Abstract
Red blood cells (RBCs) are an excellent choice for cell preparation research because of their biocompatibility, high drug loading, and long half-life. In this study, doxorubicin (DOX) was encapsulated with RBCs as the carrier. The biotin-avidin system binding principle was used to modify biotinylated cyclic arginine-glycine-aspartic acid (cRGD) onto RBC surfaces for accurate targeting, high drug loading, and sustained drug release. The RBC drug delivery system (DDS) was characterized, and the concentration of surface sulfur in the energy spectrum was 6.330%. The physical and chemical properties of RBC DDS were as follows: drug content, 0.857 mg/mL; particle size, 3339 nm; potential value, −12.5 mV; and cumulative release rate, 81.35%. There was no significant change in RBC morphology for up to seven days. The results of the targeting and cytotoxicity studies of RBC DDS showed that many RBCs covered the surfaces of U251 cells, and the fluorescence intensity was higher than that of MCF-7 cells. The IC50 value of unmodified drug-loaded RBCs was 2.5 times higher than that of targeted modified drug-loaded RBCs, indicating that the targeting of cancer cells produced satisfactory inhibition. This study confirms that the RBC DDS has the characteristics of accurate targeting, high drug loading, and slow drug release, which increases its likelihood of becoming a clinical cancer treatment in the future.
Keywords: Cytotoxicity, Red blood cells, Drug delivery, Targeting
Graphical abstract
Highlights
-
•
An innovative drug delivery system based on red blood cell is described.
-
•
The system demonstrated controlled release of doxorubicin.
-
•
The system produced excellent active targeting in vitro.
-
•
The system enhanced therapeutic effects against cancer cells.
1. Introduction
Drug delivery systems (DDSs) have always been the focus of pharmaceutical development [[1], [2], [3]]. Research on DDSs mainly focuses on carriers, construction, and effect evaluation [[4], [5], [6]]. In recent years, continuous progress has been made in cell-based therapeutic reagents, and this method of loading drugs enhances drug targeting and stability. Cell-based DDSs have the potential to revolutionize drug delivery [7,8]. Many common cell carriers, such as red blood cells (RBCs), immune cells, and stem cells, have been studied.
In recent years, RBC-based DDSs have attracted the most attention because they have the following advantages [[9], [10], [11]]. 1) The drug loading amount could meet the clinical dose, and due to the absence of nuclei and organelles, the RBC intracellular space is sufficient to hold various types of drugs, including large peptides and protein biomolecule drugs [12,13]. 2) The half-life of drugs is prolonged [14,15]. The survival time of normal RBCs in the body is long and can effectively prolong the circulation time of drugs carried by RBCs. Studies have shown that the attachment of polymer nanoparticles to the surfaces of RBCs may significantly improve their circulation life in vivo. As long as these particles remain attached to RBCs, they continue to circulate in the blood. Due to the interaction between cells, the particles are eventually separated from RBCs and then cleared by the liver and spleen. 3) The internal environment of RBCs is relatively stable, which can be regarded as an isolation space for drugs. It can increase drug stability by preventing interactions with endogenous substances, resulting in the loss or change of drug effects. Zhang et al. [16] encapsulated betamethasone phosphate (BSP) into RBCs. The circulation time of BSP in plasma was more than 9 days. 4) RBCs have good biocompatibility [17,18]. As a natural part of the circulatory system, RBCs have incomparable biocompatibility compared with synthetic drug carriers, which may effectively reduce immune responses to drugs.
RBCs can be used to transport small molecular drugs, proteins, nucleic acids, and other macromolecules [[19], [20], [21], [22]]. There are two main loading methods: intracellular entrapment and extracellular connection. Under hypotonic conditions, small molecular drugs can enter cells through the membrane pore and then encapsulate RBCs under hyperosmotic conditions, using the characteristics of the RBC membrane to control drug release [[23], [24], [25]]. Some drugs may also be encapsulated in proteins or nanoparticles and then loaded into RBCs. Magnani and Rossi [26] reported that RBCs could contain enzymes and enzyme activity.
Drugs that cannot pass through the RBC membrane are often connected to the surface of the RBC membrane. In the past few decades, biotin-avidin has been widely used in biotechnology [[27], [28], [29], [30]]. Zaltzman et al. [31] designed a reasonable drug delivery strategy using RBCs as a large carrier by studying the mechanism of biotin-avidin induction. This method is widely used in the study of extracellular ligation of drug molecules on RBCs.
The DDS carrier can increase active targeting by modifications of target molecules on carrier surfaces. The RBC DDS can also increase its targeting by modifications of the surface of RBCs [[32], [33], [34]]. Among the many targeted molecules, arginine-glycine-aspartic acid (RGD) is widely used in active targeted DDSs. RGD is the recognition site of integrin αVβ3 receptor binding to ligands, which plays an important role in cell-to-cell adhesion and transmission [[35], [36], [37]]. In tumor therapy, RGD is often used as a tumor cell target for the high expression of integrin αVβ3. By modifying RGD, the drug's efficacy can be increased by targeting the drug preparation at the tumor site.
