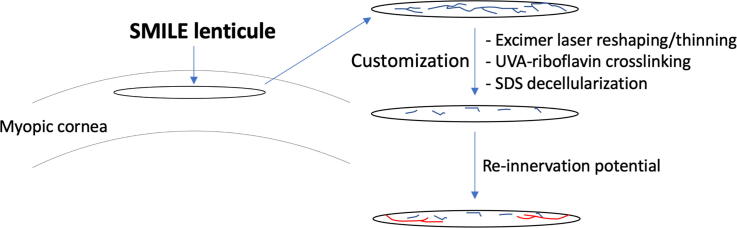
Graphical abstract
A Schematic overview of this study from the SMILE lenticule creation and extraction to lenticule customization: excimer laser reshaping/thinning, ultraviolet A (UVA)-riboflavin crosslinking and sodium dodecyl sulfate (SDS) decellularization, as well as the reinnervation of customized lenticules. Stromal neurites in lenticules (black color) sand regenerating neuritis (red color).
Keywords: Corneal refractive surgery, SMILE, Stromal lenticules, Customization, Neurite profile, Excitatory response
Highlights
-
•
Refractive SMILE-derived stromal lenticules are useful in various tissue-engineering approach for therapeutics, of which they are required to be customized before implantation.
-
•
Excimer laser-mediated reshaping, riboflavin-UVA-induced collagen crosslinking and chemical decellularization significantly removed lenticule neurites, but the residual neurites retained excitatory response.
-
•
Reinnervation occurred in the decellularized lenticules, indicating a potential of nerve regeneration.
-
•
Stromal lenticules, as a unique collagen-rich biomaterial with high transparency, refractivity and mechanically robust, together with the ability of neurite regeneration, could hold a potential for various ophthalmic applications.
Abstract
Introduction
Refractive stromal lenticules from Small Incision Lenticule Extraction (SMILE), though usually discarded, hold a potential for various ophthalmic applications, including refractive correction, stromal volume expansion, and biomechanical strengthening of the cornea.
Objectives
To investigate the effect of lenticule customization on lenticule neurite length profile and the excitatory response (calcium signaling) and the potential of reinnervation.
Methods
Human and porcine stromal lenticules were treated by (1) excimer laser reshaping, (2) ultraviolet A-riboflavin crosslinking (CXL), and (3) decellularization by sodium dodecyl sulfate (SDS), respectively. The overall neurite scaffold immuno-positive to TuJ1 (neuron-specific class III β-tubulin) expression and population of active neurite fragments with calcium response revealed by L-glutamate-induced Fluo-4-acetoxymethyl ester reaction were captured by wide-field laser-scanning confocal microscopy, followed by z-stack image construction. The NeuronJ plugin was used to measure neurite lengths for TuJ1 (NL-TuJ1) and calcium signal (NL-Ca). Reinnervation of lenticules was examined by the ex vivo grafting of chick dorsal root ganglia (DRG) to the decellularized human lenticules. Differences between groups and controls were analyzed with ANOVA and Mann-Whitney U test.
Results
The customization methods significantly eliminated neurites inside the lenticules. NL-TuJ1 was significantly reduced by 84% after excimer laser reshaping, 54% after CXL, and 96% after decellularization. The neurite remnants from reshaping and CXL exhibited calcium signaling, indicative of residual excitatory response. Re-innervation occurred in the decellularized lenticules upon stimulation of the grafted chick embryo DRG with nerve growth factor (NGF 2.5S).
Conclusion
All of the lenticule customization procedures reduced lenticule neurites, but the residual neurites still showed excitatory potential. Even though these neurite remnants seemed minimal, they could be advantageous to reinnervation with axon growth and guidance after lenticule reimplantation for refractive and volume restoration of the cornea.
Introduction
The cornea is the clear front window of the eye. It is the principal refractive apparatus where the incident light rays are bent to enter through the pupil and focus on the retina. The human cornea provides two-third of the overall refractive power of the eye, with the remaining contributed by the lens [1]. The corneal curvature is a key endophenotype for the cornea’s refractive power. An increased steepness of central corneal curvature is associated with a more myopic refractive error, a common worldwide cause of visual impairment. In 2015, a meta-analysis conducted on 288 studies involving about 4 million participants showed that the uncorrected refractive error was a leading cause of moderate to severe visual impairment, affecting 116 million people and causing blindness in 7.4 million people worldwide [2].
Glasses, contact lens or refractive surgery are means to correct refractive errors. LASer-assisted In situ Keratomileusis (LASIK) is a popular surgery to correct myopia, and astigmatism [3]. Recently, SMall Incision Lenticule Extraction (SMILE) has become clinically available as an alternative to LASIK for myopic correction. Mid-term results with SMILE have shown excellent and accurate postoperative outcomes along with painless visual rehabilitation [4], [5]. SMILE procedure involves using a femtosecond laser (FSL) to create an intrastromal lenticule, followed by manual extraction through a small incision at the corneal periphery [5]. This technique circumvents the need for flap creation and stromal ablation by an excimer laser, as performed in the LASIK. It is common practice to discard the extracted lenticule after surgery, even though it is a biocompatible collagen-rich tissue with native cyto-architectural quality, good tensile strength, and optical quality [6].
With an increasing number of patients undergoing SMILE, the extracted lenticules have become a rich source of unique biomaterial, with a high quality of transparency and refractivity. All of these features are useful in various tissue engineering approaches for ophthalmic therapeutics. Different studies have indicated the potential use of SMILE lenticules in treating hyperopia, presbyopia, aphakia, and stromal ectasia in keratoconus, as well as the closure of corneal perforation, restoration of corneal thinning, and as a patch graft for extra-corneal applications [7], [8], [9], [10], [11]. Lenticule implantation to modify the corneal stromal volume and refractive status have been reported in animal models of myopic ReLEx [12], [13]. The postoperative refractive and corneal changes were predictable, and the implanted lenticules caused minimal complications [12], [14], [15]. These promising results were reproduced in clinical studies [16], [17], [18], [19], [20], [21]. Hence, lenticule implantation is a practical approach to correct the refractive status, adjust the corneal volume and steepness, with the benefit of improving stromal biomechanics as well as high levels of safety and stability.
The cornea is the most densely innervated tissue in our body. Besides the sensations of touch, pain, and temperature, corneal nerves are important for blink reflex, wound healing, and tear production for the ocular health [22]. Corneal stromal nerve trunks arise from the limbal plexus and enter the peripheral stroma radially at the antero-mid stromal level before proceeding anteriorly towards the anterior stroma, sub-basal and epithelial layers of the cornea. Due to the flapless approach, the SMILE procedure preserves this dense anterior stromal nerve plexus and the stromal nerve severance is minimal, when compared to LASIK [23], [24]. After SMILE, patients have faster recovery of tear breakup time, reduced incidence of postoperative dry eye, and better corneal sensation recovery [24], [25], [26]. Although SMILE provokes some stromal nerve damage, the extracted lenticules still retain axotomized neurite fragments. Our recent study has shown a correlation between lenticule neurite density (LND) and thickness [27]. Thinner lenticules obtained from shallow treatments (lower dioptric powers) showed higher LND, probably due to the fact that thin lenticules are mostly resected in the anterior stromal region, where the majority of stromal nerves reside. Also, the neurite fragments retained Schwann cell (SC) support and exhibited an excitatory calcium response after the stimulation with neurotransmitters, such as L-glutamate [27]. Following lenticule implantation, whether these residual neurites and their supporting cells/matrix scaffold, assist in reinnervation or provide the topographic cues that influence neurite regeneration and pathfinding, remain to be investigated. In addition, before implantation, the stromal lenticules may need to be customized, such as; (1) reshaping to modify the thickness and achieve the desired refractive power for correction, (2) crosslinking to enhance the tissue strength and biomechanics, and (3) decellularization to reduce immunogenicity and improve survival.
