Abstract
Background
Tendon-to-bone (TtB) healing is essential for successful rotator cuff repair (RCR). This study aimed to investigate if caffeine intake impaired TtB healing in a rat RCR model.
Methods
Seventy-two rats were randomized into a caffeinated group or a noncaffeinated group. Specimens received one week of oral caffeine solution or normal saline before RCR. All rats then underwent bilateral RCR. Caffeination or saline gavages continued until rats were sacrificed at 2, 4, and 8 weeks postoperatively. Load-to-failure (primary outcomes measure), maximum stress, and stiffness of the TtB interface were measured for one shoulder of each specimen. Six random shoulders from each group underwent histological assessment of TtB healing.
Results
Load-to-failure and maximum stress of RCR did not appear to differ between groups at any time point. No difference in RCR stiffness was found between groups at 2 and 4 weeks; however, stiffness in the caffeinated group did appear to lower at 8 weeks (P = .04).
Conclusion
Perioperative caffeine intake did not appear to affect load-to-failure strength of RCR in an animal model. Although our secondary outcome measures of maximum stress and stiffness also did not appear to be influenced by perioperative caffeine intake, there did appear to be a trend toward decreased RCR stiffness at 8 weeks postoperatively in specimens that received caffeine.
Keywords: Caffeine, Rotator cuff healing, Tendon to bone healing, Rotator cuff repair, Basic science study
Rotator cuff repairs (RCRs) are among the most common orthopedic procedures performed.25 If loss of tendon-to-bone (TtB) continuity at the repair site occurs, it is often in the short to intermediate postoperative period when the RCR is vulnerable to conditions that may impair TtB healing.1 Identifying variables that inhibit TtB healing may help improve RCR outcomes.27 For example, it has been shown that tear size, age, osteoporosis, load removal, diabetes, nonsteroidal anti-inflammatory drugs (NSAIDs), fluoroquinolones, and nicotine may negatively effect the biomechanical properties, histological characteristics, and healing speed of RCRs via various mechanisms; however, many modifiable risk factors, such as perioperative caffeine use, remain unstudied.3,4,17,24,26
The ubiquitous consumption of caffeine, and its easily modifiable use, increases the importance of investigating the effects of this drug on TtB healing. Patients aged 50 to 64 years are among the most likely to undergo RCR5 and have the highest rate of caffeine consumption.20 Studies have found that caffeine can impede wound healing and epithelialization,23 inhibit collagen synthesis,8 and disturb early stages of bone healing.9 However, to our knowledge, no study has investigated the effect of caffeine on TtB healing, specifically.
The purpose of this study was to determine the effects of caffeine on TtB healing in an established rat RCR model. We hypothesized that perioperative caffeine intake would be associated with a decreased load-to-failure (LtF) strength of the repair and decreased tendon maturation on histological analysis.
Materials and methods
This is an experimental, basic science study involving a randomized control investigation to evaluate the biomechanical and histological properties of RCR in a rat model with or without exposure to perioperative caffeine. Approval for the study was obtained from the Institutional Animal Care and Use Committee. A power analysis was performed to determine a sufficient sample size based on LtF data from a previous study, assuming an alpha level of 0.05, two-tailed.4,8 Four extra rats were added to the initial sample size to account for expected perioperative complications.
Seventy-two adult male Sprague-Dawley rats (380-450 g) were randomized into two groups (caffeinated and noncaffeinated). On day 0, all rats received a daily oral gavage of either pure water or a solution of caffeine dissolved in distilled water (10 mg/kg/d), a dose which has been suggested to correspond to 3.5 mg/kg in humans (2-3 cups of coffee for a 70-kg human).11 Oral gavage was the chosen route of administration because it mimics the human route of consumption and provides controlled dosing. Additional rat maintenance techniques followed a previously published protocol.4
On day 7, all groups underwent bilateral RC detachment and immediate RCR as described in previous studies.13,14 An established bone tunnel suture fixation method of the supraspinatus was used.2,14 The surgeon was blinded to group designation at the time of surgery. Daily gavages continued until sacrifice date.
