Abstract
As an important means of communication among cells, exosomes are being studied more and more widely, especially in the context of cancer immunotherapy. In the phase of tumor immunoediting, exosomes derived from tumor cells and different immune cells have complex and changeable physiological functions, because they carry different proteins and nucleic acid from the source cells. Based on the role of exosomes in the communication between different cells, cancer treatment methods are also under continuous research. This review briefly introduces the molecular composition of exosomes, which is closely related to their secretion mechanism. Subsequently, the role of exosomes encapsulating different information molecules is summarized. The role of exosomes in the three phases of tumor immunoediting is introduced in detail, and the relevant literature of exosomes in the tumor immune microenvironment is summarized by using a novel framework for extracting relevant documents. Finally, it summarizes the various exosome-based immunotherapies currently proposed, as well as the challenges and future prospects of exosomes in tumor immunotherapy.
Keywords: Exosomes; Tumor immunoediting,; Immune escape; Immunotherapy
Graphical abstract
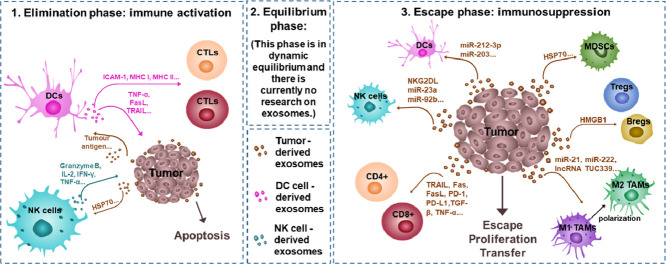
Exosomes mediate important cell communication in the process of tumor immunoediting. In the elimination phase, tumor-derived exosomes (TEs) activate the immune system, and immune cell-derived exosomes have a strong ability to inhibit tumors. The role of exosomes between the tumor and the immune system in the balance phase has not yet been studied. In the escape phase, TEs still have a significant role in the suppression of immune response cells, the activation of immunosuppressive cells and the promotion of the polarization of macrophages in the microenvironment. Different tumor immunotherapy strategies have been developed, such as cancer vaccines and various drug delivery vehicles. Although there are various obstacles to separation, purification and clinical application, as biologically derived nanovesicles, exosomes still have broad researched value and application prospects.
1. Introduction
Exosomes, a kind of nano-scale exovesicles secreted by cells, have now been widely studied as one of the communication methods among cells [1], [2], [3]. They can be derived from almost all cells and can be detected in biological fluids. The exosomes released to the outside of the cell wrap the information, such as protein and nucleic acid of the source cell, transmitted to neighboring or remote cells [4,5]. Therefore, exosomes are widely involved in physiological and pathological processes, especially in tumorigenesis and treatment [6].
American tumor biologist R.D Schreiber first proposed a hypothesis called "cancer immunoediting" in 2002 [7], which believed that the immune system not only has the ability to exclude tumor cells but also promotes tumor growth. Specifically, the three phases of cancer immunoediting, elimination, equilibrium, and escape [8], are the process of development after the tumor and the immune system fight against each other. In the past two decades, this hypothesis has been gradually refined and widely confirmed [9], [10], [11], [12]. Under this concept, there are relatively few discussions about the complex communication of exosomes between tumor cells and immune cells. Therefore, this review will use it as a key point to introduce the role of exosomes in cancer immunoediting and applications related to immunotherapy.
2. Exosomal composition
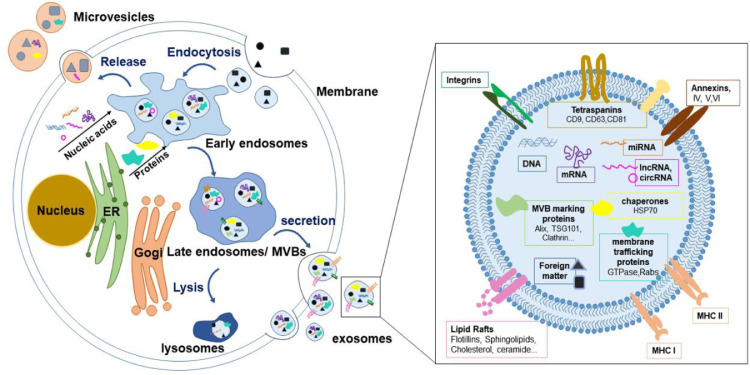
Exosomes, single-layer membrane vesicles with a size of 30–150 nm, are one of the exovesicles secreted by cells, which are heterogeneous and have no independent replication ability [13,14]. Since there is no consensus on the specific markers of several vesicles secreted by cells, according to MISEV2018, the vesicles below 200 nm are classified as small external vesicles, and those above 200 nm are medium or large external vesicles [15]. The exosomes in this review mainly discuss small extracellular vesicles including exosomes. Almost all types of cells secrete exosomes, such as epithelial cells [16], stem cells [17], immune cells [18] and various tumor cells [19]. The molecular composition of exosomes is closely related to their secretion mechanism, as shown in Fig. 1. Exosomes originate from plasma membrane or endosomal membrane budding, although many reports believe that exosomes originate from endosomal membrane budding, ignoring plasma membrane budding and considering them as microvesicles [13,20]. For exosomes originating from late endosomes, they first undergo plasma membrane invagination to form early endosomes [21]. As the endosomal membranes gradually form multivesicular bodies, the exosomes are finally released to the outside of the cell after fusion with the plasma membrane. This formation process is proved by proteomics research [22]. The rich lipid components in exosomes also imply the involvement of endosomal and plasma membranes in the whole process [23]. The cargo sorting mechanism involved in this is complicated. The endosome sorting complex required for transport (ESCRT), consisting of ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III and vacuolar protein sorting 4(VPS4)-vesicle transport 1 and some auxiliary proteins [24]. For example, it is composed of ALG-2 interacting protein X (Alix), which participates in cargo sorting and the process of endosomal membrane folding and multivesicular body (MVB) formation [25]. However, some studies have shown that eliminating the function of VPS4 has no effect on the exosome secretion of CD63 and other exosomal markers [26], which implies the existence of an exosome secretion mechanism that does not rely on the ESCRT pathway. For example, the work of Xu et al. proved that exosomes secreted by macrophages transfer phagocytic antigens to dendritic cells (DCs) in a ceramide-dependent manner [27]. Many other researchers and them have also used the neutral sphingomyelinase inhibitor, GW4869, to inhibit the secretion of exosomes, implying the fact that exosomes are secreted through the ceramide pathway. Wei et al. determined that RAB31 mediates the formation of intraluminal vesicles through flotillin protein and inhibits the lysosomal degradation of MVB, thereby promoting the release of exosomes [28]. Analysis of the composition of exosomes showed that the exosomes contained abundant types of proteins, such as tetraspanins [29], annexins [30], integrins [31], chaperones [32]. Importantly, exosomes do not contain any nuclear, mitochondrial, endoplasmic reticulum or Golgi-derived proteins, and lysosomal proteases [33,34]. Exosomes are also rich in lipids [35], DNA [36,37], mRNA [38] and non-coding RNA, including miRNA [38], lncRNA [39], circRNA [40]. MISEV2018 didn't specifically defined molecular markers that can specifically characterize each extracellular vesicle subtype, but still proposed three types of protein markers that need to be analyzed in the preparation of extracellular vesicles [15]. The first category is the transmembrane protein or GPI-anchored protein used to prove the membrane structure, such as the most commonly used tetraspanins CD63 [41], CD81 [42] and CD9 [43]. Although in addition to exosomes, they were also found in other large or small extracellular vesicles(ectosomes) [44,45]. The second category is cytoplasmic proteins, that can bind to the cytoplasmic sequence of membrane or transmembrane proteins, such as TSG101 [46] and Alix [43]. The third category is negative marker proteins that exclude contamination, such as apolipoproteins A1/2 and B and albumin, which are easily separated from extracellular vesicles [15,47]. For functional molecules, the types and contents of exosomes from different cell sources are different. For example, the miRNAs in exosomes derived from tumor cells are different from those in normal cells [48].
