in Podophyllum hexandrum, which grows in different natural habitats, varies greatly [9]. After supplementing UV-B radiation, the total content of flavonoids, kaempferol and quercetin glycosides in Brassica napus increased by 150% [10]. Panax quinquefolius grown at higher temperatures has higher ginsenoside concentrations than those grown at lower temperatures [11]. Low temperatures stimulate the synthesis of unsaturated fatty acids [12]. The synthesis and accumulation of plant metabolites are also affected by humidity, soil, microbial population and carbon dioxide concentration [13]. Hence, the environment is an important factor influencing the quality of herbal medicine.
On the other hand, hemiparasites support their growth by absorbing water, amino acids, organic acids, mineral nutrients and other substances from host plants [14,15]. For example, the higher water potential and sodium to calcium ratio (Na/Ca>1) allow mistletoe to actively absorb nutrients from the host [16]. The cardiac glycosides could be transferred from N. indicum to its parasites [17]. Piwowarczyk et al. [18] found that the metabolites and bioactivities of Cistanche armena depend on the species of its host. Vicaş et al. [19] suggested that the phenolic composition of Viscum album parasite varies on different trees. Therefore, in addition to environmental factors, host plants also largely affect the metabolite profile in hemiparasites.
At present, V. coloratum is purchased and used based on the distinction of origin, while the effect of its host on its metabolites is completely overlooked. This may lead to the uncontrollable quality of V. coloratum and affect its effectiveness and safety.
Several previous studies have been conducted to illuminate this problem. For example, Zhao et al. [20] determined ten flavonoids in V. coloratum that grow on different hosts and habitats. The common metabolites between V. coloratum and its host were analyzed by Long [21]. Vicaş et al. [19] studied the bioactive substances and antioxidant activities of V. album from different hosts. However, at present, most of researches focus on either limited numbers of metabolic compounds or merely one of these two influencing factors mentioned above. V. coloratum contains various compounds, such as flavonoids, triterpenes, lignins, glucosides, amino acid and volatile oils [[22], [23], [24], [25]]. Thus, a more comprehensive analysis is needed to evaluate the effect of the host and the environment on the metabolic profile of V. coloratum, and to determine the most influential factor. In addition, the biological activity is the most direct index to reflect the quality of V. coloratum.
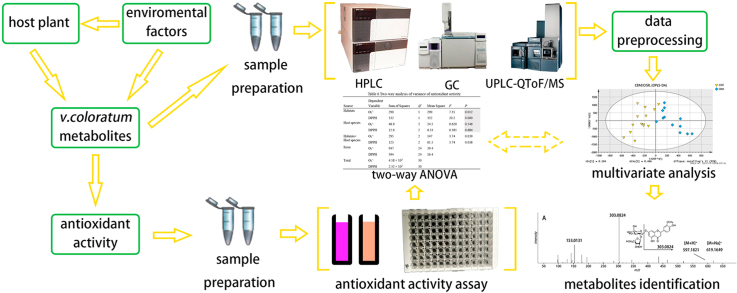
To achieve this purpose, in consideration of the characteristics of different compounds, multiple methods were applied to comprehensively analyze the differences in metabolite profile and biological activity of V. coloratum that are parasitic on three different host plants harvested from two different habitats (Fig. 1).
Fig. 1.
Integration of multiple chemical and biological approaches for the evaluation of the effects of host and environment on hemiparasite.
First, the metabolite profile of V. coloratum was globally determined by multiple methods including high-performance liquid chromatography-ultraviolet (HPLC-UV), gas chromatography-flame ionization detector (GC-FID), and ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-QTOF/MS).
Second, the differences in the metabolite profile of V. coloratum samples were analyzed by the multivariate statistical analysis. Data fusion is a technology that combines data blocks from different analytical instruments into a single data block, which provides more information than a single technology does [26]. Thus, in addition to separate analyses, a mid-level data fusion strategy was applied in this research.
Third, the antioxidant activities of V. coloratum were determined by superoxide radical scavenging and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assays.
2. Experimental
2.1. Plant materials
V. coloratum on three types of hosts was harvested from Changbai Mountain (CBM, Jilin province, China; temperate continental climate) and Chengde Mountain Resort (CISR, Hebei province, China; warm temperate humid monsoon climate). The sample details are shown in Table 1. The collected plants were placed in a ventilated and dark place, and dried at room temperature. The samples with voucher were stored in the State Key Laboratory of Traditional Chinese Medicine (Shenyang Pharmaceutical University, China).
Table 1.
Viscum coloratum grown on different hosts and environments.
| No. | Host species | Origin | No. | Host species | Origin |
|---|---|---|---|---|---|
| 1–5 | Ulmus pumila L. (UL) | CBM | 16–20 | Ulmus pumila L. (UL) | CISR |
| 6–10 | Salix babylonica L. (SA) | CBM | 21–25 | Salix babylonica L. (SA) | CISR |
| 11–15 | Populus ussuriensis Kom. (PO) | CBM | 26–30 | Populus ussuriensis Kom. (PO) | CISR |
CBM: Changbai Mountain, Jilin province, China; CISR: Chengde Mountain Resort, Hebei province, China.
2.2. Chemicals and reagents
The HPLC-grade acetonitrile and HPLC-grade methanol were bought from Fisher Scientific (Fair Lawm, NJ, USA). Tetrahydrofuran normal hexane and methanol were purchased from Tianjin Concord Co., Ltd. (Tianjin, China). Acetic acid and formic acid (HPLC-grade) were purchased from Shenyang Chemical Reagent Factory (Shenyang, China). Tris (hydroxymethyl) methyl aminomethane, pyrogallic acid, pyrogallol and benzaldehyde (HPLC-grade) were purchased from Bodi Chemical Engineering Co., Ltd. (Tianjin, China). Potassium phosphate monobasic and dipotassium phosphate were purchased from Xilong Chemical Co., Ltd. (Shantou, China). Hydrochloric acid was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). 1,1-Diphenyl-2-picrylhydrazyl free radical was purchased from TCI Chemical Industry Development Co., Ltd. (Shanghai, China). Ultrapure water was used throughout this study.
2.3. Sample preparation
V. coloratum was ground into powder that can pass through the #4 mesh sieve. An accurately weighed 1.0 g V. coloratum powder was extracted with 25 mL of 50% methanol in a conical flask with plug under sonication for 30 min. Solvent loss was compensated with 50% methanol when the conical flask was cooled. The extracts were centrifuged at 3,000 r/min for 3 min. The supernatant was then filtered using a 0.22 μm filter for HPLC analysis. The sample solution was diluted twice for UPLC-QTOF/MS.
V. coloratum powder (30 g) was immersed in a 1000 mL flask with 500 mL of distilled water and extracted for 6 h in an essential oil extractor. n-hexane was used as solvent in the essential oil extractor. The solution in the flask was kept slightly boiling during the extraction process. After cooling to ambient temperature, the extract was collected to a 5 mL volumetric flask, and the essential oil extractor was washed twice with n-hexane, which was then added to the extract. n-hexane was added to the volumetric flask until the total volume reached 5 mL. The extract was analyzed by GC after filtering through a 0.22 μm membrane.
2.4. HPLC analysis
A Shimadzu HPLC system, with an LC20ATvp pump coupled with an SPD-20Avp UV-detector (Shimadzu Corp., Kyoto, Japan), was used to analyze the 50% methanol extracts. HPLC analysis was performed according to the previous study [27].
