Abstract
Introduction
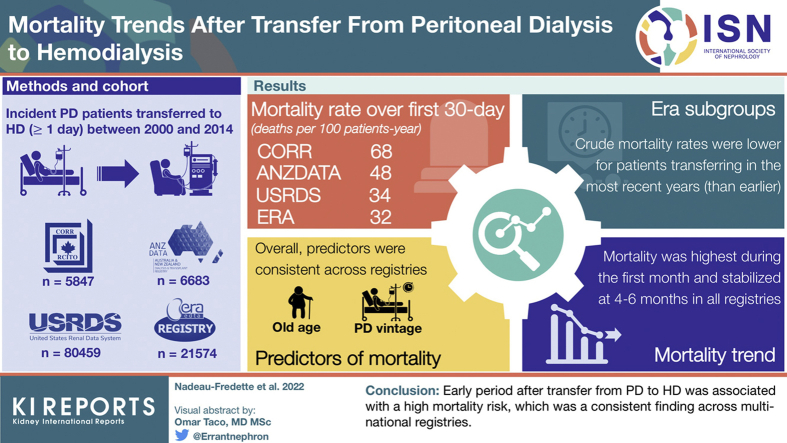
Transition to hemodialysis (HD) is a common outcome in peritoneal dialysis (PD), but the associated mortality risk is poorly understood. This study sought to identify rates of and risk factors for mortality after transitioning from PD to HD.
Methods
Patients with incident PD (between 2000 and 2014) who transferred to HD for ≥1 day were identified, using data from Australia and New Zealand Dialysis and Transplantation registry (ANZDATA), Canadian Organ Replacement Register (CORR), Europe Renal Association (ERA) Registry, and the United States Renal Dialysis System (USRDS). Crude mortality rates were calculated for the first 180 days after transfer. Separate multivariable Cox models were built for early (<90 days), medium (90–180 days), and late (>180 days) periods after transfer.
Results
Overall, 6683, 5847, 21,574, and 80,459 patients were included from ANZDATA, CORR, ERA Registry, and USRDS, respectively. In all registries, crude mortality rate was highest during the first 30 days after a transfer to HD declining thereafter to nadir at 4 to 6 months. Crude mortality rates were lower for patients transferring in the most recent years (than earlier). Older age, PD initiation in earlier cohorts, and longer PD vintage were associated with increased risk of death, with the strongest associations during the first 90 days after transfer and attenuating thereafter. Mortality risk was lower for men than women <90 days after transfer, but higher after 180 days.
Conclusion
In this multinational study, mortality was highest in the first month after a transfer from PD to HD and risk factors varied by time period after transfer. This study highlights the vulnerability of patients at the time of modality transfer and the need to improve transitions.
Keywords: hemodialysis, peritoneal dialysis, survival, technique failure, transition
Graphical abstract
See Commentary on Page 942
PD is widely promoted to treat people with kidney failure in several countries around the world.1 PD has several advantages over facility HD, including preservation of residual kidney function, preservation of potential vascular access, reduction in bacteremia, increased autonomy, and lower global socioeconomic burden.2, 3, 4, 5, 6, 7, 8, 9, 10 However, PD technique survival is often short with a majority of patients discontinuing PD within 2 to 3 years owing to death, transplantation, or transfer to HD.11, 12, 13, 14, 15, 16, 17 Several patients may also be transferred to HD as part of a planned schedule. When using a 30-day technique-failure definition, up to 25% of patients transferred to HD ultimately resumed PD in an Australian study.18
Though transitioning from PD to HD is very common, little is known about the outcomes in the immediate period after transfer from PD to HD,19 specifically regarding factors associated with enhanced mortality. Typically, adverse events occurring during the early period after transfer (up to 30–90 days) are attributed to the initial dialysis modality in most publications.18 This study aimed to assess rates, patterns, and risk factors of mortality after a transfer from PD to HD.
Methods
Study Design and Population
All adult patients who started on PD within 180 days of kidney replacement therapy (KRT) initiation between January 2000 and December 2014 (2013 in Canada) were identified from the ANZDATA, CORR, ERA Registry, and the USRDS databases. The ERA Registry included data from 17 registries from the following 11 countries: Austria, Andalusia (Spain), Asturias (Spain), Basque country (Spain), Catalonia (Spain), Dutch-speaking Belgium, French-speaking Belgium, Denmark, Finland, Greece, Iceland, Norway, Sweden, the Netherlands, United Kingdom and Valencian Region (Spain). For Spain, the coverage of the general population by the regional registries was 53%. PD patients who were recorded as transferring to HD for 1 day or more during the observation period were included in the study. Patients were only included once (first transfer only). Considering the extremely low incidence of direct transfer to home HD,20, 21, 22 home and facility HD were not differentiated. Patients undergoing kidney transplantation before treatment with PD were excluded.
Outcome and Covariates
The primary outcome was mortality after transfer from PD to HD (including dialysis withdrawal), as identified in all registries. Patients were followed from the first day of HD (after transfer from PD) until death, irrespective of any subsequent modality transfer. Data were censored at the time of kidney transplantation, loss to follow-up, or the end of the study (December 31, 2014, in CORR and December 31, 2015, in all other registries), whichever came first. Cause-specific deaths were categorized using the same definitions throughout the registries (Supplementary Annex 1).
Covariates were based on availability in all 4 registries and included age, sex, year of KRT start, cause of kidney disease, and duration of PD before transfer to HD. Age, sex, and cause of primary kidney disease were determined at time of KRT initiation. Comorbidities were not included owing to lack of availability throughout the registries (mostly ERA). The era of KRT initiation was based on the calendar year and categorized as 2000 to 2004, 2005 to 2009, and 2010 to 2014. PD vintage was defined as “the time from PD initiation until the first transfer to HD.” Primary kidney disease was categorized as glomerulonephritis, diabetes, hypertensive disease, and “other/unknown.” Temporary HD was defined as return to PD within 180 days of HD transfer.
Statistical Analysis
Crude mortality rates by 5-day periods until 30 days and by 30-day periods until 180 days after transfer were assessed using Poisson regression. Prespecified subgroup analysis was performed for cohort years of KRT initiation, patient age, and PD vintage at time of transfer to HD. For each 30-day period, patients were considered at risk if they were alive on the first day of the period, irrespective of their dialysis modality (HD/resumed PD/dialysis withdrawal), with censoring at time of kidney transplantation, loss to follow-up, or the end of the study period.