Fig. 1.
The secretion mechanism and composition of exosomes. Exosomes originate from endosomes formed by cell membrane invagination. Early endosomes gradually develop into late endosomes containing multiple vesicles after material sorting and transportation. The small vesicles finally released to the outside through membrane fusion are exosomes. Small extracellular vesicles formed by plasma membrane budding may also be considered exosomes. Exosomes have a phospholipid bilayer and rich in tetraspanins, annexins, integrins, chaperones, lipids and nucleic acids.
3. Advantages of exosomal communication
There is not only one way in which exosomes are taken up by recipient cells. It may be through endocytosis, pinocytosis, phagocytosis, or fusion with the cell membrane that their contents are internalized by the proximal or distal recipient cells [49]. This is the way exosomes serve as a means of intercellular communication. This feature of exosomes, coupled with their own nanometer-scale particle size, enables them to be used as one of the research methods of excellent materials for drug delivery [50]. Based on the consistency of structure and function, the composition and content of cargo carried by exosomes are different, which also makes them have specific cell communication functions in different environments. What the proteins highly expressed in tumor-derived exosomes (TEs) help to promote the proliferation and metastasis of tumor cells [47,51]. For example, TEs have been reported to carry a tetraspanin, Tspan8, which is taken up by recipient endothelial cells and effectively induces tumor angiogenesis [19]. High levels of integrins have been detected in exosomes derived from tumor cells, which have been proven to be one of the most effective substances for tumor metastasis [51]. Exosomes have been determined to contribute to the formation of niches before tumor metastasis. They can be taken up by cells far away from the primary tumor and eventually metastasize [49]. miRNAs change is often observed in tumor cells used as molecular markers for disease diagnosis [52]. For example, Fan et al. proposed a new platform for rapid capture of TEs to test the types and levels of miRNA in exosomes, and analyzed the specific changes of miR-21 derived from exosomes in the serum of patients with lung cancer, liver cancer and breast cancer [53]. Another study showed that the levels of miR-21, miR-27a and miR-375 can be used as potential markers for clinical diagnosis of breast cancer [54]. Similarly, Hong et al. was found that miR-4435 is a potential circulating miRNA biomarker for colorectal cancer [55].
TEs were found to carry a variety of tumor antigens and were presented by DCs to trigger cytotoxic T lymphocytes (CTLs) response [56]. As early as 1998, studies have shown that exosomes derived from DCs can trigger specific CTLs to eliminate tumors. It may be because the exosomes derived from DCs obtain abundant major histocompatibility complex (MHC) molecules from the parent cells, which activate immunity after being taken up by CTLs [57]. Additionally, DCs-derived exosomes (DEs) stimulate a stronger cytotoxic response and anti-tumor immunity than TEs, which may be closely related to the communication function of DEs to promote antigen presentation [58]. The role of immune cell-derived exosomes in resisting tumor proliferation cannot be ignored. Perforin was detected in exosomes derived from natural killer cells (NK cells), and it seems to be effective in inducing cell death after being taken up by tumor cells as receptors [59]. Exosomes derived from CTLs are also collected and used to study their potential effects [60].
However, the battle between the tumor and the immune system is not a simple process of ebb and flow, but a very complicated one. In this environment where cell-to-cell communication occurs frequently, exosomes are indispensably involved in the process. The physiological functions of exosomes in tumor immunoediting and their role in cancer immunotherapy will be described in detail below.
4. Exosomes in tumor immunoediting
Tumor immunoediting was a hypothesis first put forward by American scholar S in 2002, which was that the immune system has contradictory dual effects on tumors [7]. The dual role is reflected in three phases: elimination, equilibrium and escape [61]. In the elimination phase, the rapid growth of tumor tissues promotes the production of new blood vessels, and antigenic tumor cells are recognized and eliminated by immune effector cells [62]. However, some tumor cells with genetic mutations have lower immunogenicity and cytotoxicity to avoid being eliminated by immune cells, which is the equilibrium phase [63]. By reducing the expression of related antigens or up-regulating immunosuppressive receptors/ligands to destroy T cell responses, or even secreting immunosuppressive factors and stimulating the activation of immunosuppressive cells to change the microenvironment, tumor cells gradually proliferate and even metastasize, and develop to the escape phase [63]. In the three phases of tumor immunoediting, the communication between tumor tissues and the immune system mediated by exosomes is frequent, especially in the most studied tumor escape phase [64], [65], [66], [67], [68].
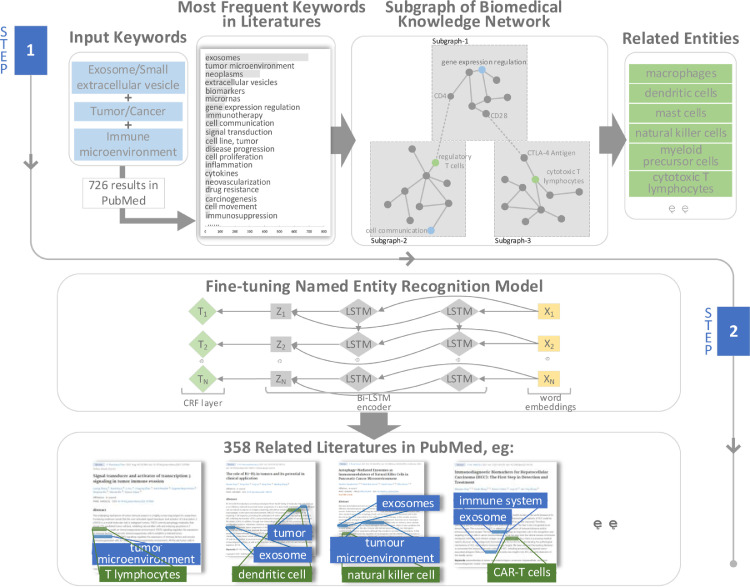
In order to accurately collect reports on exosomes and tumor immunity, we propose a novel related literature extraction framework (Fig. 2), which is based on a hybrid biomedical knowledge network and includes a variety of different knowledge and information sources to extract literature in PubMed. Entities and relationships are represented and managed in structural knowledge such as ontology, semantic web and knowledge graph, and non-structural knowledge such as research literature, medical case reports, and textbooks. We use “exosome or small extracellular vesicle”, “tumor or cancer” and “immune microenvironment” as input keywords and get 726 results through PubMed FTP server. We select some of the words according to the frequency of keywords and MeSH terms in these 726 articles. Based on the biomedical knowledge network and relationships extraction model, we will expand the entities which are related to the keywords and limit the type of related entities to cell. Multi ontology relatedness model (MORM) [69] is applied to explore indirect or implicit relationships between biological concepts. We use a scoring function to normalize the weights of each entity relation between (0–1) [70]. The scoring function is shown below:
where Wi is the credibility of entity relationship. In this paper, MORM includes 225 cell entities and 18,910 relatedness links between cells and other biomedical entities such as phenotype, disease and chemicals. Secondly, we fine-tuning a named entity recognition model based on Bi-LSTM encoder [71], which has pre-trained on 100 million PubMed abstracts and PMC full papers, to recognize the related entities accurately. We use a standard CRF layer at the end of encoder, where ZN is denoted as the output of Bi-LSTM encoder. Given the entity label Y = {y1,y2,y3,…, yN. The probability of the sequence label is calculated by:
where y′ denotes label sequence. and are weight matrix of training parameters. The final output of our framework is to return the related literature which describe the relationships between input keywords and related entities.
Fig. 2.
The framework is used to explore relevant literature on exosomes and tumor immunity. Step 1: input keywords, search all in PubMed, and count the frequency of keywords. Our keywords are “exosome/small extracellular vesicle”, “tumor/cancer” and “tumor microenvironment”. After screened, 726 articles were obtained. Based on the biomedical network and MORM model to expand and search for related entities, finding relevant entities such as “macrophages”, “dendritic cells”, “mast cells”, “natural killer cells”, “myeloid-derived suppressor cells” and “cytotoxicity T lymphocytes”; Step 2: Fine-tune the named entity recognition model to search for the most relevant documents describing the relationship between keywords and entities. In the end, we obtained 358 target documents.
