See Clinical Research on Page 1062
The use of home dialysis, specifically peritoneal dialysis (PD), is increasing in many areas of the world. Advantages of PD compared with in-center hemodialysis (HD) are myriad, including patient empowerment, preservation of residual kidney function, improvement in post-transplant outcomes, and reduction of financial health care burden.1, 2, 3 A 2019 United States (US) executive order included an ambitious goal of 80% of new patients with end-stage kidney disease (ESKD) to receive home dialysis or kidney transplantation to be reached by 2025. Because many patients on PD require transfer to HD, logically, an increased number of patients using PD for dialysis initiation will result in increasing number of transfers to HD each year.
The initial period on HD for incident ESKD has consistently been shown to have higher mortality risk than subsequent periods.4 Furthermore, the mortality risk for incident ESKD may be underestimated in the United States Renal Data System (USRDS) data o to early ascertainment bias where deaths occurring within the initial 90-day window of initiation are not always included.5 Details on mortality risk after transition from PD to HD for prevalent ESKD have not been described using large databases. A prior retrospective cohort study of prevalent PD patients in the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry found a time-dependent association between the most common causes of technique failure (defined as transfer to HD for ≥30 days) and mortality risk. The risk was highest in the first 2 years after technique failure.6
In this current issue, the study by Nadeau-Fredette et al.7 analyzes multinational registry data between 2000 and 2014 to assess mortality rates and risk factors when transfer from PD to HD is required. Patient data were sourced from the following 4 large registries representing 15 countries: ANZDATA Registry, Canadian Organ Replacement Register, European Renal Association Registry, and USRDS. Of the included patients, 70% were from the US. Patients with ESKD using PD as initial modality were included if there was recorded use of subsequent HD for ≥1 day during the specified time period. These patients were followed until the primary end point (all-cause mortality) censored for transplantation, loss to follow-up, or study end date. This group was further divided into cohorts 2000 to 2004, 2005 to 2009, and 2010 to 2014, representing 3 eras of dialysis initiation (early, medium, and late, respectively). A subset of patients transferred back to PD and were included (16%–19% of all patients who transferred to HD, data not available for USRDS). Patients with preceding kidney transplants were excluded.
In total, 114,563 patients were included in the study, representing 20% to 25% of all incident PD patients across the 4 registries. The median time on PD before transfer to HD was between 1.1 and 1.3 years. Approximately 20% to 25% of incident PD patients transferred to HD. The authors evaluated risk of death after transfer to HD by age, sex, cohort year, and PD duration in each of the 4 registries. Cause of death was available for 3 of the registries but not the USRDS.
Risk of death was high on HD in the first 30 days after transfer, but it was much higher in the Canadian and the ANZDATA (approximately 68 and 48 deaths per 100 patient-years, respectively) compared with approximately 32 to 35 deaths per 100 patient-years in the US and Europe. When evaluated, deaths over time during the first 30 days showed a peak at approximately 15 days (more than 90 deaths per 100 patient-years) in Canada and at approximately 10 days (approximately 80 deaths per 100 patient-years) in ANZDATA, whereas in the US and Europe, the rate of death gradually rose during the first 2 weeks and then plateaued, never reaching the heights of the Canadian or Australian cohorts. These findings were particularly striking for patients 60 years and older. More recent PD vintage attenuated the effect to some extent. Overall, mortality rates were lower in the late cohort (2010–2014) compared with the early cohort (2000–2004); this is consistent with findings from previous studies showing improved mortality over time.8 This improvement in early mortality was particularly striking in ANZDATA and Canadian Organ Replacement Register data.
The study also delineated other risk factors for death after transfer to HD. Not surprisingly, those with diabetic nephropathy uniformly had a higher risk of death after transfer across all time periods and all registries. Men had a lower risk of death than women during the first 90 days after transfer (hazard ratio = 0.80; CI: 0.67–0.96, P = 0.01), but higher risk of death after 180 days, although in the adjusted model this later risk disappeared. Those patients on PD 3 years or more were more likely to die after transfer to HD (again, with risk highest in the first 90 days, hazard ratio = 1.91, CI: 1.47–2.49, P < 0.001, in adjusted model) than those on PD < 6 months.
Only the ANZDATA registry contained information on cause of technique failure. Within this registry, those transferring for infectious reasons had higher early mortality than those who transferred for inadequate dialysis or mechanical causes. If the cause of transfer was social, then risk of death was particularly high between 90 and 180 days after transfer.
What take-home messages can we glean from this paper? The study raises several important questions. First, can we do more to reduce mortality after transfer from PD to HD by more preparation and planning? We do not have data on access with the transfer from PD to HD, but it seems likely that most occur with HD catheters, which are known to be high risk for infection and increased mortality risk. Although placing an arteriovenous fistula/graft at the start of PD has been shown to be frequently futile, later placement of such access while the patient is still on PD, if adequacy becomes an issue, might be worth exploring further. Second, in some cases, where the risk of early death after transfer from PD to HD is particularly high, patients should be made aware so patient-centered goals of care discussions are held before hazardous transitions. The mortality of older patients in the first 90 days is particularly high. It seems probable that older patients are more likely to withdraw from dialysis after transfer to HD. This might explain some of the variation in mortality trends in the first 30 days among the 4 registries although from the data presented this is unclear. Perhaps, in such cases, an in-depth conversation about outcomes is warranted, particularly in older frail patients, instead of a knee jerk transfer to HD, when PD cannot be continued.