In this study, RBCs loaded with the anticancer drug doxorubicin (DOX) were used as the DDS, and RBCs were modified with cyclic RGD (cRGD) to increase their active targeting. The DDS's physical and chemical properties, drug loading characteristics, drug release characteristics, and cytotoxicity were investigated. This study provides a new approach for the research and development of a new targeted DDS.
2. Materials and methods
2.1. Reagents and materials
Cyclo [RGDfK] (Biotin; 95%) was purchased from China Peptides Pty Ltd. (Shanghai, China). DOX (98%) was purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China); avidin and DSPE-PEG3400-biotin were purchased from Xi'an Ruixi Biological Technology Co., Ltd. (Xi'an, China); U251 glioma cells and MCF-7 breast cancer cells were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China).
2.2. Synthesis of the RBC-based DDS
2.2.1. Isolation and collection of RBCs
Whole blood (0.5 mL) from Balb/c mice was collected in an anticoagulant test tube. Phosphate buffer saline (PBS, 5 mL) was added, and the mixture was shaken to mix well. After centrifugation at 1,300 r/min for 5 min (4 °C), the supernatant was removed, and the precipitate was pure RBCs, which were then resuspended in PBS.
2.2.2. Preparation of cRGD-modified RBCs (R-RBC)
A coating strategy based on biotin-avidin interactions was used to modify the surfaces of RBCs [38]. To obtain surface-modified biotin RBCs (b-RBC), 1 mL of DPSE-PEG3400-biotin (0.1 mg/mL) and 1 mL of RBCs were suspended in 8 mL of PBS and shaken for 60 min. After centrifugation at 1,300 r/min for 5 min, the precipitate (b-RBC) and 1 mL of avidin (0.1 mg/mL) were suspended in 9 mL of PBS and shaken for 60 min to link avidin to biotin on RBC surfaces (A-b-RBC). According to the above method, cyclo (RGDfK (biotin)) was added to obtain RBCs with surface modification of cRGD (R-RBC).
2.2.3. Preparation of DOX-loaded R-RBC (R-RBC#DOX)
In this experiment, the drug was loaded using the hypotonic dilution-isotonic resealing method, according to a previous report [39]. The main steps were as follows: 1 mg of DOX was mixed with an RBC suspension (0.5 mL) and dialyzed in 4 °C hypotonic buffer (molecular weight cut-off = 3500) for 30 min. The RBCs encapsulating DOX were transferred to a 37 °C hyperosmotic buffer for 30 min, and then the unencapsulated DOX was removed by centrifugation. The drug was encapsulated in RBCs by the “opening and closing” of the RBC membrane.
The changes in the concentrations of C, N, O, S, and Fe on the surfaces of RBCs were analyzed using an energy spectrometer to characterize the surface modification of drug-loaded RBCs. The main steps were as follows: RBCs were suspended in PBS containing 2.5% glutaraldehyde, stirred evenly for 2 min, and then fixed at 4 °C for 12 h to obtain fixed RBCs. The sample was obtained by gradient elution with 20%, 30%, 50%, 90%, and 100% ethanol solution for 10 min, and finally freeze-dried. The freeze-dried RBC DDS was placed on the sample table, and the surface was sprayed with gold using an ion sputtering instrument for scanning electron microscope (SEM) observation. A laser particle size analyzer was used on the dispersed RBC DDS to detect any changes in particle size and zeta potential.
2.3. In vitro evaluation of R-RBC#DOX
2.3.1. Measurement of DOX
Drug loading was expressed as the amount of DOX in 1 mL of RBCs. The DOX content was determined by ultra-high-performance liquid chromatography (ACQUITY UPLC; Waters, Milford, MA, USA). The mobile phase consisted of acidified water (0.04% trifluoroacetic acid, pH 2.5)-acetonitrile-tetrahydrofuran (75:24.2:0.8, V/V/V), the flow rate was 0.4 mL/min, the injection volume was 10 μL, and the detector was a fluorescence detector (Ex: 480 nm/Em: 550 nm).
Sample preparation: DOX-loaded RBCs (1 mL) were added to ultra-pure water and ultrasonicated for 15 min. After centrifugation at 8,000 r/min for 5 min, the supernatant was extracted. DOX was extracted by adding a mixture of chloroform and methanol (4:1, V/V). The trichloromethane layer was dried with nitrogen and diluted 50 times with ultrapure water.
2.3.2. Release test
The RBC DDS was dispersed in 2.5 mL of PBS with a dialysis bag (3500D). The dialysis bag was placed in a beaker (Hanson Elite 8) with 50 mL of PBS and stirred at 37 °C. Then, 2.5 mL of samples were collected at 1, 4, 8, 12, 16, 20, 24, 36, 48, 72, 96, and 120 h, respectively. The samples were diluted 20 times to determine the DOX concentration. Simultaneously, blank PBS (2.5 mL) was added to the beaker. The concentration of DOX was determined using the method described in Section 2.3.1.
2.3.3. Cytotoxicity of R-RBC#DOX
The R-RBC#DOX system was developed for the follow-up realization of brain-targeted therapy. Therefore, we chose glioma U251 as the cell model. The experiment was divided into four groups, i.e., DOX group, R-RBC group, RBC#DOX group, and R-RBC#DOX group. The OD value of each group after incubation with U251 cells was measured using the CCK-8 method. The inhibitory effects of each group on cell proliferation were also compared. The tested samples were added to a 96-well culture plate containing U251 cells at different concentrations. After 72 h, CCK-8 solution was added to the 96-well culture plate and incubated in the incubator for 4 h. The absorbance was measured at 450 nm, and the cell inhibition rate was calculated.