4.1. Elimination phase: immune activation
4.1.1. Promote antigen presentation
Raposo et al. firstly proved that exosomes released by B lymphocytes bound to MHC class II and played the role of antigen presentation [72]. The exosomes derived from the famous antigen-presenting cell DCs are detected to promote the antigen presentation mechanism and they are quickly used as one of the strategies of cancer immunotherapy. The therapy strategy is arranged in Part 5 in detail. Andre et al. proved that DEs with MHC class I to stimulate CD8+ T cells in vitro [73]. Lysosomal-associated membrane protein 2 may help DCs internalized antigens to be enriched in exosomes to obtain high immunogenicity [74]. Except for MHC molecules, costimulatory molecules (CD86) and cell adhesion molecules (ICAM-1) have also been shown to be delivered to immature DCs, T cells or B cells through DEs [75] In more detail, the antigen presentation effect of extracellular vesicles (including exosomes) derived from antigen presenting cells was shown in the work of Lindenbergh et al. [76]. Additionally, Exosomes carrying tumor antigens were isolated and detected from tumor cell culture medium cultured in vitro or ascites and blood of tumor patients [77,78]. Lymphocyte cytosolic protein 1, a membrane-associated antigen that stimulates immune responses, has been detected in exosomes secreted by chronic lymphocytic leukemia cells as well [79]. These exosomes carrying MHC-antigen peptide complexes are recognized by CD4+ T cells or CD8+ T cells, and then activate the immune response [80]. Alternatively, they have also been found to stimulate the initial DCs to become mature DCs and indirectly promote antigen presentation [81,82]. More over some researchers have observed that immature DCs show higher exosomal internalization ability than mature DCs [82].
4.1.2. Enhance the cytotoxicity of immune cells
In addition to indirectly enhancing immune effects by promoting antigen presentation, heat shock protein 70 carried on the surface of TEs may interact with Bag-4 ligand to directly stimulate the activation of NK cells [83]. Not only activate NK cells, further, Bag-6 expressed on TEs will bind to the surface receptor NKp30 and induce the up-regulation of granzyme B, Interleukin 2 (IL-2), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ) in NK cells, triggering NK cells-mediated cytotoxicity [84,85].
DEs carry high levels of intercellular adhesion molecule 1 (ICAM-1) enhancing their capture by other immune cells [86]. In addition to ICAM-1, MHC peptide complexes and T cell costimulatory molecules found on DEs may induce antigen-dependent T cell immune responses [87]. The ability of exosomes secreted by mature DCs to induce antigen-specific T cell activation in vitro is 50 to 100 times higher than that of immature DCs [88]. More importantly, TNF-α, FasL and TNF-related apoptosis-inducing ligand (TRAIL) expressed on the surface of DEs directly induce apoptosis of tumor cells [89]. Therefore, the enhanced immune-mediated cytotoxicity by exosomes may be a potential motivation for tumor immunotherapy.
4.2. Equilibrium phase
In the equilibrium phase of immunoediting, tumor cells with weakened antigenicity will neither be recognized by the immune system, nor will they overgrow due to the strong elimination of the previous phase [63]. Therefore, it seems impossible to detect a visible tumor in the patient's body [62]. At this phase, there has been no discussion about exosomes yet. This balance between the tumor and immune system is not static. Tumor cells may undergo genetic mutations under the pressure of the immune system, which is the critical moment for immunoediting. When the accumulation of this gene mutation reaches a certain level, the immune system will be in a weak position and enter the phase of immune escape [90].
4.3. Escape phase: immunosuppression
After the equilibrium phase, the mutation effect accumulated by tumor cells for a long time makes it exhibit low immunogenicity and express specific immune ligands, so as to escape the surveillance of the immune system. TEs secreted more also transmit more complex information communication at this phase than normal cells [91]. It has been proved that exosomes are involved in inhibiting the differentiation and maturation of DCs, limiting the cytotoxicity of NK cells, inhibiting the proliferation of B cells [92], [93], [94], [95], [96], [97], [98], and possessing the immune regulation ability of CTLs [99], [100], [101]. In short, TEs may be an important way to mediate tumor immunosuppression in the escape phase.
4.3.1. Regulatory proteins or cytokines suppress immune effects
In the process of tumor development, tumor-associated antigens and specific antigens are lost under the pressure of the immune system and the recognizable proteins are down-regulated, which reduce immune recognition and clearance. The expression of transforming growth factor-β (TGF-β) in TEs induces the phenotypic differentiation of myeloid-derived suppressor cells (MDSCs) and inhibits the maturation of DCs and T lymphocyte proliferation [102]. In addition to cytokines, the exosomes of prostate cancer cells carrying miR-212-3p inhibit the expression of MHC II and induce immune tolerance in DCs [94]. The exosomes of pancreatic cancer not only mediate the down-regulation of TNF-α and IL-12 in DCs by miR-203, but also deliver miR-23a to target CD107a in NK cells to exert immunosuppressive effects [103,104]. The exosomes encapsulating miR-92b transfer from liver cancer cells to NK cells, causing the down-regulation of CD69 and the inhibition of NK cell-mediated cytotoxicity [83]. Exosomes derived from nasopharyngeal carcinoma cells have been shown to significantly down-regulate IL-2, IFN-γ and IL-17 and inhibit the proliferation and differentiation of T cells [105]. TEs express apoptosis activating factors FasL and TRAIL, which directly induce CD8+ T cell apoptosis expect for inhibiting immune cell function indirectly [100].
4.3.2. Up-regulation of inhibitory immune checkpoint molecules
Tumor cells overexpress some specific immune checkpoint molecules to inhibit the activation of T cells [106], such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) [107], programmed death 1 (PD-1) [108], T cell membrane protein 3 (TIM-3) [109] and lymphocyte activation gene 3 (LAG-3) [110], etc. Studies have shown that these inhibitory immune checkpoint molecules are also expressed on the surface of TEs [111], [112], [113]. This evidence provides a possible conviction for the immunosuppressive effect of exosomes on T cells. NKG2D is a cytotoxic receptor shared by NK cells and T cells. It has been detected that NKG2D ligand is expressed on the surface of exosomes produced by mesothelioma cells [114], and its specific binding with NKG2D to confuse NK cells and T cells and mediate tumor immune escape [115].
4.3.3. Induction of differentiation and proliferation of immunosuppressive cells
TEs may induce bone marrow-derived monocytes to differentiate into MDSCs and prevent differentiation into DCs and macrophages [116]. HSP70 highly expressed on the surface of exosomes participate in the activation of MDSC through TLR2, one of the toll-like receptor family members [117,118]. The phenotype of T lymphocytes classified as immunosuppressive regulatory T cells (Tregs) may be induced by TEs [119,120]. This upregulation of Tregs mediated by TEs is also one of the potential pathways for tumor immune escape [121]. In hepatocellular carcinoma immune escape studies, TEs also induced an increase in the number of B regulatory cells and significantly inhibited the activity of CD8+ T cells [122].
4.3.4. Inducing polarization of macrophages
TEs are involved in the remodeling of the tumor microenvironment including the induction of macrophage polarization [123]. As an important part of the tumor microenvironment, tumor-associated macrophages (TAMs) are divided into inflammatory macrophages (M1) and anti-inflammatory macrophages cells (M2), which M2 phenotype is conducive to the immunosuppressive tumor microenvironment [124], [125], [126]. TEs mediate ncRNA to regulate the M2 polarization of TAMs, such as miR-21, miR-222 [127], lncRNA TUC339 [128], etc., which helps reduce the release of pro-inflammatory factors to enhance tumor proliferation and metastasis [123].