In addition to the abovementioned mortality risk, Weinhandl9 has recently reported an increased rate of acute care encounters and health care expenditures during the period immediately before and after transitions from PD to HD. Although not addressed by Nadeau-Fredette et al.,7 further information on this period in the timeline of ESKD is very much needed to minimize hospitalizations, which may exacerbate risk in this group.
A strength of this paper is the inclusion of all patients who started on PD and then transferred to HD regardless of time on PD. The USRDS has historically only counted deaths on dialysis occurring after 90 days on therapy, thereby excluding a period of very high risk for mortality on HD (as shown again in this paper). Most registries require 30 to 90 days of any new modality in definition of technique failure and furthermore attribute a death within 30 days of transfer to the first modality. This clearly skews the results and gives a false picture of what is really happening. The current paper attempted to avoid early ascertainment bias by using transfer definition as ≥1 day on HD.
Further investigation into the first month on HD is warranted. What specifically about HD is so inherently risky in that early time period? Is it infection, bacteremia, or HD catheter related, cardiovascular events, or frequent hospitalizations leading to death? Regardless, the trend/risk is now apparent whether a patient is initiating HD or transferring to HD from PD.
Although the utilization of multinational data is one strength of the study, the analyses were limited to covariates that were available in the registries. In the largest registry (USRDS), the cause of death was often missing. In addition, race was not an analyzed covariate for risk of death. Because the USRDS constituted most of the patients and African Americans constitute a good percentage of US patients on dialysis, the impact of race would be of interest. Furthermore, regional differences exist in how variables were defined and how data were captured. For example, the study was unable to identify specific cause of the early variability in mortality between ANZDATA and Canadian Organ Replacement Register compared with European Renal Association and USRDS groups. It may be that practice variations resulted in more regionally accepted recommendations to withdrawal from dialysis. Other limitations include the inability to delve into sex-specific mortality risk and the inclusion of those transferring from PD to home HD (although this assuredly is a small number).
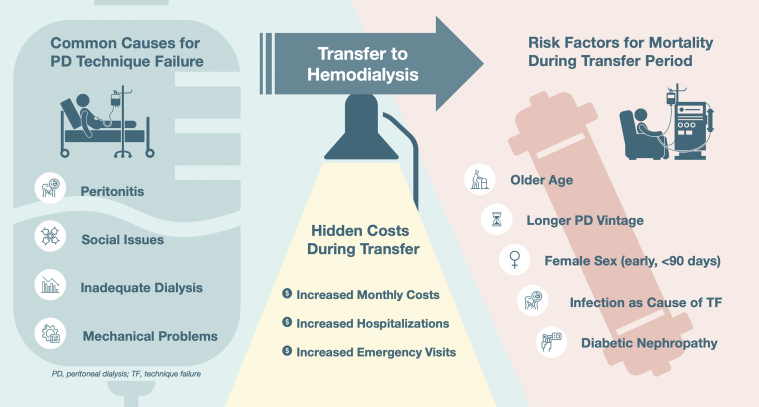
This study provides important insights into mortality trends during the period after transition from PD to HD. It particularly highlights the very high mortality risk for elderly patients who have been on PD for longer periods of time before transfer, raising the issue of futility. More research is needed to understand the mortality risk associated with transition from PD to HD. It is likely that individualized management plans considering patient-specific factors may be one way to lessen the risks and improve patient outcomes (Figure 1). Better planning may reduce the risk for some subsets of patients. As always, the patient’s goals need to be carefully discussed using real data to inform the patient of risks during necessary transitions.
Figure 1.
Common causes of PD technique failure which require temporary or permanent transfer to hemodialysis. The transfer period is fraught with increased financial costs and heightened mortality risks. PD, peritoneal dialysis; TF, technique failure.
Disclosure
All the authors declared no competing interests.
References
- 1.Chaudhary K., Sangha H., Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol. 2011;6:447–456. doi: 10.2215/CJN.07920910. [DOI] [PubMed] [Google Scholar]
- 2.Jain D., Haddad D.B., Goel N. Choice of dialysis modality prior to kidney transplantation: does it matter? World J Nephrol. 2019;8:1–10. doi: 10.5527/wjn.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klarenbach S., Manns B. Economic evaluation of dialysis therapies. Semin Nephrol. 2009;29:524–532. doi: 10.1016/j.semnephrol.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Robinson B.M., Zhang J., Morgenstern H., et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley R.N., Chen S.C., Solid C.A., et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 6.Chen J.H.C., Johnson D.W., Hawley C., Boudville N., Lim W.H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018;8:3980. doi: 10.1038/s41598-018-22335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadeau-Fredette A.-C., Sukul N., Lambie M., et al. Mortality trends after transfer from peritoneal dialysis to hemodialysis. Kidney Int Rep. 2022;7:1062–1073. doi: 10.1016/j.ekir.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsayed M.E., Morris A.D., Li X., et al. Propensity score matched mortality comparisons of peritoneal and in-centre haemodialysis: systematic review and meta-analysis. Nephrol Dial Transplant. 2020;35:2172–2182. doi: 10.1093/ndt/gfz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinhandl ED. Hidden costs associated with conversion from peritoneal dialysis to hemodialysis. Kidney360. Published online March 3, 2022. https://doi.org/10.34067/KID.0007692021 [DOI] [PMC free article] [PubMed]