2.5. GC analysis
The assay was performed on an Agilent 6890 N gas chromatograph with a FID (Agilent Corp., Palo Alto, CA, USA). Separation of volatile components was carried out on a PEG-20 M capillary gas chromatographic column (30 m × 0.32 mm × 0.6 μm). The injection volume was 2 μL. The injections were conducted in split mode with a split ratio of 5:1 under the conditions: injector at 250 °C, column oven at 60 °C for 3 min, programmed at a rate of 3 °C/min to 130 °C and then raised to 210 °C at a rate of 2.5 °C/min. Nitrogen served as the carrier gas at 1.2 mL/min.
2.6. UPLC-QTOF/MS analysis
The ACQUITY UHPLC system equipped with the Xevo G2-XS QTOF (Waters Corp., Milford, MA, USA) in ESI-positive ionisation mode was used. The separation was carried out using an ACQUITY UPLC@BEH C8 column (2.1 mm × 100 mm, 1.7 μm), and the mobile phase included 0.1% formic acid (A) and acetonitrile (B). The elution gradient program was as follows: 0–9 min, 10%–40% B; 9–15 min, 40%–85% B; 15–20 min, 85%–85% B; 20–20.5 min, 85%–10% B; 20.5–22 min, 10%–10% B; flow rate was 0.25 mL/min. The injection volume was 10 μL. The column temperature was kept at 35 °C.
For mass spectrometry detections, data were acquired using sensitivity mode with resolution >22,000 FWHM under positive electrospray ionisation. Acquisition range was 50–1000 m/z. Capillary voltage was 3 kV. Cone voltage was 30 V. The temperatures of source and desolvation gas were maintained at 150 °C and 450 °C, respectively, cone gas flow at 50 L/h and desolvation gas flow at 800 L/h. An alternation of low-energy (collision cell energy of 6 V) and elevated energy (collision cell energy ramped from 10 to 30 V) acquisition was used to obtain the precursor ion (MS) and the fragmentation ion. The QC solution was obtained by mixing equal amounts of each sample solution and was injected once every 5 samples throughout the analytical run.
2.7. Data pre-processing and statistical analyses
The original HPLC-UV data and GC-FID data were respectively normalized, which produced a data matrix including the sample name (ID), retention time (tR), and relative peak intensity.
The original multiple variables were transformed into a few comprehensive indicators (i.e., principal components (PCs)) by principal component analysis (PCA), thereby achieving feature extraction and visualization for large-scale complex data. Then, the processed data were imported in SIMCA 14.0 (Umetrics, Umea, Sweden) for further analysis through PCA with Pareto scaling mode, partial least squares discrimination analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA).
The UPLC-QTOF/MS raw data were processed on Progenesis QI 2.3 (Waters, Milford, MA, USA). The main procedures included chromatographic peak alignment, peak picking, normalisation, deconvolution, and compound identification. The 36 runs were aligned on the basis of an automatically selected QC. The selected adduct ions, including [M-H2O+H]+, [M+H]+, [M+2H]+, [M+NH4]+, [M+Na]+, [M+K]+ and [M+ACN+H]+, were used to deconvolute the data. Subsequently, a matrix was generated, which contained aligned peaks with normalized peak intensities and sample numbers.
Data fusion was carried out on Matlab R2016a. The mid-level data fusion concatenates latent variables extracted from each data source into a single data set. This data set was then used for further multivariate classification. As the purpose of this study was to investigate the factors with greater influences (better classification), the unsupervised analysis (PCA) was adopted. At first, every raw data matrix was normalized using z-scores, and PCA was then applied for latent variable extraction. The scores of PCs from each data source were concatenated to obtain the fusion data, based on which PCA was performed subsequently [28].
2.8. Identification of characteristic components
Characteristic components were selected by five criteria: variable influence in projection (VIP) >1, |p|>0.1, |p (corr)|>0.5 (correlation coefficient), ANOVA P≤0.05, and max fold change ≥2. The first three parameters were calculated by OPLS-DA model, and the last two parameters were obtained from Progenesis QI.
On the basis of the abovementioned five criteria, the characteristic components were identified using a self-established V. coloratum compounds information database, which was established through references and querying databases such as SciFinder and PubMed. This database contains information regarding compound structure, precise molecular weight, and compound mass spectrum fragment information.
2.9. Evaluation of the antioxidant activity
A total of 0.15 g V. coloratum powder was vortexed for 2 min with 4 mL of 0.1 mol/L phosphate buffer (pH 7.2). After kept at room temperature for 1 h, the mixture was sonicated for 1 min. The mixture was then centrifuged at 13,000 r/min for 5 min. The supernatant was used for superoxide radical scavenging assay.
Superoxide radical scavenging activity was determined according to pyrogallol autoxidation method as previously reported [29]. In short, 0.1 mL of the test solution and 0.4 mL of pyrogallol (2.5 mmol/L) were added into 4.5 mL of Tris-HCl buffer (0.05 mL/L, pH 8.2). Then, the mixture was incubated for 4 min at 25 °C followed by recording the absorbance at 299 nm. The free radical scavenging potential (FRSP) was calculated according to the previously reported method.
DPPH free radical scavenging assay was performed according to the method previously reported [30]. In brief, a series of the V. coloratum sample solutions (1 mL) were dispensed into a 96-well plate with 2 mL of DPPH methanolic solution (0.2 mM). The absorbance at 517 nm was recorded up to 45 min in the dark. The dose-effect curve was plotted with the clearance rate as the ordinate and the concentration of the sample solution as the abscissa. The concentration of the sample at a clearance rate of 50% (IC50, μmol/L) value was calculated based on the dose-response curve.
3. Results and discussion
3.1. HPLC-UV analysis
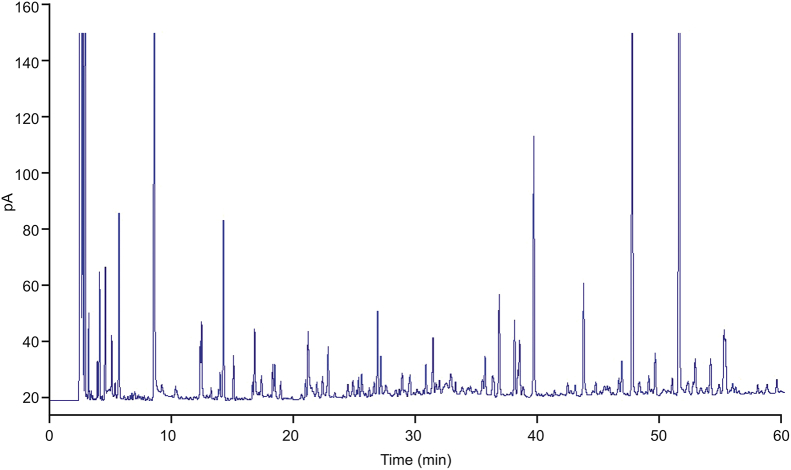
Typical chromatogram obtained from HPLC is shown in Fig. 2. The original HPLC-UV data were normalized in Excel, which generated a 51 × 30 data matrix including the sample number (ID), tR, and relative peak intensity.
Fig. 2.
Typical HPLC-UV fingerprint of Viscum coloratum.
