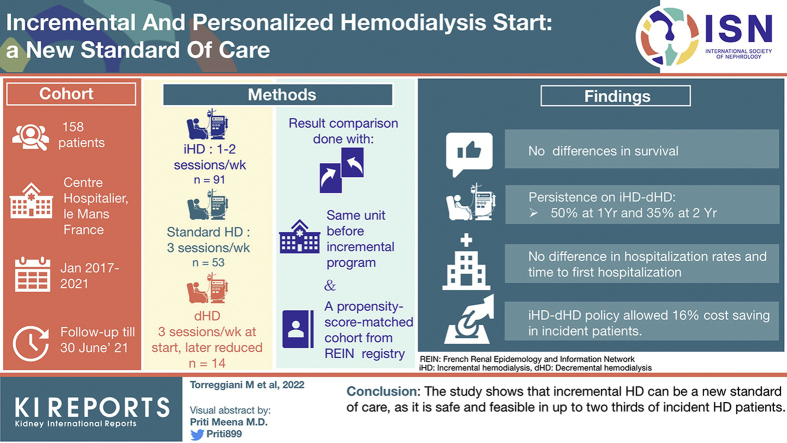
Abstract
Introduction
Incremental hemodialysis (iHD) may attenuate “dialysis shock” and reduce costs, preserving quality of life. It is considered difficult to reconcile with HD wards’ routine; fear of underdialysis and increasing mortality are additional concerns. The aim of this study was to evaluate mortality, morbidity, and costs in a large HD ward where iHD is the standard of HD start.
Methods
This observational study included all incident HD patients in 2017 to 2021, stratified according to HD start: iHD (1–2 sessions/wk), decremental HD (dHD, 3 sessions/wk at start, later reduced), or standard (3 sessions/wk). Results were compared with data recorded in the same unit before the incremental program (2015–2017) and with a propensity score-matched cohort from the French Renal Epidemiology and Information Network (REIN) registry.
Results
A total of 158 patients started HD in 2017 to 2021, 57.6% on iHD, 8.9% dHD, and 33.5% standard HD schedule. Patients on the standard schedule had lower initial estimated glomerular filtration rate (eGFR) (5 vs. 7 ml/min per 1.72 m2, P = 0.003). We found no survival differences according to period of start (same center) and propensity score matching (REIN). Patients intensively followed in the pre-HD period were more likely to start on iHD-dHD. Persistence on iHD-dHD was about 50% at 1 year and 35% at 2 years. Hospitalization rates and time to first hospitalization or death did not differ between the schedules. The iHD-dHD policy allowed a 16% cost saving, even accounting for supplemental biochemical tests.
Conclusion
Our study reveals that iHD can be a new standard of care, as it is safe and feasible in up to two-thirds of patients on incident HD.
Keywords: cost analysis, incremental hemodialysis, personalized hemodialysis, predialysis care, propensity score matching, survival
Graphical abstract
Dialysis saves lives, but at a price. Besides its intrusiveness in the patient’s life, the treatment is, in itself, not devoid of iatrogenicity, and while intensive dialysis has well-acknowledged advantages in particular situations, such as pregnancy, early start of treatment has not been associated with improvement in survival and, in the case of acute kidney injury, it has even been associated with an increase in kidney damage.1, 2, 3, 4 The concept that, on dialysis, “more is better” has also been challenged.5, 6, 7
If dialysis is seen as a “renal replacement therapy,” it is logical that it is started trying to ensure a “treatment dose” corresponding to a kidney function that comprises an acceptable degree of correction of all major metabolic derangements. It is in this way that HD is now usually started, regardless of the patient’s residual kidney function.10, 8, 9
However, apart from the few cases in which kidney function is rapidly and irreversibly lost because of surgery, or rapidly progressive kidney disease, chronic dialysis is started because of the progressive, often subtle onset of metabolic derangements, in patients whose eGFR is between 5 and 10 ml/min per 1.72 m2 or more.11
The decision on when to start dialysis is at the same time difficult and simple. Since the publication of the IDEAL study, and after its integration into several guidelines, first of all the ones of the Canadian Society of Nephrology, the concept emerged that dialysis start should follow an intent-to-defer policy, and the decision should be based on a comprehensive clinical evaluation (summarized in: first onset of a clinical indication or a decline in the eGFR to 6 ml/min per 1.73 m2 or less).12 These clinical indications are clear, but hard to quantify, as they broadly include symptoms of uremia, fluid overload, refractory hyperkalemia or acidemia, or other conditions or symptoms that are likely to be ameliorated by dialysis, and are modulated by additional factors, including, among others, patient preference, empowerment, trajectory, and severity of uremic symptoms. These concepts have been repeatedly underlined, and, indeed, the choice is deemed even more complex in elderly patients at high comorbidity.13, 14, 15
While these guidelines regard dialysis start with an “all-or-nothing” approach, the same concept of intent-to-defer and comprehensive clinical evaluation may be extended to progressively increasing dialysis dose, in the presence of residual kidney function, to attain satisfactory metabolic control. This stepwise approach is usually referred to as “incremental dialysis.” The concept is derived from peritoneal dialysis (PD), in which incremental approaches have long been used. While the experience is still limited, iHD is increasingly seen as a patient-friendly means to allow a smooth transition from predialysis care to “full-scale” renal replacement therapy, reducing what has been called “dialysis shock.”16, 17, 18, 19, 20, 21, 22
iHD has been differently defined, described, and practiced: some authors, mainly in the United States, consider it synonymous with twice-weekly HD of standard duration,16 whereas others, mainly in Europe, use the term to define a progressive increase from 1 session of short duration (2–3 hours) per week to full-dose standard HD (4 hours 3 times per week).17 Furthermore, other authors consider as iHD all schedules in which a “less-than-standard” dialysis dose is used.23 A personalized policy also allows to downscale dialysis dose (“decremental” schedules, dHD) in cases of recovery of some kidney function after dialysis start. This might also favor dialysis discontinuation beyond the 3 to 6 months conventionally considered as the limit of end-stage kidney disease reversibility.24,25
The inclusion of low-dose, “palliative dialysis” in patients at high comorbidity, including situations in which dialysis delivery is limited for economic or logistic reasons, further makes comparisons with standard schedules difficult.26
Within these different practices, a growing body of case series, reviews, registry data, and expert opinions suggests that iHD start, outside the context of economic restrictions, is at least equivalent to standard HD start in terms of mortality and morbidity.27, 28, 29 While observational studies are often affected by selection biases, randomization is difficult and may be felt to be unethical; these issues are a factor in all the “non-standard” dialysis modalities.30, 31, 32, 33
As a consequence, in spite of growing interest, the actual potential of iHD and its effect on mortality and morbidity are not clear. In a retrospective analysis, based on residual kidney function, Chin et al.34 estimated that about half of the incident patients in their center could have benefited from an iHD start. Along the same lines, some authors have tried to draw the profile of the “best” candidates for iHD, listing up to 10 characteristics, overall defining patients with a stable metabolic balance and relevant urine output.16
To the best of our knowledge, the present study is the first one to report on the results of a policy of iHD start in all patients without contraindications, and of its rapid adaptation, increasing or decreasing dialysis frequency over time. We will refer to this tailor-made policy encompassing iHD and dHD as “non-standard” HD start.
The setting of study is a large public HD unit in central France.
To account for the selection bias due to the allocation of patients with better kidney function, which can also reflect better metabolic balance and lower comorbidity, to the incremental schedules, mortality in incident patients with nonstandard HD start was compared with different cohorts: patients who started standard thrice-weekly HD in the same period; incident patients in the same center before the establishment of the incremental program; and propensity score-matched HD patients with thrice-weekly dialysis start derived from the national French dialysis registry. Duration of incremental dialysis, hospitalizations, metabolic balance, and costs for the provider and the health care system were also analyzed.