In short, exosomes from different sources affect the differentiation and maturation of a variety of immune cells, potentially affecting immune activation and immune suppression in the process of tumor immunoediting. They have been shown to mediate the delivery and function realization of different cargoes, which is extremely attractive for the consideration of tumor immunotherapy strategies.
5. Exosome-based tumor immunotherapy strategies
5.1. Cancer vaccine
Cancer vaccines that awaken the human immune system against cancer through tumor cell-related antigens are widely used in tumor treatment research [129]. In the clearance phase of tumor immunoediting, exosomes played an excellent role in immune activation. Since exosomes can cause immune activation, whether TEs carrying tumor-associated antigens or exosomes derived from DCs presenting antigens, they can be used in the field of anti-cancer vaccines [6]. After capturing the antigen, DCs seem to pack the MHC antigen peptide complex with immunostimulatory factors into exosomes [130]. These exosomes transfer MHC antigen-peptide complexes to inactive DCs that in the lymph nodes and help these DCs acquire the ability to stimulate CTLs. Exosomes from DCs may also be loaded with chaperone-rich cell lysates produced by tumor cells [131]. Under this concept, DCs-derived exosomal vaccines have been tested in phase I clinical trials in patients with advanced non-small cell lung cancer and metastatic melanoma [132]. As congenital phagocytes, macrophages are educated in the tumor microenvironment to become anti-inflammatory phenotype promoting tumor proliferation and migration. TAMs-derived exosomes have the potential as vaccine adjuvants as well. A study using exosomes derived from pro-inflammatory macrophages to act on melanoma showed that these exosomes can be used as vaccine adjuvants to enhance the response of CTLs [133]. TEs can also be studied as one of the potential anti-tumor vaccines for cancer immunotherapy [134]. They can be engineered to directly cause specific immunity as an acellular vaccine [135], or can be a cell vaccine co-incubation with DCs [136]. Compared with exosomes derived from tumor cells, exosomes derived from DCs stimulate a stronger CD8+ response and anti-tumor immunity because TEs can also cause immunosuppression [136,137]. The study of exosomes as cancer vaccines has attracted more and more attention from researchers, and has broad prospects in tumor immunotherapy. However, for clinical trials, the feasibility of large-scale production of exosomes and the safety of in vivo administration of exosomes are still emphasized.
5.2. Drug delivery vehicles for tumor immunotherapy
As a natural biological carrier, exosomes have drug delivery advantages over other non-biological nanocarriers [138], [139], [140]. Exosomes have been fully demonstrated for cargo delivery and regulation of different cellular processes in the tumor immunoediting. Due to the widespread expression of transmembrane proteins [141], it can prevent exosomes from being phagocytosed by mononuclear macrophages in the circulation, and significantly reduce the blood clearance rate than other vectors [142]. What the important points are that exosomes show excellent ability to penetrate tissue barriers due to nanoscale size and cargo delivery advantage [143], [144], [145]. Based on these advantages, exosomes, as endogenous nanocarriers, have received great attention in the research of tumor immunotherapy. As mentioned above, exosomes without modification can trigger potent antigen-specific antitumor immune responses [146], [147], [148], or use exosomes secreted by mesenchymal stem cells to induce the anti-tumor response of NKT cells [149]. The engineering modification of exosomes as a drug delivery vehicle is a common research hotspot. The first common method is to carry out structural engineering of the exosomal membrane, mainly through targeted modification of surface ligands for the delivery of drugs [150], [151], [152], [153]. Exosomes can also be applied as the carriers of both chemotherapy drugs and siRNA, which were also beneficial to improve the drug delivery efficiency [154], [155], [156]. Table. 1 lists the immunotherapy studies of some exosomes in different types of tumors. We briefly classified and summarized the strategy of the articles on engineered exosomes listed in the table, the engineered exosomes secreted by genetically modification cells can more effectively induce anti-tumor immune responses, such as co-delivery of tumor antigens and adjuvants [152,[157], [158], [159], [160], add different types of surface-displayed monoclonal antibodies [158,161], overexpress cytokines [162,163], etc. Some genetically modified membrane vesicles can also play a similar immunogenic role by specifically expressing viral antigen as a versatile antigen-delivery system [164,165]. Engineered exosomes help to enhance DCs antigen presentation [159] and promote cellular immune response based on T cells [166,167]. For example, Lu et al. verified that exosomes derived from DCs expressing alpha-fetoprotein can activate antigen-specific cellular immunity and promote the release of immune cytokines in three murine models of hepatocellular carcinoma [146]. T cells expressing chimeric antigen receptors (CAR-T) are a very promising method of cancer immunotherapy. However, CAR-T cells are prone to cause a cytokine storm in the body and cannot accumulate in the tumor microenvironment. This may Lead to poor treatment effect. Report that CAR-T cells release exosomes, which carry chimeric antigen receptors (CAR) on their surface. CAR-exosomes do not express PD-1, and recombinant PD-L1 treatment will not reduce their anti-tumor effects compared with CAR-T treatment [168]. More types of cell-derived exosomes are collected and modified for tumor immunotherapy attempts, such as macrophages [133,[169], [170], [171], [172], NK cells [173,174]. Importantly, exosomes can be obtained from patient serum, which makes it more feasible to apply them to clinical treatment of tumor immuno-therapy [100,175].
Table 1.
Cancer immunotherapy of different types of cancer with modified exosomal carriers.
| Cancer type | Exosomes | Source cells | Modification/ Effective cargo | Affected cells | Refs. |
|---|---|---|---|---|---|
| Melanoma | CpG-SAV-Exo | B16BL6 | CpG DNA-modified exosomes expressing SAV-LA | DC cells | [152] |
| CIITA-Exo | B16F1 | MHC class II molecules and CD86 | DCs/ CD4+ T cells | [160] | |
| Breast cancer | SMART-Exo | Expi293 | anti-human CD3 and anti-human HER2 antibodies | SK-BR-3, HCC, MDA-MB-468, Human PBMCs | [161] |
| Dox @ Exo-PH20-FA | HEK 293T | human hyaluronidase, folic acid, doxorubicin | PC3, 4T1, HK-2, M2 macrophages | [151] | |
| CAR-Exo—CTX or CAR-Exo-TTZ | CAR-T | cetuximab scFv or trastuzumab scFv | MCF-7 EGFR or MCF-7 HER2 | [168] | |
| PTX-M1-Exo | M1 macrophages | Paclitaxel | 4T1 | [170] | |
| Colon carcinoma | mTEx | MC38 | murine IL-12 | Immature DCs | [158] |
| Exo/IL-18 | LS-174T | AdhIL-18 | DCs/CD8+ T cells | [153] | |
| Lung cancer | CD40l-Exo | 3LL Lewis lung cancer cell | CD40 ligand gene | BMDCs | [165] |
| Exosomes | A549 | Rab27a overexpression vector | DCs/ CD4+ T cells | [147] | |
| Lymphoma | Exo/IL-2 | E.G7-OVA | IL-2 | Th1/CTLs | [135] |
| Hepatocellular | |||||
| carcinoma | HSP-Exo | HepG2 | anticancer drug | NK cells | [148] |
| Exosomes | adipose-derived mesenchymal stem cells | – | NKT-cells | [149] | |
| Pancreatic ductal adenocarcinoma | iExo—OXA | bone marrow mesenchymal stem cells | Galectin-9 siRNA | M2-TAMs, CTLs | [154] |
| Multiple myeloma | Exo-TNF-α | J558 | TNF-α | CD8+ T cells | [162] |
| Renal cell carcinoma | Exo / IL-12 | RC2 | G250, Glycolipid anchored IL-12 (GPI-IL-12) | CTLs | [155] |
| RDE | RenCa cells | GM-CSF,IL-12 | CD8+ T cells | [163] | |
| Leukemia | CTX-poly I:C-Exo | L1210 | CTX, poly I:C | spleen cells | [156] |
| glioblastoma | DEXCRCL-GL261 | DCs | Cell Lysate | CTLs | [131] |
6. Future directions