This report investigated the lenticule neurite profile and its excitatory response, assayed by calcium signaling, following different lenticule customization methods (a schematic diagram in Fig. 1). In order to minimize the result variability due to the antero-posterior distribution of stromal nerves inside the corneas, we used porcine lenticules obtained by SMILE at the defined stromal depth and with identical thickness (i.e. one lenticule per cornea) for the reshaping and crosslinking experiments. A high resemblance of stromal nerve distribution has been demonstrated between human and porcine corneas [28]. On the other hand, lenticules with similar thickness and stromal features were required to study the effect of decellularization on neurite changes. While random porcine eyes were supplied from the abattoir, we used human lenticule pairs from selected myopic patients for this comparison study. We also examined the reinnervation of decellularized human lenticules using ex vivo neurite explant culture of chick dorsal root ganglia (DRG). These data provide new evidence that SMILE lenticules can be repurposed for ophthalmic utilization while retaining the potential for reinnervation.
Fig. 1.
A Schematic overview of this study from the SMILE lenticule creation and extraction to lenticule customization: excimer laser reshaping/thinning, ultraviolet A (UVA)-riboflavin crosslinking and sodium dodecyl sulfate (SDS) decellularization, as well as the reinnervation of customized lenticules. Stromal neurites in lenticules (black color) sand regenerating neurites (red color).
Methods
Ethics statement
Human SMILE lenticules were collected from myopic patients with the study protocol (CIRB/109/A) approved by The Institutional Review Board of SingHealth, Singapore. All subjects were treated under the tenets of the Declaration of Helsinki and written informed consents were obtained before sample collection.
Collection of human and porcine corneal lenticules and experimental groups
Human SMILE lenticules (n = 3 pairs and 3 singles) were collected from myopic patients who underwent SMILE procedures with a 500-kHz VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany) as described previously [29]. The age of donors was 26–35 years old, and the gender ratio was 1:1 (male/female). The average spherical equivalent was −5.5 ± 0.7D. The lenticule pairs (n = 3) were cryopreserved at −80 °C for 5–9 weeks using a reported protocol [30], [31]. Three single lenticules freshly collected after SMILE were used for decellularization and neurite growth assay using chick dorsal root ganglia (DRG). Porcine lenticules (n = 22) were obtained from eyes enucleated within 6 h after death, followed by a SMILE procedure [27]. Lenticules with a diameter of 6.5 mm were excised with spherical equivalent at either −9D (for laser reshaping experiment, n = 10) or −3D (for crosslinking experiment, n = 12). The SMILE cap and incision programming were similar for all lenticules: 7.5-mm (diameter) cap was cut with a 2.1-mm incision positioned at 135°, and the cap thickness was 120 µm. The stromal lenticule was released with a blunt spatula (AE2403, Asico, Westmont, IL, USA) and extracted. They were stored in ice-cold sterile phosphate-buffered phosphate buffered saline (PBS, Life Technologies, Carlsbad, CA, USA) and used in different experiments. Supplementary Fig. 1 shows a schematic diagram of the overall work flow using SMILE lenticules for customization and neurite analysis.
Porcine lenticule reshaping
Fresh porcine lenticules (n = 10) were scanned with the anterior segment optical coherence tomography (AS-OCT, OptoVue, Carl Zeiss Meditec, Dublin, CA, USA). The lenticule thickness was recorded as a mean of 3 measurements taken at the center and 0.5 mm on either side, respectively [32] They were then placed in a 24-well plate with correct anteroposterior orientation and cryo-stored in Dulbecco’s modified Eagle medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Gibco) at −80 °C for 7 days [31]. After thawing, they were carefully rinsed with PBS 5 times without disturbing their orientation. The cryo-storage step was to simulate the clinical process of lenticules from specimen collection, cryo-banking, and customization before implantation. Before reshaping by laser ablation, the lenticule thickness was calibrated to the recorded thickness immediately after SMILE by partial dehydration in a moist chamber, with hourly monitoring using AS-OCT [31]. This protocol of controlled hydration prevents uneven surface topography, which may affect the overall thickness following ablation. Once the thickness was achieved, laser ablation was performed on the anterior surface of lenticules (n = 7) (Fig. 2A). The lenticules were placed on the borosilicate glass surface with the humidity maintained by normal saline. The laser procedure was performed by a single surgeon (JM) using a Technolas 217z excimer laser (Bausch & Lomb, Inc., Rochester, NY, USA) with an emission wavelength 193 nm, 50 Hz repetition rate, and laser fluence 120 μJ/cm2. The ablation depth was 50 µm and an optical zone of 6.0 mm. The lenticules were washed in ice-cold PBS 3 times and immediately processed for calcium assay. Three lenticules without excimer laser reshaping served as controls.
Fig. 2.
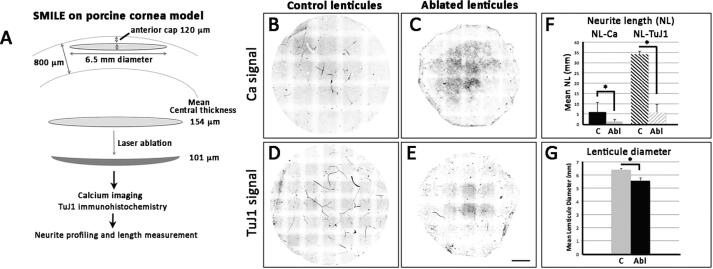
Neurite length profiling in lenticules reshaped by excimer laser. (A) Porcine lenticules (from −9D correction) were reshaped excimer laser (resembling phototherapeutic keratectomy) with an ablation depth of 50 μm. (B and C) Neurite profile with positive calcium signal for control and laser-ablated lenticules. (D and E) Neurite profile with positive TuJ1 expression for control and ablated lenticules. (F) A comparison of mean neurite length with positive calcium flux (NL-Ca) and TuJ1 signals (NL-TuJ1) in control and ablated lenticules. (G) Lenticule diameter was reduced after laser reshaping. *P < 0.05 (Mann-Whitney U test). Scale bar: 1 mm.
Porcine lenticule crosslinking
Fresh porcine lenticules (n = 12) were randomly allocated to 4 groups. Group 1: untreated control stored in PBS (n = 3); Group 2: riboflavin-treated only (n = 3); Group 3: treated with ultraviolet radiation only (n = 3); and Group 4: crosslinking treatment by riboflavin incubation and ultraviolet radiation (n = 3). Riboflavin (Avedro ParaCelTM, 0.22%, Optohellas, Katerini Pieria, Greece) was applied for 4 min, and UVA irradiation (Avedro CXL system, Waltham, MA, US) was set at a wavelength of 365 µm with a power of 30 mW/cm2 for 4 min (total dose 7.2 J/cm2) [33]. After treatment, they were washed 3 times with ice-cold PBS and processed for calcium assay.