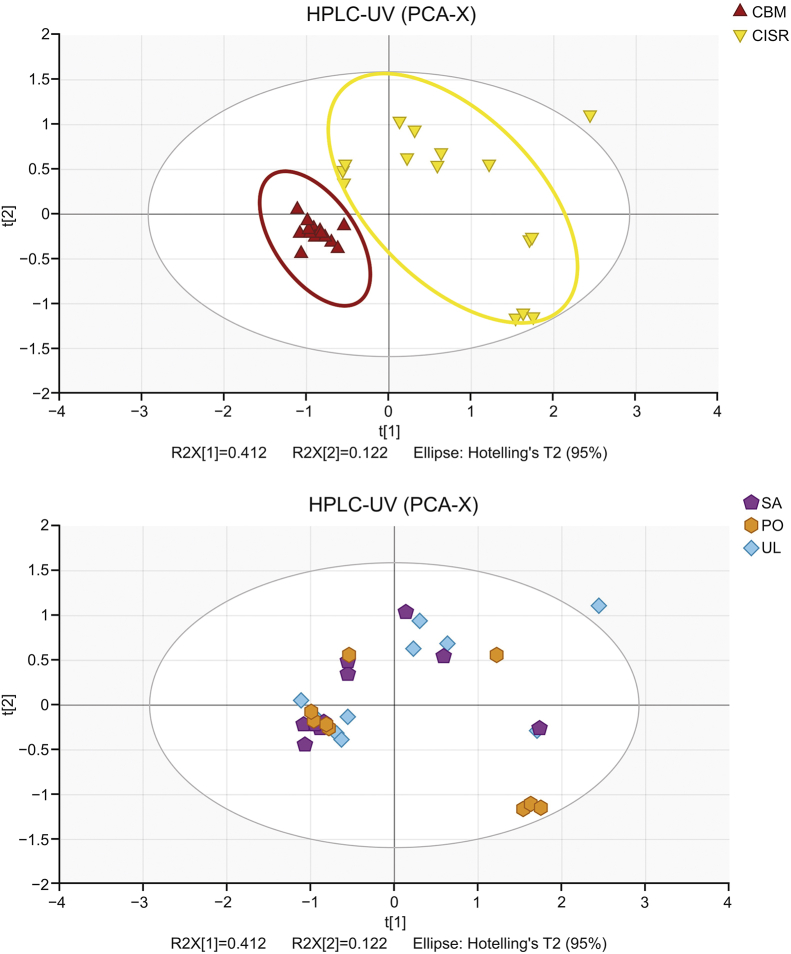
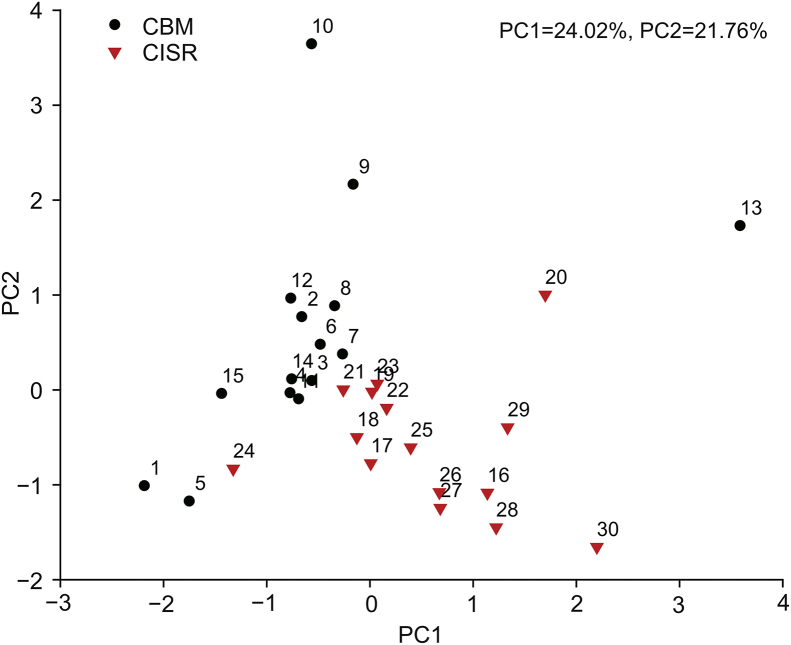
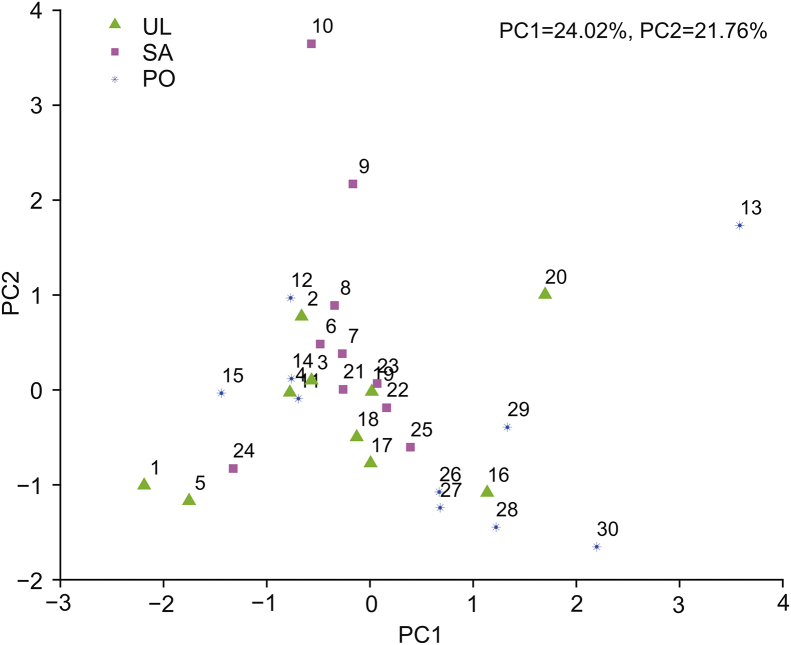
The 30 samples could be divided into two groups (CBM and CISR) according to their origin and divided into three groups (PO, SA, and UL) according to their host plant. PCA was performed to analyze the differences in metabolic profile of V. coloratum. The score plot is shown in Fig. 3.
Fig. 3.
Score plot of principal component analysis (PCA) for metabolic profile of Viscum coloratum obtained from HPLC-UV.
The result of PCA showed that the samples from CBM and CISR groups were scattered into different regions while those from PO, SA and UL groups were located together, indicating that the metabolic profile of V. coloratum is affected by more the environment than the host.
Samples in the CISR group were relatively scattered. This may be possibly attributed to the fierce competition for survival. The forests in Chengde Imperial Summer Resort are denser, and the fierce competition for survival and complex conditions of light and water resources may lead to a large difference in the growth of V. coloratum.