2.3.4. Study of cell localization and targeting
The purpose of cRGD modification of RBCs is to increase the active targeting of the DDS. Confocal microscopy was used to qualitatively describe the changes in targeting before and after cRGD modification. DOX can excite fluorescence at a certain wavelength of the excitation light. The fluorescence intensity of DOX can be used to determine the target strength indirectly. The steps were as follows: U251 and MCF-7 cells were inoculated into confocal dishes. Hoechst 33342 staining solution was added and incubated at 25 °C for 20 min. Then, 50 μL of RBC#DOX or R-RBC#DOX was added to the culture medium and incubated for 30 min. Using confocal microscopy, the distribution of DOX in cancer cells was analyzed as indicated by the fluorescence of the Hoechst reagent. The fluorescence intensity of DOX in cancer cells in each group was compared, and the targeted drug delivery performance of the RBC DDS was analyzed.
3. Results and discussion
3.1. Characterization of R-RBC#DOX
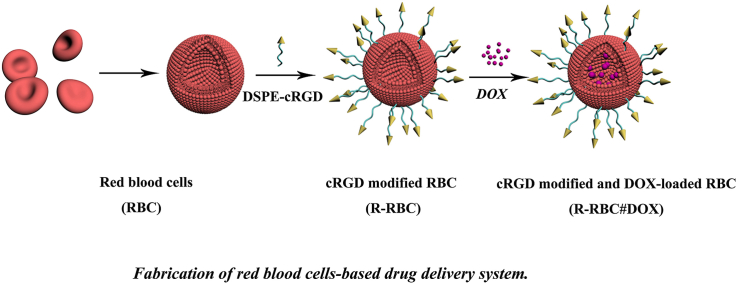
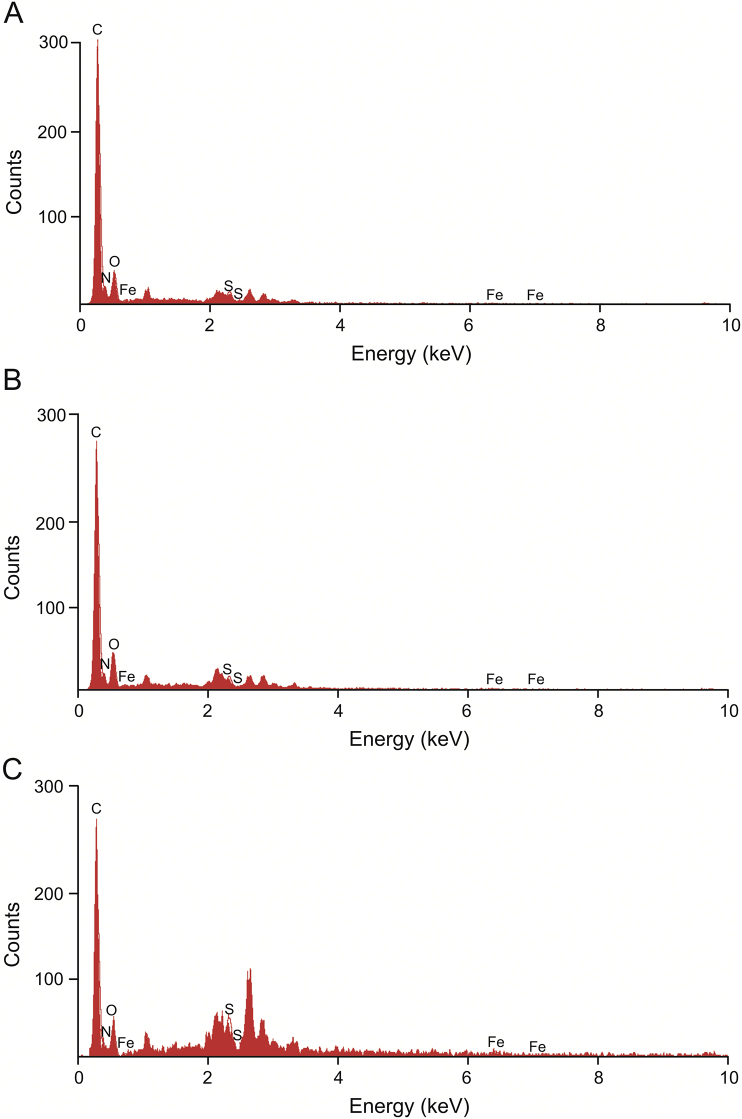
The synthesis of DDS based on RBC is shown in Fig. 1. The reagents DPSE-PEG3400-biotin and cRGD-biotin, used in the process of surface modification, contained sulfur. An energy spectrometer can be used to determine the sulfur content on the RBC surface at different stages of modification, which could verify whether the the cell surface is modified with cRGD. The results of the energy dispersive spectroscopy analyses are shown in Tables S1–S3. As shown in Fig. 2, the proportion of sulfur in b-RBC was higher than that in RBC, which indicated that DSPE-PEG3400-biotin successfully modified the RBC surfaces. After the modification of cRGD, the concentration of sulfur in R-RBC reached 6.330%. Because there is no sulfur in avidin, the added sulfur is derived from cRGD, indicating that the RBC surface has been successfully modified with cRGD.
