Abstract
Background
Various implant designs have been proposed to increase active range of motion (ROM) and avoid notching in patients treated by reverse total shoulder arthroplasty (RSA). The purpose of this study was to investigate the efficacy and safety of an onlay prosthesis design combining a 135° humeral neck-shaft angle with the glenoid component lateralized and inferiorized.
Methods
A retrospective descriptive study was conducted of the clinical and radiological outcomes at the final follow-up (≥24 months) of all RSAs performed by the same surgeon between September 2015 and December 2016 in the study center. At the last follow-up, patients were clinically assessed for ROM, Constant score, and subjective shoulder value and radiologically for scapular notching and glenoid radiolucent lines. Patients were followed up radiographically at 1 month and clinically at between 6 and 12 months (midterm) and again at between 24 and 48 months (final follow-up). Scapular notching was graded as per the Sirveaux classification at the last follow-up on anterior-posterior radiographs.
Results
Seventy-nine RSAs were included with a mean follow-up time of 31 months. The mean Constant score at the final follow-up was 42 points higher than before surgery (69 vs. 27, P < .001). There were also significant postoperative improvements in ROM (active anterior elevation, active external rotation, and active internal rotation). The final means for motions were 133° for active anterior elevation, 32° active external rotation, and level 7 for active internal rotation. The overall notching rate was 3% (2/67), and there were no cases of severe notching. Radiolucent lines were observed in 8 of 70 prostheses (11.5%) around the peg, and they were observed in 9 prostheses (13%) around the screws. Among the 79 RSAs included, there were 11 complications (13.9%) (two infections, two fractures, four cases of glenoid component loosening, and three cases of instability), 2 reoperations, and 4 prosthesis revisions.
Conclusion
This study shows that an RSA design with a 135° humeral neck-shaft angle and an inferiorized and lateralized glenoid component is associated with significant improvements in active ROM, especially in rotation, and a low notching rate. However, rates of 3.8% for dislocation and 5% for glenoid loosening are certainly a concern at such a short follow-up of two years. Future studies with a larger population are needed to confirm these rates.
Keywords: Reverse shoulder arthroplasty, Grammont, 135° humeral component, Scapular notching
Grammont’s reverse shoulder total arthroplasty (RSA) is a safe and effective treatment option for a wide range of shoulder pathologies.5,13,28,40 The original concept medialized the center of rotation (COR) of the glenohumeral joint with a glenosphere placed flush with the inferior border of the glenoid bone, whereas the arm was lengthened using a 155° inlay humeral stem.32 This Grammont design has a long track record in relieving pain and improving range of motion (ROM).4,22,23,28,34,43 However, prior studies have reported two main limitations related to this design: poor results in active rotation6 and scapular notching due to impingement of the medial aspect of the polyethylene humeral cup against the scapular pillar.8 Three strategies have been proposed to increase the distance between the humeral component and the scapular pillar, improve ROM, and, thus, prevent scapular notching: glenoid component inferiorization of the COR using a specifically designed glenosphere, for example,12,15 implanting the baseplate in a distal position,15 lateralization of the COR using a specific glenoid component28 or the bony increased-offset (BIO) technic,7 and decreasing the neck-shaft angle of the humeral component.26,35,46 In 2005, Frankle et al proposed to both lateralize the COR, using a specific glenoid component, and use a humeral stem with a lower (135°) neck-shaft angle.26 Subsequent evaluations of the latter configuration have found that it offers significant improvements in ROM, especially in external rotation (28°), without notching at 2-year follow-up18 and then stable results over time (a notching rate of 9% at 60 months).17,19
Recently, computer templating studies35,46 have suggested that the best option is to associate lateralization and inferiorization of the COR with an onlay humeral stem with a 135° neck-shaft angle. These are significant changes to Grammont’s concept, and although most studies of RSAs with BIO report no improvements in ROM but confirm the reduction in notching,21,25,31,41 other authors have found increased rates of shoulder instability11 and scapular spine fracture.2
The primary objective of this study was to evaluate ROM improvement and the notching rate in patients who underwent RSA with a 135° humeral stem and both lateral and inferior glenosphere offsetting. The secondary objective was to assess rates of clinical and radiological complications.
Materials and methods
Study design and approval
This was a retrospective study of prospectively collected data. The study was approved by the local institutional review board (COS-RGDS-2020-01-006). All patients gave informed consent to participate
Study population
All patients treated by the same surgeon (GW) who underwent primary RSA between September 2015 and December 2016 with at least 24 months of follow-up were included. All patients received a 135° neck-shaft angle onlay humeral stem, glenoid component lateralization with a humeral head bone graft (BIO-RSA described by Boileau et al7), and an eccentric glenosphere (Fig. 1). The goal of the glenoid lateralization was to create 5 mm of lateralization between the glenoid component and the native glenoid bone at the level of the post of the baseplate. The exclusion criteria were patients with congenital or post-traumatic bone deformities, requiring a specific patient stem, and patients with neoplastic sequelae.
Figure 1.