3.2. GC analysis
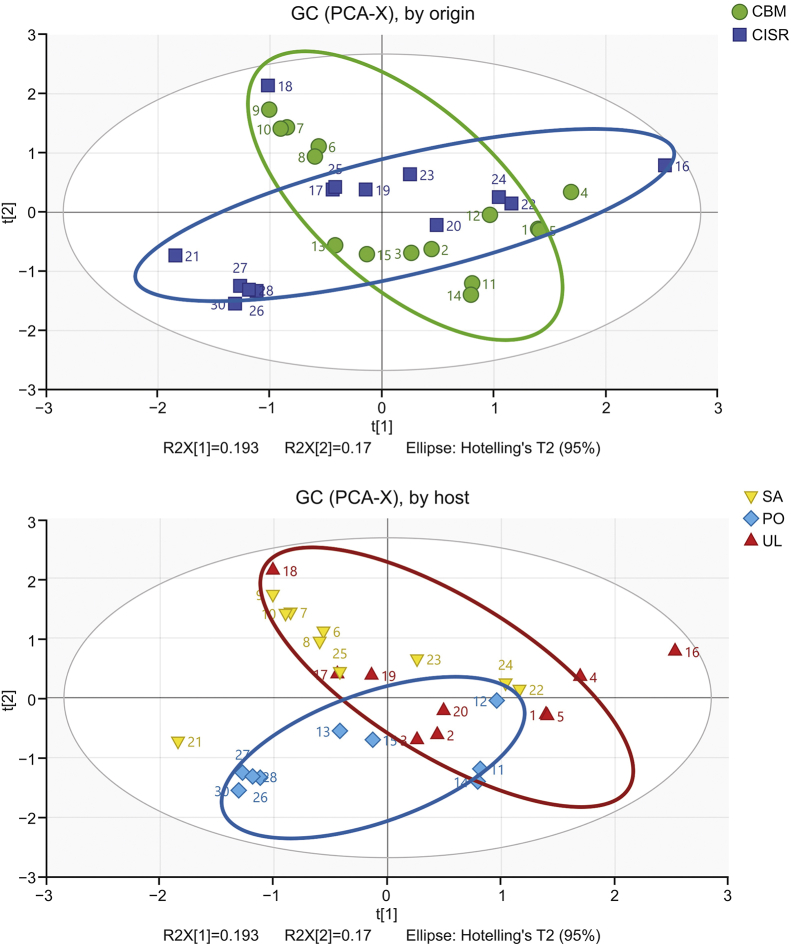
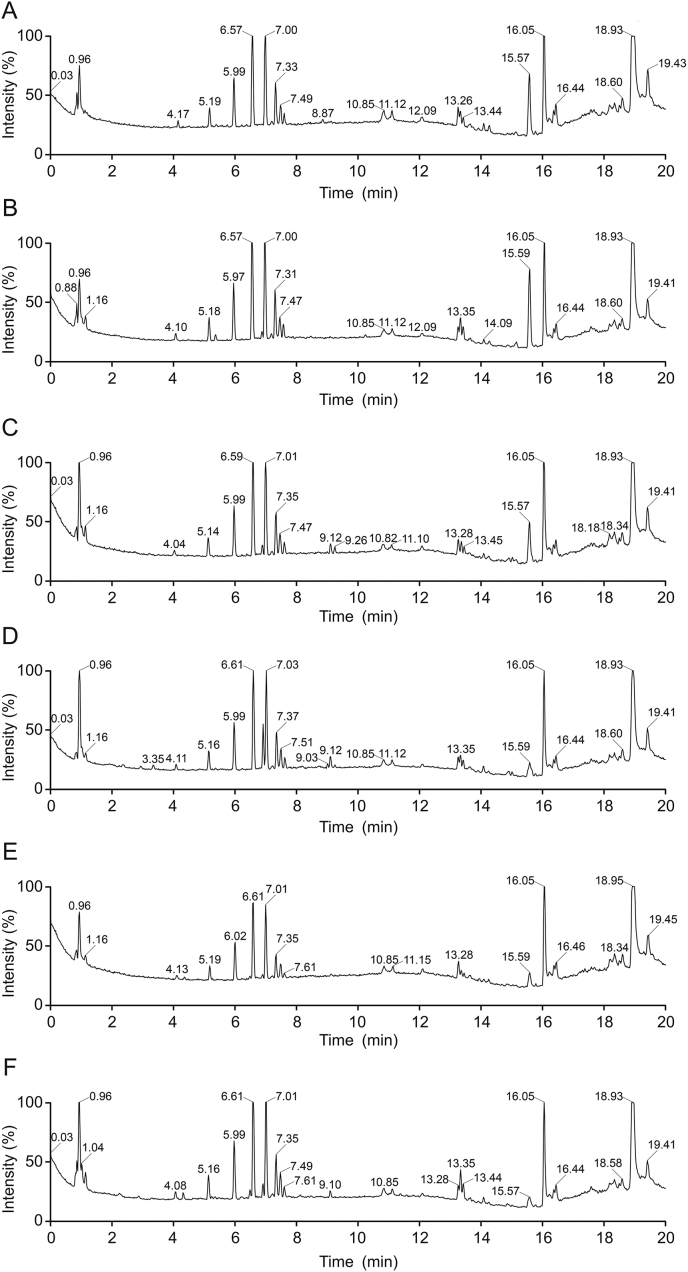
The typical chromatogram of GC is shown in Fig. 4. The original GC data were normalized in Excel, which generated a 51 × 30 data matrix including the sample name (ID), tR and relative peak intensity. PCA was performed to analyze the data in the matrix. The score plot of PCA for GC metabolic profile is shown in Fig. 5.
Fig. 4.
Typical GC fingerprint of Viscum coloratum.
Fig. 5.
Score plot of PCA for metabolic profile of Viscum coloratum obtained by GC.
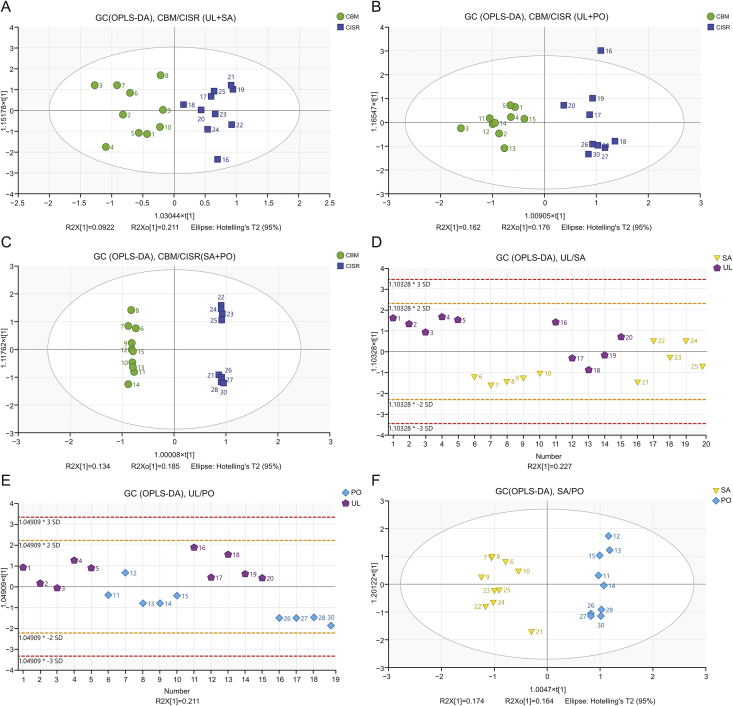
As shown in Fig. 5, neither environment nor the host could clearly distinguish V. coloratum samples, but a separation trend was observed in both score plots. Therefore, the OPLS-DA model of each pair of comparative host groups and corresponding origin groups was constructed. The score plots of six comparative groups (UL/SA, SA/PO, UL/PO and CBM/CISR in UL/SA, SA/PO and UL/PO) from OPLS-DA are shown in Fig. 6.
Fig. 6.
Score plots of OPLS-DA for six comparative groups. (A) Changbai Mountain, Jilin province, China (CBM)/Chengde Mountain Resort, Hebei province, China (CISR) comparative group in UL and SA groups. (B) CBM/CISR comparative group in PO and UL groups. (C) CBM/CISR comparative group in PO and SA groups. (D) UL/SA comparative group. (E) UL/PO comparative group. (F) SA/PO comparative group.
The effects of environment and host plants on metabolic profile were evaluated based on the quality of models, which was represented by R2X or R2Y and Q2 terms (Table 2).
Table 2.
