Abstract
Purpose
Our previous studies showed that nanotechnology improves derived adipose-derived stem cells (ADSCs) therapy for erectile dysfunction (ED). In this study, the Neuregulin-1(NRG1) gene was transfected into ADSCs with superparamagnetic iron oxide nanoparticles (SPION) further to improve the therapeutic effect of ADSCs on ED.
Materials and Methods
ADSCs were isolated from epididymal adipose tissue of Sprague–Dawley rats. The optimal concentration of PEI-SPION (SPION modified with polyethyleneimine) was selected to construct the gene complex. After electrostatic binding of PEI-SPION and DNA, a PEI layer was wrapped to make the PEI-SPION-NRG1-PEI gene transfection complex. Different groups were set up for transfection tests. Lipo2000 transfection reagent was used as the control. PEI-SPION-NRG1-PEI in the experimental group was transfected under an external magnetic field.
Results
When the concentration of PEI-SPION was 10 µg/mL, it had little cytotoxicity, and cell activity was not significantly affected. PEI-SPION-NRG1-PEI forms positively charged nanocomposites with a particle size of 72.6±14.9 nm when N/P ≥8. The PEI-SPION-NRG1-PEI gene complex can significantly improve the transfection efficiency of ADSCs, reaching 26.74%±4.62%, under the action of the external magnetic field. PCR and Western blot showed that the expression level of the NRG1 gene increased significantly, which proved that the transfection was effective.
Conclusions
PEI-SPION can be used as a vector for NRG1 gene transfection into ADSCs. PEI-SPION-NRG1-PEI packaging has the highest transfection efficiency under the external magnetic field than the other groups. These findings may provide a new strategy for ADSCs therapy for ED.
Keywords: Adipose-derived mesenchymal stem cells, Erectile dysfunction, Neuregulin-1, Superparamagnetic iron oxide nanoparticles, Transfections
Graphical Abstract
INTRODUCTION
In recent years, the cavernous nerve-injured erectile dysfunction (ED) caused by radical prostatectomy (RP) has attracted many public and academic circles. The primary pathological mechanism of ED after RP is cavernous nerve injury and subsequent vascular endothelial and smooth muscle injury. Penile rehabilitation therapy for this nerve injury ED is being widely carried out, including oral type 5 phosphodiesterase inhibitor (PDE5i), vacuum erection device, penile cavernous injection, low-energy shock wave therapy, etc. [1], However, the nerve injury after RP inhibits the NO/cGMP signal pathway. The treatment effect of first-line drug PDE5i is poor, other treatments are cumbersome, or patients’ compliance is lacking [2]. Therefore, we urgently need new and effective methods.
Adipose-derived stem cells (ADSCs) can self-renewal and multi-directional differentiation. They can also repair the lesion by migrating and secreting bioactive molecules. They show excellent application value in the treatment of a variety of diseases. Our previous studies have shown that ADSCs treatment can improve the erectile function of nerve-injured ED rats, and the application of nanotechnology can enhance this therapeutic effect [3,4]. However, there is still much room for improvement. Gene recombination of stem cells to improve the expression level of essential functional proteins and enhance the therapeutic effect of stem cells is considered a promising treatment [5].
Neuregulin-1 (NRG1) is a vital nerve repair factor. At the same time, it has a protective effect on blood vessels and smooth muscles, which is a potential target for treating nerve-injured ED. It has been confirmed that NRG1/ErbB signaling pathway plays a crucial role in nerve development and damage repair, including neuron migration, nerve differentiation, and myelination. NRG1 signal promotes peripheral nerve repair by affecting Schwann cell activity in the peripheral nervous system. In addition, NRG1 can also play an active role in vascular protection and regeneration by inhibiting RhoA and Rock1 signal pathways [6,7]. Therefore, NRG1 is a potential transfected gene to improve ADSCs in treating cavernous nerve-injured ED.
At present, several gene delivery methods have been developed, mainly divided into viral and non-viral methods. The advantage of the virus vector lies in its high delivery efficiency, but the safety problems are concerned, including cytotoxicity, cellular immune response, etc. Non-viral methods, including mechanical and electrical methods and chemical methods, are potentially safer alternative methods to introduce transgenes into cells, but the transfection efficiency is relatively low [8]. Some inorganic nanoparticles reported in recent years can be used as transfection vectors to improve gene transfection efficiency significantly. Polyethyleneimine (PEI) has a branched internal structure and can effectively bind and concentrate DNA. It is the most widely used cationic polymer to modify nanoparticles [9]. Xu et al. [10] developed novel water dispersed superparamagnetic iron oxide nanoparticles (SPION) with highly uniform size as a potential magnetic nanomaterial for effective gene transfection of stem cells. They found that SPION could efficiently transfect two reporter genes into MSCs, and proved that SPION had no adverse effect on the proliferation and differentiation of MSCs. Therefore, using SPION and external magnetic fields to assist gene delivery provides a new way for effective gene recombination of ADSCs and NRG1 [11]. This method is consistent with our previous method of using nanotechnology and external magnetic fields to improve ADSCs in treating cavernous nerve-injured ED.