Fig. 1.
Schematic illustration of the red blood cell (RBC)-based drug delivery system. cRGD: cyclic arginine-glycine-aspartic acid; DOX: doxorubicin.
Fig. 2.
Energy dispersive spectroscopy analyses of (A) RBC, (B) biotin modified RBC (b-RBC), and (C) cRGD modified RBC (R-RBC).
Avidin is tetravalent for biotin and binds to two biotinylated macromolecules or biotinylated cells. In other words, excluding the amount of sulfur in DSPE-PEG3400-biotin, the amount of sulfur in R-RBC#DOX should be increased three-fold. The test results showed that sulfur content did not increase three-fold. This result might be due to the addition of avidin to the surface of RBCs after the cRGD modification. However, the amount of C, N, O, and other elements in avidin molecules increased the total amount of surface elements on RBCs, resulting in a three-fold increase in the total amount of sulfur.
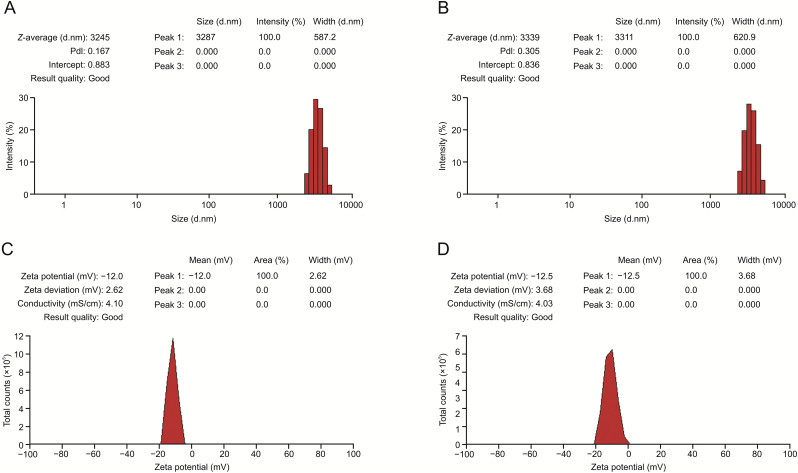
The results of the particle size and potential analyses are shown in Fig. 3 and Table S4. The particle size of the unmodified DOX-loaded RBCs was 3245 nm, and the dispersion index was 0.167. The average particle size of cRGD modified DOX-loaded RBCs was 3339 nm, and the dispersion index was 0.305. There were no changes in the sizes of the two groups. The particle size was consistent with the results of confocal microscopy and scanning electron microscopy. The results showed that the preparation did not affect RBC size. The dispersion index test results were satisfactory, indicating that the modified drug-loaded RBCs still had good dispersion characteristics with no RBC adhesion. The zeta potential of unmodified and modified RBCs were −12.0 mV and −12.5 mV.
Fig. 3.
The size distribution of (A) doxorubicin-loaded RBC (RBC#DOX) and (B) cRGD modified and DOX-loaded RBC (R-RBC#DOX); the zeta potential of (C) RBC#DOX and (D) R-RBC#DOX.
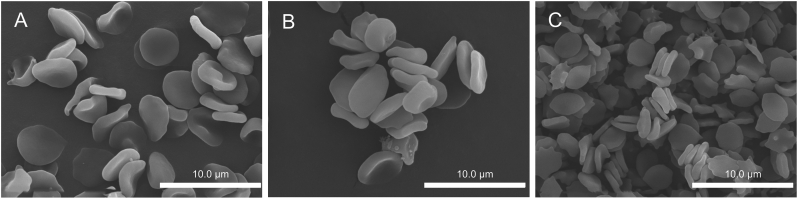
The results of scanning electron microscopy are shown in Fig. 4. There was no significant change in RBC morphology before or after drug loading, as shown in Figs. 4A and B. The results showed that drug loading did not affect RBCs. There are fine white spots visible on the surfaces of some RBCs in Figs. 4A and B, which may be due to the modification of cRGD on the red blood cell surfaces. This also confirmed the side evidence of cRGD characterization, which proved that cRGD was successfully modified on RBC surfaces. The morphological diagram of R-RBC#DOX 7 days after loading is shown in Fig. 4C. This finding shows that the stability of R-RBC#DOX samples was good, and most of the RBCs remained intact.
Fig. 4.
Scanning electron microscopy (SEM) images of RBCs (A) before and (B) after drug loading, and (C) R-RBC#DOX 7 days after loading.