Twelve rats from each group were euthanized with inhaled carbon dioxide at 2, 4, and 8 weeks after surgery to evaluate time-dependent trends among several biomechanical and histological parameters, which were hypothesized to be susceptible to perioperative caffeine intake. Time until sacrifice strategically correlated with the inflammatory, proliferative, and remodeling stages of healing.13 Immediately after sacrifice, gross observations of the repair including signs of infection, appearance of tendon-bone continuity, and reattachment site color were documented. The protocol called for one shoulder from each rat, 12 per group, to be frozen at −80°C for future biomechanical analysis. Owing to unintended specimen death and that one rat in each group was mislabeled, specimen numbers sacrificed in the caffeine and non-caffeinated group at 2, 4, and 8 weeks were not evenly distributed across the groups; however, the study was still adequately powered for assessing our primary outcomes measure based on our power analysis (Table I). The contralateral shoulder of 6 rats from each group were randomly selected and prepared for histological assessment.
Table I.
Geometric and biomechanical properties.
| Group | Shoulders | Area (mm2)∗ | Maximum stress (Mpa)∗ | Load to failure (N)∗ | Stiffness (N/mm)∗ |
|---|---|---|---|---|---|
| Caffeine group | |||||
| 2 Weeks | 13 | 6.2 (1.9) | 2.3 (1.5) | 12.4 (6.6) | 9.8 (5.6) |
| 4 Weeks | 9 | 5.3 (1.7) | 4.9 (1.8) | 23.3 (4.9) | 15.3 (6.9) |
| 8 Weeks | 11 | 5.8 (1.7) | 4.6 (2.1) | 24.1 (6.9) | 9.7 (5.9)† |
| Noncaffeine group | |||||
| 2 Weeks | 8 | 6.0 (1.7) | 2.9 (2.0) | 15.0 (8.2) | 10.3 (8.4) |
| 4 Weeks | 13 | 5.5 (1.7) | 4.4 (2.5) | 21.6 (7.9) | 13.1 (5.3) |
| 8 Weeks | 11 | 5.8 (1.7) | 4.9 (1.6) | 26.9 (7.0) | 14.1 (2.8)† |
Values are presented as means with standard deviations in parentheses.
The comparison between groups is statistically significant at P < .05.
Surgical intervention
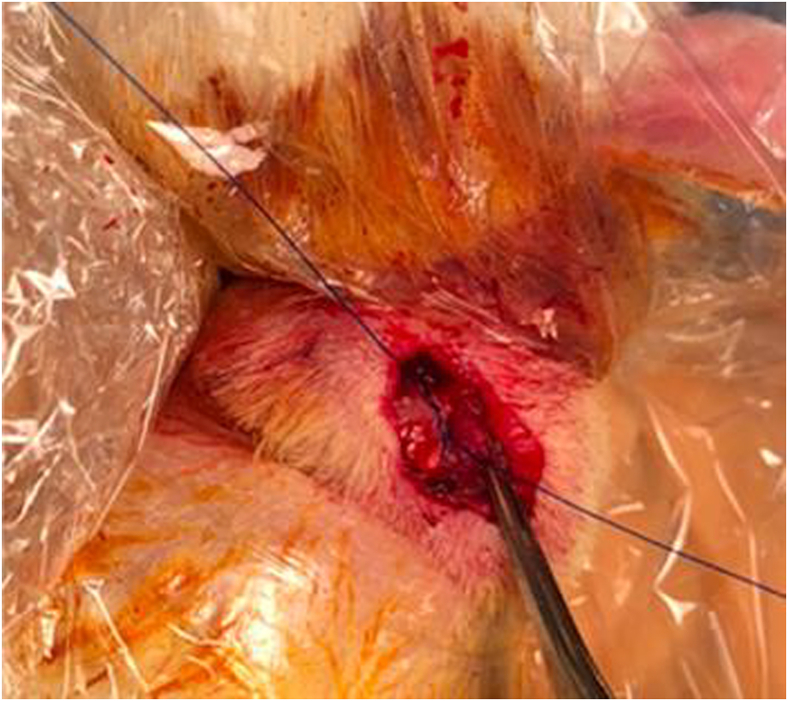
Rats were anesthetized with inhaled 2% Isoflurane. A transdeltoid surgical approach was used to visualize the supraspinatus tendon. The supraspinatus tendon margins were identified and tagged with a simple suture (5-0 Prolene; Ethicon, Johnson and Johnson, Piscataway, NJ, USA). After supraspinatus tendon detachment from the humeral greater tuberosity, its footprint was debrided of all soft tissue and fibrocartilage with a scalpel. The suture was then passed through a bone tunnel 2 mm distal to the footprint to reapproximate the anatomical insertion of the supraspinatus (Fig. 1).
Figure 1.
Transdeltoid surgical exposure and rotator cuff repair of the left shoulder in a rat.