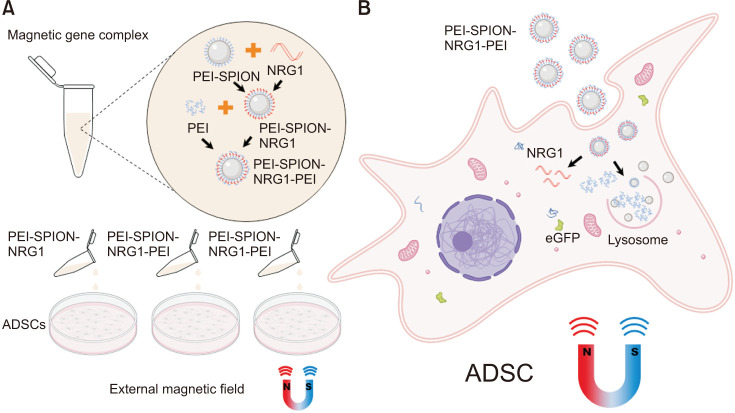
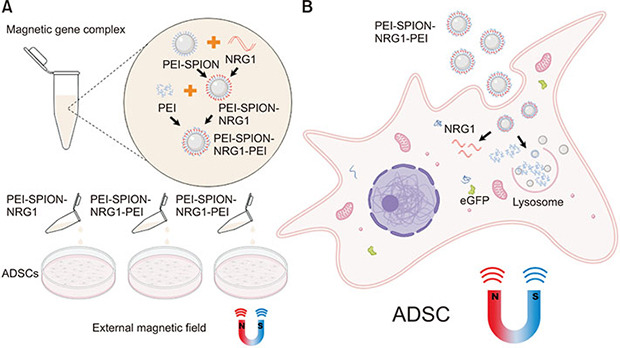
This study intends to transfect ADSCs with the NRG1 gene by SPION, compare the transfection efficiency of several different packaged nanotechnologies, and observe their cell safety (Fig. 1). It provides a basis for the follow-up treatment of nerve-injured ED.
Fig. 1. Schematic diagram of efficient delivery of NRG1 to ADSCs using SPION contained gene complexes. Gene transfection complexes were constructed in a specific order. (A) Under the action of the external magnetic field, nanoparticles with high magnetic susceptibility can effectively enter cells and promote the uptake of gene complexes containing SPION. (B) The uniquely assembled gene complex efficiently releases NRG1 plasmid DNA in cells, realizing the high-efficiency protein expression encoded by NRG1. NRG1, Neuregulin-1; ADSC, adipose-derived stem cell; PEI-SPION, superparamagnetic iron oxide nanoparticles modified with polyethyleneimine.
MATERIALS AND METHODS
1. Materials
All Sprague–Dawley male rats were from the animal experiment center, Peking University Health Science Center (PUHSC). The study was approved by the Institutional Animal Care and Use Committee (IACUC) of PUHSC (approval number: LA2017003). The following materials were used in this study: SPION modified with PEI (PEI-SPION), branched PEI (Sigma Aldrich, St. Louis, MO, USA), DMEM cell culture medium, fetal bovine serum FBS, 100 IU/mL penicillin-streptomycin (PS), 0.25% trypsin (Hyclone, Logan, UT, USA), Enhanced Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China), Lipofectamine 2000 transfection reagent (Invitrogen, Waltham, MA, USA), Gv230 NRG1 plasmid DNA (GeneChem, Shanghai, China).
2. Isolation and culture of ADSCs
Briefly, according to previous study [12], the fatty tissue from the epididymis of the rats was obtained. Then it was processed in the following steps: put in the sterile state, wash the bloodstain, wash it with phosphate buffered saline (PBS) containing 1% PS three times, transfer it into a new dish, cut the tissue into a chyme, add the digestive solution (0.1% collagenase I), digest it in a 37℃ water bath for 30 minutes, shake it every 5 minutes, filter it through a 70 µm filter screen to a centrifuge tube, centrifuge at 1,000 rpm/min at room temperature for 10 minutes, discard the supernatant, resuspend the complete medium (DMEM containing 10% fetal bovine serum and 1% PS), and incubate it in the cell culture plates.