Coupling avidin to biotinylated RBC (b-RBC) is known to sensitize biotinylated RBC to complement [[40], [41], [42], [43]], and this unintended effect can be modulated by specific approaches to anchoring biotinylated cargoes to b-RBC via avidin [[44], [45], [46]]. Avidin is also known to cause aggregation of b-RBC [47]. Therefore, agglutination activity was measured. The agglutination activity assay was performed in V-shaped agglutination plates, as described previously [40]. As a result, agglutination titer for samples of cRGD-modified avidin-biotinylated DOX-loaded RBCs (R-A-b-RBC#DOX), DOX-loaded RBCs (RBC#DOX), biotinylated DOX-loaded RBCs (b-RBC#DOX) and avidin-biotinylated DOX-loaded RBCs (A-b-RBC#DOX) were 20, 24, 12, and 5 μg/mL, respectively. The agglutination activity of R-A-b-RBC#DOX decreased significantly. This result may be because both cRGD and avidin are positively charged, which increases the possibility of mutual exclusion and reduces condensation [42].
3.2. Drug release
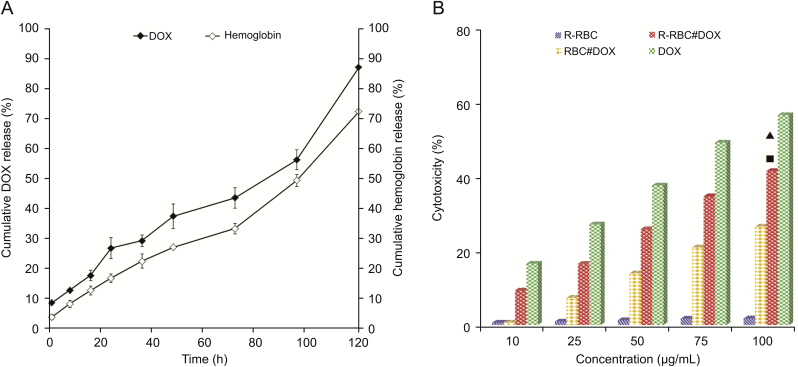
The release curves for DOX and hemoglobin are shown in Fig. 5A. These results showed that DOX in RBCs was released slowly over time. On the fifth day, the cumulative release of DOX reached 81.35%. The release curve of hemoglobin is the same as that of DOX, most likely due to the release of DOX and hemoglobin caused by the change in RBC membrane permeability. This result was similar to that reported for RBCs [39].
Fig. 5.
(A) The release profiles of DOX and hemoglobin from R-RBC#DOX in PBS solution. (B) Cytotoxicity of R-RBC, DOX, RBC#DOX and R-RBC#DOX against U251 cells. ▲ Represents P<0.001, compared with the RBC#DOX group (t-test). ■ Represents P<0.001 compared with the R-RBC group (t-test).
3.3. In vitro test
The inhibition rate of U251 cells is shown in Fig. 5B. The IC50 values of the R-RBC#DOX group and the RBC#DOX group were 155.85 ± 2.77 μg/mL and 214.34 ± 2.91 μg/mL, respectively. The cell inhibition rate of the former was higher than that of the latter. This result was probably due to the active targeting effect of cRGD. In contrast, R-RBC showed almost no cytotoxicity. According to the drug release test results, R-RBC#DOX is a slow-release DDS. Therefore, in the cytotoxicity test, R-RBC#DOX should be incubated with U251 and given sufficient time to reflect the cytotoxicity objectively.
To better explain the experimental results of R-RBC#DOX in vitro, hemolysis was tested. The hemolytic assay was performed using microtitration plates, as described previously [40]. As shown in Fig. S1, hemolysis decreased after avidin treatment combined with biotin-DSPE. This finding may be because if a similar avidin surface density is achieved by attachment of avidin through biotin-DSPE, this does not result in cell lysis by complement [45]. When cRGD was added, hemolysis decreased. This result may be due to the increased steric hindrance caused by incorporating cRGD cyclic peptides and reducing the lysis effect [45].
3.4. Targeting ability of R-RBC#DOX
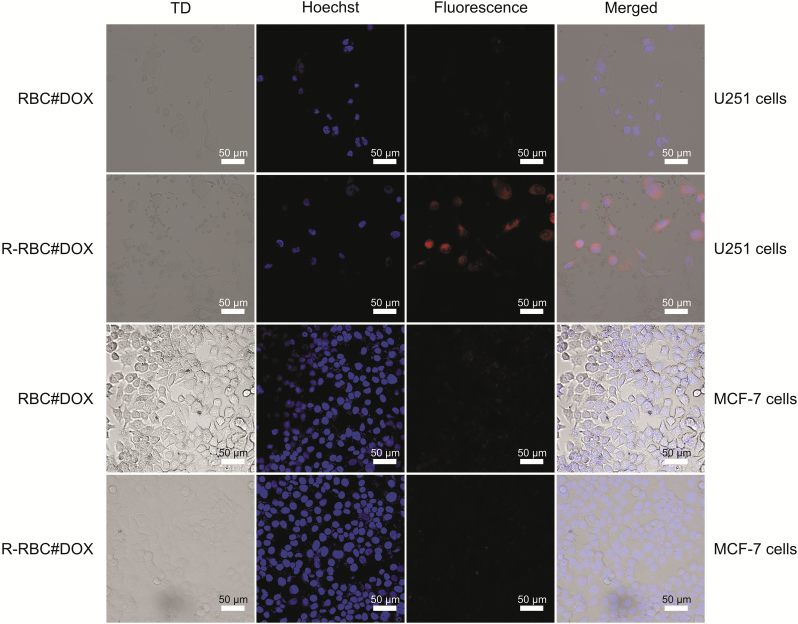
In the process of exploring the DDS in vitro, MCF-7 cells with low expression of integrin αVβ3 were compared. As shown in Fig. 6, the fluorescence intensity was weak in both the R-RBC#DOX group and the RBC#DOX group for MCF-7 cells. This finding shows that cRGD-modified RBCs had no targeting effect on MCF-7 cells. These results also confirmed that cRGD could not affect cancer cells with low expression of integrin αVβ3. However, there are different results for U251 glioma cells with high expression of integrin αVβ3. R-RBC#DOX showed strong fluorescence. This finding indicates that U251 cells have a stronger uptake of drug-loaded RBCs with surface-modified cRGD. The results showed that the modified DOX-loaded RBCs effectively targeted U251 cells with high integrin αVβ3 expression.