Human lenticule decellularization
Three pairs of cryopreserved human lenticules with the same mean power of correction were placed in 2 groups. The lenticules from the right eyes (mean refractive correction was −5.7 ± 0.7 D) were decellularized using our reported protocol [34]. The lenticules from the left eyes (mean correction was −5.3 ± 0.9 D) served as control without decellularization. After washing in sterile PBS, the right lenticules were placed in 0.1% SDS (Sigma-Aldrich) for 24 h with gentle agitation, followed by multiple PBS washes for 72 h with agitation. All steps were performed at room temperature. The left lenticules (controls) were processed similarly except for SDS treatment. After treatment, they were immediately processed for calcium assay.
Calcium assay
Lenticules after customization were rinsed with Ca2+Mg2+-free Hank’s balanced salt solution (HBSS, Invitrogen) 5 times to remove free divalent ions, then incubated in Fluo-4-acetoxymethyl ester (Fluo-4-AM, 5 μM, Invitrogen) and 0.1% pluronic F-127 (Sigma-Aldrich) in HBSS for 30 min in the dark [27]. A final concentration of 1 μM L-glutamate (Invitrogen) was added, followed by immediate examination of the lenticules under wide-field confocal microscopy using the Green Fluorescence Protein (GFP) channel.
Immunostaining
After calcium assay and imaging, the same lenticules were washed overnight at 4 °C with multiple changes of PBS and agitation. They were fixed in freshly prepared neutral-buffered 4% paraformaldehyde (Sigma-Aldrich) for 10 min at room temperature, followed by PBS washes. After quenched with ice-cold 50 mM ammonium chloride (Sigma-Aldrich) for 5 min on ice, lenticules were permeabilized and blocked with 0.15% saponin (Sigma-Aldrich), 2% bovine serum albumin (BSA, Sigma-Aldrich), and 5% normal goat serum (Sigma-Aldrich) in PBS for 30 min. Samples were incubated with mouse anti-human monoclonal antibody against βIII-tubulin (TuJ1, 0.5 μg/ml, Covance, Princeton, NJ, USA) for an hour at room temperature. After PBS rinses, the signal was detected by goat anti-mouse Alexa FluorTM 594-conjugated IgG antibody (Jackson Immunoresearch Lab) for an hour in the dark. The rinsed samples were mounted in FluoroShield containing 4′6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotech, CA, USA) and examined by Red m-Cherry channel under wide-field confocal microscopy.
Wide-field laser-scanning confocal microscopy and z-stack image construction
The entire volume of lenticules was scanned using a confocal laser spinning disk microscope (CSU W1 Spinning Disk, Nikon) with a “scan large image” protocol (NIS Elements, v. 4.4) [27]. A 10x objective was selected to mark the scan boundary with an extra 20 µm margin to account for the non-parallel distension of the plano-convex lenticule when placed as flat-mount. The pixel size was set at 0.64 µm × 0.64 µm, and the serial z-stack at 5 µm thickness. The images were processed with the “large image” method implemented in NIS Elements [35]. Mosaic image was acquired for the entire lenticule and automatically blended stitched with XY overlap set at 15%. Under maximum intensity projection, the serial z-stacks were merged into a single 2D image for neurite profiling.
Neurite length (NL) measurement
The mosaic 2D images were converted into a traceable format by the NeuronJ plugin under ImageJ/Fiji (ver 2.0.0-rc-68/1.52e, NIH, USA) to quantify the calcium and TuJ1 signals. The length of neurite fragments showing positive calcium signal (NL-Ca) and positive TuJ1 signal (NL-TuJ1) were measured as described in our previous study [27].
Lenticule reinnervation assay
Chick embryos at E10 were sacrificed by decapitation, visceral organs and tissues were removed, and lumbar DRGs were collected in ice-cold PBS [36]. After clearance of peripheral tissue, DRG were labeled with Molday ION-Evergreen reagent (2 mg/ml, BioPAL, Worcester, MA, USA) for 24 hr. After PBS rinses, they were cut into halves, and each half was placed on the surface of SDS-decellularized human lenticules (protocol in earlier section) and incubated in DMEM/F12 medium (Invitrogen) supplemented with 2% fetal bovine serum (FBS, Invitrogen) and nerve growth factor (NGF 2.5S, at 5 and 50 ng/ml, respectively; Thermo Fisher, Waltham, MA, USA) for 5 days. The samples were fixed and stained for TuJ1 and phalloidin, followed by fluorescence-conjugated secondary antibodies, and examined under laser-scanning confocal microscopy (TCP SP8, Leica, Wetzlar, Germany). Serial z-stack images (1 μm thickness) were collected and 3D-reconstructed using LAS X LSC software (Leica).
Statistical analysis
Prism statistical software (ver 6.0, GraphPad Software, La Jolla, CA, USA) was used to perform the statistical calculations. The differences between groups and controls for the reshaping experiment were analyzed with ANOVA and Mann-Whitney, and the analysis for crosslinking experiments using Kruskal-Wallis one-way ANOVA test. P values < 0.05 were considered statistically significant. The effect of decellularization on lenticule neurites was represented as the percentage of length difference between the pairwise decellularized and control lenticules. All values were presented as mean ± SD (standard deviation) unless stated otherwise.
Results
Lenticule neurite changes after lenticule reshaping by excimer laser ablation
Fresh porcine corneas (n = 10) were used to obtain stromal lenticules with the same thickness. With careful maintenance of the anteroposterior orientation, the anterior surface of porcine lenticules was reshaped by excimer laser ablation, with the ablation depth set to 50 µm. Ophthalmic imaging using anterior segment optical coherent tomography (AS-OCT) before and after ablation showed that the average lenticule thickness was reduced from 154.1 ± 19 µm (equivalent to −9D correction) to 101 ± 16.6 µm, representing a thinning of 53 μm. The final stromal thickness was equivalent to −6D correction and showed good agreement to the preset ablation depth (Fig. 2A). The lenticule diameter was 6.42 ± 0.07 μm before ablation and 5.56 ± 0.23 μm after ablation, indicating a reduction by 0.86 ± 0.14 μm (P < 0.05, Mann-Whitney U test) (Fig. 2G). Both calcium signaling and TuJ1 immunostaining showed decreased lenticule neurite populations after reshaping (Fig. 2B-E). In control lenticules, the total neurite length exhibiting TuJ1 positive signal (NL-TuJ1) was 34.2 ± 1.3 mm (Fig. 2B). This value was reduced to 5.61 ± 4.01 mm in the ablated lenticules (Fig. 2C) (P < 0.05; Mann-Whitney U test), resulting in a decrease of neurites by 84% (Fig. 2F). The mean NL exhibiting calcium signal (NL-Ca) was also significantly reduced (5.87 ± 4.57 mm in control lenticules and 1.26 ± 1.03 mm in ablated lenticules; P < 0.05, Mann-Whitney U test) (Fig. 2D, E, F). Intriguingly, the proportion of neurites with calcium response per overall TuJ1-positive neurites had no significant changes after lenticule thinning. The percentages of NL-Ca to NL-TuJ1 was 16.8 ± 13.7% for control lenticules and 24.3 ± 14.5% for ablated lenticules (P = 0.47).
