Abstract
31 years lady with complete atrioventricular canal defect, large primum atrial septal defect (ASD), inlet ventricular septal defect (VSD) and Eisenmenger syndrome, presented with atrial flutter and complete heart block. She was not suitable for corrective cardiac surgery and not yet indicated for heart-lung transplantation. She was advised single chamber permanent pacemaker and eventually Micra VR transcatheter leadless pacemaker was finalised for her. Transcatheter leadless pacemaker was deployed in her RV septum despite some unforeseen technical problems. This patient had intrahepatic interruption of IVC with Azygous continuation draining into SVC but this altered venovascular course was detected only fluoroscopically midway during the pacemaker implantation procedure and this was not detected in the preprocedural transthoracic echocardiography. This abnormal venous course was clearly demonstrated in the cardiac CT which was performed only after completion of the pacemaker implantation procedure in this patient. The technical challenges encountered mainly were mostly during the manipulation of the 27F delivery catheter of Micra through this altered cardiovascular anatomy via transfemoral approach and also due to the presence of septal defects. Thus, transcatheter leadless permanent pacemaker was implanted successfully through transfemoral access in this complex congenital heart disease with interrupted IVC and azygous continuation. Besides transthoracic echocardiography, it may be better to perform transesophageal echocardiography or even preferably radiological imaging like cardiac CT or MRI prior to transcatheter leadless pacemaker implantation in patients with complex congenital heart disease to understand the cardiovascular anatomy and plan the procedure.
Keywords: Leadless pacemaker, Micra, Permanent pacemaker, Pacemaker in congenital heart disease, Interrupted IVC, Transcatheter pacing
1. Introduction
The technology of permanent pacemaker implantation has undergone significant evolution over the years. The latest revision is the concept of leadless pacemaker which can be delivered percutaneously under fluoroscopic guidance and implanted in right ventricle (RV). Micra transcatheter leadless pacemaker (Medtronic Inc., Minneapolis, MN, USA) is the world's smallest and lightest leadless permanent pacemaker globally approved and currently available in the market for therapeutic purposes [1,2]. In this case report we would be discussing about the usage of the transcatheter leadless pacing technology in a patient with complex congenital heart disease.
2. Case report
31 years lady diagnosed to have unbalanced atrioventricular canal defect with large primum atrial septal defect (ASD), inlet ventricular septal defect (VSD), Eisenmenger syndrome presented with palpitations. She had central cyanosis with saturation of 80%, pandigital clubbing and severe pulmonary hypertension. Her electrocardiography revealed atrial flutter and complete heart block with low ventricular rate of 40bpm. She was having atrial flutter for quite some time and was receiving oral anticoagulation for prevention of stroke. Six-minute walk test and NT-pro BNP levels were not significantly altered. After evaluation and discussion with the heart team, she was deemed not suitable for corrective surgery and also not yet indicated for heart-lung transplantation. Hence permanent pacemaker implantation was decided for her. Her holter study showed atrial flutter throughout and so AV synchrony with a dual chamber pacemaker was not considered for her. Therefore, she was advised single chamber permanent pacemaker implantation and eventually Micra VR transcatheter leadless permanent pacemaker was finalised for her.
Under local anaesthesia and cover of intravenous antibiotic, 6F sheaths were placed in right and left femoral veins. Unfractionated heparin was administered intravenously to achieve an ACT around 300 s. An unexpected problem was encountered when the lead of temporary pacemaker was passed through the left femoral vein i.e., the lead could not be passed into right atrium (RA). Venogram was performed with 5F Multipurpose catheter which revealed interrupted inferior vena cava (IVC) with azygous continuation draining into superior vena cava (SVC).
Interrupted IVC was realised after accessing femoral arteries while attempting to place the lead of the temporary pacemaker. We were not sure whether we could succeed negotiating 27F outer sheath and 23F Micra deliver catheter through this interrupted IVC. Half way through the procedure, patient was also reluctant for additional vascular access via internal jugular vein. Finally, we overcame this dilemma and proceeded with transfemoral approach after explaining to the patient and her relatives about the situation.