Methods
Setting of Study
The setting of the study is Centre Hospitalier le Mans (CHM), in Le Mans in central France.32
The dialysis ward has 25 beds for treating chronic HD patients and patients with acute kidney injury needing dialysis, outside of the intensive care unit. The pool of patients on chronic HD ranged from 95 to 110 before the establishment of the iHD program, and from 105 to 130 after this, the variations in number depending on the occurrence of kidney transplantations, deaths, and transfers. The Center also runs a PD program; PD was the treatment of choice in 23% of incident patients in both periods considered, a figure much higher than the penetration of PD in France (6.5% at the last update).35 Further details are available in the Supplementary Methods.
Definitions
HD was defined as chronic when it lasted for at least 3 months, regardless of the schedule. iHD was defined as initial delivery of 1 or 2 dialysis sessions per week, independently of their duration (2 to 4 hours), lasting for at least 2 weeks, in an outpatient. The dialysis schedule during hospitalization, if any, at dialysis start was not considered.
dHD was defined as a decrease in the number of dialysis sessions to 2 or 1 session per week, independently of their duration, lasting for at least 2 weeks, occurring in an outpatient, after at least 2 weeks of outpatient HD, with 3 or more sessions per week.
Nonstandard HD included both iHD and dHD schedules, as defined previously. Standard HD was defined as dialysis start with 3 or more sessions per week, independently of their duration (usually 3–4 hours).
Intensive predialysis follow-up was defined as follow-up in the unit dedicated to the care of advanced chronic kidney disease (UIRAV: Unité pour la prise en charge de l’Insuffisance Rénale AVancée) for at least 1 month. Detailed follow-up schedules are available elsewhere; these include, for chronic kidney disease stage 5 or fragile patients with rapidly deteriorating kidney function, 1 to 4 consultations per month, usually with concomitant blood tests.36
Further definitions (dialysis discontinuation, comorbidity, planned and unplanned dialysis start) are available in the Supplementary Material.
Selection of Patients
Period of Study
All incident chronic HD patients who started HD between January 2017 and March 2021 at CHM were included in the analysis and stratified according to their iHD, dHD, or standard dialysis schedule. Follow-up was continued to June 30, 2021.
Patients were censored at the time of transfer to other dialysis centers, kidney transplantation, or kidney function recovery (after ≥3 months of HD).
Control data: period from January 2013 to December 2015. All incident chronic HD patients who started renal replacement therapy in this period at CHM, followed-up for at least 1 month as outpatients, were included in the analysis; in this period, HD was systematically started 3 times per week (4 hours). Observation was continued until March 2017.
Patients who started HD in 2016 were not considered, as 2016 was the transition year in which the incremental dialysis program was set up.
Further information on source of data is available in the Supplementary Methods.
REIN Registry
REIN is the national French dialysis registry, with full coverage of all public, associative, and private centers.37 It is organized in regional areas, and data from the Pays de la Loire region were selected. Of the 2209 patients who started dialysis in 2017 to 2021 in the 25 centers of the Pays de la Loire other than CHM, we selected 1583 patients who started with thrice-weekly HD. Patients on PD (n = 265), who had recovered kidney within 3 months (n = 75), patients on HD schedules other than thrice-weekly (n = 247, 186 on once- or twice-weekly HD and 61 on more frequent schedules), and patients with incomplete data (n = 39) were excluded.
Indications for HD Start and Changes in HD Frequency
Details on dialysis prescriptions and on control policies are reported in the Supplementary Material. The choice of when and how to start HD is determined by a comprehensive clinical and laboratory evaluation that takes into consideration clinical condition, the presence of fluid overload (in particular, in patients with cardiorenal syndrome), uremic symptoms (such as fatigue, loss of appetite, attention deficit, restless leg syndrome), and poor blood pressure control. Details on biochemical data (eGFR, urea, potassium, acidosis, anemia, phosphate, parathyroid hormone, albumin, and brain natriuretic peptide) and the thresholds considered are available in the Supplementary Materials.
The same clinical and biochemical indexes are considered when deciding whether to increase dialysis frequency. Even if creatinine and urea clearances on 24-hour urine collection, the gold standard for the assessment of the residual kidney function, are routinely prescribed, these controls are often not reliable in our elderly population and are therefore considered only as ancillary in the decision to modulate HD frequency.38, 39, 40
In addition, beta-2-microglobulin (β2m) levels are tested monthly and considered, in the absence of inflammatory status, as markers of residual kidney function in iHD and as indicators of middle-molecule depuration in patients without relevant kidney function.
Furthermore, since 2021, the formula based on β2m level and serum creatinine, urea, sex, and age proposed for the evaluation of residual kidney function, has been integrated in the monthly tests.41
Biochemical Data
Biochemical data were analyzed using standard laboratory methods. Data are reported “per protocol.” They were gathered at dialysis start (first dialysis session), regardless of hospitalization status, for urea, creatinine, hemoglobin, calcium, phosphate, and total proteins, integrated, when necessary, with data recorded in the previous or following week. Conversely, for the analysis of biochemical data at 3 months and at the last control, only patients who had been hospitalization-free for at least 1 month were considered. Data are obtained at midweek for conventional HD and at the first or only dialysis session per week in iHD-dHD patients.
Although, for the above-mentioned reasons, urea clearance was not routinely used to assess residual renal function, for the sake of this study, we took advantage of the regular assessment of β2m levels to calculate residual renal function according to the formula proposed by Jaques and Davenport41 in 2021.
Cost Analysis
The cost of dialysis was considered equivalent to the French health system’s reimbursement per session (rounded to 350 Euros per session for in-hospital dialysis).42,43 Transportation expenses are not included in the dialysis bundle; they were conservatively rounded to 50 Euros per session, in keeping with the distance covered and the average French data (13,052 Euros/patient/yr).44
The additional cost of biochemical tests for iHD and dHD patients was obtained from the hospital’s administrative offices; blood tests are included in the dialysis bundle. Giving their integration in the routine of care, we analyzed them as “production costs,” rounded to 10 additional Euros per week in the 40 weeks not included in the monthly tests.
Further details are available in the Supplementary Materials.
Statistical Analysis and Study Design
The statistical analysis was performed with JASP v.0.14.1 (JASP Team 2020, University of Amsterdam, The Netherlands) and R v.4.0.5 (R CRAN-project, 2021).
In all studies, a 2-tailed alpha risk of <5% was considered statistically significant.
Descriptive Analysis
Data were tested for normality by means of the Shapiro-Wilk test and homoscedasticity with Levene’s test and presented as mean and SDs for parametric data and median and interquartile ranges for nonparametric data as appropriate, while categorical data are presented as numbers and proportions.
Normally distributed quantitative data were compared by means of t test or analysis of variance according to the number of groups; otherwise, the Wilcoxon rank-sum test or Kruskal-Wallis test was used. Qualitative data were analyzed by means of the χ2 test or by Fisher’s test in case of a low sample size of subgroups.
Survival Analysis
Survival, time to first hospitalization or death, and time on incremental dialysis were visualized by means of Kaplan-Meier curves, and comparison between the 2 groups was performed by means of the log-rank test. Dialysis schedule was considered as intention-to-treat analysis (i.e., death was ascribed to the nonstandard schedule even after a shift to standard HD).
dHD patients were excluded in the analysis of the persistence of iHD over time.
Patient survival in the 2 cohorts pre- and postimplementation of the iHD start (start of dialysis 2013–2015 [follow-up to June 2016] and 2017–2021) was compared in the per system of care analysis.
Cox regression analysis was performed to assess the effect on survival of different combinations of dialysis schedules (standard vs. nonstandard) and period of start (2013–2016 vs. 2017–2021) with comorbidity (Charlson comorbidity index [CCI] dichotomized at ≥8), gender (male vs. female), and context of dialysis start (urgent vs. planned).