In each registry, separate multivariable Cox proportional hazard models were used for early (<90 days), medium (90–180 days), and late (>180 days) periods after the transfer to HD to examine the association between the covariates and the mortality within these periods. These 3 periods were considered owing to the nonproportional mortality hazard observed during the entire follow-up time after transfer to HD, previous publications,18,23 and clinical meaningfulness. The proportional hazards assumption was assessed with log-minus-log plots, observed (Kaplan–Meier) and predicted (Cox) graphs, and Schoenfeld residuals. Patients were followed after transfer from PD to HD from the first day (model 1), day 90 (model 2), or day 180 (model 3) until death, censoring at time of kidney transplantation, loss to follow-up, or the end of follow-up. Right-censoring was also performed in the “early” and “medium” period models if a patient was still alive after 90 or 180 days, respectively.
A sensitivity model, using the same statistical approach as presented previously, was used to assess the association between cause of transfer to HD and early mortality risk in ANZDATA. Additionally, meta-analyses were performed to combine individual results of survival models from the 4 registries using the random effect approach, with the DerSimonian-Laird estimator for variance. I2 statistic and Q test were used to assess the heterogeneity between sites. Analyses were performed locally for each registry following the same methods and using Stata SE, version 15 (StataCorp, College Station, TX), SAS 9.4 and R3.6.1.
Results
Patients with incident PD who transferred to HD for at least 1 day were included from 4 registries with respective patient numbers of 6683 (ANZDATA), 5847 (CORR), 21,574 (ERA Registry), and 80,459 (USRDS). Overall, these patients represent approximately 20%, of all patients with incident PD in the 4 registries, with the exception of the USRDS where the percentage of patients transferring to HD reached nearly 25% (Supplementary Figure S1). Frequency of transfer to HD changed minimally during the study period (2000–2014) with a small increase through years in Canada and Europe and a small decrease in Australia/New Zealand and United States. In the 4 registries, the proportion of patients who died while on PD decreased during the study period from 14% to 17% in the earliest cohort to 9% to 12% in the most recent era. Baseline characteristics of study cohorts from the 4 registries are presented in Table 1. Median duration on PD before transfer to HD was 1.1 years in Europe and the United States and 1.3 years in Australia/New Zealand and Canada. The proportion of patients for whom the transfer to HD was temporary (and who resumed PD within 180 days) was 20%, 16%, and 18% in ANZDATA, CORR, and ERA Registry, respectively, with a median duration of temporary HD ranging from 49 (26–83) to 59 (35–94) days (Supplementary Table S1).
Table 1.
Baseline characteristics of incident peritoneal dialysis patients who transferred to hemodialysis
| Characteristics | ANZDATA, n = 6683 | CORR, n = 5847 | ERA, n = 21,574 | USRDS, n = 80,459 |
|---|---|---|---|---|
| Age at KRT start (yr) | 61 [49–70] | 62 [51–71] | 61 [49–71] | 58 [46–68] |
| Male | 3880 (58) | 3473 (59) | 13,814 (64) | 57,298 (56) |
| Primary kidney disease | ||||
| Glomerulonephritis | 1721 (26) | 1102 (19) | 3355 (16) | 11,830 (15) |
| Diabetic nephropathy | 1397 (36) | 2312 (41) | 5167 (24) | 36,552 (45) |
| Hypertensive disease | 890 (13) | 1023 (18) | 3170 (15) | 19,942 (25) |
| Other | 1662 (25) | 1241 (22) | 9882 (46) | 15,746 (15) |
| Yr of KRT start | ||||
| 2000–2004 | 2274 (34) | 2225 (38) | 7188 (33) | 25,703 (32) |
| 2005–2009 | 2587 (39) | 2405 (41) | 8072 (37) | 24,573 (30) |
| 2010–2014 | 1824 (28) | 1218 (21) | 6316 (29) | 30,574 (38) |
| PD duration before transfer (yr) | 1.3 [0.5–2.5] | 1.3 [0.5–2.6] | 1.1 [0.4–2.3] | 1.1 [0.4–2.3] |
ANZDATA, Australia and New Zealand Dialysis and Transplantation registry; CORR, Canadian Organ Replacement Register; ERA, Europe Renal Association Registry; KRT, kidney replacement therapy; PD, peritoneal dialysis; USRDS, United States Renal Dialysis System databases.
Values represent number (percentage) or median [interquartile range].
Mortality Rate After Transfer From PD to HD
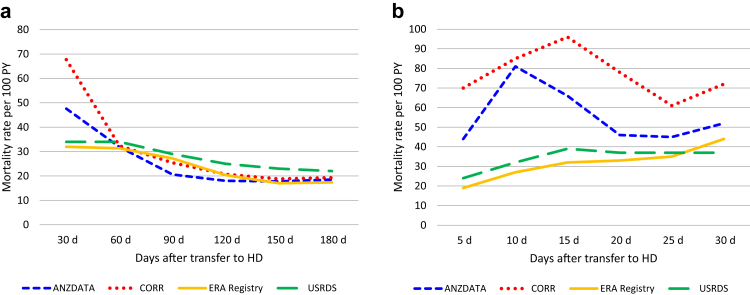
In all 4 registries, mortality rates were high after transfer from PD to HD, peaking during the first 30-day periods after transfer (Figure 1a). At their peak, crude mortality rates reached 48, 68, 32, and 34 deaths per 100 patient-years in ANZDATA, CORR, ERA Registry, and USRDS, respectively. Between-registry variability in death rates was observed over the first 30 days (Figure 1b) with rates converging to a similar level thereafter. Proportions of cause-specific deaths varied over the 30-day periods with an increase in cardiovascular-related and decrease in infection-related deaths from the earlier to later periods after transfer to HD (Supplementary Figure S2A–C).
Figure 1.
Crude mortality rates after a transfer from PD to HD (a) per 30-day period for 180 days post-transfer and (b) per 5-day period within the first 30 days post-transfer. HD, hemodialysis; PD, peritoneal dialysis; PY, person year.