After shoulder blockade with bupivacaine, the deltoid split and skin edges were reapproximated using 4-0 Vicryl (Ethicon, Johnson and Johnson, Piscataway, NJ, USA). Bupivacaine was chosen because NSAIDs have been shown to affect TtB healing.4 Postoperatively, the animals continued weight-bearing ad libitum and were monitored by animal care specialists for signs of pain and/or complications. Our postoperative pain protocol consisted of buprenorphine (0.01 to 0.05 mg/kg) administered subcutaneously every 12 hours as needed with a max of 4 doses.
Biomechanical analysis
Biomechanical testing of TtB healing was performed as described in previous studies.12,14 The supraspinatus was dissected subperiosteally from its origin on the suprascapular fossa, and the distal humerus was excised. This dissection technique provided a specimen consisting of the entire tendon, origin, and insertion that could be concomitantly subjected to mechanical testing (Fig. 2). Each specimen was thawed to room temperature on the day of testing. A micrometer was used to measure the cross-sectional area of the supraspinatus tendon at its insertion site.
Figure 2.
The muscle-tendon-bone specimen potted for biomechanical testing.
Force testing was performed using an MTS 858 Mini-Bionix Mechanical Testing System (MTS Systems Corporation, Eden Prairie, MN). The proximal supraspinatus tendon was attached to the mechanical testing system actuator using specially designed pneumatic grips and secured with ethyl cyanoacrylate. The humerus was potted in bone cement within an aluminum fixture, which was held secure to prevent fracture of the physis as seen in similar studies. This assembly allowed the shoulder components to be tested in 90 degrees of abduction. A 100 Newton (N) load cell was used to provide accurate force readings of tendon strength. The load cell was connected to a linear bearing to allow for alignment of the tendon in the direction of pull as performed in previous studies.4 Each tendon was preloaded to 1 N and then underwent ten cycles of preconditioning at a given length of displacement (5% grip-to-grip strain at a rate of 0.1%/s) to establish uniform load histories. The tendons were stretched in displacement control at 0.05 mm/sec until failure. Results allowed for calculation of maximum stress to failure, maximum force to failure, and stiffness (slope of the most linear portion of the load-deformation curve). Statistical analysis of variance, with significance set at P < .05, was used to compare data between groups and time points.
Histological analysis
Histological analysis was performed for the caffeinated and noncaffeinated groups after 2, 4, and 8 weeks of healing (6 shoulders per group). The bone-tendon-muscle sample was fixed in a 15-ml Eppendorf tube with 10% neutral buffered formalin. The sample was then decalcified with 10% ethylenediaminetetraacetic acid for two weeks and embedded in paraffin for histology. The regions of interest (TtB healing site, supraspinatus midsubstance, and greater tuberosity) were sectioned coronally. Samples were then stained with hematoxylin and eosin to examine cell morphology, tartrate-resistant acidic phosphatase and Ki67 staining to perform quantitative measurement of bone resorption and proliferation, and trichome staining followed by picrosirius red staining to analyze collagen content, organization, and maturity. Pictures of the slides were digitized and analyzed based on previously established grayscale values. Quantifying the brightness detected under polarized light allowed us to depict time-dependent changes of collagen maturation and organization. Samples were evaluated in a blinded fashion by one experienced histomorphologist using the TtB maturity score described by Ide et al.15 The scorer graded eight histological characteristics on a scale from 1 to 4, with a lower score indicating superior TtB healing.
Statistical analysis
The geometric and biomechanical measures were compared between the caffeine and noncaffeine groups at each time point using t-tests while Kruskal-Wallis tests were used for comparisons of histologic TtB healing because of the small number of observations. Analysis of variance was used to compare TtB maturation across time points, within the caffeine and noncaffeine groups. P values of <.05 (two-sided) were considered statistically significant.
Results
Biomechanical results
Gross assessment found that none of the specimens had evidence of macroscopic inflammation or purulence, all repairs were in continuity with their boney insertion, and there were no obvious tendon or muscle color differences between groups. Mean cross-sectional area of the supraspinatus tendon insertion sites was similar between groups at each time point. All specimens failed at the TtB junction during LtF testing. Mean LtF and maximum stress on the repairs were similar between groups at all time points. There was no significant difference in repair stiffness between groups at 2 and 4 weeks; however, the repair stiffness in the caffeinated group was significantly lower at 8 weeks than that in the noncaffeinated group (Table I) (P = .04).