Propensity Score Matching
Bipartite propensity score matching through the greedy nearest neighbor matching algorithm was conducted to produce a CHM-matched sample from the REIN cohort with a 1:1, 1:2, and 1:5 ratio.45, 46, 47 Matching considered the following 3 main factors classically associated with survival in dialysis patients and available in both the registry and our database: age, gender, and presence of diabetes. To ensure similar follow-up, the year of dialysis start was also matched. Matching was performed using the “Matchit” R package v.4.2.0.48
Logistic Regressions
A univariate and multivariate logistic regression analysis was performed to determine which parameters were associated with nonstandard dialysis start: CCI (dichotomized at 8), body mass index (dichotomized at 30 kg/m2), gender (female vs. male), kidney disease (diabetic and vascular kidney disease vs. others), eGFR (dichotomized at 7 ml/min per 1.72 m2), context of dialysis start (urgent vs. planned), and predialysis care (intensive vs. other). To avoid collinearity, the correlation between the explanatory variables included in the model was assessed by Pearson and Spearman correlation coefficients, and in case of 2 or more highly correlated variables, the one considered more clinically relevant was included. The accuracy of the regression model was verified by means of residual analysis.
Ethical Aspects
All patients who started dialysis in our center signed an informed consent form authorizing the anonymous use of their clinical data for research purposes. The forms were signed by tutors in cases of minors or patients with cognitive impairment.
In keeping with the French legislation, the retrospective observational study was approved by the ethics committee of the Le Mans hospital on July 10, 2021. Data from REIN were agreed on in all centers in the Pays de la Loire region.
Baseline Data
Of the 158 patients who started dialysis at CHM in the period 2017 to 2021, 91 (57.6%) started with an iHD schedule (of which, 30 on a once-weekly schedule, Supplementary Table S1), 14 (8.9%) started on a thrice-weekly schedule and decreased to a lower number of sessions, and 53 (33.5%) started and continued with a thrice-weekly schedule. No difference was found between groups regarding age, sex, CCI, and nutritional status according to Subjective Global Assessment and body mass index (Table 1). Conversely, patients on iHD had more often been followed-up in the intensive predialysis care unit, were more likely to have had a planned dialysis start, and were less frequently hospitalized when beginning HD. In the context of a relatively low frequency of late referrals (23.4% of patients with <3 months of follow-up), about two-thirds of the patients had been followed for at least 1 year before starting dialysis, with no significant differences between the groups (Table 1).
Table 1.
Baseline characteristics of patients who started hemodialysis at CHM in the period 2017 to 2021, according to dialysis modality
| Dialysis frequency |
Nonstandard |
|||||
|---|---|---|---|---|---|---|
| Standard | Nonstandard | P value | Incremental | Decremental | P value for comparison of standard, incremental, and decremental groups | |
| N | 53 | 105 | 91 | 14 | ||
| Age (yr), median (min-max) | 71 (32–90) | 69 (26–94) | 0.908 | 70 (26–94) | 69 (38–91) | 0.815 |
| Sex, n females (%) | 22 (41.5) | 42 (40.0) | 0.855 | 35 (38.5) | 7 (50.0) | 0.703 |
| CCI, median (min-max) | 8 (2–13) | 8 (2–18) | 0.619 | 8 (2–18) | 7 (3–17) | 0.208 |
| SGA, n (%) | 0.086 | 0.146 | ||||
| A | 34 (65.4) | 82 (78.9) | 69 (76.7) | 13 (92.9) | ||
| B | 17 (32.7) | 18 (17.3) | 17 (18.9) | 1 (7.1) | ||
| C | 1 (1.9) | 4 (3.8) | 4 (4.4) | 0 | ||
| BMI (kg/m-2), median (min–max) | 25.6 (16.4–79.9) | 27.0 (16.3–47.4) | 0.619 | 26.5 (16.3–47.4) | 27.9 (18.6–37.0) | 0.841 |
| BMI ≥30, n (%) | 13 (31.4) | 34 (32.4) | 0.899 | 31 (34.1) | 3 (21.4) | 0.636 |
| Start in hospitalization, n (%) | 46 (86.8) | 70 (67.3) | 0.009 | 56 (62.2) | 14 (100) | <0.001 |
| Start in emergency, n (%) | 39 (73.6) | 40 (38.1) | <0.001 | 31 (34.1) | 9 (64.3) | <0.001 |
| Origin, n (%) | <0.001 | <0.001 | ||||
| Conventional predialysis care | 25 (47.2) | 31 (29.5) | 22 (24.2) | 9 (64.3) | ||
| Intensive predialysis care | 3 (5.7) | 48 (45.7) | 47 (51.7) | 1 (7.1) | ||
| Peritoneal dialysis | 1 (1.9) | 8 (7.6) | 8 (8.8) | 0 | ||
| Transplantation | 4 (7.6) | 5 (4.8) | 5 (5.5) | 0 | ||
| Other | 20 (37.7) | 13 (12.4) | 9 (9.9) | 4 (28.6) | ||
| Time of previous follow-up, (mo) n (%) | 0.026 | 0.051 | ||||
| 0 | 17 (32.1) | 15 (14.3) | 11 (12.1) | 4 (28.6) | ||
| 3 | 0 | 5 (4.8) | 4 (4.4) | 1 (7.1) | ||
| 6 | 5 (9.4) | 8 (7.6) | 8 (8.8) | 0 | ||
| ≥12 | 31 (58.5) | 77 (73.3) | 68 (74.7) | 9 (64.3) | ||
| CKD disease, n (%) | 0.763 | 0.798 | ||||
| Diabetes, nephroangiosclerosis | 26 (49.1) | 58 (55.2) | 52 (57.1) | 6 (42.9) | ||
| Glomerulonephritis | 8 (15.1) | 14 (13.3) | 12 (13.2) | 2 (14.3) | ||
| Other | 19 (35.9) | 33 (31.4) | 27 (29.7) | 6 (42.9) | ||
| eGFR (ml/min per 1.72 m2) at dialysis start, median (min–max) (all cases) | 5 (2–29) | 7 (2–40) | 0.003 | 7 (2–40) | 7 (2–10) | 0.002 |
| eGFR (ml/min per 1.72 m2) at dialysis start, median (min-max) (only for planned start of dialysis, excluding cardiorenal syndromes) | 5 (4–8) | 7 (3–15) | 0.007 | 7 (3–15) | 7 (6–10) | 0.019a |
BMI, body mass index; CCI, Charlson comorbidity index; CHM, Centre Hospitalier le Mans; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate according to the CKD-Epidemiology Collaboration formula; max, maximum; min, minimum; SGA, subjective global assessment.
Bold value indicates significant differences.
P = 0.053 for post hoc comparison between standard and incremental.
Patients who started and continued dialysis with a thrice-weekly schedule had a significantly lower eGFR at start (5 ml/min per 1.72 m2 vs. 7 ml/min per 1.72 m2 in patients on iHD-dHD) (Table 1). However, the indication for elective dialysis start was posed at identical eGFR levels for patients being followed in the intensive predialysis care unit and for those in other outpatient facilities (median 7 ml/min per 1.72 m2).
Patients who started HD between 2013 and 2015 had a lower burden of comorbidities, as measured by CCI, and were more likely to begin renal replacement therapy during hospitalization compared with those starting HD between 2017 and 2021 (Table 2).
Table 2.
Baseline characteristics of patients who started hemodialysis in 2013 to 2015 compared with 2017 to 2021
| 2013–2015 | 2017–2021 | P value | |
|---|---|---|---|
| N | 91 | 158 | |
| Age (yr), median (min-max) | 71 (18–90) | 70 (26–94) | 0.390 |
| Gender, n females (%) | 31 (34.1) | 64 (40.5) | 0.314 |
| CCI, median (min-max) | 7 (2–15) | 8 (2–18) | 0.018 |
| Diabetes, n (%) | 32 (35.2) | 73 (46.2) | 0.089 |
| Start in hospitalization, n (%) | 79 (86.8) | 116 (73.9) | 0.017 |
CCI, Charlson comorbidity index; max, maximum; min, minimum.
Bold value indicates significant differences.