Era, Age, and PD Duration Subgroups
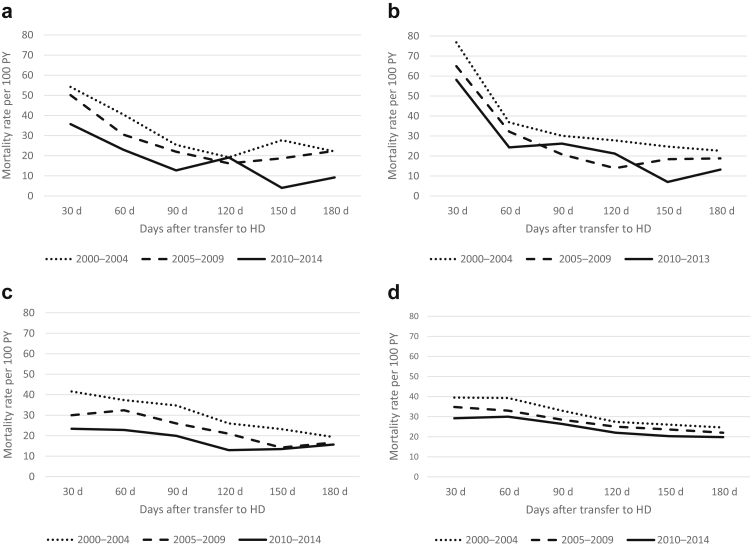
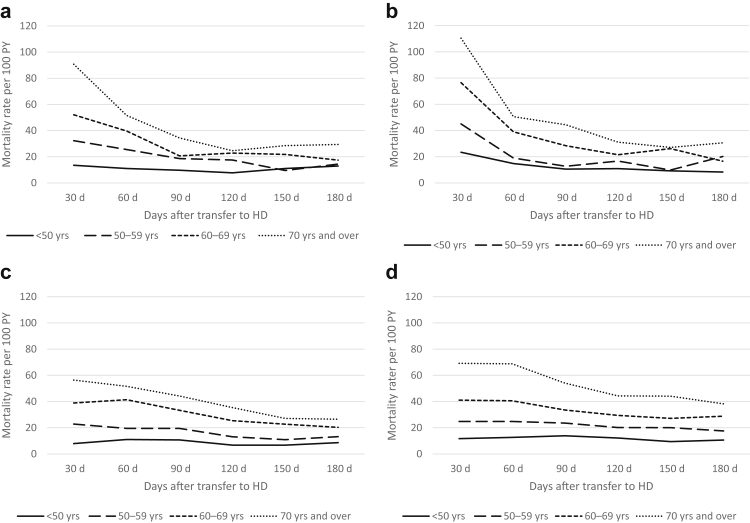
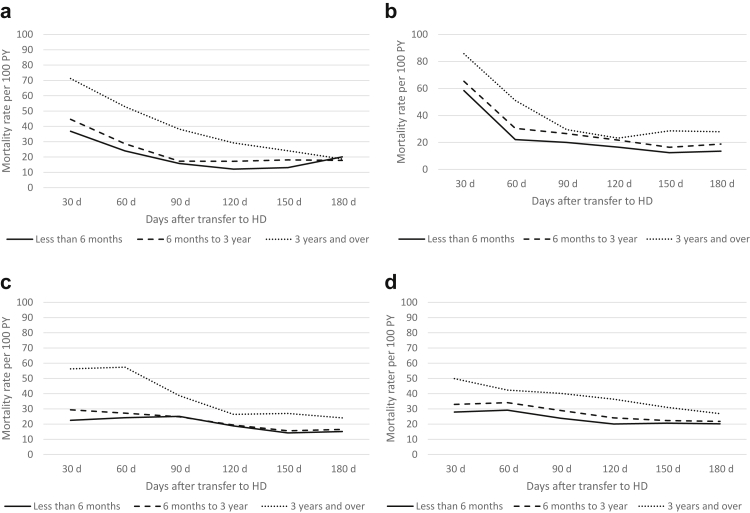
Crude mortality rates were lower in more recent cohorts (2010–2014) than earlier cohorts (2000–2004) throughout all registries. The pattern of highest mortality rates seen over the first 30-day period was, however, preserved in all eras (Figure 2a–d and Supplementary Table S2). Age-stratified mortality risk showed that although older patients were always at higher risk of death, their higher mortality risk appeared even more pronounced during the first few months after transfer to HD (Figure 3a–d), when compared with younger patients. Also, the youngest group (<50 years) appeared to have no excess mortality in the early phase, with a fairly stable crude mortality risk throughout the post-transfer periods. Similarly, patients with more than 3 years on PD before transfer had a higher mortality risk during the first months (compared with those with shorter PD duration) though this risk seemed to stabilize to a level close to patients with shorter PD vintage after 3 to 6 months post-transfer to HD (Figure 4a–d).
Figure 2.
Crude mortality rates by year of RRT initiation (2000–2004/2005–2009/2010–2014), by registry (a) ANZDATA, (b) CORR, (c) ERA Registry, and (d) USRDS. ANZDATA, Australia and New Zealand Dialysis and Transplantation registry; CORR, Canadian Organ Replacement Register; ERA, Europe Renal Association Registry; PY, person year; RRT, renal replacement therapies; USRDS, United States Renal Dialysis System databases.
Figure 3.
Crude age-stratified mortality rates after transfer from PD to HD per 30-day periods in (a) ANZDATA, (b) CORR, (c) ERA Registry, and (d) USRDS. ANZDATA, Australia and New Zealand Dialysis and Transplantation registry; CORR, Canadian Organ Replacement Register; ERA, Europe Renal Association Registry; HD, hemodialysis; PD, peritoneal dialysis; PY, person year; USRDS, United States Renal Dialysis System databases.
Figure 4.
Crude mortality rates after transfer from PD to HD per 30-day periods stratified by PD vintage before transfer, in (a) ANZDATA, (b) CORR, (c) ERA Registry, and (d) USRDS. ANZDATA, Australia and New Zealand Dialysis and Transplantation registry; CORR, Canadian Organ Replacement Register; ERA, Europe Renal Association Registry; HD, hemodialysis; PD, peritoneal dialysis; PY, person year; USRDS, United States Renal Dialysis System databases.
Predictors of Mortality During Early, Medium, and Late Periods After Transfer to HD
Risk factors for early, medium, and late periods of mortality after transfer from PD to HD are displayed in Table 2 and Supplementary Figure S3A–D. Overall, predictors were consistent across registries, both in terms of specific risk factors and change by observation period.
Table 2.