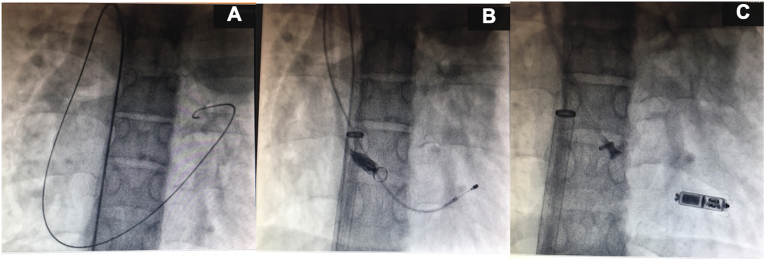
6F lead of the temporary pacemaker was finally placed in the right ventricle (RV) through left femoral vein. Course of this lead was: left femoral vein → IVC → Azygous vein → SVC → RA → RV. Using 0.035″ 150cm guide wire, 5F Multipurpose catheter was parked in SVC through right femoral venous approach and then exchanged to 0.035” 260cm Amplatz superstiff wire. Vascular access site at right groin was serially dilated with 14F, 18F and 23F dilators. Introducer sheath (23F inner diameter, 27F outer diameter) with hydrophilic coating was passed through right femoral vein till IVC. 23F delivery catheter of Micra was passed into the sheath and gradually steered through this altered cardiovascular anatomy: intrahepatic interruption of IVC with Azygous continuation draining into SVC (Fig. 1). When the delivery catheter reached RA, the next issue encountered was the presence of septal defects (ASD, VSD). Under the guidance of transthoracic echocardiography and fluoroscopy (RAO 30° and LAO 40° views), the steerable delivery catheter was gently manipulated across the tricuspid valve into RV avoiding the ASD and VSD. Micra was then navigated through the right ventricle towards the RV side of apical septum. Catheter was gently pushed against RV septum to obtain the classical gooseneck shape. Contrast angiography was performed to check the contact of the device to the trabeculae of the RV septum. Good threshold was achieved (0.3V @ 0.24 ms); tether lock was released and Micra was deployed but still connected to the tether wire. Tug test was performed and stability of Micra was confirmed. Magnified fluoroscopic view demonstrated adequate splaying of three of the four tines at the distal end of Micra. Generally, if two of the four atraumatic nitinol tines are engaged to the trabeculae within RV, there is adequate force to hold the device in place and this makes device dislodgement almost negligible with leadless pacemaker. Tether wire was then cut and Micra pacemaker was finally implanted in RV septum. Sheath was removed and there were no procedural complications.
Fig. 1.
Fluoroscopic images showing tortuous course of guidewire (A), lead of temporary pacemaker and Micra catheter [(B) IVC→Azygous vein→SVC→RA→RV) and implantation of Micra into RV septum (C).
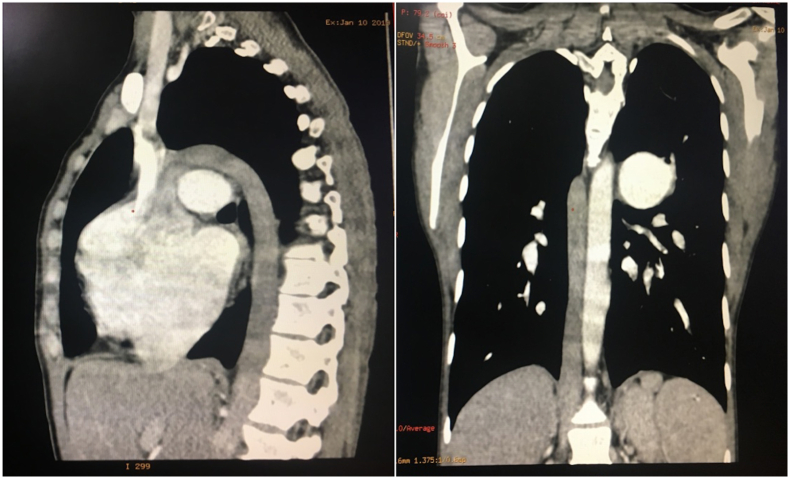
Transthoracic echocardiography and chest radiograph repeated on the following day showed that Micra device was in situ with good threshold of 0.4 V@0.24 ms, sensing of 5 mV and impedance of 430 Ohms. Neither infection nor embolic complication was reported in this patient. Cardiac CT performed after implanting Micra pacemaker retrospectively demonstrated situs ambiguous with polysplenia (bilateral left-sidedness), intrahepatic interruption of IVC with azygous continuation draining into SVC (Fig. 2). In view of atrial flutter, oral anticoagulation was restarted 24 hours after deployment of leadless pacemaker. Otherwise there is no need of routine anticoagulation for leadless pacemaker. The parameters of Micra pacemaker were stable at three months follow-up with a threshold of 0.4 V @ 0.24 ms, impedance of 440 Ohms and sensing of 5 mV.