3. CCK-8 assay
The effect of PEI-SPION on ADSC proliferation was evaluated by a CCK-8 assay. The CCK-8 analysis was carried out according to the manufacturer’s instructions. Each well was inoculated with 8000 cells in 96 well plates. The cells grew to 70% to 80% after being cultured for 12 hours. Then the cells were treated with PEI-SPION of different concentrations for 3 hours. After changing the FBS free medium, 10 µL CCK-8 solution was added to each well for incubation for 2 hours. The absorbance of A450 was measured. The experiment was repeated three times.
4. Preparation of SPION contained gene complexes
The equal volume of 1 µg NRG1 solution was mixed with PEI-SPION solution of appropriate concentration and incubated at room temperature for 15 minutes [10]. PEI-SPION combined with NRG1 to form nanocomposites through electrostatic interaction. PEI-SPION-NRG1-PEI gene complexes with different N/P ratios (a molar ratio of PEI nitrogen to DNA phosphate) [13] were obtained by adding PEI solutions of the same volume. Then they were incubated at room temperature for 15 minutes. A gel retardation test evaluated the DNA binding ability of PEI-SPION nanoparticles. The transfected complex was mixed with electrophoretic dye and added to 1% agarose gel to electrophoresis. Nanocomposites’ surface zeta potential and particle radius were measured at room temperature using a nanoparticle size and zeta potential analyzer (Zetasizer Nano ZSP, Malvern Panalytical, Malvern, UK).
5. Gene transfection
The Gene transfection procedure was followed in our previous study [10]. ADSCs were incubated into 24 well plates with 5×104 cells/well, 500 µL complete medium was added to each well, and the cells were routinely cultured in the incubator. On the second day, when the cells grew to 70% to 80%, transfection complex (containing NRG1 1 µg) was added, and the culture medium was changed after 3 hours. In the magnet group, the magnet (0.3 T) was used for 0.5 hours and replaced with a complete medium 2.5 hours later. The control group was transfected with cationic liposome Lipofectamine 2000, with three multiple pores in each group.
6. Live-dead assay
ADSCs were tested live-dead by Calcein AM/Propidium iodide (AM/PI) Assay Kit (Keygen, Nanjing, China) according to the manufacturer’s instructions to observe cell activity. To prepare the dyeing working solution, 5 µL of 16 mm PI was added into 10 mL PBS solution, 5 µL of 4 mM AM was added into 10 mL PI solution, then vortex mix well. The cells were washed gently with PBS three times, and then the working solution was added and incubated in the incubator. After 30 minutes incubation, the staining solution was discarded. The cells were washed with PBS to remove excess dyes and observed under a fluorescence microscope (LSM 720, Zeiss, Oberkochen, Germany). In this assay, live cells were stained green while dead cells were stained red.
7. RT-qPCR
After 24 hours of transfection, cells in each group were collected. The total RNA was extracted by Trizol, and the RT-PCR reaction was carried out according to the instructions of the reverted first-strand cDNA synthesis kit. The primer sequences of GDNF and their housekeeping gene β-actin were respectively 5'-CTTCGGTCAGAACGGAGCAA-3' (forward) 5'-ACAGTCGAGTGGC-3' (reverse) for NRG1, and 5'-GGCTGTATTCCCCTCCATCG-3'(forward) 5'-CCAGTTGGTAAATGCCATGT-3' (reverse). The quantitative PCR was performed according to the ABI powerup SYBR Green kit (ABI, Los Angeles, CA, USA).
8. Western blot
Forty-eight hours after transfection, the cells in each group were collected, washed with precooled PBS, added with protein lysate, and then ultrasonically lysed to extract the total protein of the cells. SDS-PAGE electrophoresis was performed routinely. After membrane transfer, the membrane was sealed in 5% skimmed milk powder solution at room temperature for 1 hour, and then incubated with antibody working solution at 4℃ overnight. The remaining steps are performed routinely.
9. Statistical analysis
The data were analyzed by SPSS 26.0 statistical analysis software (IBM Corp., Armonk, NY, USA) and the results were expressed as mean±standard deviation. One-way ANOVA was used to compare the difference. Differences among groups were considered significant at p<0.05.
RESULTS
1. The morphology of ADSCs and the effects of different concentrations of PEI-SPION on the activity of ADSCs
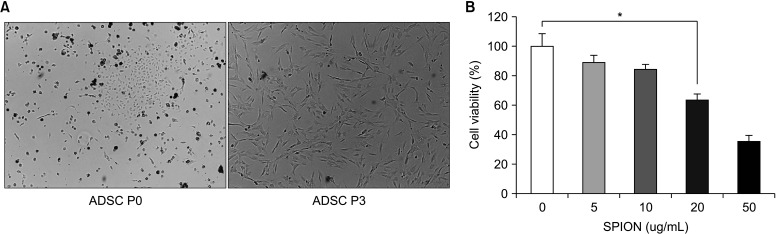
After cultured for 24 hours, the primary ADSCs cells showed round shape and adherent growth under an inverted microscope. After 4 to 6 days, the number of cells increased significantly, and the cell morphology gradually changed from round to spindle. After two passages, the cell morphology tended to be consistent, most of which were spindle-shaped. Then the cells were identified the cell surface antigen by flow cytometry, as described in our previous paper [4]. The third passage ADSCs were selected for follow-up experiments (Fig. 2A).