Survival Analysis (CHM Cohorts)
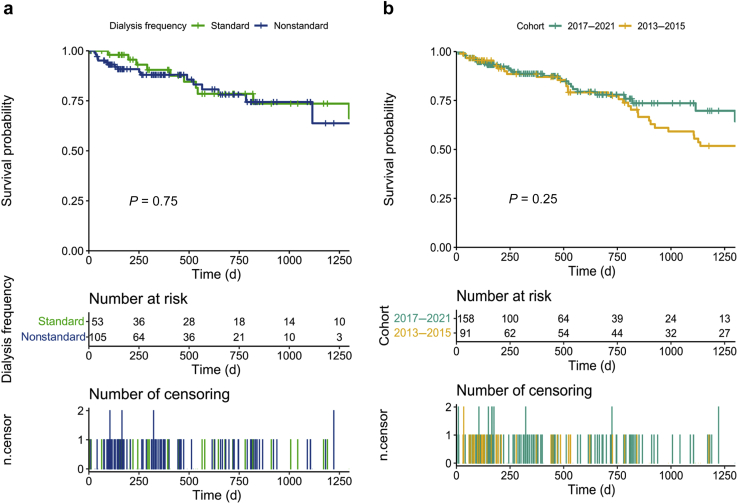
In the period 2017 to 2021, age and CCI retain the usual highly significant effect on survival, whereas initial HD schedules were not associated with survival differences (Figure 1a and Supplementary Figure S1A–D). The effect of CCI is retained in the multivariate Cox analysis (Supplementary Table S2).
Figure 1.
(a) Survival of incident hemodialysis patients at Centre Hospitalier Le Mans in the period 2017–2021, according to dialysis schedule. (b) Comparison of survival of incident hemodialysis patients at Centre Hospitalier Le Mans between the period 2017 to 2021 and 2013 to 2015.
No difference in patient survival was observed comparing mortality rates observed in the period 2017 to 2021 with those observed in the previous period, when dialysis was routinely started with 3 sessions per week (Figure 1b). Cox analysis confirmed that adding iHD to the system of care did not negatively affect its performance and only CCI remained significantly associated with risk of death (Supplementary Table S3).
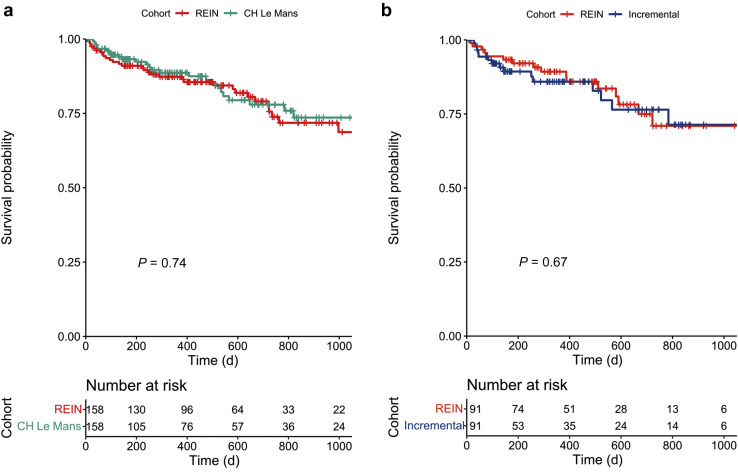
Survival Analysis With Respect to the REIN Registry, After Propensity Score Matching
The characteristics of the patients selected from the REIN Registry after propensity score matching are presented in Supplementary Table S4. No significant difference in survival was observed comparing all patients who started HD at CHM and other centers in the region (Supplementary Figure S2A–C). After matching for age, gender, year of dialysis start, and presence of diabetes, no differences in survival were found (Figure 2a and Supplementary Figure S3A and B). The same held true when the analysis was limited to patients who started HD with an incremental schedule, or considering 2 nonstandard schedules together (iHD-dHD) (Figure 2b and Supplementary Figure S3C and D).
Figure 2.
(a) Comparison of survival between incident hemodialysis patients at Centre Hospitalier Le Mans in the period 2017 to 2021 and a propensity score-matched cohort from the REIN Registry. 1:1 matching. (b) Comparison of survival between incremental hemodialysis patients at Centre Hospitalier Le Mans in the period 2017 to 2021 and a propensity score-matched cohort from REIN. 1:1 matching. REIN, Renal Epidemiology and Information Network.
Probability of iHD Start and Persistence on Incremental Dialysis
HD start with an incremental schedule was more frequent in patients with intensive predialysis follow-up of at least 1 month, while it was not affected by sex and comorbidity and type of kidney disease (Table 1). According to the multiple logistic regression analysis, an unplanned, emergency start and lower eGFR were associated with lower odds of beginning HD on a nonstandard schedule, while an intensive predialysis follow-up was associated with 10 times greater odds of starting dialysis on a nonstandard schedule (Table 3).
Table 3.
Logistical regression for the outcome nonstandard dialysis start
| Odds ratio | 95% CI |
P value | |||
|---|---|---|---|---|---|
| Lower | Higher | ||||
| CCI (≥8) | 0.863 | 0.344 | 2.150 |  |
0.752 |
| BMI (≥30 kg/m2) | 0.662 | 0.254 | 1.689 | 0.389 | |
| Gender (male) | 0.788 | 0.318 | 1.888 | 0.597 | |
| Diabetes, NAS vs. others | 1.129 | 0.442 | 2.895 | 0.799 | |
| eGFR (<7 ml/min per 1.72 m2) | 0.279 | 0.115 | 0.646 | 0.004 | |
| Urgent vs. planned start | 0.254 | 0.106 | 0.581 | 0.002 | |
| Intensive predialysis vs. conventional care | 10.826 | 3.452 | 48.220 | <0.001 | |
BMI, body mass index; CCI, Charlson comorbidity index; eGFR, estimated glomerular filtration rate according to the CKD-Epidemiology Collaboration formula; NAS, nephroangiosclerosis.
The Pearson phi coefficient64 was equal to 0.271 between urgent start and intensive predialysis care origin (both were kept in the model) and 0.57 between age and CCI, thus only the Charlson score was used.
Bold value indicates significant differences.
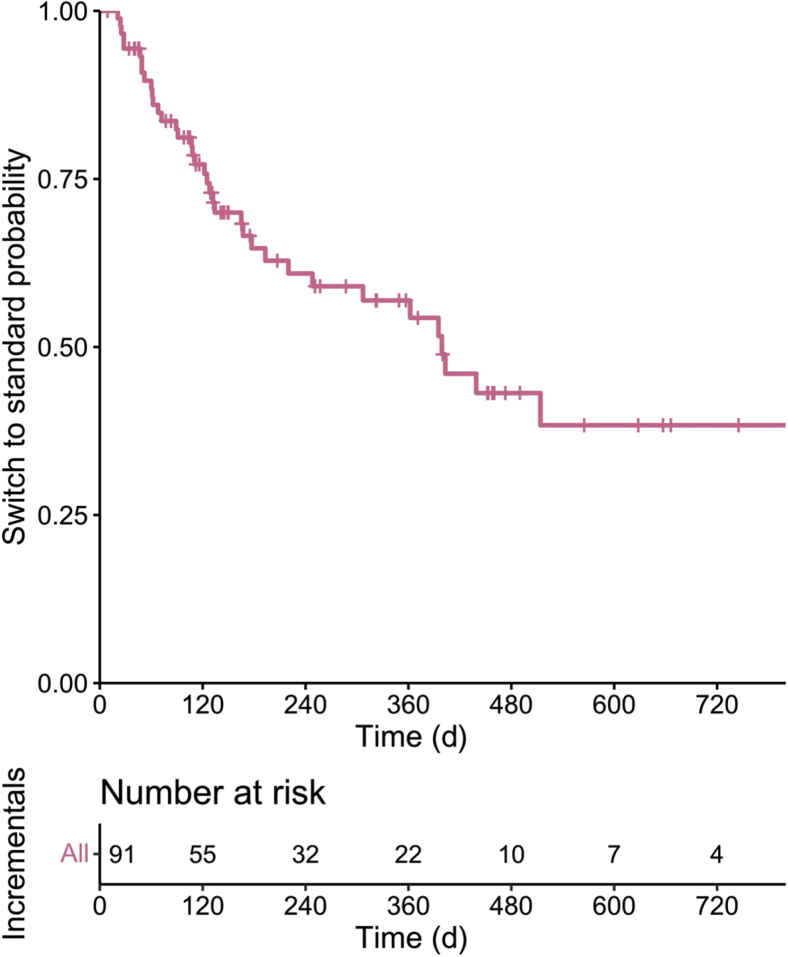
One year after iHD start, about half of the patients were still on an iHD schedule and about 35% were still on a once- or twice-weekly schedule at 2 years (Figure 3). Persistence on iHD was not associated with kidney disease (diabetes vs. other causes), body mass index, type of predialysis follow-up, and start in emergency (Supplementary Table S5). Regaining a good metabolic balance allowed us to reduce the dialysis prescription for 14 patients (dHD: about 9% of the incident dialysis cohort).