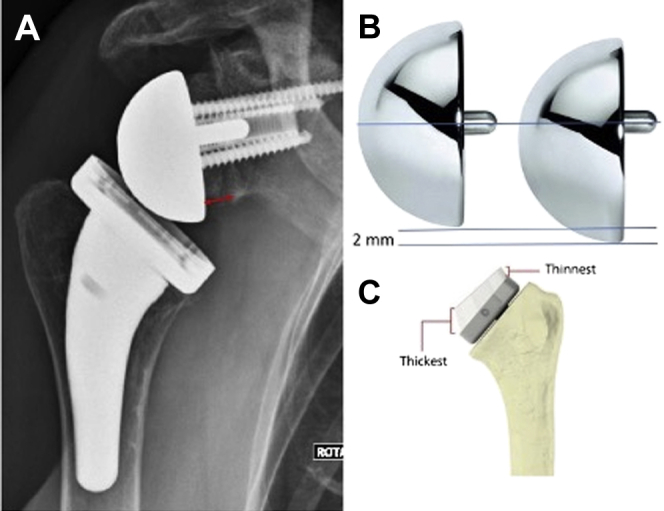
(A) Association of a lateralized glenoid component (red arrow), an eccentric glenosphere (shown in panel B), and a 135° humeral neck-shaft angle (shown in panel C).
Surgical technique
Preoperative three-dimensional planning was carried out for all patients with the software Tornier Blueprint 3D Planning (Wright Medical Inc., Memphis, TN, USA) (AMPS II) to optimize the implantation of the components, their size (diameter of the base plate and of the sphere), and the characteristics of the bone graft (25 or 29 mm diameter, 0° or 12° angle, and thickness). The objectives were to optimize the size and inclination of the glenosphere to ensure neutral tilt and version while increasing glenoid bone stock and lateralizing the COR. To prevent the polyethylene humeral cup abutting against the scapular neck, the base plate was placed flush against the inferior and posterior border of the glenoid after reaming.
The same type of the implant was used in all cases (Aequalis Ascend Flex; Tornier, Montbonnot, France), and a deltopectoral approach was used; the subscapularis tendon (if intact) was tenotomized at the anatomical neck of the humerus. The bone graft chosen in the planning stage was harvested from the humeral head using the BIO-RSA technique.7 The humeral head was cut at its anatomical neck following natural retroversion. Short compactors were locked at 127.5° and used to prepare the proximal humerus. Once the final compactor was in place, a surface planer was used to correct the resection angle and flatten the resection surface. Trial trays were used in position 6 (the most lateral tray position) to prevent excessive humeral lateralization, with 1.5-mm or 3.5-mm offsets. The offset of the tray was chosen by placing the superolateral border of the metallic tray slightly below the top of the greater tuberosity, as recommended by Läderman (Fig. 1).37 On the glenoid side, a guidewire was placed to follow the preoperative plan with either a standard or a patient-specific glenoid guide (available for 36 shoulders) and to place the glenoid base plate flush with the inferior part of the glenoid. The glenoid was reamed to the level of the pin entry point in the glenoid bone. The unreamed surface of the glenoid was drilled with a 2-mm pin to obtain bone bleeding without reaming. The aim of these procedures was to ensure that each glenoid was resurfaced in a standardized manner and thereby avoid excessive bone removal and reproduce the preoperative plan. A long-peg (25 mm) glenosphere baseplate was impacted with the bone graft. The glenosphere was fixed with three or four screws based on the available glenoid bone stock and the surgeon’s preference. A 36-, 39-, or 42-mm eccentric glenosphere was used to increase the inferior offset by 2 mm. The diameter of the base plate (28 baseplates of 25 mm and 51 baseplates of 29 mm) and the diameter of the glenosphere (36, 39, or 42 mm) were planned preoperatively for each patient and were not randomized (in total: 53, 7, and 19 glenosphere implants, respectively). The humeral component with a 135° neck-shaft angle (a 127.5° stem, a 1.5- or 3.5-mm offset tray, and a 6-mm thick, 7.5° angled polyethylene cup in the lateral position [position 6]) was placed in the standard fashion. The subscapularis tendon was repaired in all possible cases via transosseous sutures (52 repairs and 19 partial repairs).
Assessment criteria
Patients were followed up radiographically and clinically at 1 month, 3 months, 6 months, and 12 months (midterm) and again at 24 and 48 months (final follow-up).
ROM and Constant scores16 were measured preoperatively and at the last follow-up, whereas subjective shoulder values (SSVs)29 were determined at the last follow-up only. The motions considered were passive and active motions in anterior elevation, external rotation arm at side, and internal rotation hand to back.
Scapular notching was graded as per the Sirveaux classification39 at the last follow-up on anterior-posterior (AP) radiographs of the glenohumeral joint on three views (shoulder in internal, neutral, and external rotation) after fluoroscopy framing, as per a standardized protocol at a radiology center specialized in musculoskeletal imaging. Notching severity was evaluated as low (stage 1) or high grade (stage 2 or higher). When osteolysis of the inferior part of the graft was observed, the images were independently reviewed by a second investigator to differentiate between classical graft osteolysis (oblique erosion) and notching (double contour erosion) (Fig. 2). The radiographs were also assessed for radiolucent lines around the post and screws and for any other obvious signs of glenosphere loosening or component disassembly. Radiolucencies >2 mm in width were considered radiological signs of loosening. Patients with poor-quality radiographs (too oblique incidence because of thoracic kyphosis, for example) and nonreoperated patients with glenosphere loosening were included in the analysis of clinical outcomes but excluded from the radiographic analysis.