Parameters of six models.
| No. | Type | N | R2X (cum) | R2Y (cum) | Q2 (cum) | Model |
|---|---|---|---|---|---|---|
| 1 | OPLS-DA | 20 | 0.227 | 0.477 | 0.129 | UL/SA |
| 2 | OPLS-DA | 20 | 0.304 | 0.816 | 0.375 | CBM/CISR in (UL + SA) |
| 3 | OPLS-DA | 19 | 0.457 | 0.952 | 0.829 | SA/PO |
| 4 | OPLS-DA | 19 | 0.859 | 0.998 | 0.856 | CBM/CISR in (SA + PO) |
| 5 | OPLS-DA | 19 | 0.211 | 0.627 | 0.358 | UL/PO |
| 6 | OPLS-DA | 19 | 0.337 | 0.905 | 0.72 | CBM/CISR in (UL/PO) |
OPLS-DA: orthogonal partial least squares discriminant analysis.
In Table 2, Q2 represents the predictability of the model. R2X or R2Y represents the explanation proportion of variance. For all parameters, the closer to 1, the better the model.
An obvious separation between the CBM and CISR groups was observed in the score plots as shown in Figs. 6A–C, but no distinct separation could be seen between the UL and SA groups (Fig. 6D) or between the UL and PO groups (Fig. 6E). Although the samples from SA and PO groups were separated according to principal component 1 (t[1]) (Fig. 6F), they were separated better based on the sample origin (Fig. 6C).
The parameters (Table 2) of every CBM/CISR model were greater than those of the model constructed between host groups. Overall, the comparison results indicate that both environment and host influence the metabolic profile of V. coloratum, while different environments largely contribute to the changes of metabolic profile.
The volatile components, most of which are unstable and evaporable, were examined by GC-FID. Due to the fact that the current result can be affected by sample acquisition and storage time, the effect of the host plants and environment on the volatile components of V. coloratum requires further study.
3.3. UPLC-QTOF/MS analysis
3.3.1. Multivariate statistical analysis
A typical UPLC-QTOF/MS chromatogram is shown in Fig. 7. The raw data were imported in Progenesis QI 2.3 (Waters, Milford, MA, USA) for data processing, in which a 10,383 × 36 data block including the sample number (ID), tR, and relative peak intensity was generated.
Fig. 7.
Typical positive TIC chromatograms of Viscum coloratum. (A) Host on PO from CISR, (B) host on UL from CISR, (C) host on SA from CISR, (D) host on UL from CBM, (E) host on PO from CBM, and (F) host on SA from CBM.
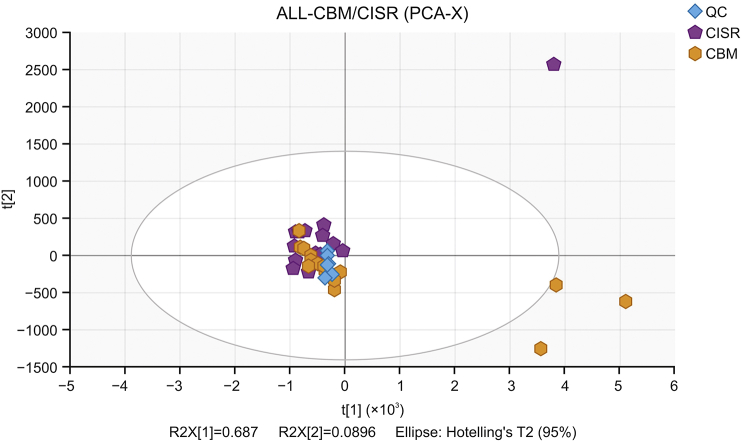
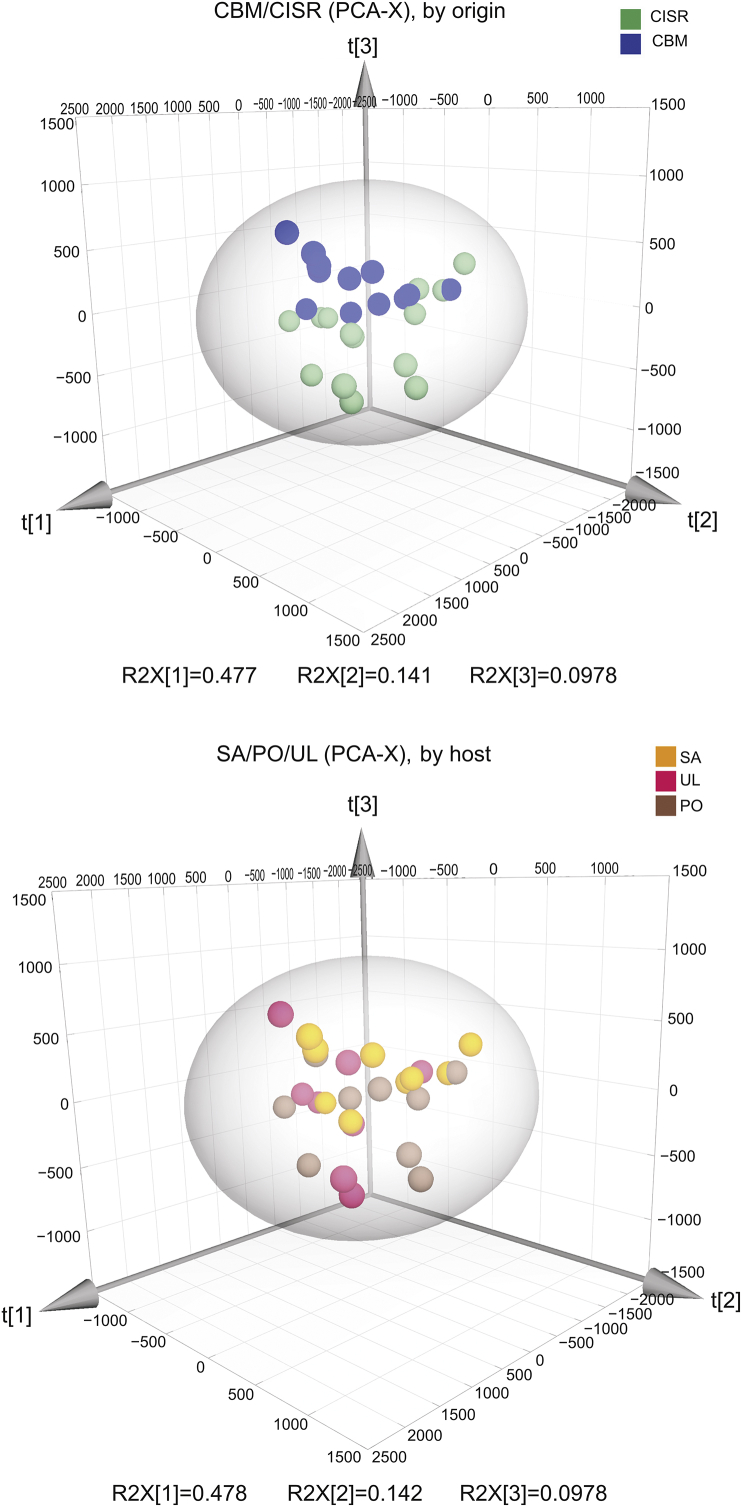
PCA was used to analyze the data. The score plot from PCA (Fig. 8) shows that the QC samples were clustered closely together located at the centre, indicating that the system stability was excellent. Moreover, four samples (Nos. 1, 5, 15, and 24) were outside the range of the ellipse, indicating that these samples were outliers. Therefore, the remaining samples excluding Nos. 1, 5, 15 and 24 were used in further analyses.
Fig. 8.
Score plot of PCA for metabolic profile of Viscum coloratum obtained from UPLC-QTOF/MS.
The score plot of PCA excluding outliers (Fig. 9) showed a good separation pattern between the CISR and CBM groups, while no obvious separation was observed among the UL, SA and PO groups, indicating that the metabolites are more affected by the environment than by the host.