Figure 3.
Time on incremental dialysis before switching to thrice-weekly dialysis.
The main reasons why 53 patients were not able to start dialysis in an incremental manner or to decrease the session frequency within 3 months from the start are reported in Supplementary Table S6. While severe acute injury, postpartum cortical necrosis, nephrectomy, and rapidly progressive glomerulonephritides account for 7 (13%) of the cases, the most common situation associated with start and persistence on a thrice-weekly schedule was beginning HD in a condition of fluid overload (34%) and decompensated uremic syndrome (17%), requiring intensive HD at start, possibly contributing to a rapid loss of residual kidney function.
Hospitalizations
During the period 2017 to 2021, the number and duration of hospitalizations following the start of dialysis were not significantly different, with a median of 3 hospitalizations per patient on standard HD start and 2 in patients on nonstandard schedules, all with a wide range (0–17 hospitalizations) (Supplementary Table S7). Likewise, the time to first hospitalization or death did not differ according to type of dialysis start (Supplementary Figure S4).
Biochemical Profiles
The biochemical profiles at start of dialysis, at 3 months, and at the last update are available in Supplementary Tables S1, S8, and S9. The flowchart of the patients is given in Supplementary Figure S5.
The biochemical signature is different at the start of dialysis, with once- or twice-weekly dialysis being proposed to patients with better metabolic control, as witnessed by significantly higher hemoglobin (9.9 vs. 8.8 g/dl, P = 0.007), albumin (3.2 vs. 2.9 g/dl, P < 0.001), eGFR (7 vs. 6 ml/min per 1.72 m2, P = 0.035), and urinary output (≥1000 ml/24 h: 82.8% vs. 44.4%, P < 0.001) and lower phosphates (1.73 vs. 1.90 mmol/l, P = 0.002) and NT-proBNP (4640 vs. 11092 ng/l, P = 0.008) (Supplementary Table S1).
At 3 months and at the last update, the recorded differences essentially confirmed the selection criteria, allowing only patients with stable biochemical profiles to continue on once- or twice-weekly HD (Supplementary Table S8). Of note, β2m, a marker of residual kidney function, was lowest in patients on once-weekly HD and highest on thrice-weekly HD (P < 0.001) (Supplementary Tables S8 and S9).
Cost Analysis
Table 4 reports on the number of dialysis sessions “saved,” when compared with a standard dialysis schedule. If the 105 patients on incremental and decremental dialysis had been on thrice-weekly schedules, they would have needed 12,199 dialysis sessions over their follow-up; because they received only 6780 sessions, we calculated that 5419 HD sessions were “saved.”
Table 4.
Cost analysis
| Nonstandard |
Total among group | ||
|---|---|---|---|
| Incremental | Decremental | ||
| n | 91 | 14 | 105 |
| 1 dialysis per week | |||
| Patient-years of observation | 21.4 | 4.5 | 25.9 |
| Number of dialysis | 1115 | 234 | 1349 |
| 2 dialysis per week | |||
| Patient-years of observation | 37.8 | 14.5 | 52.2 |
| Number of dialysis | 3926 | 1505 | 5431 |
| Total among nonstandard dialysis frequency | |||
| Patient-years of observation | 59.2 | 19.0 | 78.2 |
| Number of dialysis | 5041 | 1739 | 6780 |
| Summary | |||
|---|---|---|---|
| Patient-years of observation on nonstandard HD | Number of sessions observed on nonstandard HD | Number of sessions expected on thrice-weekly HD | “Saved” HD sessions |
| 78.2 | 6780 | 12,199 | 5419a |
HD, hemodialysis.
The hypothetic dialysis sessions were calculated using the following equation: ,
where Wy is the number of weeks per year (i.e., 52), Dfreq is the dialysis frequency considered, and Pyear is the patient-years of observation.
With an overall follow-up of 219 patient-years in the total incident population of 158 patients (median follow-up per patient: 1.03 years), 5419 dialysis sessions correspond to 15.86% of the theoretical 34,164 sessions calculated with respect to the standard thrice-weekly HD.
Bold value indicates significant differences.
Equivalent to 1,896,850 Euros for dialysis costs (each session calculated at 350 Euros) and 270,950 Euros for transportation (calculated at 50 Euros for each dialysis session).
With an overall follow-up of 219 patient-years in the total incident population of 158 patients, 5419 dialysis sessions correspond to 16% of the sessions that would have been needed if all patients were on thrice-weekly HD (Table 4).
Considering the French reimbursement system and the cost of transportation, we calculated a savings of >2 million Euros. This consistent savings for society is however counterbalanced by an increase in the cost of controls (evaluated at 400 Euros/yr/patient for additional biochemical tests).
Discussion
The main result of our study is that it demonstrates that HD can be safely started in a “non-standard” way, combining an incremental and a decremental schedule, in the large majority of patients (105/158 cases, 66%). This prevalence is higher than the already high potential calculated a posteriori in a recent study in the United States, that sets it at about 50% of incident cases.34 It is worth noting that in the setting in which the study was carried out, dialysis is started in accordance with an intent-to-defer policy (median eGFR at dialysis start: 7 ml/min per 1.72 m2) (Table 1).
In line with the European incident dialysis population, the study cohort is elderly and at high comorbidity (median age 70 years, median CCI: 8, Table 1).49
It is also worth noticing that the prevalence of patients without previous follow-up was in the lower range recorded in France (about 30%) and that over 60% of the cases had follow-up of at least 1 year before the start of HD.50,51 Likewise, the prevalence of new patients who started dialysis with a PD modality was well above the French range (23% vs. 6.3%).35
The systematic introduction of an iHD schedule, associated with the possibility of reducing the number of dialysis sessions for patients who had started on a standard schedule (dHD), was not associated with lower survival.
Drawing clear conclusions from observational studies is difficult; however, these studies directly reflect the clinical practice and may complement randomized controlled trials. Interestingly, our data are in keeping with those of a recently published randomized controlled trial, demonstrating superimposable survival in the 2 arms (26 and 29 patients randomized to thrice- and twice-weekly dialysis).52
Because we felt that randomizing HD start would not be considered acceptable by our patients, we wanted to ensure that survival was at least equivalent to the one on standard schedules. We did so in the following 3 complementary ways, each affected by a different bias: per protocol, comparing patients with standard and nonstandard dialysis in the period of study (selection bias, according to residual kidney function); per system of care, comparing the period of study with a previous one in which HD was started with thrice-weekly schedules in all cases (potentially biased by further concomitant treatment changes); and comparison with propensity score-matched patients from other regional centers obtained from the REIN Registry (potential “center effect”). In all analyses, results observed on nonstandard HD were not inferior to those observed on standard schedules (Figure 1, Figure 2, Table 3).
In a setting in which patients come from a large area (up to about 80 km from the HD center), and social isolation is common, hospitalization was frequent. In this context, no difference was recorded in incidence and duration of hospitalization after HD start for patients on standard and nonstandard dialysis schedules, or in time to first hospitalization or death (Supplementary Table S6 and Supplementary Figure S4).