Fig. 2. (A) The isolated primary ADSCs began to form colonies after two days of culture and showed uniform stellate cell morphology when passed to the third passage. Bright field, ×20. (B) The effect of PEI-SPION on cell viability was determined by CCK-8 method. When the concentration of PEI-SPION was 10 ug/mL, it would not have an apparent toxic effect on ADSC. ADSC, adipose-derived stem cell; PEI-SPION, superparamagnetic iron oxide nanoparticles modified with polyethyleneimine. *p<0.05.
Although different assembly methods of SPION gene complexes have a particular impact on biocompatibility, the concentration of ions is the primary factor determining cytotoxicity [14]. Our results showed that the living cells were decreased by increasing the iron concentration of PEI-SPION. The ratio of living cells was 90.48%, 83.62%, 69.33%, and 38.14% at concentration of 5, 10, 20, and 50 µg/mL, respectively (Fig. 2B). According to the experimental results, we chose the concentration of low cytotoxicity 10 µg/mL for follow-up experiments.
2. Gel retardation assay and particle size and surface potential of transfection complex
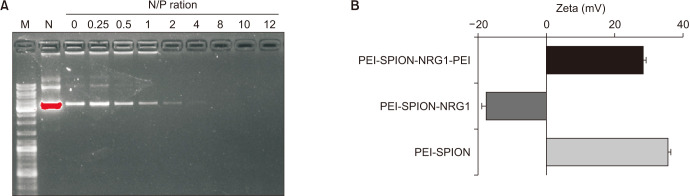
The physical and chemical properties of SPION have a significant impact on gene delivery efficiency and the biocompatibility of stem cells [15]. After PEI surface modification, the surface of PEI-SPION nanoparticles was positively charged with an average diameter of 10.3±3.7 nm. After binding with DNA, the complex potential changed from positive to negative, and the particle size increased to 85.4±12.3 nm. After PEI was added for incubation, the complex potential became positive again. The particle size decreased slightly to 72.6±14.9 nm, indicating that PEI successfully compressed the DNA adsorbed on the surface of PEI-SPION (Fig. 3B).
Fig. 3. (A) Agarose gel electrophoresis showed that when N/P was above 8, the gene complex began to be positively charged, and the PEI-SPION gene complex was utterly complex. (B) The measurement of surface zeta potential showed that the surface potential of gene complex containing PEI-SPION became negative after binding with the plasmid, and the potential turned positive after adding PEI, which was conducive to the entry of gene complex into cells. N/P, a molar ratio of PEI nitrogen to DNA phosphate; PEI-SPION, superparamagnetic iron oxide nanoparticles modified with polyethyleneimine; NRG1, Neuregulin-1.
The agarose gel electrophoresis results showed that with the increase of the N/P ratio, the complexes gradually shifted from negative to positive. When the N/P of the PEI-SPION-NRG1-PEI gene complex was above 8, positively charged nanocomposite could be formed, which could effectively compress and protect the DNA carried (Fig. 3A).