Fig. 9.
3D score plot of PCA for metabolic profile of Viscum coloratum analyzed by UPLC-QTOF/MS.
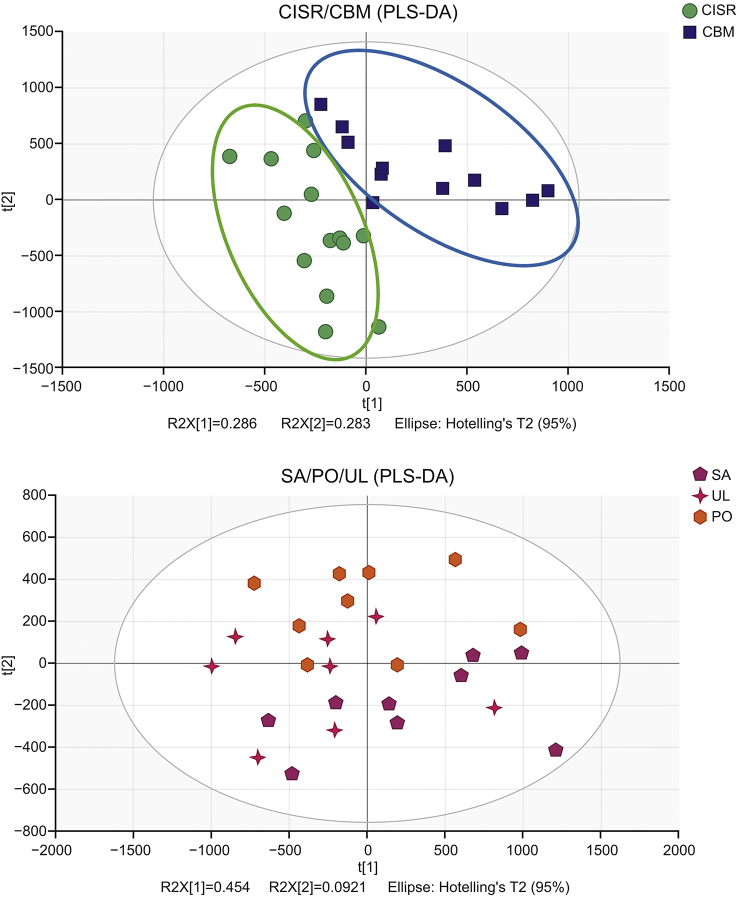
PLS-DA was used to differentiate the metabolic profiles between the CBM and CISR groups and among the PO, SA and UL groups. As shown in Fig. 10, samples were divided into two groups according to habitats, while the samples were clustered when grouped by hosts. All parameters of these models are summarised in Table 3. Obviously, the model parameters of CBM/CISR had significantly higher values, whereas the model of SA/PO/UL had lower values of goodness of fit, with Q2 (cum) < 0 and R2Y = 0.336. This result indicated that the metabolites in V. coloratum were more affected by the environment than the host plant. Therefore, differential metabolites were screened between CBM and CISR.
Fig. 10.
Score plot of partial least squares discrimination analysis (PLS-DA) for metabolic profile of Viscum coloratum obtained from UPLC-QTOF/MS.
Table 3.
Parameters of five models.
| No. | Type | N | R2X (cum) | R2Y (cum) | Q2 (cum) | Title |
|---|---|---|---|---|---|---|
| 1 | PCA-X | 36 | 0.777 | 0.619 | ALL | |
| 2 | PCA-X | 26 | 0.618 | 0.574 | / | |
| 3 | PLS-DA | 26 | 0.569 | 0.709 | 0.603 | CBM/CISR |
| 4 | PLS-DA | 26 | 0.547 | 0.336 | −0.00074 | SA/PO/UL |
| 5 | 0PLS-DA | 26 | 0.569 | 0.709 | 0.572 | CBM/CISR |
PCA: principal component analysis; PLS-DA: partial least squares discrimination analysis.
3.3.2. Identification of characteristic components
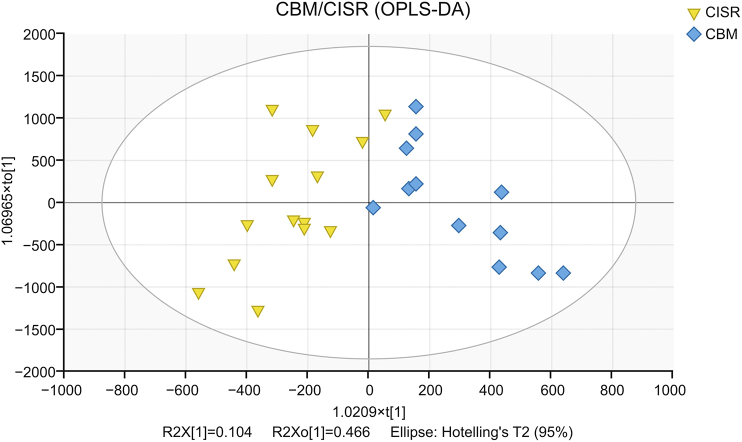
Different metabolites between CBM and CISR groups were analyzed using OPLS-DA. Score plots are presented in Fig. 11.
Fig. 11.
Score plot of OPLS-DA for metabolic profile of Viscum coloratum obtained from UPLC-QTOF/MS.
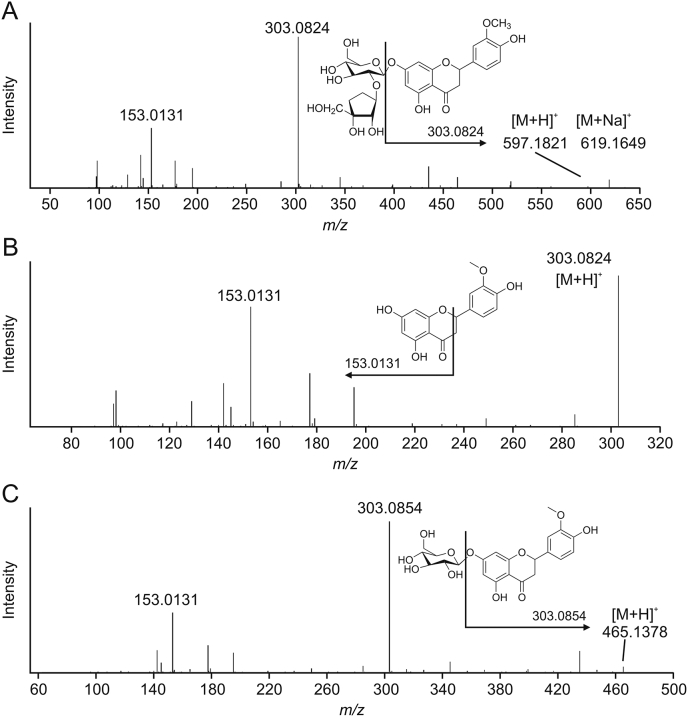
Characteristic components were screened based on the criteria described in Section 2.8. Ten differential metabolites were screened according to the five standards, and three compounds were identified using a self-established V. coloratum compounds information database (Table 4 and Fig. 12).
Table 4.
UHPLC-QTOF/MS data of the characteristic components in Viscum coloratum between CBM and CISR groups.
| No. | Compounds | Retention time (min) | [M+H]+ and fragments (m/z) |
Elemental composition | ||
|---|---|---|---|---|---|---|
| Calculated | Observed | Error (ppm) | ||||
| 1 | Homoeriodictyol-7-O-β-D-apiosiyl-(1→ 2)-O-β-D- glucoside | 6.89 | 597.1819 | 597.1830 | 1.84 | C27H33O15 |
| 303.0869 | 303.0844 | −8.25 | C16H15O6 | |||
| 2 | Homoeriodictyol | 6.89 | 303.0869 | 303.0856 | −4.29 | C16H15O6 |
| 153.0188 | 153.0171 | −11.11 | C7H5O4 | |||
| 3 | Homoeriodictyol-7-Ο-β-D-glucoside | 6.89 | 465.1397 | 465.1379 | −3.87 | C22H25O11 |
| 303.0869 | 303.0854 | −4.95 | C16H15O6 | |||
Fig. 12.