Exosomes are heterogeneous extracellular vesicles with a variety of cargo, including lipids, proteins, nucleic acids and carbohydrates. The secretion of exosomes is related to the transport of endosomal and plasma membranes, and the sorting of goods depends on ESCRT or other pathways, such as lipid rafts. The secreted external vesicles are internalized by the recipient cells through endocytosis and receptor binding to mediate information transmission. Exosomes have abundant cell communication functions, including proximal and distal communication. It plays an important role in the biogenesis and development of tumors. A large number of exosomes secreted by tumor cells seem to have a dual role, which can not only activate immunity, but also inhibit the function of immune cells, promote immune suppression, and form a tumor-promoting immune microenvironment. Based on the concept of tumor immunoediting, we have sorted out the role of exosomes in it, including immune activation that promotes antigen presentation and enhanced response, immune escape that reduces immune clearance by regulating cytokines and immune checkpoint proteins, or stimulates the proliferation of immunosuppressive cells, or promote tumor-related macrophage polarization. Taking advantage of the flexible and rich information transmission functions of exosomes, many researchers have proposed methods for cancer immunotherapy based on exosomes, such as cancer vaccines and various drug delivery vehicles. Exosomes derived from DCs, macrophages, tumor cells and even T cells have been studied for cancer immunotherapy, and there are many promising works. For example, an exciting study proved that exosomes derived from CAR-T cells expressed CAR but not PD-1 and they showed stronger anti-tumor ability and are safer as a cellular vesicles [168]. Despite great achievements in exosome research, challenges remain. The separation and purification technology of exosomes is still a major challenge. The current methods for obtaining exosomes include: ultracentrifugation [176], size exclusion [177], precipitation [178], immunoaffinity capture [177]. However, these methods have different disadvantages, such as high time cost, low yield, and impurity contamination [179]. The recent application of microfluidic technology to the separation of exosomes may be a more promising method because it is more efficient [180]. Related content was recently published in the review by Ding et al. [14]. Another challenge is the complexity of the cargo carried by engineering exosomes. The methods for loading drugs on exosomes include co-incubation [181], electroporation [182], ultrasound [183], liposome-based loading [184] and loading of gene products based on biogenesis [185]. The storage conditions of exosomes are also a point that needs to be considered in research and application. The routine storage is at -80 °C, while lyophilization may be a consideration that does not rely on ultra-low temperature [186]. Overcoming these challenges and standardizing them is the basis for improving the possibility of achieving precise treatment of exosomes in tumor immunity and other diseases. Of course, the exciting thing is that there are more and more researches to try to solve these problems and exosomes still have broad research value and application prospects as biologically derived nanovesicles.
Conflicts of interest
There are no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors acknowledge the financial support received from National Natural Science Foundation of China (No. 82073784), Jilin Province Science and Technology Development Program (No. 20200801012GH) and Industrial Technology Research and Development Projects from the Development and Reform Commission of Jilin Province (2019C050-4).
Contributor Information
Tian Bai, Email: baitian@jlu.edu.cn.
Lesheng Teng, Email: tenglesheng@jlu.edu.cn.
References
- 1.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Li C., Xu X. Biological functions and clinical applications of exosomal non-coding RNAs in hepatocellular carcinoma. Cell Mol Life Sci. 2019;76(21):4203–4219. doi: 10.1007/s00018-019-03215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Bu H., He D., He X., Wang K. Exosomes: isolation, analysis, and applications in cancer detection and therapy. Chembiochem. 2019;20(4):451–461. doi: 10.1002/cbic.201800470. [DOI] [PubMed] [Google Scholar]
- 5.Beuzelin D., Kaeffer B. Exosomes and mirna-loaded biomimetic nanovehicles, a focus on their potentials preventing type-2 diabetes linked to metabolic syndrome. Front Immunol. 2018;9(1):2711. doi: 10.3389/fimmu.2018.02711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie F., Zhou X., Fang M., Li H., Su P., Tu Y., et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24) doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.8 Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K., Smyth M.J., Martinet L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood. 2020;136(24):2731–2740. doi: 10.1182/blood.2020006540. [DOI] [PubMed] [Google Scholar]
- 11.Anichini A., Perotti V.E., Sgambelluri F., Mortarini R. Immune escape mechanisms in non-small cell lung cancer. Cancers. 2020;12(12):3605–3625. doi: 10.3390/cancers12123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.12 O'donnell J.S., MWL Teng, Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 13.Pegtel D.M., Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 14.Ding L., Yang X., Gao Z., Effah C.Y., Zhang X., Wu Y., et al. A holistic review of the state-of-the-art microfluidics for exosome separation: an overview of the current status, existing obstacles, and future outlook. Small. 2021;17(29) doi: 10.1002/smll.202007174. [DOI] [PubMed] [Google Scholar]
- 15.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathivanan S., Lim J.W., Tauro B.J., Ji H., Moritz R.L., Simpson R.J. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9(2):197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Mittelbrunn M., Gutierrez-Vazquez C., Villarroya-Beltri C., Gonzalez S., Sanchez-Cabo F., Gonzalez M.A., et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webber J., Steadman R., Mason M.D., Tabi Z., Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 20.Shen B., Wu N., Yang J.M., Gould S.J. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tetta C., Ghigo E., Silengo L., MC Deregibus, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44(1):11–19. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo Y., HS Kim, IS Hong. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019 doi: 10.1155/2019/5126156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin X., Zeng W., Wu B., Wang L., Wang Z., Tian H., et al. PPARalpha inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020;33(3) doi: 10.1016/j.celrep.2020.108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spellicy S.E., Stice S.L. Tissue and stem cell sourced extracellular vesicle communications with microglia. Stem Cell Rev Rep. 2021;17(2):357–368. doi: 10.1007/s12015-020-10011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju Y., Bai H., Ren L., Zhang L. The role of exosome and the escrt pathway on enveloped virus infection. Int J Mol Sci. 2021;22(16):9060. doi: 10.3390/ijms22169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Liu K., Li Q., Yao Y., Wang Y. Exosomes function in tumor immune microenvironment. Adv Exp Med Biol. 2018;1056:109–122. doi: 10.1007/978-3-319-74470-4_7. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y., Liu Y., Yang C., Kang L., Wang M., Hu J., et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4(+) T-cell responses. Immunology. 2016;149(2):157–171. doi: 10.1111/imm.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei D., Zhan W., Gao Y., Huang L., Gong R., Wang W., et al. Rab31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31(2):157–177. doi: 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SW Ferguson, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Thery C., Boussac M., Veron P., Ricciardi-Castagnoli P., Raposo G., Garin J., et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Mark M.T., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Corbett A.L., Taatizadeh E., Tasnim N., Little J.P., Garnis C., et al. Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. Apl Bioeng. 2019;3(1) doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 34.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donoso-Quezada J., Ayala-Mar S., Gonzalez-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics, and applications. Traffic. 2021;22(7):204–220. doi: 10.1111/tra.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 37.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–672. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 39.Lan F.M., Zhang X.D., Li H.B., Yue X., Sun Q.H. Serum exosomal lncRNA xist is a potential non-invasive biomarker to diagnose recurrence of triple-negative breast cancer. J Cell Mol Med. 2021;25(16):7602–7607. doi: 10.1111/jcmm.16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B., Teng F., Chang L., Wang J., Liu D.L., Cui Y.S., et al. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging-Us. 2021;13(9):13264–13286. doi: 10.18632/aging.203011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuo B., Qi H., Lu Z., Chen L., Sun B., Yang R., et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat Commun. 2020;11(1):1790. doi: 10.1038/s41467-020-15569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv Y.N., Chen J., Hu J.F., Qian Y.S., Kong Y., Fu L.S. Nonmuscle myosin heavy chain IIA-mediated exosome release via regulation of the rho-associated kinase 1/myosin light chains/actin pathway. Front Pharmaco. 