Adjusted hazard ratios and their 95% CIs for mortality after transfer from peritoneal dialysis to hemodialysis during early, medium, and late periods and in 4 international registries
| Characteristics | ANZDATA |
CORR |
ERA Registry |
USRDS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early <90 d |
Medium 91–180 d |
Late >180 d |
Early <90 d |
Medium 91–180 d |
Late >180 d |
Early <90 d |
Medium 91–180 d |
Late >180 d |
Early <90 d |
Medium 91–180 d |
Late >180 d |
|
| Age, ref. <50 yr | ||||||||||||
| 50–59 | 2.05 (1.43–2.93) | 1.18 (0.76–1.81) | 1.56 (1.17–1.79) | 1.49 (1.03–2.15) | 1.50 (0.91–2.48) | 1.37 (1.16–1.62) | 1.99 (1.61–2.48) | 1.62 (1.23–2.12) | 1.63 (1.49–1.78) | 1.85 (1.69–2.03) | 1.68 (1.51–1.87) | 1.69 (1.65–1.75) |
| 60–69 | 2.95 (2.11–4.10) | 1.73 (1.17–2.54) | 1.99 (1.75–2.24) | 2.71 (1.97–3.73) | 2.20 (1.39–3.48) | 1.85 (1.59–2.14) | 3.62 (2.99–4.39) | 2.96 (2.34–3.75) | 2.26 (2.08–2.44) | 3.00 (2.75–3.27) | 2.53 (2.28–2.79) | 2.43 (2.36–2.51) |
| ≥70 | 4.94 (3.58–6.80) | 2.55 (1.74–3.71) | 2.93 (2.59–3.32) | 4.26 (3.13–5.80) | 3.23 (2.08–5.03) | 2.69 (2.33–3.11) | 5.25 (4.35–6.33) | 4.25 (3.37–5.35) | 3.19 (2.95–3.44) | 5.36 (4.94–5.82) | 4.15 (3.76–4.57) | 3.86 (3.74–3.98) |
| Sex, ref. female | ||||||||||||
| Male | 0.79 (0.66–0.95) | 0.76 (0.58–0.97) | 1.05 (0.97–1.14) | 0.95 (0.80–1.14) | 1.03 (0.78–1.35) | 1.17 (1.07–1.28) | 0.81 (0.73–0.90) | 1.06 (0.91–1.22) | 1.17 (1.12–1.23) | 0.94 (0.90–0.99) | 1.01 (0.95–1.07) | 1.08 (2.06–1.10) |
| Primary kidney disease, ref. diabetes | ||||||||||||
| GN | 0.66 (0.51–0.85) | 0.49 (0.34–0.70) | 0.58 (0.52–0.64) | 0.56 (0.42–0.75) | 0.55 (0.36–0.85) | 0.54 (0.47–0.62) | 0.48 (0.40–0.59) | 0.44 (0.34–0.57) | 0.50 (0.46–0.54) | 0.72 (0.65–0.78) | 0.67 (0.60–0.75) | 0.57 (0.55–0.59) |
| HTN | 0.97 (0.75–1.25) | 0.88 (0.62–1.25) | 0.76 (0.67–0.85) | 0.82 (0.66–1.03) | 0.76 (0.52–0.92) | 0.72 (0.64–0.82) | 0.90 (0.77–1.05) | 0.65 (0.53–0.80) | 0.67 (0.62–0.72) | 0.86 (0.81–0.92) | 0.79 (0.73–0.85) | 0.74 (0.72–0.76) |
| Other | 0.89 (0.71–1.11) | 0.55 (0.39–0.77) | 0.64 (0.58–0.71) | 0.76 (0.60–0.95) | 0.77 (0.54–1.10) | 0.66 (0.58–0.74) | 0.73 (0.65–0.83) | 0.65 (0.53–0.77) | 0.60 (0.56–0.63) | 0.91 (0.85–0.99) | 0.84 (0.76–0.92) | 0.66 (0.64–0.69) |
| Yr, ref. 2000–2004 | ||||||||||||
| 2005–2009 | 0.91 (0.75–1.11) | 0.85 (0.65–1.10) | 0.81 (0.74–0.88) | 0.83 (0.69–1.00) | 0.70 (0.52–0.92) | 0.99 (0.90–1.09) | 0.76 (0.67–0.85) | 0.71 (0.61–0.83) | 0.76 (0.72–0.80) | 0.84 (0.79–0.90) | 0.88 (0.82–0.95) | 0.88 (0.86–0.91) |
| 2010–2014 | 0.68 (0.52–0.87) | 0.48 (0.33–0.70) | 0.79 (0.69–0.90) | 0.79 (0.61–1.01) | 0.66 (0.44–1.01) | 0.81 (0.65–0.99) | 0.61 (0.53–0.70) | 0.59 (0.49–0.71) | 0.75 (0.69–0.80) | 0.77 (0.73–0.82) | 0.79 (0.73–0.85) | 0.94 (0.91–0.96) |
| PD duration ref. <6 mo | ||||||||||||
| 6 mo–3 yr | 1.26 (0.99–1.58) | 1.2 (0.88–1.65) | 1.16 (1.06–1.27) | 1.31 (1.04–1.64) | 1.45 (1.02–2.07) | 1.07 (0.97–1.19) | 1.18 (1.04–1.34) | 1.11 (0.94–1.31) | 1.03 (0.98–1.09) | 1.35 (1.27–1.43) | 1.25 (1.16–1.34) | 1.10 (1.08–1.13) |
| ≥3 yr | 2.20 (1.70–2.85) | 1.57 (1.08–2.28) | 1.34 (1.19–1.52) | 2.00 (1.53–2.59) | 2.12 (1.40–3.21) | 1.31 (1.13–1.52) | 2.04 (1.76–2.35) | 1.61 (1.32–1.96) | 1.21 (1.12–1.30) | 2.14 (1.98–2.30) | 1.87 (1.70–2.05) | 1.35 (1.31–1.40) |
ANZDATA, Australia and New Zealand Dialysis and Transplantation registry; CORR, Canadian Organ Replacement Register; d, day; ERA, Europe Renal Association Registry; GN, glomerulonephritis; HD, hemodialysis; HTN, hypertensive nephrosclerosis; PD, peritoneal dialysis; ref, reference category; ref., reference; USRDS, United States Renal Dialysis System.
Adjusted for age, sex, primary kidney disease, year of kidney replacement therapy initiation, and PD duration before transfer to HD.
Older age was consistently associated with higher risk of death, although the strength of the association was much more pronounced during the early post-transfer period (<90 days) than the medium and late periods. Similarly, longer PD vintage (>3 years) before transfer was associated with higher mortality especially during the early (<90 days) and medium (90–180 days) periods after transfer with a lower increase in mortality risk in the late transfer period (>180 days). In all 4 registries, males had a lower risk of death during the early post-transfer period (compared with females) whereas their mortality risk was higher in the late post-transfer period. Survival curves for the early post-transfer period are presented in Supplementary Figure S4A–C, comparing “50 to 59 years” versus “70 years and over” with fixed values of other covariates (male, diabetic kidney disease, PD vintage >3 years, and most recent era).
Results of the meta-analysis combining the 4 registries are presented in Figure 5a–d. Although effect sizes varied across the registries, the meta-analysis showed a global consistency between the registries. It confirmed the order of magnitude of association between mortality risk and risk factors during early, medium, and late periods after transfer. There was variability in I2 statistic for heterogeneity assessment through the different predictors and periods assessed. Of note, there was no statistically different heterogeneity for age and PD vintage during the early and medium periods after transfer, whereas the heterogeneity was statistically significantly different during the late post-transfer period. Sex and era predictors showed a statistically significant heterogeneity in most subgroups and periods.