Fig. 6.
Cellular uptake of U251 cells and MCF-7 cells after treatment with RBC#DOX and R-RBC#DOX.
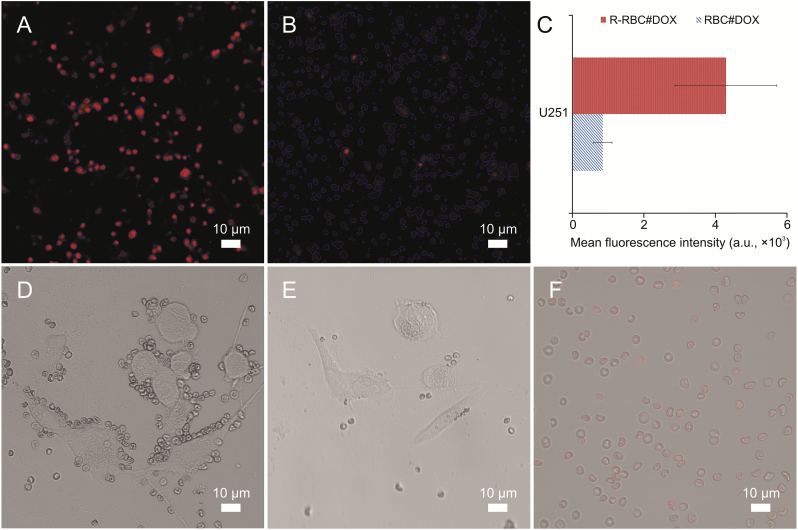
cRGD-modified RBCs actively target cancer cells, as shown by our high-content cellomics analysis. As shown in Figs. 7A and B, red represents DOX fluorescence. The red color of RBCs modified by cRGD was quite apparent. The qualitative analysis of fluorescence intensity provided by the high-content cellomics analysis reader is shown in Fig. 7C. Based on the above data, it can be concluded that RBCs modified by cRGD are more likely to target cancer cells actively than unmodified RBCs.
Fig. 7.
(A and B) High-content cellomics analysis of R-RBC#DOX (A) and RBC#DOX (B). (C) The mean fluorescence intensity of different samples determined in U251 cells. (D and E) Confocal microscopy field photographs of U251 cells after treatment with R-RBC#DOX (D) and RBC#DOX (E). (F) Confocal microscopy photographs of RBC after DOX loading.
The relationship between the RBC DDS and cancer cells is shown in Figs. 7D and E. Many RBCs were gathered at the site of the cancer cells in the R-RBC#DOX group. There was no apparent coverage in the RBC#DOX group. This shows that the RBC drug carrier modified by cRGD has a strong ability to target cancer cells with a high expression of integrin αVβ3 and can gather at the site of cancer cells. This coverage is more conducive to the subsequent release of DOX into cancer cells. Fig. 7F shows the confocal microscopy photographs of drug-loaded RBCs, showing that DOX was successfully loaded into RBCs.
4. Conclusions
In this study, we successfully prepared a DDS based on RBCs. The surface of RBCs was modified, and DOX was encapsulated in RBCs to confer the characteristics of slow-release, high drug loading, and accurate targeting. The DDS's physical and chemical properties, targeting characteristics, and tumor cell inhibition were verified. cRGD was successfully modified on RBC surfaces using the biotin-avidin reaction. There were no significant changes in the structure, size, and potential characteristics of RBCs before or after drug loading. The RBC DDS also showed the characteristics of sustained-release and good stability for 7 days and proved to have good targeting characteristics of integrin αVβ3 and inhibition of cell proliferation. In summary, this study provides a basis for developing follow-up targeted agents and a new strategy for clinical cancer treatment.