2020;11 doi: 10.3389/fphar.2020.598592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dou D., Ren X., Han M., Xu X., Ge X., Gu Y., et al. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front Immunol. 2020;11(1):2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Haraszti R.A., Didiot M.C., Sapp E., Leszyk J., Shaffer S.A., Rockwell H.E., et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathieu M., Nevo N., Jouve M., Valenzuela J.I., Maurin M., Verweij F.J., et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N., Wang Y.Y., Xu H.Y., Wang H.X., Gao Y.Y., Zhang Y. Exosomes derived from RM-1 cells promote the recruitment of MDSCs into tumor microenvironment by upregulating CXCR4 via TLR2/NF-kappa B pathway. J Oncol. 2021;2021 doi: 10.1155/2021/5584406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X., Corbett A.L., Taatizadeh E., Tasnim N., Little J.P., Garnis C., et al. Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. Apl Bioeng. 2019;3(1) doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kharaziha P., Ceder S., Li Q., Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012;1826(1):103–111. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Milane L., Singh A., Mattheolabakis G., Suresh M., Amiji M.M. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 50.Kim H., Kim E.H., Kwak G., Chi S.G., SH Kim, Yang Y. Exosomes: cell-derived nanoplatforms for the delivery of cancer therapeutics. Int J Mol Sci. 2020;22(1):14. doi: 10.3390/ijms22010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabinowits G., Gercel-Taylor C., Day J.M., Taylor D.D., Kloecker G.H. Exosomal microrna: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 53.Fan Z., Yu J., Lin J., Liu Y., Liao Y. Exosome-specific tumor diagnosis via biomedical analysis of exosome-containing microRNA biomarkers. Analyst. 2019;144(19):5856–5865. doi: 10.1039/c9an00777f. [DOI] [PubMed] [Google Scholar]
- 54.Wang H., He D., Wan K., Sheng X., Cheng H., Huang J., et al. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst. 2020;145(9):3289–3296. doi: 10.1039/d0an00393j. [DOI] [PubMed] [Google Scholar]
- 55.Roman-Canal B., Moiola C.P., Gatius S., Bonnin S., Ruiz-Miro M., Gonzalez E., et al. EV-associated miRNAs from pleural lavage as potential diagnostic biomarkers in lung cancer. Sci Rep-Uk. 2019;9:15057. doi: 10.1038/s41598-019-51578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 57.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto A., Asuka M., Takahashi Y., Takakura Y. Antitumor immunity by small extracellular vesicles collected from activated dendritic cells through effective induction of cellular and humoral immune responses. Biomaterials. 2020;252 doi: 10.1016/j.biomaterials.2020.120112. [DOI] [PubMed] [Google Scholar]
- 59.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 60.Li L., Jay S.M., Wang Y., Wu S.W., Xiao Z. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci Rep. 2017;7(1):13365. doi: 10.1038/s41598-017-14000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desai R., Coxon A.T., Dunn G.P. Therapeutic applications of the cancer immunoediting hypothesis. Semin Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.03.002. org/10.1016/j.semcancer 2021.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Koebel C.M., Vermi W., Swann J.B., Zerafa N., Rodig S.J., Old L.J., et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 63.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benito-Martin A., Di Giannatale A., Ceder S., Peinado H. The new deal a potentia role for secretec vesicles in innate immunity and tumor progression. Front Immunol. 2015;6(1):1–13. doi: 10.3389/fimmu.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinha D., Roy S., Saha P., Chatterjee N., Bishayee A. Trends in research on exosomes in cancer progression and anticancer therapy. Cancers (Basel) 2021;13(2):326. doi: 10.3390/cancers13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie F., Zhou X.X., Fang M.Y., Li H.Y., Tu Y.F., Su P., et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24) doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Droste M., Thakur B.K., Eliceiri B.P. Tumor-derived extracellular vesicles and the immune system-lessons from immune-competent mouse-tumor models. Front Immunol. 2020;11(1) doi: 10.3389/fimmu.2020.606859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jafari R., Rahbarghazi R., Ahmadi M., Hassanpour M., Rezaie J. Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J Transl Med. 2020;18(1):474. doi: 10.1186/s12967-020-02662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai T., Gong L.G., Wang Y., Wang Y., CA Kulikowski, Huang L. A method for exploring implicit concept relatedness in biomedical knowledge network. BMC Bioinformatics. 2016;17:265. doi: 10.1186/s12859-016-1131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai T., Ge Y., Yang C.Q., Liu X.H., Gong L.G., Wang Y., et al. Berst: an engine and tool for exploring biomedical entities and relationships. Chinese J Electron. 2019;28(4):797–804. [Google Scholar]
- 71.Zhu C., Lin S.H., Jiang X.Q., Xiang Y., Belal Z., Jun G., et al. A novel deep learning model using dosimetric and clinical information for grade 4 radiotherapy-induced lymphopenia prediction. Phys Med Biol. 2020;65(3) doi: 10.1088/1361-6560/ab63b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andre F., Chaput N., Schartz N.E.C., Flament C., Aubert N., Bernard J., et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 74.Leone D.A., Peschel A., Brown M., Schachner H., Ball M.J., Gyuraszova M., et al. Surface LAMP-2 is an endocytic receptor that diverts antigen internalized by human dendritic cells into highly immunogenic exosomes. J Immunol. 2017;199(2):531–546. doi: 10.4049/jimmunol.1601263. [DOI] [PubMed] [Google Scholar]
- 75.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4) doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindenbergh S., Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. 2018;36:435–459. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 77.Logozzi M., De Milito A., Lugini L., Borghi M., Calabro L., Spada M., et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16(4):782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dubovsky J.A., Chappell D.L., Harrington B.K., Agrawal K., Andritsos L.A., Flynn J.M., et al. Lymphocyte cytosolic protein 1 is a chronic lymphocytic leukemia membrane-associated antigen critical to niche homing. Blood. 2013;122(19):3308–3316. doi: 10.1182/blood-2013-05-504597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu D.H., Paz P., Villaflor G., Rivas A., Mehta-Damani A., Angevin E., et al. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26(5):440–450. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Leone D.A., Rees A.J., Kain R. Dendritic cells and routing cargo into exosomes. Immunol Cell Biol. 2018;96(7):683–693. doi: 10.1111/imcb.12170. [DOI] [PubMed] [Google Scholar]
- 82.Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.L., Stolz D.B., Papworth G.D., et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 83.Reiners K.S., Dassler J., Coch C., Von Strandmann E.P. Role of exosomes released by dendritic cells and/or by tumor targets: regulation of NK cell plasticity. Front Immunol. 2014;5(1):91. doi: 10.3389/fimmu.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zech D., Rana S., Buchler M.W., Zoller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10(1):37–54. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duray A., Demoulin S., Hubert P., Delvenne P., Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010 doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Segura E., Guerin C., Hogg N., Amigorena S., Thery C. CD8(+) dendritic cells use IFA-1 to capture mhc-peptide complexes from exosomes in vivo. J Immunol. 2007;179(3):1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 87.Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Indirect activation of naïve CD4(+) T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 88.Segura E., Nicco C., Lombard B., Veron P., Raposo G., Batteux F., et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 89.Munich S., Sobo-Vujanovic A., Buchser W.J., Beer-Stolz D., Vujanovic N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tavakoli F., Sartakhti J.S., Manshaei M.H., Basanta D. Cancer immunoediting: a game theoretical approach. In Silico Biol. 2021;14(1–2):1–12. doi: 10.3233/ISB-200475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinata M., Kunita A., Abe H., Morishita Y., Sakuma K., Yamashita H., et al. Exosomes of Epstein-Barr virus-associated gastric carcinoma suppress dendritic cell maturation. Microorganisms. 2020;8(11):1776. doi: 10.3390/microorganisms8111776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ning Y., Shen K., Wu Q., Sun X., Bai Y., Xie Y., et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36–43. doi: 10.1016/j.imlet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Yu S., Sha H., Qin X., Chen Y., Li X., Shi M., et al. EGFR E746-A750 deletion in lung cancer represses antitumor immunity through the exosome-mediated inhibition of dendritic cells. Oncogene. 2020;39(13):2643–2657. doi: 10.1038/s41388-020-1182-y. [DOI] [PubMed] [Google Scholar]