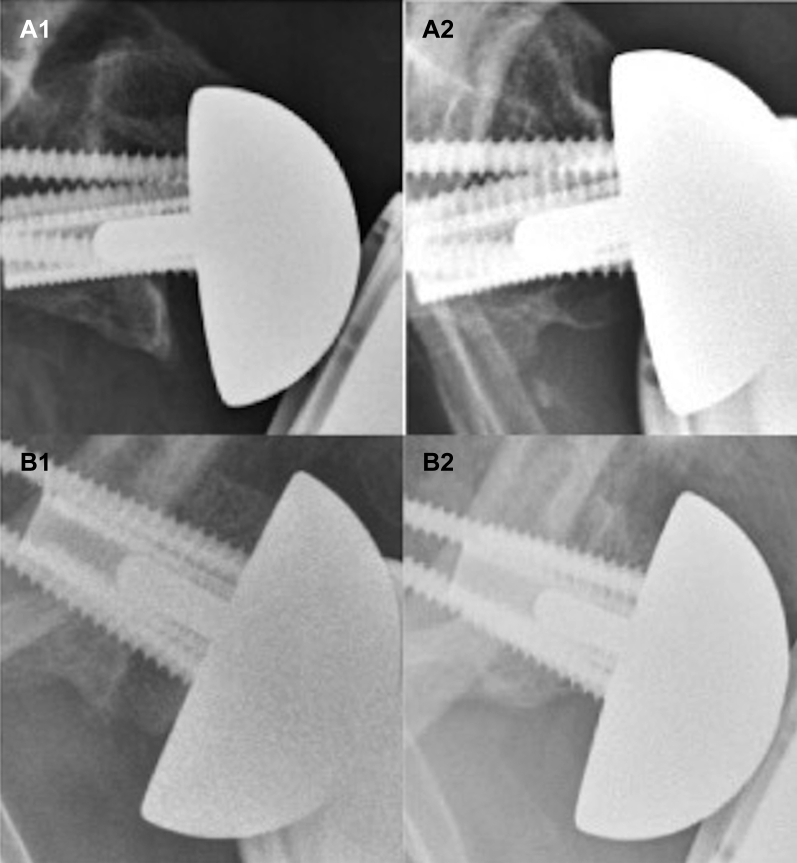
Figure 2.
Bone graft osteolysis vs. notching. (A1 and B1) AP postoperative radiographs. (A2) Notch with double contour erosion. (B2) graft osteolysis with oblique erosion. AP, anterior-posterior.
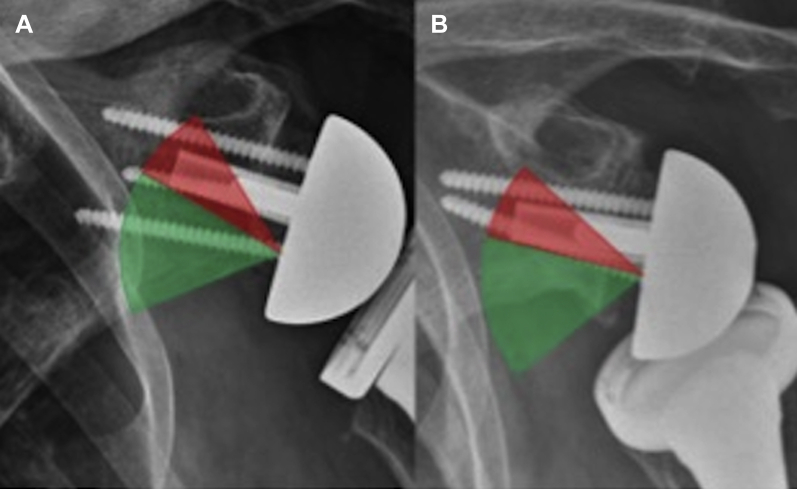
The orientation of the inferior baseplate screw was carefully analyzed on 1-month postoperative radiographs. Two areas were defined by drawing a line from the head of the inferior screw to the inferomedial angle of the post (Fig. 3). Patients in whom the axis of the inferior screw lay above this line were compared with those in whom the axis was below the line.
Figure 3.
AP radiographs showing (A) an RSA prosthesis with a safely positioned inferior screw (in the green area) and (B) an RSA prosthesis with a badly positioned inferior screw (in the red area) at the risk of glenoid bone loosening. AP, anterior-posterior; RSA, reverse total shoulder arthroplasty.
Statistical analysis
All calculations were made with SAS for Windows (v 9.4; SAS Institute Inc.). Statistical significance was defined as P < .05. Descriptive statistics are provided as per the nature of the criterion considered. Quantitative variables are presented as mean (range), and categorical variables are described as number (percentage). Preoperative and postoperative values were compared using paired t-tests.
Results
Study population
Eighty-four primary RSAs were performed between September 2015 and December 2016. Two patients were excluded because they had post-traumatic humeral bone deformation and, thus, received a specific humeral stem implant. Three patients (3.5% of shoulders) were lost to follow-up, so that the final study population consisted of 79 RSAs (73 patients; 48 women and 25 men). The mean age at inclusion was 73 years (range: 28-90), and the mean follow-up time was 31 months (range: 24-44 months). The reasons for surgery were primary osteoarthritis in 26 of 79 cases (33%), massive rotator cuff tear in 23 cases (29%), cuff tear arthropathy in 20 cases (25%), fracture sequelae in 4 cases (5%), rheumatoid arthritis in 4 cases (5%), and instability arthropathy in 2 cases (3%). No revision arthroplasties were included (Table I).