3. Detection of cell transfection efficiency and live-dead staining experiment
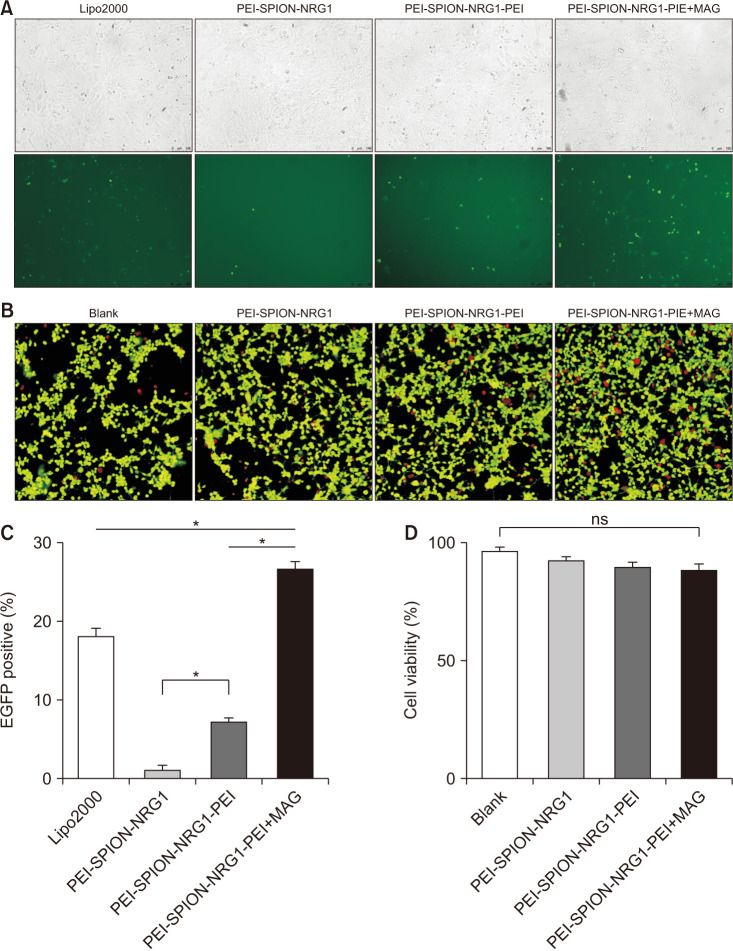
Groups were divided according to different transfection methods: lipo2000 group, PEI-SPION-NRG1 group, PEI-SPION-NRG1-PEI group, and PEI-SPION-NRG1-PEI+MAG (external magnetic field group). The expression of eGFP in the PEI-SPION-NRG1-PEI group was significantly higher than that in the PEI-SPION-NRG1 group but considerably lower than that in the lipo2000 group. However, after adding an external magnetic field, the expression of green fluorescent protein in the PEI-SPION-NRG1-PEI+MAG group was significantly higher than that in the lipo2000 PEI-SPION-NRG1 and the other groups (Fig. 4A, C).
Fig. 4. PEI-SPION can efficiently deliver genes to ADSCs with the help of the external magnetic field. (A) 24 hours after transfection, the eGFP expression of ADSCs in each group was observed by fluorescence microscope, ×20. (B) Live/dead assay showed that most ADSCs survived after treatment with gene complexes containing SPION, ×20. (C) The eGFP expression level assessed by flow cytometry showed that PEI-SPION efficiently transferred genes to ADSCs in the presence of external magnetic field (*p<0.05). (D) Cell viability evaluation showed that the SPION gene complex had hardly any harmful effect on the proliferation of ADSCs with or without the external magnetic field. eGFP, enhanced green fluorescent protein; PEI-SPION, superparamagnetic iron oxide nanoparticles modified with polyethyleneimine; ADSC, adipose-derived stem cell; NRG1, Neuregulin-1; ns, not significant.
The cytotoxicity of each group was compared by counting the dead cell ratio after live-dead staining. It was found that the percentage of dead cells in the blank group, the PEI-SPION-NRG1 group, the PEI-SPION-NRG1-PEI group, and PEI-SPION-NRG1-PEI+MAG group were 4.2%, 8.7%, 10.3%, and 10.9%, respectively. There was no significant difference (p=0.1693) among groups (Fig. 4B, D).
4. RNA and protein expression levels of NRG1 in adipose stem cells in each group
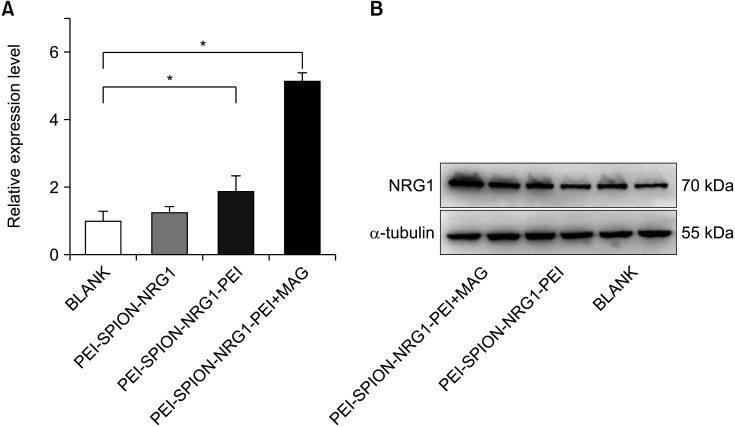
PCR showed the NRG1 mRNA expression of ADSCs in each group 24 hours after transfection: the ratio of NRG1 mRNA in PEI-SPION-NRG1 group, PEI-SPION-NRG1-PEI group, and PEI-SPION-NRG1-PEI+MAG group to the control group was 1.23±0.06, 1.87±0.28, and 5.19±0.34 respectively (Fig. 5A). There was a significant difference among the groups (p=0.0224). The results showed that the expression of NRG1 mRNA was the highest in the PEI-SPION-NRG1-PEI+MAG group.