Figure 5.
Meta-analysis with adjusted hazard ratios for mortality by (a) age groups, (b) male sex, (c) cohort years, and (d) peritoneal dialysis vintage during early (<90 days), medium (90–180 days), and late (>180 days) periods post-transfer from PD to HD. Reference groups: age <50 years, female, years 2000 to 2004, PD vintage <6 months. Adjusted for age, sex, primary kidney disease, year of kidney replacement therapy initiation, and duration of PD before transfer to HD. HD, hemodialysis; HR, hazard ratio; PD, peritoneal dialysis.
Additional Analysis
Assuming cause of transfer from PD to HD could be a significant confounder, the main survival analysis was repeated in ANZDATA registry with inclusion of the cause of transfer. Overall, the associations between other baseline characteristics and mortality remained highly similar (Supplementary Table S3). During the early period, risk of death was lower for patients transferred to HD owing to inadequate dialysis (hazard ratio 0.66, 95% CI 0.50–0.88) and mechanical causes (hazard ratio 0.37, 95% CI 0.25–0.53) than those transferred for infection-related reasons. After the first 90 days, having a social cause for transfer to HD was associated with the highest risk of death, especially between 90 and 180 days after transfer (hazard ratio 1.78, 95% CI 1.27–2.47, compared with infectious causes).
Discussion
This study is one of the first robust descriptions of the mortality risk after transferring from PD to HD, with similar results found from 4 registries covering 21 countries. This multicenter, multiregistry study reported consistently higher mortality rates in the first 60 to 90 days after transfer to HD, stabilizing thereafter. Across the 4 registries, crude mortality rates after transfer were lower in the more recent era as compared with earlier eras, younger versus older patients, and in patients with shorter versus longer PD vintage before transfer to HD. In addition, adjusted multivariable models showed similar trends in all 4 registries, with more pronounced association between risk factors (older age, PD vintage) and mortality risk during the early post-transfer period. Differences in sex-related mortality risk were also consistent across registries with males experiencing lower mortality risk than females during the early period and higher risk during the late periods.
The early period after transition from chronic kidney disease to dialysis has been repeatedly shown to be associated with higher risk of death than the subsequent dialysis periods.24, 25, 26 Various causes may be involved in this increased risk, including acute events precipitating chronic kidney disease progression and cardiovascular stress associated with dialysis (especially HD),27 or even selection bias, possibly leading to survival of the fittest. Modifiable risk factors include predialysis referral and education, timely vascular access planning, and timely initiation of dialysis (avoiding “crash” start).28, 29, 30, 31 Although little data can be found on transitioning between dialysis modalities, this research group postulated that similar phenomena may also exist at time of modality transfer.19 Recently, a study from the ANZDATA observed heightened mortality risk after transfer from home HD to in-center HD.32 In consequence, the increased mortality risk seen during the first 30-day period after transfer from PD to HD is perhaps not surprising.
Although our observations may not be a revelation for clinicians, these novel, current data have not been reported before. This is mostly owing to the technical analytical approaches of most registries to define technique failure by commonly requiring up to 30 and even 90 days of any new modality.18 In these cases, any event (including death) occurring during that time interval is typically attributed to the previous modality (here, PD) which may artificially minimize the very early mortality risk associated with any modality transfer.
In this multiregistry study, a higher mortality risk during the first month as compared with base rate was consistently observed in all 4 registries and in all eras. There was, however, a variation in the magnitude of this increased risk with ANZDATA and CORR displaying very high crude mortality rates during the first 30 days and the ERA Registry and USRDS more moderate increases. Interestingly, all 4 registries had almost identical risks of death during the second month after transfer.
The nature of the study makes it difficult to identify specific reasons behind this early rate divergence, though several hypotheses can be postulated, including differences in data capture. For patients with a very short time on HD, this may not always be systematically documented in all jurisdictions, especially when hospitalization or HD occurs outside their usual dialysis care system. Alternatively, practice patterns may differ internationally. More specifically, some might have a policy of transferring patients who are not doing well on PD, to HD, though not necessarily for PD-related reasons, rather than advising withdrawal from dialysis. These 2 hypotheses are partially reflected by Figure 1b where 5-day crude death rates increase slowly in the ERA Registry and USRDS and more steeply over 10 to 15 days in ANZDATA and CORR. Differences in dialysis withdrawal practices overall (during PD and early after transfer to HD) may also exist between regions and could have contributed to this different pattern. For instances, dialysis withdrawal was identified as the cause of death in 34% of dialysis patient in Australia in 2019 whereas <15% were classified as such in United States. Caution should however be used when comparing these proportions as classification practices may differ internationally and the proportion of missing/unknown cause of death was much higher in USRDS than ANZDATA. Other reasons potentially involved in this early crude rate-disparity include differences in characteristics of patients transferred from PD to HD, with slightly shorter PD vintages in United States and Europe, for instance. These measured and unmeasured differences in patients’ characteristics may also translate into variation in the total risk and causes of transfer to HD.
A previous study from the ANZDATA registry showed that reasons for transfer from PD to HD modulated the risk of early mortality after transfer.33 The authors found that patients transferred owing to infections and social reasons had a higher crude rate of death and higher adjusted risk of death during the first 2 years after transfer to HD than those transferred owing to inadequate dialysis and mechanical issues.33 Differences in outcome may also reflect whether the transfer was anticipated and planned for, with, for instance, pre-emptive arteriovenous fistula creation. The potential different outcomes associated with planned modality transfer are important when considering any PD-first, integrated dialysis34 (or home dialysis)35, 36, 37 approaches, acknowledging that this first modality may well be temporary.
Causes of death also varied in proportion during 30-day periods after transfer to HD with a pattern where infections were more frequently involved during the early days after transfer whereas cardiovascular-related deaths increased in proportion during later periods after transfer. It remains difficult to perform any between-registry comparison because certainty of reason for transfer varied greatly with a much higher proportion of “unknown” causes in Canadian and European data than in Australian/New Zealand data.
The unexpected association between lower mortality risk in males than females early after transfer to HD and higher risk during the later period after transfer was consistent in the 4 registries. Sex is not a traditional mortality risk factor in patients with kidney failure, in contrast with the general population.38, 39, 40 The pattern and underlying causes of sex-differences in mortality risk found here are likely complex, raising the possibility that the overall lack of sex effect is simply an average of different situations where risk is higher and others where it is lower.