Table I.
Demographic characteristics of the study group.
| Variable | Total | |
|---|---|---|
| Age (yr) | N | 79 RSA/73 patients |
| Mean (SD) | 72.6 (9.9) | |
| Median [Min; Max] | 74 [28; 90] | |
| Follow-up (mo) | Mean (SD) | 30.08 (6.1) |
| Median [Min; Max] | 30 [24; 44] | |
| Sex | Male:female | 25:48 |
| Dominant side arthroplasty | Yes | 44 (56%) |
| No | 35 (44%) | |
| RSA indication | Cuff tear arthropathy | 20 (25%) |
| Primary osteoarthritis | 26 (33%) | |
| Massive rotator cuff tear | 23 (29%) | |
| Fracture sequelae | 4 (5%) | |
| Rheumatoid arthritis | 4 (5%) | |
| Instability arthropathy | 2 (3%) |
SD, standard deviation; RSA, reverse total shoulder arthroplasty.
Complications and revisions
Revision surgeries were defined as replacement of the intraosseous components and/or modification of the sizes of the mobile implants. Reoperations were surgeries that did not involve implant exchange and/or any surgeries involving change of the mobile parts without changing the sizes or position of the mobile implants. There were 11 postoperative complications in 10 patients (12.5%): two cases of Cutibacterium acnes infection (2.5% of prostheses), three cases of shoulder instability (3.8%) with recurrent dislocations in two cases (2.5%), four cases of glenoid loosening (5%), and two post-traumatic humeral fractures.
There were two reoperations (2.5%; 1 case of infection and 1 case of osteosynthesis on periprosthetic fracture) and four prosthesis revisions (5%; 1 case of infection, 1 case of glenoid loosening, and 2 cases of persistent instability).
The two humeral fractures occurred, one in the patient with C acnes infection described previously and was treated accordingly (débridement of the periprosthetic tissue, synovectomy, and irrigation), whereas the other, a type D (mid-diaphysis) fracture (24), occurred 15 months after surgery and was treated by open reduction and internal fixation. There were no cases of scapular spine fracture.
Clinical outcomes
The clinical outcomes at the final follow-up are summarized in Table II. The ROM improved significantly (active anterior elevation, active external rotation at 0° of abduction, and active internal rotation at 0° of abduction, P < .001). The mean final Constant score was 69 (+42 compared with the preoperative evaluation, P < .001), and the mean SSV was 80% at the last follow-up (Table III).
Table II.
Functional scores before surgery and at the last follow-up.
| Variable | Preoperative N = 79 |
Postoperative N = 79 |
P value |
|---|---|---|---|
| aAE (°) | <.001 | ||
| Mean (SD) | 84 (34.6) | 133 (34.8) | |
| Median | 90 | 145 | |
| [Min; Max] | [20; 180] | [30; 180] | |
| Missing data | 1 | 3 | |
| aER1 (°) | <.001 | ||
| Mean (SD) | 5 (18.3) | 32 (26) | |
| Median | 0 | 30 | |
| [Min; Max] | [−30; 90] | [−15; 90] | |
| Missing data | 1 | 3 | |
| aIR1 (level) | <.001 | ||
| Mean (SD) | 4 (2) | 7 (3) | |
| Median | 2 | 8 | |
| [Min; Max] | [0; 10] | [2; 10] | |
| Missing data | 1 | 3 |
SD, standard deviation; aAE, active anterior elevation; aER1, active external rotation at 0° of abduction; aIR1, active internal rotation at 0° of abduction. Levels for IR1: 0 = lateral thigh, 2 = buttock, 4 = lumbosacral junction, 6 = L3, 8 = T12, 10 = T7.
Table III.
Constant scores and subjective shoulder values before surgery and at the last follow-up.
| Variable | Preoperative N = 79 |
Postoperative N = 79 |
P value |
|---|---|---|---|
| Constant score | |||
| Total | |||
| Mean (SD) | 27 (11.9) | 69 (16.5) | <.001 |
| Median | 28 | 73 | |
| [Min; Max] | [9; 68] | [34; 95] | |
| Missing data | 2 | 3 | |
| Pain | |||
| Mean (SD) | 4 (2.4) | 13 (3) | <.001 |
| Median | 3 | 15 | |
| [Min; Max] | [2; 12] | [5; 15] | |
| Missing data | 2 | 3 | |
| Activity | |||
| Mean (SD) | 6 (3.1) | 39 (13.5) | <.001 |
| Median | 6 | 40.5 | |
| [Min; Max] | [2; 20] | [16; 83] | |
| Missing data | 2 | 3 | |
| Mobility | |||
| Mean (SD) | 15 (8.2) | 52 (14.4) | <.001 |
| Median | 14 | 53 | |
| [Min; Max] | [2; 34] | [24; 98] | |
| Missing data | 2 | 3 | |
| Strength | |||
| Mean (SD) | 1 (2) | 8 (6.2) | |
| Median | 1 | 7.5 | <.001 |
| [Min; Max] | [0; 10] | [0; 24] | |
| Missing data | 2 | 3 | |
| SSV | |||
| Mean (SD) | 77 (18.1) | ||
| Median | 80 | ||
| [Min; Max] | [20; 100] | ||
| Missing data | 6 |
SD, standard deviation.