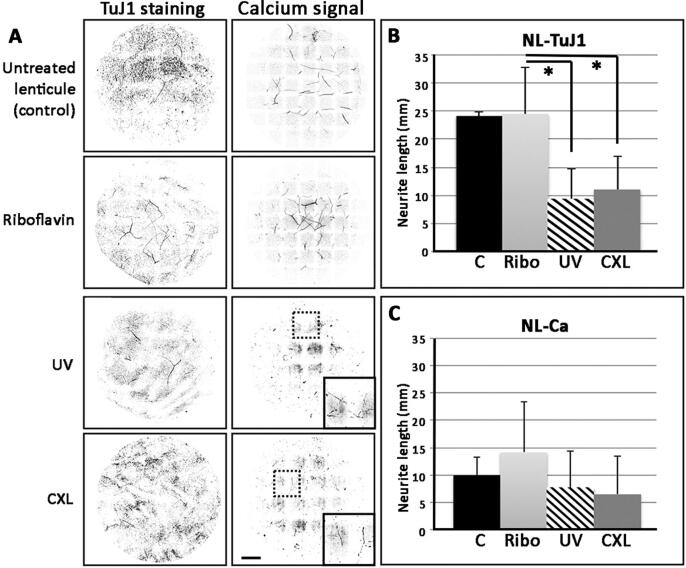
Lenticule neurite detection after crosslinking by riboflavin and UVA
Fresh porcine lenticules (n = 12) with the same thickness were used to achieve similar riboflavin penetration, ensuring similar crosslinking effects. Lenticules (-3D spherical equivalent) were treated with riboflavin- or UVA-only or both (CXL samples), followed by TuJ1 immunostaining and calcium assay. Our results clearly showed neurite structures present in lenticules treated with riboflavin-only and in control lenticules, but their detection was faint in UVA-treated and CXL lenticules (Fig. 3A; original fluorescence images in Supplementary Fig. 2). The mean NL-TuJ1 of the riboflavin group was 24.52 ± 8.27 mm, and the control group was 24.13 ± 0.81 mm. Both were significantly greater than that of UVA-only (9.40 ± 5.26 mm) and CXL lenticules (11.02 ± 5.96 mm) (P < 0.05, Kruskal-Wallis) (Fig. 3B). Compared to control lenticules, NL-TuJ1 was decreased by 61% after UVA treatment and by 54.3% after CXL, while minimal changes were noted for riboflavin-only. Notably, the calcium signal was readily detectable in riboflavin-only and control lenticules, while UVA-only and CXL lenticules had faint signals, but the difference was not significant (P > 0.05) (Fig. 3C). The proportion of NL-Ca to the overall NL-TuJ1 was higher in the UVA-only group (72 ± 40%) than other groups (riboflavin-only: 54.8 ± 19.2%; CXL: 46.2 ± 36.7% and untreated control: 41.1 ± 14%).
Fig. 3.
Neurite length profiling in lenticules crosslinked by riboflavin and ultraviolet (UV) irradiation. Porcine lenticules (from −3D SMILE correction) were treated with riboflavin only (Ribo), UV-irradiation only (UV), or riboflavin and UV crosslinked (CXL). (A) Neurite length profile after TuJ1 immunostaining or excitatory calcium signal. (B) Mean neurite length with TuJ1 signals (NL-TuJ1) and (C) calcium signals (NL-Ca) in control and treated lenticules. *P < 0.05 (Kruskal-Wallis one-way ANOVA). Scale bar: 1 mm.
Lenticule neurite detection after decellularization
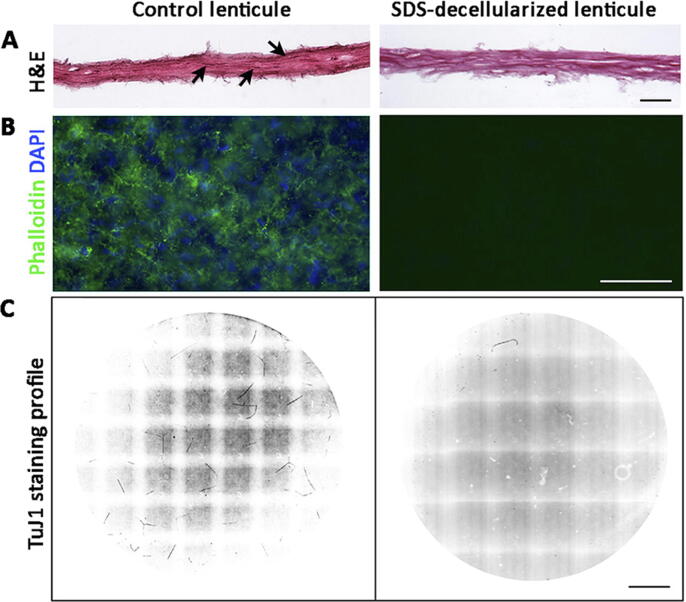
Lenticule decellularization was performed using our previously reported protocol with 0.1% SDS treatment [34]. The choice of human lenticules instead of porcine was to ensure high efficiency of cell material depletion using the method optimized with human stromal tissue. Different intermolecular spacing and stromal hydration content and partitioning between human and porcine corneal stromal tissue have been reported, affecting the decellularization results [37]. Three pairs of cryopreserved human lenticules obtained from myopic patients who underwent SMILE procedures were treated with 0.1% SDS, followed by multiple washes with PBS. There was no apparent difference in the average lenticule thickness and gross fibrillar structure with or without SDS treatment. Histology revealed an absence of hematoxylin-positive nuclei in the decellularized lenticules compared to controls (Fig. 4A). The negligible phalloidin staining unequivocally illustrated the removal of cellular and nuclear materials for cellular actin and DAPI staining for nucleic acids in SDS-treated lenticules (Fig. 4B). After TuJ1 immunostaining, the decellularized lenticules showed an almost absence of neurites compared to control lenticules (Fig. 4C). Only short fragments (<0.5 mm length) were detectable. Neurite tracing and quantification showed that NL-TuJ1 was 0.85 ± 0.14 mm for the decellularized lenticules, compared to 18.71 ± 4.93 mm in control lenticules. This resulted in a significant reduction of 96% NL-TuJ1 after SDS decellularization (P < 0.05, Mann-Whitney U test). Similarly, the treatment completely removed the NL-Ca signal.
Fig. 4.
Neurite profiling in porcine lenticules decellularized by 0.1% SDS. (A) Hematoxylin and eosin histochemistry of lenticules showed no distinct lenticule alteration. Hematoxylin-stained nuclei (arrows) were present in control lenticule but not in decellularized lenticule. (B) Phalloidin and DAPI staining of lenticules showed removal of cellular and nuclear materials after SDS treatment. (C) Neurite profile by TuJ1 immunostaining in control and treated lenticules. Scale bars: 100 μm (A), 25 μm (B), and 1 mm (C).