In this study, the risk of early mortality was worse for patients who had a longer PD vintage before they transferred to HD. Numerous studies have demonstrated that duration of dialysis is a predictor of mortality, and this study is no exception. It may also reflect differences in the cause of transfer to HD, as this varies with duration of PD,12,41,42 but our subanalysis of ANZDATA including the cause of transfer to HD did not mitigate the higher mortality risk association with longer dialysis vintage. It should, however, be noted that crude mortality rates were higher during the first few months after transfer, even in subgroups of patients with less than 6 months of PD vintage, highlighting the fact that modality transfer can be hazardous at any time.
This study also found lower risks of death in patients transferred to HD during the most recent versus earlier years. This association was found in all 4 registries and is consistent with the global improvement of survival of people with kidney failure.38,43 It could also be postulated that a component of this lower post-transfer mortality is related to changes in practice, which might include more “pre-emptive” or planned transfers to HD, improvement of transitioning programs, or a decrease in futile transfers. Despite this era effect, the pattern of peaking mortality risk during the first 60 days after transfer to HD remains present in the most recent cohorts.
Ultimately, in some cases, early mortality after modality transfer could be directly related to an acute health issue that would have been fatal irrespective of the dialysis modality transfer. It remains difficult to identify what proportion of very early deaths falls into this category. However, this also brings up the question about the potential futility of dialysis modality transfer in extremely unstable patients who would potentially be a candidate for dialysis withdrawal.44,45 Results of the present study may help clinicians identify patients who are most likely to die after transfer to HD and prevent potentially futile interventions.
This study has several strengths. It is the first study to report outcomes of one of the most common modality transfers, without the bias of the traditional 30 to 90 days where outcomes are often attributed to the initial modality. It includes data from 4 well-known multinational registries, used consistent definitions and statistical analyses, and consistently found similar patterns in all registries.
These strengths are balanced against some limitations. The most important is related to the multiregistry nature of the study, limited by using only covariates that were available in all registries, and preventing adjustment for most comorbidities and other potentially important factors with risk of residual confounding. In addition, although using consistent outcomes definitions, there may be persistent differences between registries in data capture and how variables are defined. For example, modality transfer in the context of prolonged hospitalization might be missing in registries and could lead to underestimation of transitions rates and outcomes. Ultimately, this study cannot make any causal inference between the transfer to HD and increased mortality risk.
In conclusion, this study showed that the early period after transfer from PD to HD was associated with an increased mortality risk as compared with baseline risk, which was a consistent finding across multinational registries. This highlights the need to increase the awareness of potential hazards of such transfers and to potentially consider the eventual futility of transfer for non–PD-related reasons. Most importantly, it calls for efforts to improve transitioning care pathways to hopefully improve patient outcomes and reduce suffering.
Acknowledgments
This study was supported by a Baxter Health Care Extramural Grant. We acknowledge the contribution of the following “INTEGRATED research initiative” additional members: Gill Combes, Catherine Firanek, Rafael Gomez, Vivek Jha George, Magdalena Madero, Ikuto Masakane, Madhukar Misra, Stephen McDonald, Sandip Mitra, Thyago Moraes, Puma Mukhopadhyay, James Sloand, Allison Tong, and Cheuk-Chun Szeto. For the ANZDATA: The authors gratefully acknowledge the substantial contribution of the entire Australia and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the ANZDATA registry database.
For the CORR: The authors thank the staff at CORR for maintaining the database and the dialysis units throughout Canada for submitting information to CORR. For the ERA Registry: The authors thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry (R. Kramar); Dutch speaking Belgian Society of Nephrology (M. Couttenye, F. Schroven, and J. De Meester); French speaking Belgian Society of Nephrology (JM. des Grottes and F. Collart); Danish Nephrology Registry (J.G. Heaf); Finnish Registry for Kidney Diseases (P. Finne, J. Helve and P.H. Groop); Hellenic Renal Registry (G. Moustakas); Icelandic End-Stage Renal Disease Registry (R. Pálsson); Norwegian Renal Registry (A.V. Reisæter and A. Åsberg); Swedish Renal Registry (M. Stendahl, H. Rydell, M. Evans, K.G. Prütz, T. Lundgren, and M. Segelmark); Dutch Renal Registry (L. Heuveling, S. Vogelaar, and M. Hemmelder); UK Renal Registry (all staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (all of the Scottish renal units); and the regional registries of Andalusia (P. Castro de la Nuez [on behalf of all users of SICATA]), Asturias (P. Beltrán, J.R. Quirós, and RERCA Working Group), Basque country [UNIPAR] (Á. Magaz, J. Aranzabal, M. Rodrigo, and I. Moina), Catalonia [RMRC] (E. Arcos, J. Comas, and J. Tort), and Valencian regions [REMRENAL] (M. Ferrer Alamar, N. Fuster Camarena and J. Pérez Penadés); and A. Kramer and R. Boenink in the AMC Registry office for data collection and management. The ERA Registry is funded by the European Renal Association. This article was written by NAMES of ALL AUTHORS on behalf of the ERA Registry which is an official body of the ERA (European Renal Association). For USRDS: This study was performed under a National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases USRDS Coordinating Center contract HHSN276201400001C. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. At the time of this writing, the USRDS Coordinating Center was located at the University of Michigan Kidney Epidemiology and Cost Center, in partnership with Arbor Research Collaborative for Health, Ann Arbor, MI. The USRDS director was Rajiv Saran, MBBS, MD, MRCP, MS, Professor of Medicine and Epidemiology at the University of Michigan, and the co-deputy directors were Vahakn B. Shahinian, MD, MS, Associate Professor of Medicine at the University of Michigan and Bruce M. Robinson, MD, Vice President Clinical Research, Arbor Research Collaborative for Health. The National Institute of Diabetes and Digestive and Kidney Diseases project officers were Kevin C. Abbott, MD, MPH, and Lawrence Y.C. Agodoa, MD. Any views or opinions expressed are solely those of the authors and do not necessarily represent those of the UK Renal Registry. The data reported here have been supplied by the ANZDATA. The interpretation and reporting of these data are the responsibility of the Editors and in no way should be seen as an official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry.
Footnotes
Annex 1. Cause-specific death categories.
Figure S1. Crude rates of transfer to HD (≥1 day) and mortality in all incident PD patients in each registry per 100 patient-years, by cohort year of kidney replacement therapy initiation.
Figure S2. Cause of death after transfer from PD to HD, by 30-day period in (A) ANZDATA, (B) CORR, and (C) ERA Registry. Data unavailable in USRDS cohort.
Figure S3. Forest plots of the adjusted hazard ratios for mortality after transfer from PD to HD, stratified by early (<90 d), medium (90-180 d), and late (>180 d) period in (A) ANZDATA, (B) CORR, (C) ERA Registry, and (D) USRDS. Adjusted for age, sex, primary kidney disease, year of kidney replacement therapy initiation, and PD duration before transfer to HD.