Histological results
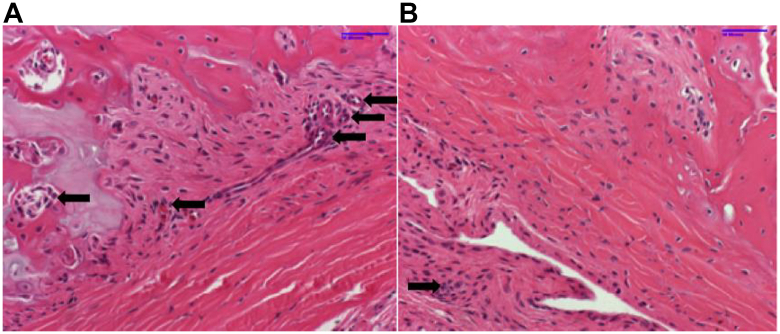
The TtB maturation scores were similar between caffeinated and noncaffeinated groups at 2, 4, and 8 weeks (Table II). Staining with picrosirius red to analyze collagen content, tartrate-resistant acidic phosphatase to measure quantitative measurements of bone resorption via osteoclasts activity, and Ki67 to evaluate the degree of cell proliferation did not show any remarkable qualitative differences between groups based on the observations of one experienced histomorphologist. Preliminarily, there did appear to be attenuated osteoclast activity in specimens among the caffeinated group (Fig. 3).
Table II.
Comparison of tendon-to-bone maturation between time points.
| Group | 2 Weeks | 4 Weeks | 8 Weeks | P value |
|---|---|---|---|---|
| Caffeine | 24.3 ± 1.2 | 26.3 ± 1.2 | 26.0 ± 1.0 | .150 |
| Noncaffeine | 25.3 ± 1.2 | 27.0 ± 2.0 | 27.7 ± 2.1 | .258 |
Figure 3.
Osteoclasts are denoted by small arrows. (A) H&E stain of bone-tendon junction at 4 weeks after RCR in a noncaffeinated specimen demonstrating abundant osteoclast activity. (B) H&E stain of bone-tendon junction at 4 weeks after RCR in a caffeinated specimen demonstrating attenuated osteoclast response.
Discussion
To our knowledge, this is the first study to investigate the effects of caffeine on TtB healing. This investigation found that perioperative caffeine intake did not appear to influence the LtF strength (primary outcome measure), cross-sectional area, and maximum stress of the supraspinatus repair site within 8 weeks in a rat RCR model. However, the stiffness of the repair did appear to be influenced by perioperative caffeine intake at 8 weeks postoperatively. Histologically, the overall TtB maturation scores were similar between groups based on underpowered, pilot data.
The stiffness of RCR at 8 weeks appeared lower in rats that received perioperative caffeine. Stiffness of a tendon is the amount of force required to stretch the tendon per unit of distance. It has been suggested that stiffness increases with collagen synthesis and integrity.18 Therefore, a reduction in stiffness is thought to indicate microfailure and irreversible plastic deformation.19 It should be recognized that our biomechanical assessment of stiffness may have been underpowered because the sample size for this study was determined based on our primary outcome measure. However, our primary outcomes measure, load to failure, did not appear to be influenced by perioperative caffeine intake at 8 weeks postoperatively, indicating that the difference in stiffness may not correlate with repair failure, when failure is defined as retear at the RCR site. Further studies should be performed to determine the clinical significance of RCR stiffness in the postoperative period and how tendon stiffness correlates with clinical outcomes.