- 94.Ding G., Zhou L., Qian Y., Fu M., Chen J., Chen J., et al. Pancreatic cancer-derived exosomes transfer mirnas to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6(30):29877–29888. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vulpis E., Soriani A., Cerboni C., Santoni A., Zingoni A. Cancer exosomes as conveyors of stress-induced molecules: new players in the modulation of NK cell response. Int J Mol Sci. 2019;20(3):611. doi: 10.3390/ijms20030611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Briand J., Garnier D., Nadaradjane A., Clement-Colmou K., Potiron V., Supiot S., et al. Radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits natural killer cells cytotoxicity. Epigenomics. 2020;12(5):397–408. doi: 10.2217/epi-2019-0193. [DOI] [PubMed] [Google Scholar]
- 97.Liu C.R., Yu S.H., Zinn K., Wang J.H., Zhang L.M., Jia Y.J., et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 98.Schroeder J.C., Puntigam L., Hofmann L., Jeske S.S., Beccard I.J., Doescher J., et al. Circulating exosomes inhibit b cell proliferation and activity. Cancers. 2020;12(8):2110. doi: 10.3390/cancers12082110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iorgulescu J.B., Ivan M.E., Safaee M., Parsa A.T. The limited capacity of malignant glioma-derived exosomes to suppress peripheral immune effectors. J Neuroimmunol. 2016;290:103–108. doi: 10.1016/j.jneuroim.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 100.Sharma P., Diergaarde B., Ferrone S., Kirkwood J.M., Whiteside T.L. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci Rep. 2020;10(1):92. doi: 10.1038/s41598-019-56542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clayton A., Mitchell J.P., Court J., MD Mason, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 102.Othman N., Jamal R., Abu N. Cancer-derived exosomes as effectors of key inflammation-related players. Front Immunol. 2019;10(1):2103. doi: 10.3389/fimmu.2019.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou M., Chen J., Zhou L., Chen W., Ding G., Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292(1–2):65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Berchem G., Noman M.Z., Bosseler M., Paggetti J., Baconnais S., Le Cam E. Hypoxic tumor-derived microvesicles negatively regulate nk cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5(4) doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye S.B., Li Z.L., Luo D.H., Huang B.J., Chen Y.S., Zhang X.S., et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen L.J., Zhou T.J., Fan Y.T., Chang X., Wang Y., Sun J.G., et al. Recent progress in tumor photodynamic immunotherapy. Chinese Chem Lett. 2020;31(7):1709–1716. [Google Scholar]
- 107.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating t cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao J.W., Qiu X.Y., Li X.Y., Fan H., Zhang F., Lv T.F., et al. Expression profiles and clinical value of plasma exosomal TIM-3 and Galectin-9 in non-small cell lung cancer. Biochem Bioph Res Co. 2018;498(3):409–415. doi: 10.1016/j.bbrc.2018.02.114. [DOI] [PubMed] [Google Scholar]
- 110.Long L., Zhang X., Chen F., Pan Q., Phiphatwatchara P., Zeng Y., et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9(5–6):176–189. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie F., Xu M., Lu J., Mao L., Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146. doi: 10.1186/s12943-019-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Whiteside T.L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41(1):245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clayton A., Mitchell J.P., Court J., Linnane S., MD Mason, Tabi Z. Human tumor-derived exosomes down-modulate NKG2d expression. J Immunol. 2008;180(11):7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 115.Lundholm M., Schroder M., Nagaeva O., Baranov V., Widmark A., Mincheva-Nilsson L., et al. Prostate tumor-derived exosomes down-regulate NKG2d expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J.P., et al. Membrane-associated HSP72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiang X., Poliakov A., Liu C., Liu Y., Deng Z.B., Wang J., et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gobbo J., Marcion G., Cordonnier M., Dias A.M.M., Pernet N., Hammann A., et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst. 2016;108(3):djv330. doi: 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]
- 119.Muller L., Mitsuhashi M., Simms P., Gooding W.E., Whiteside T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller L., Simms P., Hong C.S., Nishimura M.I., Jackson E.K., Watkins S.C., et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6(8):e1261243–e1261249. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szajnik M., Czystowska M., Szczepanski M.J., Mandapathil M., Whiteside T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS ONE. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ye L., Zhang Q., Cheng Y., Chen X., Wang G., Shi M., et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. J Immunother Cancer. 2018;6(1):145. doi: 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baig M.S., Roy A., Rajpoot S., Liu D., Savai R., Banerjee S., et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69(5):435–451. doi: 10.1007/s00011-020-01318-0. [DOI] [PubMed] [Google Scholar]
- 124.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar A.T., Knops A., Swendseid B., Martinez-Outschoom U., Harshyne L., Philp N., et al. Prognostic significance of tumor-associated macrophage content in head and neck squamous cell carcinoma: a meta-analysis. Front Oncol. 2019;9:656. doi: 10.3389/fonc.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu Z.X., Xu L.F., He N.Y., Huang F.Y., Xu T.F., Li L., et al. Cy5.5-MSA-G250 nanoparticles (CMGNPs) induce M1 polarity of RAW264. 7 macrophage cells via TLR4-dependent manner. Chinese Chem Lett. 2019;30(6):1320–1324. [Google Scholar]
- 127.Kanlikilicer P., Bayraktar R., Denizli M., Rashed M.H., Ivan C., Aslan B., et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018;38:100–112. doi: 10.1016/j.ebiom.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng L., Liu J.T., Liu Q.Q., Liu Y., Fan L.L., Wang F., et al. Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through STAT3 pathway in macrophages. Int J Biol Sci. 2017;13(6):723–734. doi: 10.7150/ijbs.19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeng L.M., Liao Z.L., Li W.W., Yuan Q.J., Wu P., Gu Z.P., et al. Non-covalent glycosylated gold nanoparticles/peptides nanovaccine as potential cancer vaccines. Chinese Chem Lett. 2020;31(5):1162–1164. [Google Scholar]
- 130.Taieb J., Chaput N., Zitvogel L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol. 2005;25(3):215–223. doi: 10.1615/critrevimmunol.v25.i3.30. [DOI] [PubMed] [Google Scholar]
- 131.Bu N., Wu H., Zhang G., Zhan S., Zhang R., Sun H., et al. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent T cell immune response against intracranial glioma in mice. J Mol Neurosci. 2015;56(3):631–643. doi: 10.1007/s12031-015-0506-9. [DOI] [PubMed] [Google Scholar]
- 132.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng L., Wang Y., Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25(7):1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Y., Xiu F., Cai Z., Wang J., Wang Q., Fu Y., et al. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol. 2007;133(6):389–399. doi: 10.1007/s00432-006-0184-7. [DOI] [PubMed] [Google Scholar]
- 136.Gu X., Erb U., Buchler M.W., Zoller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136(4):74–84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- 137.Hao S., Bai O., Yuan J., Qureshi M., Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8(+) CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3(3):205–211. [PubMed] [Google Scholar]
- 138.Chinnappan M., Srivastava A., Amreddy N., Razaq M., Pareek V., Ahmed R., et al. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 2020;486:18–28. doi: 10.1016/j.canlet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y., Zhang Y.R., Cai G., Li Q. Exosomes as actively targeted nanocarriers for cancer therapy. Int J Nanomed. 2020;15:4257–4273. doi: 10.2147/IJN.S239548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu C.P., Chen Z.D., Ye Z.Y., He D.Y., Dang Y., Li Z.W., et al. Therapeutic applications of functional nanomaterials for prostatitis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.685465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koh E., Lee E.J., Nam G.H., Hong Y., Cho E., Yang Y., et al. Exosome-sirpalpha, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 142.Syn N.L., Wang L., Chow E.K., Lim C.T., Goh B.C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Li X., Tsibouklis J., Weng T., Zhang B., Yin G., Feng G., et al. Nano carriers for drug transport across the blood-brain barrier. J Drug Target. 2017;25(1):17–28. doi: 10.1080/1061186X.2016.1184272. [DOI] [PubMed] [Google Scholar]