Fig. 2.
Cardiac CT images (sagittal and coronal sections) showing interrupted IVC continuing as azygous vein and draining into SVC.
3. Discussion
Micra VR transcatheter leadless pacemaker was successfully implanted in this patient with complex congenital heart disease with altered venous anatomy and septal defects.
Micra transcatheter leadless pacing procedure involves the use of large sized sheath (27F) and stiffer delivery cable (23F); it may be technically challenging at times in the presence of complex venous anatomy. The uniqueness of this case is that the large sized introducer sheath and stiffer delivery catheter were manipulated in this challengingly tortuous cardiovascular anatomy (intrahepatic interruption of IVC with Azygous continuation draining into SVC and also the presence of septal defects like large ASD, VSD) and Micra pacemaker was deployed successfully in RV septum in this underweight patient with complex congenital heart disease. The altered venovascular course in this patient was detected only fluoroscopically midway during the pacemaker implantation procedure and this was not detected in the preprocedural transthoracic echocardiography.
Conventional pacemaker requires a mini surgical procedure associated with issues of lead and chest wall pocket related complications, removal of stitches, infection, wound healing etc. Micra pacemaker is the world's smallest and lightest leadless pacemaker currently available. It is delivered percutaneously via transfemoral venous approach through a catheter and implanted in the right ventricle. It is just the size of a large pill/coin (Fig. 3), 93% smaller (25.9 × 6.7mm) and also lighter (1.75g) than conventional pacemaker, with an average battery longevity of 12-years and MRI compatibility. Micra occupies less than 1% of the volume of a normal right ventricle in an adult. It has >99% higher implant success rate and 63% fewer long-term complications than conventional pacemaker. Complications related to venous thrombosis, infection and lead extraction are all eliminated by using leadless pacemakers [1,2]. This procedure, unlike conventional pacemaker, is a no-scalpel no-stitch technique, cosmetically good because there is no scar in chest wall, can be performed as a day-care procedure in straightforward cases and patient can be mobilised on the same day.
Fig. 3.
Comparison of the sizes of Micra and Indian coin.
The following are the reasons for choosing the Micra leadless pacemaker in this particular case: (i) removing the risks of infection because of the complex nature of her congenital heart disease, (ii) single chamber pacing only needed in view of atrial flutter, (iii) she was a young girl from abroad who was very much concerned about her cosmetic appearance and insisted on avoiding surgical scar on her upper chest wall, (iv) she was already on oral anticoagulation which was interrupted only for 24–48 hours for implanting leadless pacemaker, unlike transvenous pacemaker where it needs to be interrupted relatively for a longer duration to reduce bleeding complications, (v) minimally invasive percutaneous procedure, (vi) post-procedure pocket/lead related complications were avoided with leadless pacemaker, (vii) she was also keen on having the shortest stay possible at hospital which was 24–48 hours with leadless pacemaker procedure, (viii) early mobilisation without limitation of upper limb activity, (ix) lead of transvenous pacemaker can increase the incidence of tricuspid regurgitation on long-term, but leadless pacemakers do not have this risk, and (x) she is a case of Eisenmenger syndrome who will eventually need heart lung transplantation, until then a simple pacing procedure was intended for her.