Figure S4. Adjusted survival curves for the initial 90 days after transfer from PD to HD in (A) ANZDATA, (B) CORR, and (C) ERA Registry. Data adjusted for male sex, diabetic nephropathy, PD vintage > 3 years, era 2010-2014, and age as displayed (≥70 years versus 50-59 years).
Table S1. Baseline characteristics of incident PD patients transferred to HD who did or did not resume PD within 180 days of transfer to HD.
Table S2. Crude mortality rates per 100 patient-years.
Table S3. Adjusted hazard ratios for mortality, and their 95% confidence intervals in ANZDATA during early, medium, and late periods, with adjustment for cause of transfer, age, sex, primary kidney disease, year of kidney replacement therapy initiation, and PD duration before transfer to HD.; mortality rates per 100 patient-years.
STROBE Checklist.
Contributor Information
Annie-Claire Nadeau-Fredette, Email: ac.nadeau-fredette@umontreal.ca.
INTEGRATED Study Group:
Gill Combes, Catherine Firanek, Rafael Gomez, Vivek Jha George, Magdalena Madero, Ikuto Masakane, Madhukar Misra, Stephen McDonald, Sandip Mitra, Thyago Moraes, Puma Mukhopadhyay, James Sloand, Allison Tong, and Cheuk-Chun Szeto
Appendix
INTEGRATED Study Group Additional Collaborators
Gill Combes, Catherine Firanek, Rafael Gomez, Vivek Jha George, Magdalena Madero, Ikuto Masakane, Madhukar Misra, Stephen McDonald, Sandip Mitra, Thyago Moraes, Puma Mukhopadhyay, James Sloand, Allison Tong, and Cheuk-Chun Szeto.
Disclosure
ACNF has a junior 1 scholarship from Fonds de Recherche du Québec - Santé and a previous research grant from Baxter Healthcare. ML has received honoraria from Fresenius Medical Care, Baxter Healthcare, and NxStage and a research grant from Baxter Healthcare. DWJ has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca and AWAK, speaker’s honoraria and travel sponsorships from ONO, and travel sponsorships from Amgen. He is a current recipient of an Australian National Health and Medical Research Council Practitioner Fellowship. WVB has received speaker fees and travel funds from Fresenius Medical Care and Baxter. BR has received consultancy fees or travel reimbursement in the last 3 years from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin Co. all paid directly to his institution of employment. SD has received grant funding from Baxter HealthCare and Fresenius Medica Care and advisory board fees from Baxter HealthCare and Ellen Medical. All the other authors declared no competing interests.
Supplementary Material
Annex 1. Cause-specific death categories
Figure S1. Crude rates of transfer to HD (≥1 day) and mortality in all incident PD patients in each registry per 100 patient-years, by cohort year of kidney replacement therapy initiation.
Figure S2. Cause of death after transfer from PD to HD, by 30-day period in (A) ANZDATA, (B) CORR, and (C) ERA Registry. Data unavailable in USRDS cohort.
Figure S3. Forest plots of the adjusted hazard ratios for mortality after transfer from PD to HD, stratified by early (<90 d), medium (90-180 d), and late (>180 d) period in (A) ANZDATA, (B) CORR, (C) ERA Registry, and (D) USRDS. Adjusted for age, sex, primary kidney disease, year of kidney replacement therapy initiation, and PD duration before transfer to HD.
Figure S4. Adjusted survival curves for the initial 90 days after transfer from PD to HD in (A) ANZDATA, (B) CORR, and (C) ERA Registry. Data adjusted for male sex, diabetic nephropathy, PD vintage > 3 years, era 2010-2014, and age as displayed (≥70 years versus 50-59 years).
Table S1. Baseline characteristics of incident PD patients transferred to HD who did or did not resume PD within 180 days of transfer to HD.
Table S2. Crude mortality rates per 100 patient-years.
Table S3. Adjusted hazard ratios for mortality, and their 95% confidence intervals in ANZDATA during early, medium, and late periods, with adjustment for cause of transfer, age, sex, primary kidney disease, year of kidney replacement therapy initiation, and PD duration before transfer to HD.; mortality rates per 100 patient-years.
STROBE Checklist.
References
- 1.United States Renal Data System . NIH, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2020. USRDS Annual Data Report: End-Stage Renal Disease (ESRD) in the United States, Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. [Google Scholar]
- 2.Jansen M.A., Hart A.A., Korevaar J.C., et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 3.Bargman J.M., Thorpe K.E., Churchill D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A., Collins A.J., Herzog C.A., Foley R.N. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int. 2005;68:311–318. doi: 10.1111/j.1523-1755.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Fan S.L., Sathick I., McKitty K., Punzalan S. Quality of life of caregivers and patients on peritoneal dialysis. Nephrol Dial Transplant. 2008;23:1713–1719. doi: 10.1093/ndt/gfm830. [DOI] [PubMed] [Google Scholar]
- 6.Kutner N.G., Zhang R., Barnhart H., Collins A.J. Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transplant. 2005;20:2159–2167. doi: 10.1093/ndt/gfh973. [DOI] [PubMed] [Google Scholar]
- 7.Chui B.K., Manns B., Pannu N., et al. Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am J Kidney Dis. 2013;61:104–111. doi: 10.1053/j.ajkd.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Karopadi A.N., Mason G., Rettore E., Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary K., Sangha H., Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol. 2011;6:447–456. doi: 10.2215/CJN.07920910. [DOI] [PubMed] [Google Scholar]
- 10.Coentrao L.A., Araujo C.S., Ribeiro C.A., Dias C.C., Pestana M.J. Cost analysis of hemodialysis and peritoneal dialysis access in incident dialysis patients. Perit Dial Int. 2013;33:662–670. doi: 10.3747/pdi.2011.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaar B.G., Plantinga L.C., Crews D.C., et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol. 2009;10:3. doi: 10.1186/1471-2369-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolesnyk I., Dekker F.W., Boeschoten E.W., Krediet R.T. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30:170–177. doi: 10.3747/pdi.2008.00277. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V.A., Sidell M.A., Yang W.T., Jones J.P. Predictors of peritonitis, hospital days, and technique survival for peritoneal dialysis patients in a managed care setting. Perit Dial Int. 2014;34:171–178. doi: 10.3747/pdi.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan P.G., Clayton P.A., Saunders J., Polkinghorne K.R., Snelling P.L. Predictors and outcomes of transfers from peritoneal dialysis to hemodialysis. Perit Dial Int. 2015;35:306–315. doi: 10.3747/pdi.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perl J., Wald R., Bargman J.M., et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol. 2012;7:1145–1154. doi: 10.2215/CJN.01480212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan S., Cho Y., Koh Y.H., et al. Association of socio-economic position with technique failure and mortality in Australian non-indigenous peritoneal dialysis patients. Perit Dial Int. 2017;37:397–406. doi: 10.3747/pdi.2016.00209. [DOI] [PubMed] [Google Scholar]