Reinnervation in decellularized lenticules
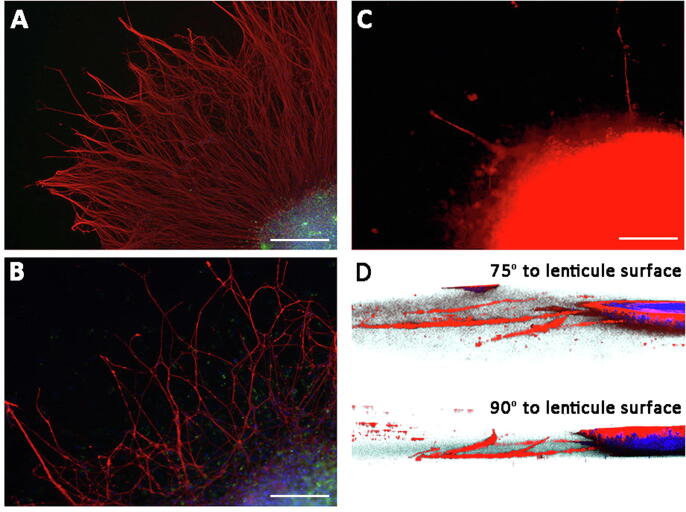
As mentioned above, there are differences between intermolecular spacing and stromal water content between human and porcine corneal stroma, and these might also influence neurite growth and extension. Thus we used decellularized human lenticules to demonstrate if reinnervation could occur after lenticule implantation. Chick embryo DRG cultured on the surface of SDS-decellularized human lenticules in medium supplemented with NGF 2.5S at 50 ng/ml concentration for 5 days generated a dense network of neurites which were TuJ1 positive (Fig. 5A). Fewer neurites were detected in culture when the NGF level was reduced to 5 ng/ml (Fig. 5B). Without NGF, the growth of DRG neurites appeared minimal (Fig. 5C). Hence, the neurite growth and extension on the decellularized lenticules were dose-dependent on NGF.
Fig. 5.
Reinnervation of decellularized human lenticules was NGF dependent. Explant culture of chick embryonic dorsal root ganglia (DRG) for 5 days, followed by TuJ1 immunostaining showed dense neurite network in culture with 50 ng/ml NGF 2.5S (A), and much reduced neurite extension with 5 ng/ml (B). Culture without NGF (C) had only sparse neurite growth. (D) 3D reconstruction from z-stack images showed neurite penetration through lenticule matrix. Scale bars: 0.5 mm.
Next, we examined if the regenerating neurites extended into the lenticule matrix. As a proof-of-concept study, we chose SDS-decellularized lenticules rather than lenticules after excimer laser reshaping or CXL for this neurite growth study. As shown earlier, SDS decellularization almost completely removed NL-TuJ1 signal (>95% reduction). The absence of pre-existing neurite structures could reduce any interference in the extension of regenerating neurites. Moreover, we performed DRG explant culture with 5 ng/ml NGF, which generated lower densities of neurites for precise tracing. The 5-day explant culture was immunolabeled for TuJ1, and the 3D reconstructed images from z-stacked confocal pictures illustrated that some neurites were present inside the lenticule matrix (Fig. 5D). The observations at 75° and 90° to the lenticule surface, respectively, showed that the regenerating neurites penetrated the lenticule stroma. The neurites extended to a distance of 192 ± 36 μm (n = 13 neurites) and a depth of 31 ± 16 μm (n = 9 neurites) after 5-day explant culture (data in Supplementary Table 1).
Discussion
In this study, we examined the effects of three different techniques for stromal lenticule customization on the residual neurite profile following: (1) reshaping and thinning using an excimer laser-induced ablation; (2) riboflavin-UVA-induced collagen CXL; and (3) decellularization by 0.1% SDS treatment. These customization methods significantly eliminated neurites inside the lenticules; however, the remaining neurite fragments after ablation and CXL had detectable Ca++ response. In the chick DRG explant assay, the acellular lenticule matrix allowed neurite regeneration, indicating that the lenticules still retained a potential for reinnervation, following NGF stimulation. Our results provide relevant information about the customization of SMILE lenticules with the consequences of neurite removal and the capacity of neurite regrowth. Our data and previous studies, thus, support the use of SMILE lenticules for various ophthalmic applications, owing to their biocompatibility, good optical and biomechanical properties [31], and the ability of neurite regeneration.
Lenticule extraction by SMILE is an increasingly popular procedure for the refractive correction of myopia and myopic astigmatism [5], [38]. The lenticules are the by-product of surgery and are usually discarded, but this surplus tissue could pave the path for new therapeutic possibilities or be used for various purposes. The thin lenticule is a native collagen-rich, mechanically robust, optically clear, and highly innervated tissue [27], [31]. Intrastromal lenticule implantation is useful for stromal volume expansion, refractive power modulation, and biomechanical strengthening in corneal disorders. The lenticules can be used either for autologous reimplantation or allogenic grafting. Surgeons can harness lenticules as (i) fresh (with a voluntary donor from SMILE surgery), or (ii) from short-term storage, e.g., from an eye bank, or (iii) from long-term storage under appropriate banking conditions until the time of need [39]. Different cryo-storage protocols reported the maintenance of lenticule clarity and structural integrity to establish proper lenticule banking [30], [40]. Under Good Manufacturing Protocol (www.optiq.asia), the development facilitates long-term cryopreservation of lenticules. However, before implantation, lenticules usually require some degree of modification to have the appropriate thickness for refractive correction or be mechanically strengthened for ectasia treatment [31]. Allogenic lenticule implantation, used in most clinical cases, may also benefit from decellularization to eliminate immune-prone biomolecules [34].
Our previous work has characterized the stromal neurite profiling, glial association, and residual functional capacity in lenticules of different thicknesses and storage conditions (fresh versus cryopreserved) [27]. Axotomized neurite fragments inside lenticules are subject to degenerative responses, owing to modifications in electrophysiological changes, depolarization, and calcium homeostasis, by axonal and retrograde transport of trophic factors, as well as the survival and metabolic changes of Schwann cells (SC). The latter will have altered trophic factor production, causing a local inflammatory response [41], [42], [43]. Most, if not all, could affect the trophic support with respect to nerve regeneration after lenticule implantation [26], [44], [45]. Different studies have examined the post-surgical corneal changes and lenticule integration (stromal lenticule addition keratoplasty) [21], [46], [47], [48]. However, the influence of residual neurites and the abortive neurite scaffold inside the implanted lenticules on the neurite regeneration and stromal responses is not known. Adjuvant mediators, e.g. low-frequency electricity, treatment with a cyclic adenosine monophosphate agonist (rolipram) [49], topical pergolide (a dopamine D1 and D2 receptor agonist) that increases NGF synthesis and release) [50], may also aid to innervation.
This study showed that various customization methods (reshaping/thinning, crosslinking, and decellularization) drastically reduced the neurite fragment population. However, the remaining short neurite fragments maintained detectable excitatory activity. Excimer laser reshaping on the anterior side of lenticule resulted in substantial removal of neurite scaffold by 84%. This drastic reduction was due to the excimer laser-caused photoablation of neurite structures, secondary to the direct breakdown of organic molecular bonds [51]. Since the SMILE lenticules are extracted from the anterior to mid-level of the corneal stroma (the cap thickness is set at 110–120 μm) and laser ablation was applied to the anterior side, the ablated region would have contained densely ramified neurites [52]. Ablation on the posterior aspect may damage fewer neurites. Interestingly, we still detected glutamate-induced Ca++ signal changes after ablation, and the ratios of NL-Ca to NL-TuJ1 were similar to that in control lenticules. This residual activity might be explained by the superficial photoablation, which removed non-viable neurites at and close to the lenticule surface, already damaged by the SMILE procedure, and partial dehydration following lenticule extraction.