While the metabolic profile is different at the start of dialysis in patients on thrice-weekly HD or less frequent schedules, at 3 months, and at the last update, the only differences regard residual kidney function and β2m levels, considered to be reliable markers of residual kidney function, thus confirming the feasibility of iHD-dHD schedules, provided strict control is assured.41,53
While the duration of follow-up in patients followed-up for at least 1 month predialysis had no significant effect on the choice of the initial schedule, the quality of follow-up did, and patients who were exposed to at least 1 month of intensive predialysis care had a higher chance of starting dialysis in a nonstandard, more gradual way. Conversely, obesity, comorbidity, age, or sex did not significantly affect this choice, while needing dialysis as an emergency measure was associated with higher odds of starting and continuing treatment with a thrice-weekly schedule.
With a median duration of 1 year, and with about 1 patient of 4 still on an incremental schedule 2 years after dialysis start, this policy made possible consistent savings with respect to standard schedules, corresponding to roughly a 15% reduction in the number of dialysis sessions in incident patients over the period of study. The amount saved, calculated using French data, works out to be over 2 million Euros in 4 years. This is clearly a relevant sum for the health care system, but it may not be favorable for the individual structure, as organizing dialysis sessions can be more complex and the additional cost of blood tests has to be met by the dialysis providers. This consideration supports the need for dedicated investments to avoid penalizing an overall favorable, more physiological, and logical dialysis option.
This study has limitations. Information on patients’ preferences and on quality of life is lacking and, while it is intuitive that they prefer having fewer HD sessions, we have no direct evidence to show that they do. This knowledge gap will hopefully be filled by a planned prospective study.
Furthermore, even if creatinine and urea clearances on 24-hour urine collection, the gold standard for the assessment of the residual kidney function, are routinely prescribed, these controls are often not reliable (incomplete collection, errors in sampling, refusal of the patient to perform the collection). This problem is not unusual; while, to the best of our knowledge, no study specifically addressed the issue of the reliability of 24-hour urine collection in dialysis patients (and in particular in elderly populations at high comorbidity), data gathered in younger populations, mainly with lithiasis, suggest that at least half of the 24-hour urine collections are unreliable.38, 39, 40 Similar problems are well known in the context of the care of advanced chronic kidney disease and make the application of the formulae based on 24-hour urine collection not applicable on the overall chronic kidney disease population; hence, several alternative evaluations are proposed in this population.11 As a consequence, we did not model dialysis schedule on urea clearance in our elderly population and considered the data, whenever available, as ancillary in the decision to modulate HD frequency, and based the decision on blood tests. Furthermore, we included in the monthly evaluations of the outpatients the residual renal function calculated on the levels of β2m. While this formula is not free of interferences with inflammation and myeloproliferative diseases, this was a precious integration to the monthly data (Supplementary Tables S8 and S9).
Indeed, we feel that this limitation may also become a strength, indicating incremental schedules can be safely controlled without the need for the periodic, cumbersome 24-hour urine collection.41,53, 54, 55, 56
Most importantly, as it is a proof-of-concept study, we deal only with 1 center, the one in which this “prototype” experience was developed. The limits of the observational design are at least partly counterbalanced by the advantage of dealing with the whole dialysis population, thus avoiding the bias of selective recruitment, which is common in randomized studies dealing with different dialysis schedules (about 20% in the most important daily dialysis trial; about 50% in the recent randomized controlled trial comparing twice and thrice-weekly dialysis).31,32,52,57, 58, 59, 60
While the different analyses regarding the risk of death were performed on larger cohorts (per period and with respect to the REIN Registry), the analysis on hospitalizations was limited to the last period, and numbers are still relatively low, so that large-scale confirmation on a longer period is needed. Furthermore, the effect of changes in practices over time and the “center effect” could not be ruled out.
A further characteristic of our study, which might be seen as a limitation, is the fact that both the choice of dialysis start and the modulation of dialysis frequency are not based on single measured items or on a rigid algorithm, but on a comprehensive evaluation that weighs different factors (metabolic balance, weight gain, blood pressure, uremic symptoms). While we tried to capture this individualized approach in the tables and in the clinical examples, we are aware of the fact that it may render comparisons among different settings more difficult. However, this nuanced approach is in line with a large body of literature and is in keeping with the guidelines that suggest basing the intent-to-defer strategy, in the context of low residual kidney function, on a vast array of symptoms, contextualized to the clinical, social, and educational characteristics and to patients’ preferences. We considered that the logical corollary of basing dialysis start on an integrated evaluation was to use the same approach for dialysis modulation. This, of course, needs further prospective evaluations, also aimed at assessing the “relative weight” of each indication and their combination.
The cohort studied is one of the largest of iHD so far available, yet it is limited with respect to registry data or multicenter studies. This bias is in common with pilot experiences, and we recognize, of course, that validation on a larger scale is needed.27 The present study was possible thanks to a particular situation, that is, the reorganization of a large nephrology unit, with the recruitment of a new medical team, experienced in personalized dialysis and predialysis treatments.61, 62, 63 Transforming routine practice is often difficult and time consuming, and this is why we feel that this experience of a rapid shift from standardized to personalized HD offered us, within the limits of a single-center experience, a unique occasion to describe the potential of implementing iHD.
In conclusion, the main result is having found that iHD start may represent a new standard of care, as it is safe and feasible in up to two-thirds of patients, at least in a European population with advanced age and high comorbidity. We therefore suggest that instead of trying to identify ideal candidates for iHD, we should focus on reducing contraindications, mainly through strict predialysis follow-up, to this more physiological, gradual, and flexible modality of HD start.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Susan Finnel for her careful language editing. The authors thank all those contributing information to the REIN registry, a full list of whom is available at https://www.agence-biomedecine.fr/R-E-I-N-Reseau-Epidemiologique-et-Information-en-Nephrologie?lang=fr.
Footnotes
Supplementary Methods.
Table S1. Biochemical profile at dialysis start in the period 2017–2021 according to dialysis schedule.
Table S2. Survival analysis. Cox model.
Table S3. Survival analysis. Cox regression model for the 2 periods (2013–2015 and 2017–2021).
Table S4. Baseline characteristics of incident hemodialysis patients at Centre Hospitalier Le Mans in the period 2017–2021, of patients in the REIN Registry, and of patients in the REIN Registry after propensity score matching.
Table S5. Time on incremental dialysis. Cox model.
Table S6. Reasons for starting and continuing dialysis at 3 or more sessions/wk.
Table S7. Number of hospitalizations according to initial dialysis schedule.
Table S8. Biochemical profile at 3 months from dialysis start in the period 2017–2021 according to dialysis schedule.
Table S9. Biochemical profile at the last available control in June 2021 for the 2017–2021 cohort according to dialysis schedule.
Figure S1. Survival of incident hemodialysis patients in the period 2017–2021 at Centre Hospitalier Le Mans, according to gender (A), age (B), predialysis care (C), and Charlson comorbidity index (D).
Figure S2. Comparison of survival between incident hemodialysis patients at Centre Hospitalier Le Mans in the periods 2017–2021, 2013–2015 and the REIN Registry: overall (A); patients aged ≥70 years (B); patients younger than 70 years (C).
Figure S3. Comparison of survival between incident hemodialysis patients (A, 1:2 matching; B, 1:5 matching) or incremental hemodialysis patients (C, 1:2 matching; D, 1:5 matching) at Centre Hospitalier Le Mans in the period 2017–2021 and a propensity score-matched cohort from the REIN Registry.
Figure S4. Time to first event (hospitalization or death) in the 2017–2021 cohort after the start of renal replacement therapy according to dialysis schedule.
Figure S5. Flowchart of patients hemodialyzed at Centre Hospitalier Le Mans in the period 2017–2021 included in the study.
STROBE Statement.
Supplementary Material
Supplementary Methods.