Radiographic outcomes
Standardized radiographs at the final follow-up were performed in the study center for 65 of 79 prostheses (82.5%) and externally for the remaining 14.
Four of the 79 prostheses (5%) had loosening around the glenoid component (radiolucent lines >2 mm thick) with migration of the implant. Among these 4 cases of glenoid loosening, 2 cases presented a complete resorption of the bone graft. In one of the cases, the analysis of the integration of the bone graft was not possible. In addition, in the last case, the integration of the bone graft had a good aspect. Although it is a high rate of glenoid loosening, there are too few events to conclude on the relationship between bone graft resorption and glenoid loosening.
In two of these cases, loosening did not have a significant negative impact on functional outcomes (Constant scores, 85 and 68, and SSVs, 90% and 80%, respectively). One patient underwent revision surgery to replace the glenoid implant. Analysis of the postoperative radiographs revealed two types of technical error. In one patient, the baseplate was implanted with a superior tilt. In all four patients, the inferior cortical unlocking screw was too close to the central peg of the baseplate and oriented superiorly with the axis of the lower screw crossing the inferior border of the central peg (critical zone, Fig. 3). In this series, four of the eight patients with an inferior screw in the critical zone had glenoid bone loosening compared with none of those (0/60) with an inferior screw in the safety zone (P = .0001).
Twelve prostheses were excluded from the radiographic analysis of scapular notching: five patients with kyphosis because the incidence was too oblique, the four patients revised within 24 months of the initial surgery, and the three patients with radiographic loosening and migration of the baseplate who were not reoperated. Scapular notching was observed in 2 of 67 prostheses (3%), and both cases were assessed as grade 1 (mild). In the first case, notching may have been caused by the glenoid implant having been placed in a superior tilt (Fig. 4), but this did not have a negative effect on functional outcomes (Constant score, 83; SSV, 90%). The second patient had bone graft osteolysis with bone remodeling in the inferior part of the native glenoid (Fig. 5). In spite of this atypical presentation, the diagnosis of notching was retained on review.
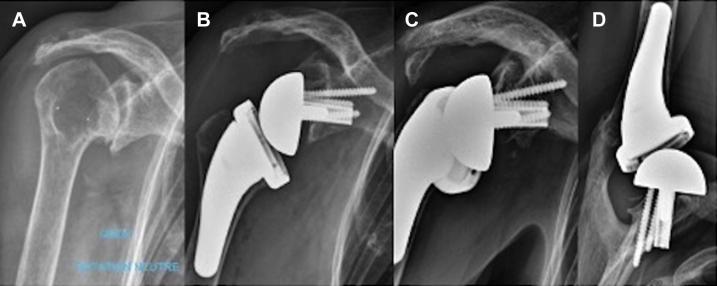
Figure 4.
Scapular notching (case 1). (A) Preoperative AP radiograph showing massive superior glenoid erosion. (B) Postoperative AP view showing the superior tilt of the glenoid implant. (C) AP and (D) axillary views at 2 years’ follow-up showing grade I notching and a bony spur with osteolysis of the bone graft. A preoperative os acromiale tilted about 90° inferiorly without any consequence. AP, anterior-posterior.
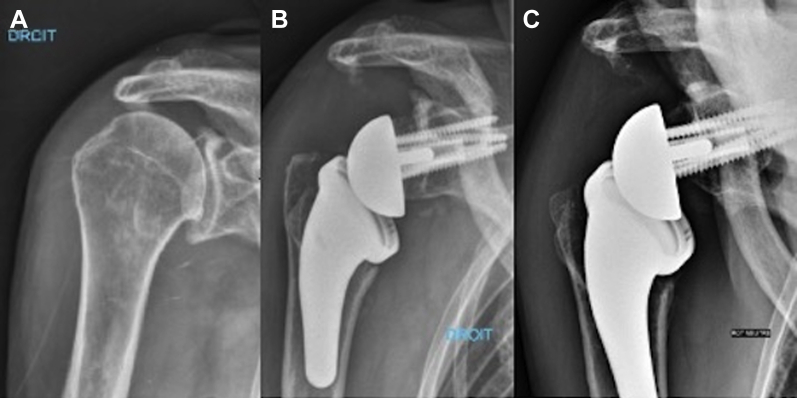
Figure 5.
Scapular notching (case 2). (A) Preoperative AP radiograph, showing Hamada stage 2 massive rotator cuff tear. (B) Postoperative AP view. (C) AP view at 2 years’ follow-up showing osteolysis at the inferior part of the bone graft that was interpreted as notching grade 1.
Nine prostheses were excluded from the radiographic analysis of radiolucent lines: the five patients with kyphosis, because of poor-quality images, the two patients who were reoperated for C acnes infection, and the two patients who were reoperated for instability. A glenoid spur was observed in 8 of 70 prostheses (11.5%), and glenohumeral ossification was observed in three prostheses (4%). Radiolucent lines were observed in 8 of 70 prostheses (11.5%) around the peg and in 9 prostheses (13%) around the screws.