Collagen CXL by riboflavin-UVA protocol is a safe and effective procedure to stabilize the stromal tissue in ectatic diseases [53]. The treatment stiffens the corneal stroma by a photochemical reaction that increases the chemical bonds between collagen fibers [54]. Our previous work has shown that CXL resulted in a two-fold additive stiffening of lenticules without reducing their transparency [55]. In this study, we found that CXL reduced both neurite scaffold (54% by TuJ1 expression) and its excitatory activity (46% by calcium assay). In corneas after CXL, similar stromal nerve changes have been reported, including the localized swelling of stromal nerves with a disruption of axonal membrane and a loss of axonal continuity [56]. Other clinical studies have shown a transient disappearance of sub-basal and anterior stromal nerves and impaired corneal sensation after CXL [57], [58], [59]. We analyzed the independent effects of crosslinking components on lenticule neurites. Based on NL-TuJ1 and NL-Ca measurements, there were minor differences between control and riboflavin-treated lenticules. Conversely, UVA irradiation significantly reduced the NL-TuJ1 signal in lenticule scaffold with or without riboflavin instillation, while NL-Ca had no distinct changes. Delayed sensation after UVA exposure has been demonstrated in a cutaneous sensation model [60]. UVA treatment generates free nerve endings, terminal enlargement and mediates the degeneration of nerves, non-myelinated SC, and perineural cells [61]. The precipitation of amorphous materials around non-myelinated SC could affect neurite-SC interaction (e.g., insulation and depolarization activities). In our study, the lenticule neurites were directly exposed to UVA irradiation, causing microstructural changes. Paradoxically, we found that the neurite remnants still maintained glutamate-induced Ca++ signaling after UVA exposure. Whether this is related to the release of neurotransmitters, such as substance P and calcitonin gene-related peptides [62], from the surviving SC remains to be investigated.
Lenticule decellularization eliminates cellular molecules that could be immunogenic to reduce the risk of rejection and circumvent the need for immunosuppression after implantation [6]. Our previous work using 0.1% SDS treatment removed almost all stromal cells from human lenticules without affecting the light transmittance and ECM (fibrous and non-fibrous) components [34]. The decellularized lenticules were non-toxic, biocompatible, and did not elicit any signs of rejection after xenotransplantation to rabbit corneas. In this study, SDS decellularization drastically reduced the neurite scaffold, and the treated lenticules had negligible TuJ1 staining in addition to the absence of phalloidin and DAPI signals. These findings also confirmed the elimination of excitatory neurite activity. Similarly, SDS treatment removed nuclei and axons in a rat sciatic nerve model [63].
In order to examine if reinnervation was possible, we used the chick embryo DRG neurite growth model and performed explant culture on the surface of SDS-decellularized human lenticules. We chose to use decellularized tissue because the SDS yielded the most significant (almost absolute) neurite reduction among different customized groups. Furthermore, it is uncertain that human or porcine neurite remnants can provide cues for other neural growth or extension with neural grafts from other species. Interestingly, the DRG neurites ramified on the lenticule surface. Some were able to grow and extend through the lenticule matrix and even penetrated the deeper stroma. The growth and ramification of DRG neurites suggest that reinnervation inside lenticule is possible even following massive neurite depletion. Whether the regenerated neurites explore new paths through lenticule matrix or can follow the cues of remaining SC needs to be investigated.
Conclusions
The lenticule neurite profile and its residual excitatory activity were affected by tissue customization: excimer laser reshaping, UVA-riboflavin CXL, and decellularization. The regrowth of neurites inside lenticules under appropriate conditions indicates the potential of reinnervation after lenticule implantation, and this may promote the recovery of corneal sensation recovery, ocular reflexes and aid in the maintenance or restoration of corneal epithelial homeostasis. Whether such reinnervation occurs in vivo and if the nerve growth ability varies between fresh/cryopreserved and customized/native lenticules remains to be investigated. New studies will provide important information on corneal sensation recovery after lenticule reimplantation to treat corneal refractive and ectatic disorders.
Ethics approval and consent to participate
The study was approved by Institutional Review Board of SingHealth, Singapore (CIRB/109/A) and all subjects were treated in accordance with tenets of the Declaration of Helsinki.
CRediT authorship contribution statement
Gary Hin-Fai Yam: Data curation, Investigation, Methodology, Writing – review & editing. Francisco Bandeira: Conceptualization, Supervision, Data curation, Investigation, Methodology, Writing – review & editing. Yu-Chi Liu: Conceptualization, Supervision, Data curation, Investigation, Methodology, Funding acquisition, Writing – review & editing. Kavya Devarajan: Data curation, Investigation, Methodology. Nur Zahirah Binte M. Yusoff: Data curation, Investigation. Hla-Myint Htoon: Statistics. Jodhbir S. Mehta: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank Laser Vision Centre, Singapore National Eye Centre, Singapore for human lenticule collection; Experimental Microscopy Platform, Singapore Eye Research Institute for microscopies, and SingHealth Bioimaging Core Facilities, The Academia, Singapore, for laser scanning confocal microscopies. This work was supported by SingHealth Academic Clinical Program (ACP) funding R1396/82/2016, Singapore.
Data availability
All data are included in the text.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.09.004.
Contributor Information
Gary Hin-Fai Yam, Email: yamg@pitt.edu.
Jodhbir S. Mehta, Email: jodmehta@gmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.DelMonte D.W., Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision Loss Expert Group of the Global Burden of Disease. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e1221-e1234. [DOI] [PubMed]
- 3.Sutton G., Lawless M., Hodge C. Laser in situ keratomileusis in 2012: a review. Clin Exp Optom. 2014;97(1):18–29. doi: 10.1111/cxo.12075. [DOI] [PubMed] [Google Scholar]
- 4.Liu YC, Riau AK, Mehta JS. Small incision lenticule extraction (SMILE). In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. Philadelphia: Elsevier; 2016, pp. 1317–1327.