- 17.van de Luijtgaarden M.W., Jager K.J., Segelmark M., et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant. 2016;31:120–128. doi: 10.1093/ndt/gfv295. [DOI] [PubMed] [Google Scholar]
- 18.Lan P.G., Clayton P.A., Johnson D.W., et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int. 2016;36:623–630. doi: 10.3747/pdi.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan C., Combes G., et al. Transition between different renal replacement modalities: gaps in knowledge and care—the integrated research initiative. Integrated. Perit Dial Int. 2019;39:4–12. doi: 10.3747/pdi.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbokl M.A., Kennedy C., Bargman J.M., McGrath Chong M., Chan C.T. Home-to-home dialysis transition: a 24-year single-centre experience. Perit Dial Int. 2021 doi: 10.1177/08968608211029213. [DOI] [PubMed] [Google Scholar]
- 21.Cina D.P., Dacouris N., Kashani M., et al. Use of home hemodialysis after peritoneal dialysis technique failure. Perit Dial Int. 2013;33:96–99. doi: 10.3747/pdi.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadeau-Fredette A.C., Hawley C., Pascoe E., et al. Predictors of transfer to home hemodialysis after peritoneal dialysis completion. Perit Dial Int. 2016;36:547–554. doi: 10.3747/pdi.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho Y., Badve S.V., Hawley C.M., et al. Peritoneal dialysis outcomes after temporary haemodialysis transfer for peritonitis. Nephrol Dial Transplant. 2014;29:1940–1947. doi: 10.1093/ndt/gfu050. [DOI] [PubMed] [Google Scholar]
- 24.Foley R.N., Chen S.C., Solid C.A., et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 25.Lukowsky L.R., Kheifets L., Arah O.A., Nissenson A.R., Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35:548–558. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson B.M., Zhang J., Morgenstern H., et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal N., Roy J., Chen H.Y., et al. Evolution of echocardiographic measures of cardiac disease from CKD to ESRD and risk of all-cause mortality: findings from the CRIC study. Am J Kidney Dis. 2018;72:390–399. doi: 10.1053/j.ajkd.2018.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley R.N. Epidemiology and risk factors for early mortality after dialysis initiation. Semin Nephrol. 2017;37:114–119. doi: 10.1016/j.semnephrol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Lok C.E., Foley R. Vascular access morbidity and mortality: trends of the last decade. Clin J Am Soc Nephrol. 2013;8:1213–1219. doi: 10.2215/CJN.01690213. [DOI] [PubMed] [Google Scholar]
- 30.Fischer M.J., Stroupe K.T., Kaufman J.S., et al. Predialysis nephrology care and dialysis-related health outcomes among older adults initiating dialysis. BMC Nephrol. 2016;17:103. doi: 10.1186/s12882-016-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Jaishi A.A., Lok C.E., Garg A.X., Zhang J.C., Moist L.M. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol. 2015;10:418–427. doi: 10.2215/CJN.06220614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semple D.J., Sypek M., Ullah S., Davies C., McDonald S. Mortality after home hemodialysis treatment failure and return to in-center hemodialysis. Am J Kidney Dis. 2022;79:15–23e1. doi: 10.1053/j.ajkd.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Chen J.H.C., Johnson D.W., Hawley C., Boudville N., Lim W.H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018;8:3980. doi: 10.1038/s41598-018-22335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biesen W.V., Vanholder R.C., Veys N., Dhondt A., Lameire N.H. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol. 2000;11:116–125. doi: 10.1681/ASN.V111116. [DOI] [PubMed] [Google Scholar]
- 35.Nadeau-Fredette A.C., Chan C.T., Cho Y., et al. Outcomes of integrated home dialysis care: a multi-centre, multi-national registry study. Nephrol Dial Transplant. 2015;30:1897–1904. doi: 10.1093/ndt/gfv132. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H., Hoshi H., Inoue T., Kikuta T., Tsuda M., Takenaka T. New modality of dialysis therapy: peritoneal dialysis first and transition to home hemodialysis. Adv Perit Dial. 2012;28:106–111. [PubMed] [Google Scholar]
- 37.Nadeau-Fredette A.C., Bargman J.M., Chan C.T. Clinical outcome of home hemodialysis in patients with previous peritoneal dialysis exposure: evaluation of the integrated home dialysis model. Perit Dial Int. 2015;35:316–323. doi: 10.3747/pdi.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Renal Data System . NIH, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2018. USRDS Annual Data Report: End-Stage Renal Disease (ESRD) in the United States, Mortality. [Google Scholar]
- 39.Statistics Canada Report in the demographic situation in Canada. Statistics Canada. https://www150.statcan.gc.ca/n1/pub/91-209-x/91-209-x2018001-eng.htm
- 40.Carrero J.J., de Jager D.J., Verduijn M., et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol. 2011;6:1722–1730. doi: 10.2215/CJN.11331210. [DOI] [PubMed] [Google Scholar]
- 41.See E.J., Johnson D.W., Hawley C.M., Hawley C.M., et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) study. Am J Kidney Dis. 2018;72:188–197. doi: 10.1053/j.ajkd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Bechade C., Guittet L., Evans D., Verger C., Ryckelynck J.P., Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant. 2014;29:2127–2135. doi: 10.1093/ndt/gft055. [DOI] [PubMed] [Google Scholar]
- 43.ANZDATA. Chapter 3: Mortaliry in end stage kidney disease. Australia and New Zealand Dialysis and Transplant Registry. ANZDATA. Published 2019. Accessed December 14, 2020. https://www.anzdata.org.au/wp-content/uploads/2019/09/c03_mortality_2018_ar_2019_v1.0_20191202.pdf.
- 44.Chan S., Marshall M.R., Ellis R.J., et al. Haemodialysis withdrawal in Australia and New Zealand: a binational registry study. Nephrol Dial Transplant. 2020;35:669–676. doi: 10.1093/ndt/gfz160. [DOI] [PubMed] [Google Scholar]
- 45.Agunbiade A., Dasgupta A., Ward M.M. Racial/ethnic differences in dialysis discontinuation and survival after hospitalization for serious conditions among patients on maintenance dialysis. J Am Soc Nephrol. 2020;31:149–160. doi: 10.1681/ASN.2019020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.