Without TtB healing, the strength of an RCR relies on the force required to pull the suture through the tendon, which has been shown to be the most common mode of RCR failure.6 Therefore, it is important to understand the physiology behind TtB healing and the variables that can adversely effect this process. As with any damaged tissue, blood supply is essential for healing. Because neovascularization rarely occurs from the tuberosity bone into the reattached tendon, the nutrients and healing factors are provided to the repair site in an antegrade fashion via epitendinous blood vessels and diffusion into the synovial fluid through the circulus articuli vasculosis around the joint capsule.2 These healing factors foster chemotaxis of fibroblasts and the proliferation of a collagen and extracellular matrix around the repair site.21 Oguma et al showed that this collagen is then anchored to the repair interface via the formation of woven bone.22
Several drugs have been found to effect the biomechanical properties of RCR in murine models, including but not limited to NSAIDs, fluoroquinolones, and nicotine.4,10,14 Cohen et al performed a similar investigation as reported herein on 180 rats that underwent RCR.4 Their study found that perioperative intake of either indomethacin or celecoxib was associated with significant changes in collagen organization and lower LtF rates.4 Fox et al performed a similar controlled laboratory study that compared rats that underwent RCR and were given perioperative fluoroquinolones vs. saline.10 It appeared that preoperative and/or postoperative fluoroquinolone use was associated with significantly less fibrocartilage and poorly organized collagen at the repair site. In addition, rats that received the drug preoperatively and postoperatively had significantly lower LtF strength than controls.10 Moreover, Galatz et al performed a similar study that investigated the effects of nicotine on RCR healing in rats.14 They found that perioperative nicotine exposure was associated with lower type-I collagen expression and load to failure at 28 days after surgery, helping them conclude that decreased cell proliferation at the repair site may explain inferior biomechanical properties of RCR in rats exposed to perioperative nicotine.14
Caffeine has a demonstrated effect on wound healing in an ex vivo model of human skin. Ojeh et al found that topical caffeine impeded epithelialization of damaged tissue, slowed keratinocyte proliferation, and slowed keratinocyte migration to the injury site.23 In a separate ex vivo model of human skin, Donejko et al found caffeine inhibited collagen synthesis in fibroblasts via inhibiting DNA biosynthesis and the expression of β1-integrin and insulin-like growth factor receptor, both of which are integral to tissue healing.8 Furthermore, Duarte et al performed a comparative study that assessed the effects of oral caffeine administration on early bone healing in rats with a traumatic cortical defect in the tibia.9 They found that the tibiae of rats that received caffeine daily presented a significantly lower area of new bone formation on histometric analysis, which may be in line with our preliminary findings of an attenuated osteoclastic response at the TtB interface of RCR repair.9 Interestingly, despite the findings of the aforementioned studies, our hypothesis that caffeine would adversely effect the LtF strength at the TtB interface of RCR was not supported by the results of this study.
This study had several limitations. The study was designed to be underpowered for histological analysis because of restricted resources; however, owing to unintended specimen death as described in the methods, the study may have also become underpowered for biomechanical analysis of stiffness and stress at the repair site but fortunately remained adequately powered to assess our primary outcome measure, load to failure, of which our anticipated effect size was far from what was observed in biomechanical testing. Also, our histological analysis was performed by only one histologist, leading to potential bias; however, this is the first investigation into the relationship between caffeine intake and TtB healing, providing preliminary data for future studies. In addition, it should be noted that caffeine metabolism in humans and rats is different. In our study design, we dosed the rats once daily with a concentration of caffeine that corresponded to the consumption of 2-3 cups of coffee by an average American, at a single sitting, based on weight.11 This does not take into account differences in caffeine metabolism and does not account for multiple caffeinated beverages consumed throughout the course of a day. In addition, caffeine metabolism is variable within individuals based on genetics, concurrent medication use, tobacco consumption, and age.7 Although obtaining blood or urine samples could have provided an objective measurement of caffeine absorption, an investigation by Ikeda et al has shown that rats receiving caffeine via gavage, as performed in this study, had reliably higher blood concentrations than rats that sipped a caffeine solution over an extended period of time.16
Conclusion
Perioperative caffeine intake did not appear to effect the LtF strength of RCR in an animal model. Although our secondary outcome measures of maximum stress and stiffness also did not appear to be influenced by perioperative caffeine intake, there did appear to be a trend toward decreased RCR stiffness at 8 weeks postoperatively in specimens that received caffeine. Further investigations into the effects of caffeine on TtB healing and the clinical significance of RCR stiffness are recommended.