- 5.Ang M., Farook M., Htoon H.M., Mehta J.S. Randomized clinical trial comparing Femtosecond LASIK and Small-Incision Lenticule Extraction. Ophthalmology. 2020;127(6):724–730. doi: 10.1016/j.ophtha.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Riau A.K., Liu Y.C., Yam G.H.F., Mehta J.S. Stromal keratophakia: Corneal inlay implantation. Prog Retin Eye Res. 2020;75:100780. doi: 10.1016/j.preteyeres.2019.100780. [DOI] [PubMed] [Google Scholar]
- 7.Kunert K.S., Blum M., Duncker G.I.W., Sietmann R., Heichel J. Surface quality of human corneal lenticules after femtosecond laser surgery for myopia comparing different laser parameters. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1417–1424. doi: 10.1007/s00417-010-1578-4. [DOI] [PubMed] [Google Scholar]
- 8.Ziebarth N.M., Lorenzo M.A., Chow J., Cabot F., Spooner G.J.R., Dishler J., et al. Surface quality of human corneal lenticules after SMILE assessed using environmental scanning electron microscopy. J Refract Surg. 2014;30(6):388–393. doi: 10.3928/1081597X-20140513-01. [DOI] [PubMed] [Google Scholar]
- 9.Bhandari V., Ganesh S., Brar S., Pandey R. Application of the SMILE-derived glued lenticule patch graft in microperforations and partial-thickness Corneal Defects. Cornea. 2016;35:408–412. doi: 10.1097/ICO.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 10.Song Y.J., Kim S., Yoon G.J. Case series: Use of stromal lenticule as patch graft. Am J Ophthalmol Case Rep. 2018;12:79–82. doi: 10.1016/j.ajoc.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Zhou Y., Zhao H., Xue J., Jiang Q. Application of the SMILE-derived lenticule in therapeutic keratoplasty. Int Ophthalmol. 2020;40(3):689–695. doi: 10.1007/s10792-019-01229-y. [DOI] [PubMed] [Google Scholar]
- 12.Angunawela R.I., Riau A.K., Chaurasia S.S., Tan D.T., Mehta J.S. Refractive lenticule re-implantation after myopic ReLEx: a feasibility study of stromal restoration after refractive surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2012;53(8):4975. doi: 10.1167/iovs.12-10170. [DOI] [PubMed] [Google Scholar]
- 13.Riau A.K., Angunawela R.I., Chaurasia S.S., Lee W.S., Tan D.T., Mehta J.S., et al. Reversible femtosecond laser-assisted myopia correction: a non-human primate study of lenticule re-implantation after refractive lenticule extraction. PLoS ONE. 2013;8(6):e67058. doi: 10.1371/journal.pone.0067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T., Sun Y., Liu M., Zhou Y., Wang D., Chen Y., et al. Femtosecond laser-assisted endokeratophakia using allogeneic corneal lenticule in a rabbit model. J Refract Surg. 2015;31(11):775–782. doi: 10.3928/1081597X-20151021-07. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y.C., Wen J., Teo E.P.W., Williams G.P., Lwin N.C., Mehta J.S. Higher-order aberrations following hyperopia treatment: Small Incision Lenticule Extraction, Laser-Assisted In Situ Keratomileusis and Lenticule Implantation. Transl Vis Sci Technol. 2018;7(2):15. doi: 10.1167/tvst.7.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan K.R., Reinstein D.Z., Carp G.I., Archer T.J., Gobbe M., Gurung R. Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refract Surg. 2013;29(11):777–782. doi: 10.3928/1081597X-20131021-07. [DOI] [PubMed] [Google Scholar]
- 17.Ganesh S., Brar S., Rao P.A. Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea. 2014;33:1355–1362. doi: 10.1097/ICO.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdev M.S., Gupta D., Sachdev G., Sachdev R. Tailored stromal expansion with a refractive lenticule for crosslinking ultrathin cornea. J Cataract Refract Surg. 2015;41:918–923. doi: 10.1016/j.jcrs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Sun L., Yao P., Li M., Shen Y., Zhao J., Zhou X. The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia. J Refract Surg. 2015;31(6):374–379. doi: 10.3928/1081597X-20150521-03. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J., Sun L., Shen Y., Tian M.i., Yao P., Zhou X. Using donor lenticules obtained through SMILE for an epikeratophakia technique combined with phototherapeutic keratectomy. J Refract Surg. 2016;32(12):840–845. doi: 10.3928/1081597X-20160920-01. [DOI] [PubMed] [Google Scholar]
- 21.Mastropasqua L., Nubile M., Salgari N., Mastropasqua R. Femtosecond laser-assisted stromal lenticule addition keratoplasty for the treatment of advanced keratoconus: a preliminary study. J Refract Surg. 2018;34(1):36–44. doi: 10.3928/1081597X-20171004-04. [DOI] [PubMed] [Google Scholar]
- 22.Shaheen B.S., Bakir M., Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed-Noriega K., Riau A.K., Lwin N.C., Chaurasia S.S., Tan D.T., Mehta J.S. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Invest Ophthalmol Vis Sci. 2014;55(3):1823. doi: 10.1167/iovs.13-13324. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y.C., Jung A.S.J., Chin J.Y., Yang L.W.Y., Mehta J.S. Cross-sectional Study on Corneal Denervation in Contralateral Eyes Following SMILE Versus LASIK. J Refract Surg. 2020;36(10):653–660. doi: 10.3928/1081597X-20200730-01. [DOI] [PubMed] [Google Scholar]
- 25.Li M., Chen Y., Miao H., Yang D., Ni K., Zhou X. Five-year results of small incision lenticule extraction (SMILE) and femtosecond laser LASIK (FS-LASIK) for myopia. Acta Ophthalmol. 2019;97:e373–e380. doi: 10.1111/aos.14017. [DOI] [PubMed] [Google Scholar]
- 26.Bandeira F., Yusoff N.Z., Yam G.H.F., Mehta J.S. Corneal re-innervation following refractive surgery treatments. Neural Regen Res. 2019;14(4):557. doi: 10.4103/1673-5374.247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandeira F., Yam G.H.F., Liu Y.C., Devarajan K., Mehta J.S. Three-dimensional neurite characterization of Small Incision Lenticule Extraction derived lenticules. Invest Ophthalmol Vis Sci. 2019;60(13):4408. doi: 10.1167/iovs.19-27566. [DOI] [PubMed] [Google Scholar]
- 28.Lagali N.S., Griffith M., Shinozaki N., Fagerholm P., Munger R. Innervation of tissue-engineered corneal implants in a porcine model: a 1-year in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48(8):3537. doi: 10.1167/iovs.06-1483. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y.C., Jayasinghe L., Ang H.P., Lwin N.C., Yam G.H.F., Mehta J.S. Effect of Intraoperative Corneal Stromal Pocket Irrigation in Small Incision Lenticule Extraction. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/928608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.C., Williams G.P., George B.L., Soh Y.Q., Seah X.Y., Peh G.S., et al. Corneal lenticule storage before reimplantation. Mol Vis. 2017;23:753–764. [PMC free article] [PubMed] [Google Scholar]
- 31.Damgaard I.B., Riau A.K., Liu Y.C., Tey M.L., Yam G.H.F., Mehta J.S. Reshaping and customization of SMILE-derived biological lenticules for intrastromal implantation. Invest Ophthalmol Vis Sci. 2018;59(6):2555. doi: 10.1167/iovs.17-23427. [DOI] [PubMed] [Google Scholar]
- 32.Yam G.H.F., Fuest M., Yusoff N.Z., Goh T.W., Bandeira F., Setiawan M., et al. Safety and feasibility of intrastromal injection of cultivated human corneal stromal keratocytes as cell-based therapy for corneal opacities. Invest Ophthalmol Vis Sci. 2018;59(8):3340. doi: 10.1167/iovs.17-23575. [DOI] [PubMed] [Google Scholar]