- 144.EL Andaloussi S., Lakhal S., Mager I., Wood M.J.A. Exosomes for targeted sirna delivery across biological barriers. Adv Drug Deliver Rev. 2013;65(3):391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 145.Jia G., Han Y., An Y., Ding Y., He C., Wang X., et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 146.Lu Z., Zuo B., Jing R., Gao X., Rao Q., Liu Z., et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 147.Li W., Mu D., Tian F., Hu Y., Jiang T., Han Y., et al. Exosomes derived from Rab27a-overexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep. 2013;8(6):1876–1882. doi: 10.3892/mmr.2013.1738. [DOI] [PubMed] [Google Scholar]
- 148.Lv L.H., Wan Y.L., Lin Y., Zhang W., Yang M., Li G.L., et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ko S.F., Yip H.K., Zhen Y.Y., Lee C.C., Lee C.C., Huang C.C., et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. 2015;2015 doi: 10.1155/2015/853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu J., Ye Z., Xiang M., Chang B., Cui J., Ji T., et al. Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials. 2019;223 doi: 10.1016/j.biomaterials.2019.119475. [DOI] [PubMed] [Google Scholar]
- 151.Feng C., Xiong Z., Wang C., Xiao W., Xiao H., Xie K., et al. Folic acid-modified exosome-PH20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact Mater. 2021;6(4):963–974. doi: 10.1016/j.bioactmat.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 153.Dai S., Zhou X., Wang B., Wang Q., Fu Y., Chen T., et al. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med. 2006;84(12):1067–1076. doi: 10.1007/s00109-006-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou W., Zhou Y., Chen X., Ning T., Chen H., Guo Q., et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y., Luo C.L., He B.C., Zhang J.M., Cheng G., Wu X.H. Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int J Oncol. 2010;36(1):133–140. [PubMed] [Google Scholar]
- 156.Guo F., Chang C.K., Fan H.H., Nie X.X., Ren Y.N., Liu Y.Y., et al. Anti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic-polycytidylic acid. J Int Med Res. 2008;36(6):1342–1353. doi: 10.1177/147323000803600623. [DOI] [PubMed] [Google Scholar]
- 157.Zhang P.F., Zhang L., Qin Z.E., Hua S.H., Guo Z.D., Chu C.C., et al. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv Mater. 2018;30(7) doi: 10.1002/adma.201705350. [DOI] [PubMed] [Google Scholar]
- 158.Rossowska J., Anger N., Wegierek K., Szczygiel A., Mierzejewska J., Milczarek M., et al. Antitumor potential of extracellular vesicles released by genetically modified murine colon carcinoma cells with overexpression of interleukin-12 and shRNA for TGF-beta 1. Front Immunol. 2019;10(1):211. doi: 10.3389/fimmu.2019.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morishita M., Takahashi Y., Nishikawa M., Ariizumi R., Takakura Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol Pharm. 2017;14(11):4079–4086. doi: 10.1021/acs.molpharmaceut.7b00760. [DOI] [PubMed] [Google Scholar]
- 160.Lee Y.S., Kim S.H., Cho J.A., Kim C.W. Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med. 2011;43(5):281–290. doi: 10.3858/emm.2011.43.5.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shi X., Cheng Q., Hou T., Han M., Smbatyan G., Lang J.E., et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28(2):536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xie Y., Bai O., Zhang H., Li W., Xiang J. Tumor necrosis factor gene-engineered J558 tumor cell-released exosomes stimulate tumor antigen P1A-specific CD8 + CTL responses and antitumor immunity. Cancer Biotherapy Radio. 2010;25(1):21–28. doi: 10.1089/cbr.2009.0714. [DOI] [PubMed] [Google Scholar]
- 163.163 Xu H.Y., Li N., Yao N., Xu X.F., Wang H.X., Liu X.Y., et al. CD8(+) T cells stimulated by exosomes derived from renca cells mediate specific immune responses through the Fasl/Fas signaling pathway and, combined with GM-CSF and IL-12, enhance the anti-renal cortical adenocarcinoma effect. Oncol Rep. 2019;42(2):866–879. doi: 10.3892/or.2019.7208. [DOI] [PubMed] [Google Scholar]
- 164.Zhang P.F., Chen Y.X., Zeng Y., Shen C.G., Li R., Guo Z.D., et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. P Natl Acad Sci USA. 2015;112(45):6129–6138. doi: 10.1073/pnas.1505799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J., Wang L., Lin Z., Tao L., Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40l gene-modified lung tumor cells. Mol Med Rep. 2014;9(1):125–131. doi: 10.3892/mmr.2013.1759. [DOI] [PubMed] [Google Scholar]
- 166.Phung C.D., Pham T.T., Nguyen H.T., Nguyen T.T., Ou W., Jeong J.H., et al. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–382. doi: 10.1016/j.actbio.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 167.Liu X., Yuan L., Zhang L., Mu Y., Li X., Liu C., et al. Bioinspired artificial nanodecoys for hepatitis B virus. Angew Chem Int Ed Engl. 2018;57(38):12499–12503. doi: 10.1002/anie.201807212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fu W., Lei C., Liu S., Cui Y., Wang C., Qian K., et al. Car exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10:4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Behzadi E., Hosseini H.M., Halabian R., Fooladi A.A.I. Macrophage cell-derived exosomes/staphylococcal enterotoxin B against fibrosarcoma tumor. Microb Pathogenesis. 2017;111:132–138. doi: 10.1016/j.micpath.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 170.Wang P., Wang H., Huang Q., Peng C., Yao L., Chen H., et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choo Y.W., Kang M., Kim H.Y., Han J., Kang S., Lee J.R., et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12(9):8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 172.Fan Z., Xiao K., Lin J., Liao Y., Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15(47) doi: 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- 173.Di Pace A.L., Tumino N., Besi F., Alicata C., Conti L.A., Munari E., et al. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel) 2020;12(3):661. doi: 10.3390/cancers12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kang Y.T., Niu Z.Q., Hadlock T., Purcell E., Lo T.W., Zeinali M., et al. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv Sci. 2021;8(6) doi: 10.1002/advs.202003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peng X., Yu R., Wu X. Correlation of plasma exosomal micrornas with the efficacy of immunotherapy in EGFR /ALK wild-type advanced non-small cell lung cancer. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thery C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006:22. doi: 10.1002/0471143030.cb0322s30. chapter 3: unit 3. [DOI] [PubMed] [Google Scholar]
- 177.Greening D.W., Xu R., Ji H., BJ Tauro, Simpson R.J. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 178.Lobb R.J., Becker M., Wen S.W., Wong C.S., Wiegmans A.P., Leimgruber A., et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Das C.K., Jena B.C., Banerjee I., Das S., Parekh A., Bhutia S.K., et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharmaceut. 2019;16(1):24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 180.Wang J., Ma P., Kim D.H., Liu B.F., Demirci U. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today. 2021;37 doi: 10.1016/j.nantod.2020.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun D.M., Zhuang X.Y., Grizzle W., Miller D., Zhang H.G. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Cancer Res. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wahlgren J., Karlson T.D., Brisslert M., Sani F.V., Telemo E., Sunnerhagen P., et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed-Nanotechnol. 2016;12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sun L.N., Fan M.R., Huang D., Li B.Q., Xu R.T., Gao F., et al. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120761. [DOI] [PubMed] [Google Scholar]
- 185.Wang L., Zhou X., Zou W., Wu Y., Zhao J., Chen X., et al. Exosomes containing mirnas targeting HER2 synthesis and engineered to adhere to HER2 on tumor cells surface exhibit enhanced antitumor activity. J Nanobiotechnol. 2020;18(1):153. doi: 10.1186/s12951-020-00711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamashita T., Takahashi Y., Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–842. doi: 10.1248/bpb.b18-00133. [DOI] [PubMed] [Google Scholar]