Discussion
The main finding of this study is that patients with a lateralized and inferiorized glenoid component and a 135° humeral stem had significant improvements in functional scores postoperatively, especially in internal and external rotation, with a very low notching rate and no cases of severe notching. The second finding is that this prosthesis design is safe, especially in terms of the severe clinical complications reported in the literature for other RSA designs, notably shoulder instability and spine fracture. Glenoid loosening was observed in 5% of cases, but this was most likely due to misplacement of the inferior screw—too close to the peg of the baseplate—rather than to the prosthesis design itself.
In theory, medializing the COR decreases the efficiency of any remaining rotator cuff in external and internal rotations, as reflected in practice by the very low rotational ROM of patients treated with Grammont-design prostheses.4,23,45 The strategy initially proposed by Frankle et al26 to increase impingement-free motion was to lateralize the COR and use a 135° humeral stem. Significant improvements in ROM have since been reported with this design.17, 18, 19 An alternative approach7 involves lateralizing the glenoid implant using bone harvested from the humeral head, with several studies highlighting the benefits of lateralization.21,25,31,41 In particular, Franceschetti et al25 have recently reported encouraging results in a series of patients treated with this type of the glenoid implant and a 145° humeral component. However, in a recent digital templating study, Werner et al46 found that better theoretical impingement-free ROM could be achieved by combining a 135° humeral stem with glenoid lateralization and inferiorization (using an eccentric glenosphere, Fig. 1). The present study of this approach shows that it significantly improves functional outcomes in both anterior flexion and in external and internal rotation at 0° and 90° abduction. The improvements in external rotation are similar to those reported for patients treated using 145° lateralized RSA25,41 or the design by Frankle et al.26 However, the present study’s outcomes regarding internal rotation—hand to back—are among the best in the literature (up to L1 vs. between the sacrum and L5 in patients treated with a lateralized RSA and a 145° humeral stem).25,41 This is possibly because of the increased distance between the medial aspect of the humeral component and the scapula pillar afforded by the increased lateral and inferior offset of the glenoid component and the 135° humeral neck-shaft angle, which may prevent impingement between the humerus and scapula. This would be reflected radiologically by a lower notching rate and clinically by improved ROM. Comparative studies are required, however, to confirm the superiority of this design.
Another aim of increasing the distance between the medial aspect of the humeral component and the scapular pillar is to prevent scapular notching.39,42 Existing results indicate that notching rates are lower (<10%) with 135° humeral stems than with 145° or 155° humeral neck-shaft angles.3,14,17,19,21,25,31,33,41 Combining glenoid lateralization and inferiorization with a 135° humeral neck-shaft angle should reduce the friction between the polyethylene insert and the scapular pillar, and the low rate of notching in our study (3%) suggests that this is an effective way of preventing scapular notching, at least in the short term. Previous authors report an increase in the notching rate over time.17,19 Longer follow-up studies are therefore required to confirm our findings.
Shoulder instability is a common postoperative complication of RSA.9,10,27 Several risk factors have been identified, but the mechanism is often unclear,9,10,20,27,44,48 and the influence of the humeral neck shaft angle remains to be clarified.10,21,24 The low instability rate in the present study (3.8%) suggests that 135° BIO-RSA with glenoid lateralization and inferiorization is a safe treatment option. The two patients with recurrent shoulder instability had clear risk factors, namely, male gender and a subscapularis deficiency. One of the two patients was also noncompliant with postoperative instructions and had a traumatic injury. Early recovery of postoperative ROM, as in the second patient, may be a risk factor for dislocation, but the single case in this study is insufficient evidence to conclude.
In a biomechanical study of RSA implant parameters, COR lateralization was found to increase deltoid muscle force during elevation of the arm,47 which could increase the risk of spine stress fracture. A recent study of RSA using an onlay-design 145° humeral stem identified an increased prevalence of scapular spine fracture compared with the standard Grammont design (∼4%-5% vs. ∼1%).2 Further studies are required to determine risk factors for scapula spine fractures and how they can be prevented.
Lateralization and inferiorization of the glenoid component and humeral lengthening all increase soft-tissue tension and, thus, joint load30,36,38 and the risk of glenoid component failure.1 Although just one of our 79 patients (1.5%) underwent revision surgery for glenoid component loosening, radiographic loosening with migration of the glenoid component was observed in four patients (5% of prostheses). In their first study of RSAs with glenoid lateralization and 135° humeral stems, Frankle et al26 reported a glenoid loosening rate of 13.5%. In the present study, radiographic analysis of all four cases of glenoid loosening showed that the inferior screw was implanted too close to the peg of the baseplate, with their axes crossing (Fig. 3). This may have reduced bone ingrowth around the peg and thereby also reduced the primary stability and bony integration of the baseplate. To avoid this complication, we recommend implanting the inferior screw horizontally or oriented inferiorly to keep it away from the peg.