Fig. 5. (A) NRG1 mRNA expression of ADSCs in each group 24 hours after transfection was significantly different from that in the control group (*p<0.05). (B) Western blot results of NRG1 protein in each group after gene transfection complex treatment for 48 hours. PEI-SPION, superparamagnetic iron oxide nanoparticles modified with polyethyleneimine; NRG1, Neuregulin-1.
Western blot showed the expression of NRG1 protein 48 hours after gene transfection complex treatment: the band gray of NRG1 protein in the control group, PEI-SPION-NRG1-PEI group, and PEI-SPION-NRG1-PEI+MAG group was 53.47%, 65.21%, and 83.18% respectively compared with the β-actin (Fig. 5B). The results showed that the expression of NRG1 protein was the highest in the PEI-SPION-NRG1-PEI+MAG group.
DISCUSSION
The application of ADSCs in the treatment of various diseases has shown great value. Compared with other types of stem cells, ADSCs can be obtained from autologous fat without complex ethical issues. Appropriate gene modification of ADSCs can improve the therapeutic effect of cells. Magnetic nanoparticles have been used for the transfection of cell lines and primary cells [16]. In the process of gene transfer by PEI-SPION, the gene complex first passes through the cell membrane and protects DNA and its subsequent release [17]. Magnetic nanoparticles mediated gene transfection has significant advantages over other gene delivery methods, such as high efficiency, low cytotoxicity, low cost, directional and remote controllability, effective in vivo application, and lack of immune response [18]. Yamoah et al. [19] successfully transfected human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (hiPSC-CMs) with magnetic nanoparticles under an external magnetic field. However, literatures of the SPION mediated gene transfection of ADSCs are very limited. In this study, NRG1 was firstly used as the transfection gene of ADSCs. It was proved that the transfection efficiency of PEI-SPION-NRG1-PEI was the highest under the action of the external magnetic field compared with the other groups. The cytotoxicity test showed that there was no significant difference between the gene complex and the control group.
The physical and chemical properties of SPION have a significant impact on the gene efficiency and the biocompatibility of stem cells [15]. Arsianti et al. [20] found that the assembly sequence of the transfection complex had a significant impact on cell uptake and intracellular escape ratio. The adsorption of DNA on PEI-SPION is attributed to the interaction between DNA phosphate and PEI amino groups through electrostatic and hydrogen bonds. The increased ability of PEI-coated SPION to bind DNA may also be attributed to the fact that PEI-SPION provides a larger DNA binding surface area than naked SPION. When the PEI was added to the PEI-SPION-NRG1 vector, the amount of unbound DNA detected was negligible, further stabilizing the gene complex and preventing premature loss of DNA [21]. Our study found that the PEI-SPION-NRG1-PEI gene complex can form positively charged nanocomposites when N/P ≥8, which can effectively compress and protect the carried DNA (Fig. 3A). Other studies have shown that the higher the N/P ratio of PEI to DNA, the easier the complex is to induce cell damage through cationic charge [22]. Thus, we constructed a PEI-SPION-NRG1-PEI gene complex with an N/P ratio of 10, making the complex positively charged and minimizing the damage to DNA in the following study. Our results also showed that PEI-SPION has no apparent harmful effect on the activity and growth of ADSCs when the concentration is 10 ug/mL and can efficiently transfer genes to stem cells.
As a vector of gene transfection, the transfection efficiency of nanoparticles is affected by many factors. Appropriate DNA packaging methods and external magnetic fields may significantly improve the transfection efficiency. According to references, we selected the external magnetic field of 0.3 T and the time of 0.5 hours for the experiment [23]. Our study found that although the transfection efficiency of the PEI-SPION-NRG1-PEI group was significantly higher than that of the PEI-SPION-NRG1 group, it was considerably lower than that of the lipo2000 group. However, after adding an external magnetic field, the PEI-SPION-NRG1-PEI+MAG group (26.74%±4.62%) was significantly higher than the lipo2000 group (18.35%±3.94%). This shows that the addition of an appropriate external magnetic field and exposure time is essential to improve the transfection efficiency. Therefore, we proved that the gene transfer efficiency of PEI-SPION-NRG1-PEI nanocomposite packaging was the highest under a specific external magnetic field. The improvement of cell transfection efficiency under an external magnetic field may relate to the increase of cell uptake complex caused by superparamagnetism of SPION [24].