CRediT author statement
Chen Wang: Conceptualization, Writing - Reviewing and Editing; Min Wang: Data curation; Yan Zhang: Writing - Original draft preparation, Investigation; Hongxin Jia: Data curation; Binbin Chen: Software, Validation.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are grateful for the financial support provided by the General Program of the Natural Science Foundation of Fujian Province of China (Grant No.: 2019D016), the Program of the Institute of Respiratory Diseases of Xiamen Medical College (Program No.: HXJB-04), the New Century Excellent Talent Support Program of Higher Education Institutions of Fujian Province (Program No.: MinJiaoKe [2018] 47), and the Innovation and Entrepreneurship Training Program for College Students (Program No.: 201912631017).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.06.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Anselmo A.C., Gupta V., Zern B.J., et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehryab F., Rabbani S., Shahhosseini S., et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi: 10.1016/j.actbio.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 3.da Silva C.C.P., Owoyemi B.C.D., Alvarenga Jr. B.R., et al. Synthesis and solid-state characterization of diclofenac imidazolium monohydrate: an imidazolium pharmaceutical ionic liquid. CrystEngComm. 2020;22:5345–5354. [Google Scholar]
- 4.Yan G., Chen B., Zeng X., et al. Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr. Polym. 2020;244:116492. doi: 10.1016/j.carbpol.2020.116492. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H., Fan T., Chen W., et al. Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact. Mater. 2020;5:1071–1086. doi: 10.1016/j.bioactmat.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang T., Yin Q., Li Y. Progress of cell-derived biomimetic drug delivery systems for cancer therapy. Adv. Ther. 2018;1:1800053. [Google Scholar]
- 7.Kremer L., Schultz-Fademrecht C., Baumann M., et al. Discovery of a novel inhibitor of the hedgehog signaling pathway through cell-based compound discovery and target prediction. Angew. Chem. Int. Ed. Engl. 2017;56:13021–13025. doi: 10.1002/anie.201707394. [DOI] [PubMed] [Google Scholar]
- 8.Patel N. Validation of CRISPR/Cas9 off-target discovery profiles from in silico prediction, cell-based and biochemical-based assays with targeted off-target sequencing. Mol. Ther. 2020;28:99. [Google Scholar]
- 9.Sun D., Chen J., Wang Y., et al. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics. 2019;9:6885–6900. doi: 10.7150/thno.36510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koleva L., Bovt E., Ataullakhanov F., et al. Erythrocytes as carriers: from drug delivery to biosensors. Pharmaceutics. 2020;12:276. doi: 10.3390/pharmaceutics12030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzounakas V.L., Karadimas D.G., Papassideri I.S., et al. Erythrocyte-based drug delivery in transfusion medicine: wandering questions seeking answers. Transfus. Apher. Sci. 2017;56:626–634. doi: 10.1016/j.transci.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Dong X., Niu Y., Ding Y., et al. Formulation and drug loading features of nano-erythrocytes. Nanoscale Res. Lett. 2017;12:202. doi: 10.1186/s11671-017-1980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu E., Wu X., Zhang X., et al. Study on the protection of dextran on erythrocytes during drug loading. Colloids Surf. B Biointerfaces. 2020;189:110882. doi: 10.1016/j.colsurfb.2020.110882. [DOI] [PubMed] [Google Scholar]
- 14.Glassman P.M., Villa C.H., Ukidve A., et al. Vascular drug delivery using carrier red blood cells: focus on RBC surface loading and pharmacokinetics. Pharmaceutics. 2020;12:440. doi: 10.3390/pharmaceutics12050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J., Agola J.O., Serda R., et al. Biomimetic rebuilding of multifunctional red blood cells: modular design using functional components. ACS Nano. 2020;14:7847–7859. doi: 10.1021/acsnano.9b08714. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Qiu M., Guo P., et al. Autologous red blood cell delivery of betamethasone phosphate sodium for long anti-inflammation. Pharmaceutics. 2018;10:286. doi: 10.3390/pharmaceutics10040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J., Liu B., Long Y., et al. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554–569. doi: 10.1016/j.actbio.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Wang T., Luo Y., Lv H., et al. Aptamer-based erythrocyte-derived mimic vesicles loaded with siRNA and doxorubicin for the targeted treatment of multidrug-resistant tumors. ACS Appl. Mater. Interfaces. 2019;11:45455–45466. doi: 10.1021/acsami.9b16637. [DOI] [PubMed] [Google Scholar]
- 19.Dey P., Banerjee S., Mandal S., et al. Design and evaluation of anti-fibrosis drug engineered resealed erythrocytes for targeted delivery. Drug Deliv. Transl. Res. 2019;9:997–1007. doi: 10.1007/s13346-019-00642-1. [DOI] [PubMed] [Google Scholar]
- 20.Riaz M.I., Sarwar H.S., Rehman M., et al. Study of erythrocytes as a novel drug carrier for the delivery of artemether. Braz. J. Pharm. Sci. 2019;55 [Google Scholar]
- 21.Seghatchian J. A joint narrative on methodological/standardization aspects of the lupus anticoagulant and erythrocyte-based drug delivery in transfusion medicine. Transfus. Apher. Sci. 2017;56:611. doi: 10.1016/j.transci.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Villa C.H., Seghatchian J., Muzykantov V. Drug delivery by erythrocytes: “Primum non nocere”. Transfus. Apher. Sci. 2016;55:275–280. doi: 10.1016/j.transci.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarrin A., Foroozesh M., Hamidi M. Carrier erythrocytes: recent advances, present status, current trends and future horizons. Expet Opin. Drug Deliv. 2014;11:433–447. doi: 10.1517/17425247.2014.880422. [DOI] [PubMed] [Google Scholar]