The estimated average longevity of single chamber Micra leadless pacemaker is about 12 years. The optimal end-of-life strategy would be either to place an additional leadless pacemaker adjacent to the non-functioning device in right ventricle, or to retrieve the non-functioning Micra and subsequently implant a new pacemaker. In the current case, although the patient is young, she has complex congenital heart disease with Eisenmenger syndrome and there is very high chance that patient may undergo heart lung transplantation prior to the end-of-life of her leadless pacemaker.
In general, implantation of transvenous pacemaker in patients with complex congenital heart disease may be technically demanding due to altered venous anatomy and increased risk of infection and embolization, etc [3]. Transcatheter leadless pacemaker may be a better choice in this context because they eliminate complications related to lead extraction and have lower rate of venous thrombosis and infection compared to conventional pacemakers both at short-term and long-term follow-up [4,5]. When transthoracic echocardiography does not completely delineate the altered cardiovascular anatomy in patients with complex congenital heart disease it may be better to perform transesophageal echocardiography or even preferably radiological imaging like cardiac CT or MRI prior to transcatheter leadless pacemaker implantation to understand the cardiovascular anatomy and plan the procedure accordingly. Unfortunately, altered venous anatomy was not detected in the preprocedural echocardiography in this case and cardiac CT was performed only after the completion of the pacemaker implantation procedure. In patients with limitations of lower venous system like this patient, approach through internal jugular vein or subclavian vein can be tried for implanting leadless pacemaker.
Transcatheter leadless pacemaker is an option for patients not suitable for either conventional pacemaker with endocardial lead implantation or epicardial pacing. The possibility to deploy the device from the femoral vein, without the need to create a pocket and tunneling the pacing leads, is a major advantage of this transcatheter leadless pacemaker system. However, there are some potential risk for occasional complications associated with implanting leadless pacemaker such as pericardial effusion, vascular access site complications and very rarely device embolization. To conclude, the decision to choose between conventional and leadless pacemaker in every patients should be individualized.
4. Conclusion
Transcatheter leadless permanent pacemaker was implanted successfully through transfemoral access in this complex congenital heart disease with interrupted IVC and azygous continuation. Besides transthoracic echocardiography, it may be better to perform transesophageal echocardiography or even preferably radiological imaging like cardiac CT or MRI prior to transcatheter leadless pacemaker implantation in patients with complex congenital heart disease to understand the cardiovascular anatomy and plan the procedure.
Funding
No funding/grant was obtained for this study.
Declaration of competing interest
None to be declared for all the authors of this study.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Ferrero P., Yeong M., D'Elia E., Duncan E., Graham Stuart A. Leadless pacemaker implantation in a patient with complex congenital heart disease and limited vascular access. Indian Pacing Electrophysiol J. 2016;16(6):201–204. doi: 10.1016/j.ipej.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCanta A.C., Morchi G.S., Tuozo F., Berdjis F., Starr J.P., Batra A.S. Implantation of a leadless pacemaker in a pediatric patient with congenital heart disease. HeartRhythm Case Rep. 2018;4(11):506–509. doi: 10.1016/j.hrcr.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira M., Mesquita D., Cunha P.S., Delgado A.S., Ferreira R.C. Leadless pacemaker implantation via azygos vein in a patient with absence of the hepatic segment of the inferior vena cava. Europace. 2019;21(4):547. doi: 10.1093/europace/euy274. [DOI] [PubMed] [Google Scholar]
- 4.Mohananey D., Jobanputra Y., Kumar A., et al. Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: meta-analysis and meta-regression. Circ Cardiovasc Interv. 2017;10(7) doi: 10.1161/CIRCINTERVENTIONS.117.005046. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Sande J.L., García-Seara J., Rodríguez-Mañero M., et al. The Micra leadless transcatheter pacemaker. Implantation and mid-term follow-up results in a single center. Rev Esp Cardiol. 2017;70(4):275–281. doi: 10.1016/j.rec.2016.11.027. [DOI] [PubMed] [Google Scholar]