Disclaimers
Funding: This study was sponsored by UAB DEPARTMENT Animal Project Number (APN): IACUC-20206.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1.Barth J., Andrieu K., Fotiadis E., Hannink G., Barthelemy R., Saffarini M. Critical period and risk factors for retear following arthroscopic repair of the rotator cuff. Knee Surg Sports Traumatol Arthrosc. 2017;25:2196–2204. doi: 10.1007/s00167-016-4276-x. [DOI] [PubMed] [Google Scholar]
- 2.Bunker D.L., Ilie V., Ilie V., Nicklin S. Tendon to bone healing and its implications for surgery. Muscles Ligaments Tendons J. 2014;4:343–350. [PMC free article] [PubMed] [Google Scholar]
- 3.Chung S.W., Oh J.H., Gong H.S., Kim S.H. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39:2099–2107. doi: 10.1177/0363546511415659. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D.B., Kawamura S., Ehteshami J.R., Rodeo S.A. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 5.Colvin A.C., Egorova N., Harrison A.K., Moskowitz A., Flatow E. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227–233. doi: 10.2106/jbjs.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins C.A., Murrell G.A. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg. 2003;12:128–133. doi: 10.1067/mse.2003.21. [DOI] [PubMed] [Google Scholar]
- 7.Debry G. John Libbey Eurotext; Paris, France: 1994. Coffee and health. [Google Scholar]
- 8.Donejko M., Przylipiak A., Rysiak E., Gluszuk K., Surazynski A. Influence of caffeine and hyaluronic acid on collagen biosynthesis in human skin fibroblasts. Drug Des Devel Ther. 2014;8:1923–1928. doi: 10.2147/dddt.S69791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte P.M., Marques M.R., Bezerra J.P., Bastos M.F. The effects of caffeine administration on the early stage of bone healing and bone density A histometric study in rats. Arch Oral Biol. 2009;54:717–722. doi: 10.1016/j.archoralbio.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Fox A.J., Schär M.O., Wanivenhaus F., Chen T., Attia E., Binker N., et al. Fluoroquinolones impair tendon healing in a rat rotator cuff repair model: a preliminary study. Am J Sports Med. 2014;42:2851–2859. doi: 10.1177/0363546514545858. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm B.B., Bättig K., Holmén J., Nehlig A., Zvartau E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 12.Galatz L.M., Charlton N., Das R., Kim H.M., Havlioglu N., Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009;18:669–675. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Galatz L.M., Sandell L.J., Rothermich S.Y., Das R., Mastny A., Havlioglu N., et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 14.Galatz L.M., Silva M.J., Rothermich S.Y., Zaegel M.A., Havlioglu N., Thomopoulos S. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006;88:2027–2034. doi: 10.2106/jbjs.E.00899. [DOI] [PubMed] [Google Scholar]
- 15.Ide J., Kikukawa K., Hirose J., Iyama K., Sakamoto H., Fujimoto T., et al. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elbow Surg. 2009;18:391–398. doi: 10.1016/j.jse.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda G.J., Sapienza P.P., McGinnis M.L., Bragg L.E., Walsh J.J., Collins T.F. Blood levels of caffeine and results of fetal examination after oral administration of caffeine to pregnant rats. J Appl Toxicol. 1982;2:307–314. doi: 10.1002/jat.2550020609. [DOI] [PubMed] [Google Scholar]
- 17.Itoigawa Y., Suzuki O., Sano H., Anada T., Handa T., Hatta T., et al. The role of an octacalcium phosphate in the re-formation of infraspinatus tendon insertion. J Shoulder Elbow Surg. 2015;24:e175–e184. doi: 10.1016/j.jse.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Kannus P., Józsa L., Natri A., Jarvinen M. Effects of training, immobilization and remobilization on tendons. Scand J Med Sci Sports. 1997;7:67–71. doi: 10.1111/j.1600-0838.1997.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 19.LaCroix A.S., Duenwald-Kuehl S.E., Lakes R.S., Vanderby R., Jr. Relationship between tendon stiffness and failure: a metaanalysis. J Appl Physiol (1985) 2013;115:43–51. doi: 10.1152/japplphysiol.01449.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell D.C., Knight C.A., Hockenberry J., Teplansky R., Hartman T.J. Beverage caffeine intakes in the U.S. Food Chem Toxicol. 2014;63:136–142. doi: 10.1016/j.fct.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Molloy T., Wang Y., Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Oguma H., Murakami G., Takahashi-Iwanaga H., Aoki M., Ishii S. Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res. 2001;19:873–880. doi: 10.1016/S0736-0266(01)00021-3. [DOI] [PubMed] [Google Scholar]
- 23.Ojeh N., Stojadinovic O., Pastar I., Sawaya A., Yin N., Tomic-Canic M. The effects of caffeine on wound healing. Int Wound J. 2016;13:605–613. doi: 10.1111/iwj.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugaya H., Maeda K., Matsuki K., Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/jbjs.F.00512. [DOI] [PubMed] [Google Scholar]
- 25.Tashjian R.Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Thomopoulos S., Genin G.M., Galatz L.M. The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10:35–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Wildemann B., Klatte F. Biological aspects of rotator cuff healing. Muscles Ligaments Tendons J. 2011;1:161–168. [PMC free article] [PubMed] [Google Scholar]