The limitations of this study include its retrospective nature and the fact that the Sirveaux classification used to evaluate notching only considers inferior notching in the AP view, with no assessment of posterior notching or glenoid osteolysis. Moreover, cases of posterior notching may have been missed because these are difficult to assess on AP radiographs. Standardized glenoid lateralization and inferiorization may be difficult to obtain, but as explained, we standardized our glenoid reaming technique to reproduce the preoperative plan and achieve the desired lateralization and inferiorization. In addition, variability depending on the incidence of the radiographs could be a source of measurement method bias; however, patients were systematically reviewed at 1 month, 3 months, and 6 months with a standardized radiographic protocol at the clinic. The strengths of this study are that it is the first to report clinical and radiological results for the optimal RSA design by Werner et al,46 associating glenoid lateralization and inferiorization with a 135° humeral stem. The present study is relatively large, and the surgical indication, technique, and implant were the same for all patients. All operations were performed by the same surgeon, reducing variability, and all clinical and radiological assessments were standardized.
Conclusion
This study shows that an RSA design with a 135° humeral neck-shaft angle and an inferiorized and lateralized glenoid component is associated with significant improvements in active ROM, especially in rotation, and a low notching rate. However, rates of 4% for dislocation and 5% for glenoid loosening are certainly a concern at such a short follow-up of two years. Future studies with a larger population are needed to confirm these rates.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: G.W. and M-O.G. receive royalties from Wright Company. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional Review Board approval was received from the Conseil d’Orientation Scientifique Ramsay Santé : Comité d’Ethique (IRB COS-RGDS-2020-01-006-COLLOTTE-P).
References
- 1.Ackland D.C., Patel M., Knox D. Prosthesis design and placement in reverse total shoulder arthroplasty. J Orthop Surg. 2015;10:101. doi: 10.1186/s13018-015-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascione F., Kilian C.M., Laughlin M.S., Bugelli G., Domos P., Neyton L., et al. Increased scapular spine fractures after reverse shoulder arthroplasty with a humeral onlay short stem: an analysis of 485 consecutive cases. J Shoulder Elbow Surg. 2018;27:2183–2190. doi: 10.1016/j.jse.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Athwal G.S., MacDermid J.C., Reddy K.M., Marsh J.P., Faber K.J., Drosdowech D. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg. 2015;24:468–473. doi: 10.1016/j.jse.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Bacle G., Nové-Josserand L., Garaud P., Walch G. Long-Term outcomes of reverse total shoulder arthroplasty: a follow-up of a Previous study. J Bone Jt Surg. 2017;99:454–461. doi: 10.2106/JBJS.16.00223. [DOI] [PubMed] [Google Scholar]
- 5.Bassens D., Decock T., Van Tongel A., De Wilde L. Long-term results of the Delta Xtend reverse shoulder prosthesis. J Shoulder Elbow Surg. 2019;28:1091–1097. doi: 10.1016/j.jse.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Berliner J.L., Regalado-Magdos A., Ma C.B., Feeley B.T. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:150–160. doi: 10.1016/j.jse.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Boileau P., Moineau G., Roussanne Y., O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop. 2011;469:2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers P.N., Rahman Z., Romeo A.A., Nicholson G.P. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:737–744. doi: 10.1016/j.jse.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Cheung E., Willis M., Walker M., Clark R., Frankle M.A. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19:439–449. [PubMed] [Google Scholar]
- 11.Cheung E.V., Sarkissian E.J., Sox-Harris A., Comer G.C., Saleh J.R., Diaz R., et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:1946–1952. doi: 10.1016/j.jse.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Chou J., Malak S.F., Anderson I.A., Astley T., Poon P.C. Biomechanical evaluation of different designs of glenospheres in the SMR reverse total shoulder prosthesis: range of motion and risk of scapular notching. J Shoulder Elbow Surg. 2009;18:354–359. doi: 10.1016/j.jse.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Collin P., Betz M., Herve A., Walch G., Mansat P., Favard L., et al. Clinical and structural outcome 20 years after repair of massive rotator cuff tears. J Shoulder Elbow Surg. 2020;29:521–526. doi: 10.1016/j.jse.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Collin P., Liu X., Denard P.J., Gain S., Nowak A., Lädermann A. Standard versus bony increased-offset reverse shoulder arthroplasty: a retrospective comparative cohort study. J Shoulder Elbow Surg. 2018;27:59–64. doi: 10.1016/j.jse.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Collotte P., Erickson J., Vieira T.D., Domos P., Walch G. Midterm clinical and radiologic results of reverse shoulder arthroplasty with an eccentric glenosphere. J Shoulder Elbow Surg. 2020;29:976–981. doi: 10.1016/j.jse.2019.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Constant C.R., Murley A.H. A clinical method of functional assessment of the shoulder. Clin Orthop. 1987:160–164. [PubMed] [Google Scholar]
- 17.Cuff D., Clark R., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff Deficiency: a Concise follow-up, at a minimum of five Years, of a Previous report∗. J Bone Jt Surg. 2012;94:1996–2000. doi: 10.2106/JBJS.K.01206. [DOI] [PubMed] [Google Scholar]
- 18.Cuff D., Pupello D., Virani N., Levy J., Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff Deficiency. J Bone Jt Surg. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 19.Cuff D.J., Pupello D.R., Santoni B.G., Clark R.E., Frankle M.A. Reverse shoulder arthroplasty for the treatment of rotator cuff Deficiency: a Concise follow-up, at a minimum of 10 Years, of Previous reports∗. J Bone Jt Surg. 2017;99:1895–1899. doi: 10.2106/JBJS.17.00175. [DOI] [PubMed] [Google Scholar]