- 33.Konstantopoulos A., Liu Y.C., Teo E.P., Nyein C.L., Yam G.H., Mehta J.S. Corneal stability of LASIK and SMILE when combined With collagen cross-linking. Transl Vis Sci Technol. 2019;8(3):21. doi: 10.1167/tvst.8.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yam G.H.F., Yusoff N.Z., Goh T.W., Setiawan M., Lee X.W., Liu Y.C., et al. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci Rep. 2016;6(1) doi: 10.1038/srep26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piotrowska-Nitsche K., Caspary T. Ex vivo live imaging of single cell divisions in mouse neuroepithelium. J Vis Exp. 2013 doi: 10.3791/4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yam G.H.F., Williams G.P., Setiawan M., Yusoff N.Z., Lee X.W., Htoon H.M., et al. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci Rep. 2017;7(1) doi: 10.1038/srep45396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes S., White T., Boote C., Kamma-Lorger C.S., Bell J., Sorenson T., et al. The structural response of the cornea to changes in stromal hydration. J R Soc Interface. 2017;14(131):20170062. doi: 10.1098/rsif.2017.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu R., Zhao J., Xu Y.e., Li M., Niu L., Liu H., et al. Femtosecond laser-assisted corneal small incision allogenic intrastromal lenticule implantation in monkeys: a pilot study. Invest Ophthalmol Vis Sci. 2015;56(6):3715. doi: 10.1167/iovs.14-15296. [DOI] [PubMed] [Google Scholar]
- 39.Riau A.K., Boey K.P., Yusoff N.Z., Goh T.W,. Yam G.H., Tang K.F., et al. Experimental-based validation of corneal lenticule banking in a health authority-licensed facility. Tissue Eng Part A. 2021. doi: 10.1089/ten.TEA.2021.0042. Online ahead of print. [DOI] [PubMed]
- 40.Xia F., Zhao J., Fu D., Xu Y.e., Yao P., Li M., et al. Optical transmittance and ultrastructure of SMILE-derived lenticules subjected to three different preservative methods. Exp Eye Res. 2020;201:108357. doi: 10.1016/j.exer.2020.108357. [DOI] [PubMed] [Google Scholar]
- 41.Chang Q., Pereda A., Pinter M.J., Balice-Gordon R.J. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci. 2000;20(2):674–684. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sulaiman W., Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J. 2013;13:100–108. [PMC free article] [PubMed] [Google Scholar]
- 43.Jessen K.R., Arthur-Farraj P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67(3):421–437. doi: 10.1002/glia.23532. [DOI] [PubMed] [Google Scholar]
- 44.Müller L.J., Marfurt C.F., Kruse F., Tervo T.M.T. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar J., Milani B., Kim E., An S., Kwon J., Jain S., et al. Corneal nerve healing after in situ laser nerve transection. PLoS ONE. 2019;14(6):e0218879. doi: 10.1371/journal.pone.0218879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedrotti E., Cozzini T., Fasolo A., Bonacci E., Bonetto J., Merz T., et al. Small-incision lenticule addition in ex vivo model of ectatic human corneas. Int Ophthalmol. 2019;39(11):2575–2581. doi: 10.1007/s10792-019-01106-8. [DOI] [PubMed] [Google Scholar]
- 47.Mastropasqua L., Salgari N., D'Ugo E., Lanzini M., Alio Del Barrio J.L., Alio J.L., et al. In Vivo Confocal Microscopy of Stromal Lenticule Addition Keratoplasty for Advanced Keratoconus. J Refract Surg. 2020;36:544–550. doi: 10.3928/1081597X-20200527-01. [DOI] [PubMed] [Google Scholar]
- 48.Nubile M., Salgari N., Mehta J.S., Calienno R., Erroi E., Bondì J., et al. Epithelial and stromal remodelling following femtosecond laser-assisted stromal lenticule addition keratoplasty (SLAK) for keratoconus. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-81626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon T. Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury, Neurosurgery 2009; 65: A132-144. [DOI] [PubMed]
- 50.Zhang X, Muddana S, Kumar SR, Burton JN, Labroo P, Shea J, et al. Topical Pergolide enhance corneal nerve regrowth following induced corneal abrasion, Invest Ophthalmol Vis Sci 2020; 61: 4. [DOI] [PMC free article] [PubMed]
- 51.Trokel S.L., Srinivasan R., Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96(6):710–715. doi: 10.1016/s0002-9394(14)71911-7. [DOI] [PubMed] [Google Scholar]
- 52.Eguchi H., Hiura A., Nakagawa H., Kusaka S., Shimomura Y. Corneal nerve fiber structure, its role in corneal function, and its changes in corneal diseases. Biomed Res Int. 2017;2017:1–15. doi: 10.1155/2017/3242649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spoerl E., Mrochen M., Sliney D., Trokel S., Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 54.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 55.Damgaard I.B., Liu Y.C., Riau A.K., Teo E.P., Tey M.L., Nyein C.L., et al. Corneal remodelling and topography following biological inlay implantation with combined crosslinking in a rabbit model. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-39617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Aqaba M., Calienno R., Fares U., Otri A.M., Mastropasqua L., Nubile M., et al. The effect of standard and transepithelial ultraviolet collagen cross-linking on human corneal nerves: an ex vivo study. Am J Ophthalmol. 2012;153(2):258–266.e2. doi: 10.1016/j.ajo.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Mazzotta C., Traversi C., Baiocchi S., Caporossi O., Bovone C., Sparano M.C., et al. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008;146(4):527–533.e1. doi: 10.1016/j.ajo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 58.Kymionis G.D., Diakonis V.F., Kalyvianaki M., Portaliou D., Siganos C., Kozobolis V.P., et al. One-year follow-up of corneal confocal microscopy after corneal cross-linking in patients with post laser in situ keratosmileusis ectasia and keratoconus. Am J Ophthalmol. 2009;147(5):774–778.e1. doi: 10.1016/j.ajo.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Knappe S., Stachs O., Zhivov A., Hovakimyan M., Guthoff R. Results of confocal microscopy examinations after collagen cross-linking with riboflavin and UVA light in patients with progressive keratoconus. Ophthalmologica. 2011;225(2):95–104. doi: 10.1159/000319465. [DOI] [PubMed] [Google Scholar]
- 60.Legat F.J. The antipruritic effect of phototherapy. Front Med (Lausanne) 2018;5:333. doi: 10.3389/fmed.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumakiri Masanobu, Hashimoto Ken, Willis Isaac. Biological changes of human cutaneous nerves caused by ultraviolet irradiation: an ultrastructural study. Br J Dermatol. 1978;99(1):65–75. doi: 10.1111/j.1365-2133.1978.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 62.Scholzen T.E., Steinhoff M., Bonaccorsi P., Klein R., Amadesi S., Geppetti P., et al. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol. 2001;166(2):1285–1291. doi: 10.4049/jimmunol.166.2.1285. [DOI] [PubMed] [Google Scholar]
- 63.Han L.W., Xu G., Guo M.Y., Chang Y.A., Zhang Y.U., Zhao Y.T., et al. Comparison of SB-SDS and other decellularization methods for the acellular nerve graft: Biological evaluation and nerve repair in vitro and in vivo. Synapse. 2020;74(5) doi: 10.1002/syn.v74.510.1002/syn.22143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the text.