- 24.Harisa G.I., Ibrahim M.F., Alanazi F., et al. Engineering erythrocytes as a novel carrier for the targeted delivery of the anticancer drug paclitaxel. Saudi Pharm. J. 2014;22:223–230. doi: 10.1016/j.jsps.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biagiotti S., Paoletti M.F., Fraternale A., et al. Drug delivery by red blood cells. IUBMB Life. 2011;63:621–631. doi: 10.1002/iub.478. [DOI] [PubMed] [Google Scholar]
- 26.Magnani M., Rossi L. Approaches to erythrocyte-mediated drug delivery. Expet Opin. Drug Deliv. 2014;11:677–687. doi: 10.1517/17425247.2014.889679. [DOI] [PubMed] [Google Scholar]
- 27.Villa C.H., Pan D.C., Johnston I.H., et al. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2018;2:165–176. doi: 10.1182/bloodadvances.2017011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C.M., Fang R.H., Zhang L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 2012;1:537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 29.Magnani M., Chiarantini L., Vittoria E., et al. Red blood cells as an antigen delivery system. Biotechnol. Appl. Biochem. 1992;16:188–194. [PubMed] [Google Scholar]
- 30.Muzykantov V.R., Murciano J.C., Taylor R.P., et al. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal. Biochem. 1996;241:109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 31.Zaltzman A.B., van den Berg C.W., Muzykantov V.R., et al. Enhanced complement susceptibility of avidin-biotin-treated human erythrocytes is a consequence of neutralization of the complement regulators CD59 and decay accelerating factor. Biochem. J. 1995;307:651–656. doi: 10.1042/bj3070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasconcellos F.C., Swiston A.J., Beppu M.M., et al. Bioactive polyelectrolyte multilayers: hyaluronic acid mediated B lymphocyte adhesion. Biomacromolecules. 2010;11:2407–2414. doi: 10.1021/bm100570r. [DOI] [PubMed] [Google Scholar]
- 33.Doshi N., Swiston A.J., Gilbert J.B., et al. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 2011;23:H105–H109. doi: 10.1002/adma.201004074. [DOI] [PubMed] [Google Scholar]
- 34.Brenner J.S., Pan D.C., Myerson J.W., et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y., Ji Y. RGD-modified polymer and liposome nanovehicles: recent research progress for drug delivery in cancer therapeutics. Eur. J. Pharm. Sci. 2019;128:8–17. doi: 10.1016/j.ejps.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Peng J.Q., Fumoto S., Suga T., et al. Targeted co-delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J. Control Release. 2019;302:42–53. doi: 10.1016/j.jconrel.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Song Z., Lin Y., Zhang X., et al. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomed. 2017;12:1941–1958. doi: 10.2147/IJN.S125573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Guan L., Mu Y. Preparation of arginine-glycine-aspartic acid (RGDS)-urokinase-carrying targeting ultrasound contrast agent by avidin-biotin system and its impacts on thrombus-targeting affinity. Int. J. Clin. Exp. Med. 2017;10:15160–15167. [Google Scholar]
- 39.Hamidi M., Zarrin A.H., Foroozesh M., et al. Preparation and in vitro evaluation of carrier erythrocytes for RES-targeted delivery of interferon-alpha 2b. Int. J. Pharm. 2007;341:125–133. doi: 10.1016/j.ijpharm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Muzykantov V.R., Smirnov M.D., Samokhin G.P. Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement. Blood. 1991;78:2611–2618. [PubMed] [Google Scholar]
- 41.Muzykantov V.R., Smirnov M.D., Samokhin G.P. Streptavidin-induced lysis of homologous biotinylated erythrocytes. Evidence against the key role of the avidin charge in complement activation via the alternative pathway. FEBS Lett. 1991;280:112–114. doi: 10.1016/0014-5793(91)80216-p. [DOI] [PubMed] [Google Scholar]
- 42.Muzykantov V.R., Smirnov M.D., Samokhin G.P. Avidin-induced lysis of biotinylated erythrocytes by homologous complement via the alternative pathway depends on avidin's ability of multipoint binding with biotinylated membrane. Biochim. Biophys. Acta. 1992;1107:119–125. doi: 10.1016/0005-2736(92)90336-k. [DOI] [PubMed] [Google Scholar]
- 43.Muzykantov V.R., Smirnov M.D., Klibanov A.L. Avidin attachment to biotinylated amino groups of the erythrocyte membrane eliminates homologous restriction of both classical and alternative pathways of the complement. FEBS Lett. 1993;318:108–112. doi: 10.1016/0014-5793(93)80002-c. [DOI] [PubMed] [Google Scholar]
- 44.Muzykantov V.R., Smirnov M.D., Samokhin G.P. Avidin acylation prevents the complement-dependent lysis of avidin-carrying erythrocytes. Biochem. J. 1991;273:393–397. doi: 10.1042/bj2730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muzykantov V.R., Smirnov M.D., Klibanov A.L. Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes. J. Immunol. Methods. 1993;158:183–190. doi: 10.1016/0022-1759(93)90212-p. [DOI] [PubMed] [Google Scholar]
- 46.Muzykantov V.R., Smirnov M.D., Zaltzman A.B., et al. Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement. Anal. Biochem. 1993;208:338–342. doi: 10.1006/abio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 47.Muzykantov V.R., Seregina N., Smirnov M.D. Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes. Int. J. Artif. Organs. 1992;15:622–627. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.