Table S1. Biochemical profile at dialysis start in the period 2017–2021 according to dialysis schedule.
Table S2. Survival analysis. Cox model.
Table S3. Survival analysis. Cox regression model for the 2 periods (2013–2015 and 2017–2021).
Table S4. Baseline characteristics of incident hemodialysis patients at Centre Hospitalier Le Mans in the period 2017–2021, of patients in the REIN Registry, and of patients in the REIN Registry after propensity score matching.
Table S5. Time on incremental dialysis. Cox model.
Table S6. Reasons for starting and continuing dialysis at 3 or more sessions/wk.
Table S7. Number of hospitalizations according to initial dialysis schedule.
Table S8. Biochemical profile at 3 months from dialysis start in the period 2017–2021 according to dialysis schedule.
Table S9. Biochemical profile at the last available control in June 2021 for the 2017–2021 cohort according to dialysis schedule.
Figure S1. Survival of incident hemodialysis patients in the period 2017–2021 at Centre Hospitalier Le Mans, according to gender (A), age (B), predialysis care (C), and Charlson comorbidity index (D).
Figure S2. Comparison of survival between incident hemodialysis patients at Centre Hospitalier Le Mans in the periods 2017–2021, 2013–2015 and the REIN Registry: overall (A); patients aged ≥70 years (B); patients younger than 70 years (C).
Figure S3. Comparison of survival between incident hemodialysis patients (A, 1:2 matching; B, 1:5 matching) or incremental hemodialysis patients (C, 1:2 matching; D, 1:5 matching) at Centre Hospitalier Le Mans in the period 2017–2021 and a propensity score-matched cohort from the REIN Registry.
Figure S4. Time to first event (hospitalization or death) in the 2017–2021 cohort after the start of renal replacement therapy according to dialysis schedule.
Figure S5. Flowchart of patients hemodialyzed at Centre Hospitalier Le Mans in the period 2017–2021 included in the study.
STROBE Statement (PDF).
References
- 1.Gaudry S., Hajage D., Martin-Lefevre L., et al. Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet. 2021;397:1293–1300. doi: 10.1016/S0140-6736(21)00350-0. [DOI] [PubMed] [Google Scholar]
- 2.Gaudry S., Hajage D., Schortgen F., et al. Initiation strategies for renal-replacement therapy in the Intensive Care Unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 3.Cooper B.A., Branley P., Bulfone L., et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 4.STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian and New Zealand Intensive Care Society Clinical Trials Group, et al. Timing of initiation of renal-replacement therapy in acute kidney injury [published correction appears in N Engl J Med. 2020;383:502] N Engl J Med. 2020;383:240–251. doi: 10.1056/NEJMoa2000741. [DOI] [PubMed] [Google Scholar]
- 5.Tattersall J. Residual renal function in incremental dialysis. Clin Kidney J. 2018;11:853–856. doi: 10.1093/ckj/sfy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tattersall J., Farrington K., Gentile G., et al. Is Kt/V useful in elderly dialysis patients? Pro and con arguments. Nephrol Dial Transplant. 2018;33:742–750. doi: 10.1093/ndt/gfy042. [DOI] [PubMed] [Google Scholar]
- 7.Tattersall J. Hemodialysis time and Kt/V: less may be better. Semin Dial. 2017;30:10–14. doi: 10.1111/sdi.12555. [DOI] [PubMed] [Google Scholar]
- 8.Lertdumrongluk P., Tantisattamo E., Obi Y., et al. Estimated glomerular filtration rate at dialysis initiation and subsequent decline in residual kidney function among incident hemodialysis patients. Nephrol Dial Transplant. 2020;35:1786–1793. doi: 10.1093/ndt/gfaa05. [DOI] [PubMed] [Google Scholar]
- 9.Hsu C.Y., Parikh R.V., Pravoverov L.N., et al. Implication of trends in timing of dialysis initiation for incidence of end-stage kidney disease. JAMA Intern Med. 2020;180:1647–1654. doi: 10.1001/jamainternmed.2020.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong J., Davies M., Mount P. The importance of residual kidney function in haemodialysis patients. Nephrology. 2018;23:1073–1080. doi: 10.1111/nep.13427. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 12.Nesrallah G.E., Mustafa R.A., Clark W.F., et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186:112–117. doi: 10.1503/cmaj.130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberek T., Warzocha A., Galgowska J., Taszner K., Clark W.F., Rutkowski B. When to initiate dialysis--is early start always better? Nephrol Dial Transplant. 2011;26:2087–2091. doi: 10.1093/ndt/gfr181. [DOI] [PubMed] [Google Scholar]
- 14.Leurs P., Machowska A., Lindholm B. Timing of dialysis initiation: when to start? Which treatment? J Ren Nutr. 2015;25:238–241. doi: 10.1053/j.jrn.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Chen T., Lee V.W., Harris D.C. When to initiate dialysis for end-stage kidney disease: evidence and challenges. Med J Aust. 2018;209:275–279. doi: 10.5694/mja18.00297. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K., Unruh M., Zager P.G., et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caria S., Cupisti A., Sau G., Bolasco P. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014;15:172. doi: 10.1186/1471-2369-15-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libetta C., Esposito P., Dal Canton A. Once-weekly hemodialysis: a single-center experience. Am J Kidney Dis. 2015;65:343. doi: 10.1053/j.ajkd.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Bolasco P., Cupisti A., Locatelli F., Caria S., Kalantar-Zadeh K. Dietary management of incremental transition to dialysis therapy: once-weekly hemodialysis combined with low-protein diet [published correction appears in J Ren Nutr. 2017;27:74] J Ren Nutr. 2016;26:352–359. doi: 10.1053/j.jrn.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Wolley M.J., Hawley C.M., Johnson D.W., Marshall M.R., Roberts M.A. Incremental and twice weekly haemodialysis in Australia and New Zealand. Nephrol (Carlton) 2019;24:1172–1178. doi: 10.1111/nep.13556. [DOI] [PubMed] [Google Scholar]
- 21.Yan Y., Ramirez S., Anand S., Qian J., Zuo L. Twice-weekly hemodialysis in China: can it be a better option for initiation or maintenance dialysis therapy? Semin Dial. 2017;30:277–281. doi: 10.1111/sdi.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer T.W., Hostetter T.H., Watnick S. Twice-weekly hemodialysis is an option for many patients in times of dialysis unit stress. J Am Soc Nephrol. 2020;31:1141–1142. doi: 10.1681/ASN.2020030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murea M. Precision medicine approach to dialysis including incremental and decremental dialysis regimens. Curr Opin Nephrol Hypertens. 2021;30:85–92. doi: 10.1097/MNH.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 24.Piccoli G.B., Guzzo G., Vigotti F.N., et al. Chronic dialysis discontinuation: a systematic narrative review of the literature in the new millennium. Int J Artif Organs. 2014;37:556–562. doi: 10.5301/ijao.5000321. [DOI] [PubMed] [Google Scholar]
- 25.Piccoli G.B., Guzzo G., Vigotti F.N., et al. Tailoring dialysis and resuming low-protein diets may favor chronic dialysis discontinuation: report on three cases. Hemodial Int. 2014;18:590–595. doi: 10.1111/hdi.12168. [DOI] [PubMed] [Google Scholar]
- 26.Tentori F., Hunt A., Nissenson A.R. Palliative dialysis: addressing the need for alternative dialysis delivery modes. Semin Dial. 2019;32:391–395. doi: 10.1111/sdi.12820. [DOI] [PubMed] [Google Scholar]
- 27.Garofalo C., Borrelli S., De Stefano T., et al. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol. 2019;32:823–836. doi: 10.1007/s40620-018-00577-9. [DOI] [PubMed] [Google Scholar]