Fragmentation trace for (A) compounds 6.89_596.1757n, (B) 6.89_302.0625n, and (C) 6.89_464.1294n.
3.3.3. Data fusion
The latent variables (PCs) were obtained by PCA analysis on three data blocks from HPLC-UV, GC-FID, and UPLC-QTOF/MS. As a result, PC1 and PC2 in HPLC-UV, GC-FID, and UPLC-QTOF/MS explained 56.19%, 52.24%, and 69.64% variances, respectively.
Mid-level data fusion was carried out according to the combination of PCs scores from each data block. Subsequently, PCA was performed on the fused data. The model generated similar results compared with those of the separate analysis. Most samples were clearly classified according to origin (Fig. 13), while they were fused together in accordance with their hosts (Fig. 14). The results of the comprehensive analysis of the data from three platforms indicated that origin accounts for the main influential source, which is consistent with the results from the separate analysis.
Fig. 13.
Scores plot from PCA group by origin for mid-level data fusion.
Fig. 14.
Scores plot from PCA group by host for mid-level data fusion.
3.4. Antioxidant activity
The results from the antioxidant assay of the 30 samples are listed in Table 5. The effects of environment and host plants on the antioxidant activity of V. coloratum were determined by two-way ANOVA using IBM SPSS Statistics (IBM, Armonk, NY, USA). The results (Table 6) suggest that the environment exerts a significant impact on the antioxidant activity (P<0.05). This finding is consistent with the results from metabolic analysis, which indicated that the quality of V. coloratum in the CBM group might be better.
Table 5.
Antioxidant activity of Viscum coloratum grown on different hosts and origins.
| No. | O2−· FRSP (%) | DPPH IC50 (μg/mL) | No. | O2−· FRSP (%) | DPPH IC50 (μg/mL) |
|---|---|---|---|---|---|
| 1 | 54.26 | 4.746 | 16 | 28.81 | 11.893 |
| 2 | 34.34 | 4.480 | 17 | 35.22 | 11.728 |
| 3 | 29.70 | 0.996 | 18 | 32.07 | 1.106 |
| 4 | 51.96 | 11.321 | 19 | 36.80 | 4.325 |
| 5 | 50.79 | 3.200 | 20 | 34.19 | 7.286 |
| 6 | 31.38 | 1.166 | 21 | 34.29 | 6.789 |
| 7 | 33.25 | 1.193 | 22 | 34.00 | 22.962 |
| 8 | 36.31 | 1.194 | 23 | 33.45 | 8.609 |
| 9 | 37.35 | 1.686 | 24 | 35.13 | 13.225 |
| 10 | 38.28 | 2.952 | 25 | 52.94 | 16.905 |
| 11 | 45.62 | 6.837 | 26 | 35.22 | 11.321 |
| 12 | 38.97 | 4.301 | 27 | 32.91 | 10.506 |
| 13 | 50.00 | 8.580 | 28 | 37.84 | 13.115 |
| 14 | 45.17 | 1.105 | 29 | 33.50 | 6.144 |
| 15 | 44.89 | 1.002 | 30 | 32.61 | 8.657 |
FRSP: free radical scavenging potential.
Table 6.
Two-way analysis of variance of antioxidant activity.
| Source | Dependent variable | Sum of squares | df | Mean square | F | P |
|---|---|---|---|---|---|---|
| Habitats | O2−· | 290 | 1 | 290 | 7.35 | 0.012 |
| DPPH | 332 | 1 | 332 | 20.2 | 0.000 | |
| Host species | O2−· | 48.9 | 2 | 24.5 | 0.620 | 0.546 |
| DPPH | 12.6 | 2 | 6.33 | 0.385 | 0.684 | |
| Habitats × Host species | O2−· | 295 | 2 | 147 | 3.74 | 0.039 |
| DPPH | 123 | 2 | 61.5 | 3.74 | 0.038 | |
| Error | O2−· | 947 | 24 | 39.4 | ||
| DPPH | 394 | 24 | 16.4 | |||
| Total | O2−· | 4.58 × 103 | 30 | |||
| DPPH | 2.32 × 103 | 30 |
Türe et al. [31] studied the nutritional relationships between mistletoes and its hosts in different habitats, and found that the nutrient absorption of mistletoes from the hosts is mainly influenced by the type of habitat. Scalon et al. [32] found that temperature and humidity significantly affect nitrogen concentration and carbon isotopic composition of leaves in mistletoes and host plants. Moreover, they also suggested that nutrient absorption by mistletoes is closely related to the type of habitat.
Aside from the nutritional characteristics, the numbers of V. coloratum on hosts reveal important information for evaluating environmental factors. Improvements in V. coloratum greatly depends on the ability to absorb water and minerals from their host plants [33]. Hosts may make an attempt to make up for the increased nutritional requirements of V. coloratum by acquiring more nutrients from the environment [34]. Generally, nutrients are easily absorbed by host plants in areas with abundant water resources; therefore, the nutrition potential of V. coloratum from the hosts is mainly affected by the type of habitat. CBM is covered with original dense forests and rich water resources, whereas CISR is with a semi-arid habitat. Considering the findings from this study, we believe that host plants and environment can have influence on the secondary metabolism and antioxidant activity of V. coloratum, while the influence of environment is greater than that of host plants.
4. Conclusions
The metabolite profile of V. coloratum is simultaneously affected by host plant and environment, and the environment factors play a key role in the synthesis and accumulation of the metabolites. Meanwhile, three main differential metabolites between two environment groups are identified. The results of antioxidant activity assay indicated that the biological activity of V. coloratum is more affected by the environment factors as well, and the quality of V. coloratum in CBM may be better.
Overall, various chemical analysis methods can provide multi-dimensional metabolite profile information for the integral quality evaluation. This study may provide new ideas and references for the quality control of semi-parasitic herbal medicine.