Many studies have proved the role of NRG1 in nerve repair, regeneration, and vascular protection [25,26]. Soluble NRG1 is strongly up-regulated during axon regeneration, accompanied by the up-regulation of the ErbB receptor. This precise regulation shows that this molecule can be used to improve nerve regeneration clinically [27]. Recent studies have shown that NRG1/ErbB3 signal plays a vital role in vascular remodeling and blood-brain barrier regulation [28]. Endothelial NRG is necessary for angiogenesis induced by ischemic injury. Besides, exogenous administration of NRG can enhance this process [29]. This study successfully constructed ADSCs overexpressing NRG1 by using SPION special packaging NRG1. SPION also entered ADSCs as a gene transfection vector, which provides a basis for the subsequent use of the external magnetic field in animal models to improve the treatment efficiency. This is a continuation of our previous experimental methods, and overexpression of NRG1 was expected to enhance the effect of ADSCs in the treatment of nerve injury ED.
Although the current study presents limitations such as an in vitro study and needs future evaluation of in vivo, it is original and verified that the PEI-SPION can be used as a vector for NRG1 gene transfection into ADSCs. Based on our previous studies [3,4], further studies will focus on investigating the effect of PEI-SPION-NRG1-PEI on the cavernous nerve-injured ED model and the underlying mechanisms.
CONCLUSIONS
Our study shows that PEI-SPION can be used as a vector for NRG1 gene transfection into ADSCs. PEI-SPION-NRG1-PEI packaging has the highest transfection efficiency under the action of an external magnetic field compared with the other three groups. These findings may provide a new strategy for ADSCs therapy for ED.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: This study was supported by National Natural Science Foundations of China (#81601272), Natural Science Foundation of Beijing (General #7212134), Peking University Clinical Medicine+X Youth Special Program (PKU#2018LCXQ019) and National Key Research & Developmental Program of China (2018YFC1003600).
- Research conception and design: Haocheng Lin and Hui Jiang.
- Data acquisition: Jianxing Cheng and Zhongjie Zheng.
- Statistical analysis: Jianxing Cheng.
- Data analysis and interpretation: Zhongjie Zheng and Haocheng Lin.
- Drafting of the manuscript: Jianxing Cheng.
- Critical revision of the manuscript: Haocheng Lin, Zhongjie Zheng, Wenhao Tang, and Jichun Shao.
- Obtaining funding: Haocheng Lin.
- Administrative, technical, or material support: Wenhao Tang and Jichun Shao.
- Supervision: Haocheng Lin and Hui Jiang.
- Approval of the final manuscript: all authors.
References
- 1.Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osadchiy V, Eleswarapu SV, Mills SA, Pollard ME, Reiter RE, Mills JN. Efficacy of a preprostatectomy multi-modal penile rehabilitation regimen on recovery of postoperative erectile function. Int J Impot Res. 2020;32:323–328. doi: 10.1038/s41443-019-0187-y. [DOI] [PubMed] [Google Scholar]
- 3.Lin H, Dhanani N, Tseng H, Souza GR, Wang G, Cao Y, et al. Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol. 2016;195:788–795. doi: 10.1016/j.juro.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Tang WH, Zhao LM, Liu DF, Yang YZ, Zhang HT, et al. Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: in vivo cell tracking, optimized injection dosage, and functional evaluation. Asian J Androl. 2018;20:442–447. doi: 10.4103/aja.aja_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. 2013;45:1083–1086. doi: 10.1016/j.biocel.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Kang W, Cheng Y, Zhou F, Wang L, Zhong L, Li HT, et al. Neuregulin-1 protects cardiac function in septic rats through multiple targets based on endothelial cells. Int J Mol Med. 2019;44:1255–1266. doi: 10.3892/ijmm.2019.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bakker DEM, Bouwman M, Dronkers E, Simões FC, Riley PR, Goumans MJ, et al. Prrx1b restricts fibrosis and promotes Nrg1-dependent cardiomyocyte proliferation during zebrafish heart regeneration. Development. 2021;148:dev198937. doi: 10.1242/dev.198937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigante A, Li M, Junghänel S, Hirschhäuser C, Knauer S, Schmuck C. Non-viral transfection vectors: are hybrid materials the way forward? Medchemcomm. 2019;10:1692–1718. doi: 10.1039/c9md00275h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Y, Gao Y, Yang C, Guo R, Shi X, Cao X. Low-molecular-weight poly(ethylenimine) nanogels loaded with ultrasmall iron oxide nanoparticles for T1-weighted MR imaging-guided gene therapy of sarcoma. ACS Appl Mater Interfaces. 2021;13:27806–27813. doi: 10.1021/acsami.1c04081. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Zhang T, Wang Q, Jiang X, Li A, Li Y, et al. Uniformly sized iron oxide nanoparticles for efficient gene delivery to mesenchymal stem cells. Int J Pharm. 2018;552:443–452. doi: 10.1016/j.ijpharm.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Kalyane D, Kumar N, Anup N, Rajpoot K, Maheshwari R, Sengupta P, et al. Recent advancements and future submissions of silica core-shell nanoparticles. Int J Pharm. 2021;609:121173. doi: 10.1016/j.ijpharm.2021.121173. [DOI] [PubMed] [Google Scholar]