- 28.Murea M., Moossavi S., Garneata L., Kalantar-Zadeh K. Narrative review of incremental hemodialysis. Kidney Int Rep. 2020;5:135–148. doi: 10.1016/j.ekir.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew A.T., Obi Y., Rhee C.M., Chou J.A., Kalantar-Zadeh K. Incremental dialysis for preserving residual kidney function-Does one size fit all when initiating dialysis? Semin Dial. 2018;31:343–352. doi: 10.1111/sdi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosdriesz J.R., Stel V.S., van Diepen M., et al. Evidence-based medicine—when observational studies are better than randomized controlled trials. Nephrol (Carlton) 2020;25:737–743. doi: 10.1111/nep.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Israni A.K., Halpern S.D., McFadden C., et al. Willingness of dialysis patients to participate in a randomized controlled trial of daily dialysis. Kidney Int. 2004;65:990–998. doi: 10.1111/j.1523-1755.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 32.Twardowski Z.J., Misra M., Singh A.K. Randomized controlled trials (RCT) have failed in the study of dialysis methods. Nephrol Dial Transplant. 2013;28:826–832. doi: 10.1093/ndt/gfs307. [DOI] [PubMed] [Google Scholar]
- 33.Halpern S.D., Berns J.S., Israni A.K. Willingness of patients to switch from conventional to daily hemodialysis: looking before we leap. Am J Med. 2004;116:606–612. doi: 10.1016/j.amjmed.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Chin A.I., Appasamy S., Carey R.J., Madan N. Feasibility of incremental 2-times weekly hemodialysis in incident patients with residual kidney function. Kidney Int Rep. 2017;2:933–942. doi: 10.1016/j.ekir.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassalle M., Monnet E., Ayav C., et al. Annual report digest of the Renal Epidemiology Information Network (REIN) registry. Transpl Int. 2019;32:892–902. doi: 10.1111/tri.13466. [DOI] [PubMed] [Google Scholar]
- 36.Fois A., Torreggiani M., Trabace T., et al. Quality of life in CKD patients on low-protein diets in a multiple-choice diet system. Comparison between a French and an Italian experience. Nutrients. 2021;13:1354. doi: 10.3390/nu13041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couchoud C., Stengel B., Landais P., et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411–418. doi: 10.1093/ndt/gfi198. [DOI] [PubMed] [Google Scholar]
- 38.Boyd C., Wood K., Whitaker D., et al. Accuracy in 24-hour urine collection at a tertiary center. Rev Urol. 2018;20:119–124. doi: 10.3909/riu0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miler M., Simundić A.M. Low level of adherence to instructions for 24-hour urine collection among hospital outpatients. Biochem Med (Zagreb) 2013;23:316–320. doi: 10.11613/bm.2013.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghiraldi E.M., Reddy M., Li T., Lawler A.C., Friedlander J.I. Factors associated with compliance in submitting 24-hour urine collections in an underserved community. J Endourol. 2017;31:S64–S68. doi: 10.1089/end.2016.0594. [DOI] [PubMed] [Google Scholar]
- 41.Jaques D.A., Davenport A. Serum beta2-microglobulin as a predictor of residual kidney function in peritoneal dialysis patients. J Nephrol. 2021;34:473–481. doi: 10.1007/s40620-020-00906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couillerot-Peyrondet A.L., Sambuc C., Sainsaulieu Y., Couchoud C., Bongiovanni-Delarozière I. A comprehensive approach to assess the costs of renal replacement therapy for end-stage renal disease in France: the importance of age, diabetes status, and clinical events. Eur J Health Econ. 2017;18:459–469. doi: 10.1007/s10198-016-0801-6. [DOI] [PubMed] [Google Scholar]
- 43.Piccoli G.B., Cabiddu G., Breuer C., Jadeau C., Testa A., Brunori G. Dialysis reimbursement: what impact do different models have on clinical choices? J Clin Med. 2019;8:276. doi: 10.3390/jcm8020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allenbach D., Pereira O. Analyse de la demande de transport des patients dialysés en Lorraine. Sante Publique. 2015;27:S155–S165. [PubMed] [Google Scholar]
- 45.Austin P.C. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austin P.C. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172:1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 48.Ho D.E., Imai K., King G., Stuart E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Pol Anal. 2007;15:199–236. doi: 10.1093/pan/mpl013. [DOI] [Google Scholar]
- 49.Kramer A., Pippias M., Noordzij M., et al. The European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: a summary. Clin Kidney J. 2018;11:108–122. doi: 10.1093/ckj/sfx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michel A., Pladys A., Bayat S., Couchoud C., Hannedouche T., Vigneau C. Deleterious effects of dialysis emergency start, insights from the French REIN registry [published correction appears in BMC Nephrol. 2018;19:266] BMC Nephrol. 2018;19:233. doi: 10.1186/s12882-018-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padilla C.M., Raffray M., Pladys A., Vigneau C., Bayat S. Geographic variations in the risk of emergency first dialysis for patients with end stage renal disease in the Bretagne region, France. Int J Environ Res Public Health. 2018;16:18. doi: 10.3390/ijerph16010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilar E., Kaja Kamal R.M., Fotheringham J., et al. A multicenter feasibility randomized controlled trial to assess the impact of incremental versus conventional initiation of hemodialysis on residual kidney function. Kidney Int. 2022;101:615–625. doi: 10.1016/j.kint.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Davenport A. Measuring residual renal function for hemodialysis adequacy: is there an easier option? Hemodial Int. 2017;21(suppl 2):S41–S46. doi: 10.1111/hdi.12592. [DOI] [PubMed] [Google Scholar]
- 54.Fry A.C., Singh D.K., Chandna S.M., Farrington K. Relative importance of residual renal function and convection in determining beta-2-microglobulin levels in high-flux haemodialysis and on-line haemodiafiltration. Blood Purif. 2007;25:295–302. doi: 10.1159/000104870. [DOI] [PubMed] [Google Scholar]
- 55.Argyropoulos C.P., Chen S.S., Ng Y.H., et al. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med (Lausanne) 2017;4:73. doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong J., Sridharan S., Berdeprado J., et al. Predicting residual kidney function in hemodialysis patients using serum β-trace protein and β2-microglobulin. Kidney Int. 2016;89:1090–1098. doi: 10.1016/j.kint.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 57.FHN Trial Group. Chertow G.M., Levin N.W., et al. In-center hemodialysis six times per week versus three times per week [published correction appears in N Engl J Med. 2011;364:93] N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pipkin M., Eggers P.W., Larive B., et al. Recruitment and training for home hemodialysis: experience and lessons from the Nocturnal Dialysis Trial. Clin J Am Soc Nephrol. 2010;5:1614–1620. doi: 10.2215/CJN.02440310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natale P., Gutman T., Howell M., et al. Recruitment and retention in clinical trials in chronic kidney disease: report from national workshops with patients, caregivers and health professionals. Nephrol Dial Transplant. 2020;35:755–764. doi: 10.1093/ndt/gfaa044. [DOI] [PubMed] [Google Scholar]
- 60.Piccoli G.B., Magnano A., Perrotta L., Piccoli G. Daily dialysis, nocturnal dialysis, and randomized controlled trials: are we asking the right questions? Kidney Int. 2005;68:2913–2914. doi: 10.1111/j.1523-1755.2005.00583_11.x. [DOI] [PubMed] [Google Scholar]
- 61.Torreggiani M., Chatrenet A., Fois A., et al. Elderly patients in a large nephrology unit: who are our old, old-old and oldest-old patients? J Clin Med. 2021;10:1168. doi: 10.3390/jcm10061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torreggiani M., Chatrenet A., Fois A., et al. Unmet needs for CKD care: from the general population to the CKD clinics. How many patients are we missing? Clin Kidney J. 2021;14:2246–2254. doi: 10.1093/ckj/sfab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piccoli G.B., Lippi F., Fois A., et al. Intradialytic nutrition and hemodialysis prescriptions: a personalized stepwise approach. Nutrients. 2020;12:785. doi: 10.3390/nu12030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cernovsky Z.Z. A frequent misunderstanding associated with point biserial and phi coefficients. Psychol Rep. 2002;90:65–66. doi: 10.2466/pr0.2002.90.1.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.