CRediT author statement
Rui-Zhen Zhang: Conceptualization, Methodology, Software, Formal analysis, Writing - Original draft preparation; Jing-Tao Zhao: Investigation, Formal analysis; Wei-Qing Wang: Investigation, Methodology; Rong-Hua Fan: Formal analysis; Rong Rong: Methodology; Zhi-Guo Yu: Resources, Supervision; Yun-Li Zhao: Writing - Reviewing and Editing, Supervision, Funding acquisition, Project administration.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Grant No.: 30901967), the Natural Science Foundation of Liaoning Province (Grant No.: 2013020223), and Shenyang Pharmaceutical University Student Science and Technology Innovation Project (Grant No.: 12). We would like to thank TopEdit (www.topeditsci.com) for English language editing as well.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Rodríguez-Cruz M.E., Pérez-Ordaz L., Serrato-Barajas B.E., et al. Endothelium-dependent effects of the ethanolic extract of the mistletoe Psittacanthus calyculatus on the vasomotor responses of rat aortic rings. J. Ethnopharmacol. 2003;86:213–218. doi: 10.1016/s0378-8741(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 2.Radenkovic M., Ivetic V., Popovic M., et al. Effects of mistletoe (Viscum Album L., Loranthaceae) extracts on arterial blood pressure in rats treated with atropine sulfate and hexocycline. Clin. Exp. Hypertens. 2009;31:11–19. doi: 10.1080/10641960802409820. [DOI] [PubMed] [Google Scholar]
- 3.Suveren E., Baxter G.F., Iskit A.B., et al. Cardioprotective effects of Viscum album L. subsp. album (European misletoe) leaf extracts in myocardial ischemia and reperfusion. J. Ethnopharmacol. 2017;209:203–209. doi: 10.1016/j.jep.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Orhan D.D., Aslan M., Sendogdu N., et al. Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J. Ethnopharmacol. 2005;98:95–102. doi: 10.1016/j.jep.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Majeed M., Hakeem K.R., Rehman R.U. Mistletoe lectins: from interconnecting proteins to potential tumour inhibiting agents. Phytomedicine Plus. 2021;1 [Google Scholar]
- 6.Giudici M., Poveda J.A., Molina M.L., et al. Antifungal effects and mechanism of action of viscotoxin A3. FEBS J. 2006;273:72–83. doi: 10.1111/j.1742-4658.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 7.Coulon A., Berkane E., Sautereau A.M., et al. Modes of membrane interaction of a natural cysteine-rich peptide: viscotoxin A3. Biochim. Biophys. Acta. 2002;1559:145–159. doi: 10.1016/s0005-2736(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 8.Enesel M.B., Acalovschi I., Grosu V., et al. Perioperative application of the Viscum album extract Isorel in digestive tract cancer patients. Anticancer Res. 2005;25:4583–4590. [PubMed] [Google Scholar]
- 9.Alam M.A., Naik P.K. Impact of soil nutrients and environmental factors on Podophyllotoxin content among 28 Podophyllum Hexandrum populations of northwestern Himalayan region using linear and nonlinear approaches. Commun. Soil Sci. Plant Anal. 2009;40:2485–2504. [Google Scholar]
- 10.Olsson L.C., Veit M., Weissenböck G., et al. Differential flavonoid response to enhanced UV-B radiation in brassica napus. Phytochemistry. 1998;49:1021–1028. [Google Scholar]
- 11.Jochum G.M., Mudge K.W., Thomas R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae) Am. J. Bot. 2007;94:819–826. doi: 10.3732/ajb.94.5.819. [DOI] [PubMed] [Google Scholar]
- 12.Li Q., Lei S., Du K., et al. RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci. Rep. 2016;6 doi: 10.1038/srep36463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Kong D., Fu Y., et al. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Yoder J.I. Parasitic plant responses to host plant signals: a model for subterranean plant–plant interactions. Curr. Opin. Plant Biol. 1999;2:65–70. doi: 10.1016/s1369-5266(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 15.Bowie M., Ward D. Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. J. Arid Environ. 2004;56:487–508. [Google Scholar]
- 16.Okubamichael D.Y., Griffiths M.E., Ward D. Host specificity, nutrient and water dynamics of the mistletoe Viscum rotundifolium and its potential host species in the Kalahari of South Africa. J. Arid Environ. 2011;75:898–902. [Google Scholar]
- 17.Liu R., Su B., Huang F., et al. Identification and analysis of cardiac glycosides in Loranthaceae parasites Taxillus chinensis (DC.) Danser and Scurrula parasitica Linn. and their host Nerium indicum Mill. J. Pharm. Biomed. Anal. 2019;174:450–459. doi: 10.1016/j.jpba.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 18.Piwowarczyk R., Ochmian I., Lachowicz S., et al. Phytochemical parasite-host relations and interactions: a Cistanche armena case study. Sci. Total Environ. 2020;716 doi: 10.1016/j.scitotenv.2020.137071. [DOI] [PubMed] [Google Scholar]
- 19.Vicaş S.I., Rugină D., Leopold L., et al. HPLC fingerprint of bioactive compounds and antioxidant activities of Viscum album from different host trees. Not. Bot. Hort. Agrobot. Cluj. 2011;39:48–57. [Google Scholar]
- 20.Zhao Y., Yu Z., Fan R., et al. Simultaneous determination of ten flavonoids from Viscum coloratum grown on different host species and different sources by LC-MS. Chem. Pharm. Bull. (Tokyo) 2011;59:1322–1328. doi: 10.1248/cpb.59.1322. [DOI] [PubMed] [Google Scholar]
- 21.Long C., Fan R., Zhang Q., et al. Simultaneous identification and quantification of the common compounds of Viscum coloratum and its corresponding host plants by ultra-high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry and triple quadrupole mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1061−1062:176–184. doi: 10.1016/j.jchromb.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Qian C.-D., Fu Y.-H., Jiang F.-S., et al. Lasiodiplodia sp. ME4-2, an endophytic fungus from the floral parts of Viscum coloratum, produces indole-3-carboxylic acid and other aromatic metabolites. BMC Microbiol. 2014;14 doi: 10.1186/s12866-014-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi S., Miyamoto E., Kudo K., et al. Comparison of the volatile components of three mistletoes. J. Essent. Oil Res. 1996;8:619–626. [Google Scholar]
- 24.Li Y., Zhao Y.-L., Yang Y.-P., et al. Chemical constituents of Viscum album var. meridianum. Biochem. Systemat. Ecol. 2011;39:849–852. [Google Scholar]
- 25.Fan R., Ma Y., Yuan H., et al. A new flavonoid glycoside and four other chemical constituents from Viscum coloratum and their antioxidant activity. Heterocycles. 2014;89:1455–1462. [Google Scholar]
- 26.Borràs E., Ferré J., Boqué R., et al. Data fusion methodologies for food and beverage authentication and quality assessment - a review. Anal. Chim. Acta. 2015;891:1–14. doi: 10.1016/j.aca.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y.-L., Fan R.-H., Yuan H.-X., et al. Development of the fingerprints for the quality evaluation of Viscum coloratum by high performance liquid chromatography. J. Pharm. Anal. 2011;1:113–118. doi: 10.1016/S2095-1779(11)70020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai S., Lin Z., Xu B., et al. Metabolomics data fusion between near infrared spectroscopy and high-resolution mass spectrometry: a synergetic approach to boost performance or induce confusion. Talanta. 2018;189:641–648. doi: 10.1016/j.talanta.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Meng F., Chen R., Zhang M., et al. Extraction and antioxidant activity of polysaccharides from Acanthopanacis senticosi leaves. Food Sci. 2010;31:168–174. [Google Scholar]
- 30.Sun L., Wang D., Zhang Z. 2,2-Diphenyl-1-picrylhydrazyl radical scavenging activites of eleven species of natural plant extracts. Food Sci. 2009;30:45–47. [Google Scholar]
- 31.Türe C., Böcük H., Aşan Z. Nutritional relationships between hemi-parasitic mistletoe and some of its deciduous hosts in different habitats. Biologia. 2010;65:859–867. [Google Scholar]
- 32.Scalon M.C., Wright I.J. A global analysis of water and nitrogen relationships between mistletoes and their hosts: broad-scale tests of old and enduring hypotheses. Funct. Ecol. 2015;29:1114–1124. [Google Scholar]
- 33.Mathiasen R.L., Nickrent D.L., Shaw D.C., et al. Mistletoes: pathology, systematics, ecology, and management. Plant Dis. 2008;92:988–1006. doi: 10.1094/PDIS-92-7-0988. [DOI] [PubMed] [Google Scholar]
- 34.Miura O., Kuris A.M., Torchin M.E., et al. Parasites alter host phenotype and may create a new ecological niche for snail hosts. Proc. Biol. Sci. 2006;273:1323–1328. doi: 10.1098/rspb.2005.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]