- 12.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adiposederived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Q, Huang T, Chen Z, Zhang T. Uniform iron oxide nanoparticles reduce the required amount of polyethylenimine in the gene delivery to mesenchymal stem cells. Nanotechnology. 2021;33:125101. doi: 10.1088/1361-6528/ac4066. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Peng E, Zhao F, Li J, Xue J. Supramolecular surface functionalization of iron oxide nanoparticles with α-cyclodextrin-based cationic star polymer for magnetically-enhanced gene delivery. Pharmaceutics. 2021;13:1884. doi: 10.3390/pharmaceutics13111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Workalemahu B, Jiang X. The effects of physicochemical properties of nanomaterials on their cellular uptake in vitro and in vivo. Small. 2017;13:1701815. doi: 10.1002/smll.201701815. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Palavicini JP, Wang H, Maiti P, Bianchi E, Xu S, et al. RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer's disease. Neurobiol Dis. 2014;69:169–179. doi: 10.1016/j.nbd.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidyanathan S, Orr BG, Banaszak Holl MM. Role of cell membrane-vector interactions in successful gene delivery. Acc Chem Res. 2016;49:1486–1493. doi: 10.1021/acs.accounts.6b00200. [DOI] [PubMed] [Google Scholar]
- 18.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 19.Yamoah MA, Moshref M, Sharma J, Chen WC, Ledford HA, Lee JH, et al. Highly efficient transfection of human induced pluripotent stem cells using magnetic nanoparticles. Int J Nanomedicine. 2018;13:6073–6078. doi: 10.2147/IJN.S172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsianti M, Lim M, Marquis CP, Amal R. Assembly of polyethylenimine-based magnetic iron oxide vectors: insights into gene delivery. Langmuir. 2010;26:7314–7326. doi: 10.1021/la9041919. [DOI] [PubMed] [Google Scholar]
- 21.Arsianti M, Lim M, Marquis CP, Amal R. Polyethylenimine based magnetic iron-oxide vector: the effect of vector component assembly on cellular entry mechanism, intracellular localization, and cellular viability. Biomacromolecules. 2010;11:2521–2531. doi: 10.1021/bm100748p. [DOI] [PubMed] [Google Scholar]
- 22.Park W, Yang HN, Ling D, Yim H, Kim KS, Hyeon T, et al. Multi-modal transfection agent based on monodisperse magnetic nanoparticles for stem cell gene delivery and tracking. Biomaterials. 2014;35:7239–7247. doi: 10.1016/j.biomaterials.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Siene Ng W, Binley K, Song B, Morgan JE. Use of magnetic nanoparticles and oscillating magnetic field for non-viral gene transfer into mouse cornea. Lancet. 2015;385 Suppl 1:S75. doi: 10.1016/S0140-6736(15)60390-7. [DOI] [PubMed] [Google Scholar]
- 24.Yu B, Dong B, He J, Huang H, Huang J, Wang Y, et al. Bimodal imaging-visible nanomedicine integrating CXCR4 and VEGFa genes directs synergistic reendothelialization of endothelial progenitor cells. Adv Sci (Weinh) 2020;7:2001657. doi: 10.1002/advs.202001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geissler A, Ryzhov S, Sawyer DB. Neuregulins: protective and reparative growth factors in multiple forms of cardiovascular disease. Clin Sci (Lond) 2020;134:2623–2643. doi: 10.1042/CS20200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataria H, Alizadeh A, Karimi-Abdolrezaee S. Neuregulin-1/ErbB network: an emerging modulator of nervous system injury and repair. Prog Neurobiol. 2019;180:101643. doi: 10.1016/j.pneurobio.2019.101643. [DOI] [PubMed] [Google Scholar]
- 27.Ronchi G, Haastert-Talini K, Fornasari BE, Perroteau I, Geuna S, Gambarotta G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur J Neurosci. 2016;43:351–364. doi: 10.1111/ejn.12974. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Islam MR, Lee J, Takase H, Guo S, Andrews AM, et al. ErbB3 is a critical regulator of cytoskeletal dynamics in brain microvascular endothelial cells: implications for vascular remodeling and blood brain barrier modulation. J Cereb Blood Flow Metab. 2021;41:2242–2255. doi: 10.1177/0271678X20984976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedhli N, Dobrucki LW, Kalinowski A, Zhuang ZW, Wu X, Russell RR, 3rd, et al. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res. 2012;93:516–524. doi: 10.1093/cvr/cvr352. [DOI] [PMC free article] [PubMed] [Google Scholar]