- 20.Edwards T.B., Williams M.D., Labriola J.E., Elkousy H.A., Gartsman G.M., O’Connor D.P. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Erickson B.J., Frank R.M., Harris J.D., Mall N., Romeo A.A. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24:988–993. doi: 10.1016/j.jse.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ernstbrunner L., Andronic O., Grubhofer F., Camenzind R.S., Wieser K., Gerber C. Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: a systematic review of longitudinal outcomes. J Shoulder Elbow Surg. 2019;28:774–781. doi: 10.1016/j.jse.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Favard L., Levigne C., Nerot C., Gerber C., De Wilde L., Mole D. Reverse prostheses in Arthropathies with cuff tear: are Survivorship and function Maintained over time? Clin Orthop Relat Res. 2011;469:2469–2475. doi: 10.1007/s11999-011-1833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferle M., Pastor M.-F., Hagenah J., Hurschler C., Smith T. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg. 2019;28:966–973. doi: 10.1016/j.jse.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Franceschetti E., Ranieri R., Giovanetti de Sanctis E., Palumbo A., Franceschi F. Clinical results of bony increased-offset reverse shoulder arthroplasty (BIO-RSA) associated with an onlay 145° curved stem in patients with cuff tear arthropathy: a comparative study. J Shoulder Elbow Surg. 2020;29:58–67. doi: 10.1016/j.jse.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Frankle M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff DeficiencyA minimum two-year follow-up study of Sixty patients. J Bone Jt Surg Am. 2005;87:1697. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 27.Gallo R.A., Gamradt S.C., Mattern C.J., Cordasco F.A., Craig E.V., Dines D.M., et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20:584–590. doi: 10.1016/j.jse.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Gerber C., Canonica S., Catanzaro S., Ernstbrunner L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: results after 15 years. J Shoulder Elbow Surg. 2018;27:831–838. doi: 10.1016/j.jse.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Gilbart M.K., Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 30.Giles J.W., Langohr G.D.G., Johnson J.A., Athwal G.S. Implant design Variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res. 2015;473:3615–3626. doi: 10.1007/s11999-015-4526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gobezie R., Shishani Y., Lederman E., Denard P.J. Can a functional difference be detected in reverse arthroplasty with 135° versus 155° prosthesis for the treatment of rotator cuff arthropathy: a prospective randomized study. J Shoulder Elbow Surg. 2019;28:813–818. doi: 10.1016/j.jse.2018.11.064. [DOI] [PubMed] [Google Scholar]
- 32.Grammont P.M., Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 33.Greiner S., Schmidt C., Herrmann S., Pauly S., Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24:1397–1404. doi: 10.1016/j.jse.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 34.Guery J., Favard L., Sirveaux F., Oudet D., Mole D., Walch G. Reverse total shoulder arthroplasty: Survivorship analysis of eighty replacements followed for five to ten Years. J Bone Jt Surg. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez S., Comiskey C.A., Luo Z.-P., Pupello D.R., Frankle M.A. Range of impingement-free abduction and Adduction Deficit after reverse shoulder arthroplasty: Hierarchy of surgical and implant-design-related factors. J Bone Jt Surg.-Am. 2008;90:2606–2615. doi: 10.2106/JBJS.H.00012. [DOI] [PubMed] [Google Scholar]
- 36.Henninger H.B., Barg A., Anderson A.E., Bachus K.N., Burks R.T., Tashjian R.Z. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21:1128–1135. doi: 10.1016/j.jse.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Lädermann A., Denard P.J., Boileau P., Farron A., Deransart P., Terrier A., et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39:2205–2213. doi: 10.1007/s00264-015-2984-3. [DOI] [PubMed] [Google Scholar]
- 38.Lädermann A., Williams M.D., Melis B., Hoffmeyer P., Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Lévigne C., Boileau P., Favard L., Garaud P., Molé D., Sirveaux F., et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Lindbloom B.J., Christmas K.N., Downes K., Simon P., McLendon P.B., Hess A.V., et al. Is there a relationship between preoperative diagnosis and clinical outcomes in reverse shoulder arthroplasty? An experience in 699 shoulders. J Shoulder Elbow Surg. 2019;28:S110–S117. doi: 10.1016/j.jse.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Merolla G., Walch G., Ascione F., Paladini P., Fabbri E., Padolino A., et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27:701–710. doi: 10.1016/j.jse.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Simovitch R.W., Zumstein M.A., Lohri E., Helmy N., Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg. Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 43.Sirveaux F., Favard L., Oudet D., Huquet D., Walch G., Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg. Br. 2004;86:388–395. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 44.Trappey G.J., O’Connor D.P., Edwards T.B. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–2511. doi: 10.1007/s11999-010-1686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wall B., Nové-Josserand L., O’Connor D.P., Edwards T.B., Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg. Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 46.Werner B.S., Chaoui J., Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1726–1731. doi: 10.1016/j.jse.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Wong M.T., Langohr G.D.G., Athwal G.S., Johnson J.A. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg. 2016;25:1889–1895. doi: 10.1016/j.jse.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]