Abstract
AIM
To evaluate the therapeutic effect of amniotic membrane (AM) for covering high myopic macular hole associated with retinal detachment following failed primary surgery.
METHODS
Seventeen eyes of 17 patients whose axial length was more than 29 mm suffered from macular hole (MH) or MH associated with retinal detachment (RD), and had previously surgery of pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling and silicone oil (SO) tamponade. Half a year after the surgery, optical coherence tomography (OCT) showed that MH did not heal in all 17 eyes and RD was still maintained in 13 eyes of these 17 eyes. We performed SO removal combined with AM covering on macular area and C3F8 tamponade, and phacoemulsification combined with intraocular lens implantation simultaneously cataract eyes. We followed up these patients for one year.
RESULTS
In all 17 eyes, SO was removed successfully, MHs were healed and RDs were reattached. One eye (5.89%, 1/17) had AM shifted half a month after surgery and underwent a second surgery to adjust the position of the AM and supplement C3F8. After surgery, the visual acuity (VA) improved in 15 eyes (88.24%, 15/17), no change in two eyes (11.76%, 2/17). No serious complications occurred in all eyes.
CONCLUSION
AM covering is helpful to rescue the previous failure surgery of high myopic MH.
Keywords: amniotic membrane, high myopia, macular hole, retinal detachment
INTRODUCTION
Myopia is a global public health problem. The population of myopia in China ranking first in the world is nearly 600 million, of which about 300 million are teenagers[1]–[3]. However, the further challenge of myopia is the increased risk of secondary diseases, including cataract, glaucoma, retinal detachment (RD), macular hole (MH), and macular degeneration. These secondary fundus lesions may lead to an irreversible loss of vision[4]–[5].
Secondary fundus lesions caused by pathological myopia (high myopia) is the second major cause of low vision and blindness after cataract in China[6]. Pathological myopia macular hole (PMMH) and its related complications are difficult points in current clinical treatment.
Patients with PMMH always have the characteristics of long ocular axis, complicated with retinal choroid atrophy, posterior scleral staphyloma, tight adhesion of vitreous body and abnormal peripheral retina, so that the difficulty of operation is much higher than that of ordinary MH operation, which poses a challenge to the doctors of fundus disease[7]. The standard treatment of PMMH was pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling and endotamponade[8]. And the healing rate of PMMH in reports from different countries and regions is between 60% and 70%[9]–[11].
The low success rate of first surgery necessarily requires a second operation. It is one of the problems that need to be solved urgently for operators that how to improve the success rate in more complex secondary surgery. To solve this problem, Capoross et al[7] invented a technique named human amniotic membrane (hAM) tamponade to treat recurrent MH and had achieved perfect effect in clinical application. However, this technique still has disadvantages of complicated operation and iatrogenic injury. Inspired by this study, we improved this technique. The improved technique of covering AM for rescuing failed primary surgery of PMMH had gained good effect.
SUBJECTS AND METHODS
Ethical Approval
This is a prospective non-control case study. The case data were obtained from Wanjiang Eye Hospital of Mianyang. All subjects signed informed consent. And the study was approved by the Medical Ethics Committee of Wanjiang Eye Hospital of Mianyang and in accordance with the ethical requirements of clinicl trials and the Declaration of Helsinki.
Inclusion Criteria
The primary disease of all cases was PMMH associated with RD and the axial length was more than 29 mm. The first surgery of PPV with ILM peeling and silicone oil (SO) tamponade was performed in our hospital or other hospitals. The MH was not healed or accompanied by RD more than half a year.
Exclusion Criteria
The ocular axial length was less than 29 mm. The cases had undergone the surgery of macular buckling or posterior scleral reinforcement. The cases could not, or had no value for second surgery because of systemic disease, ocular inflammation, or already blindness.
Preoperative Examination
All patients underwent comprehensive ophthalmologic examination before surgery, including visual acuity (VA), intraocular pressure (IOP), slit lamp microscopy examination, fundus examination, optical coherence tomography (OCT; Heidelberg Spectralis OCT) examination of macular area, fundus photography. The best-corrected visual acuity (BCVA) was converted to logMAR VA.
Surgical Technique
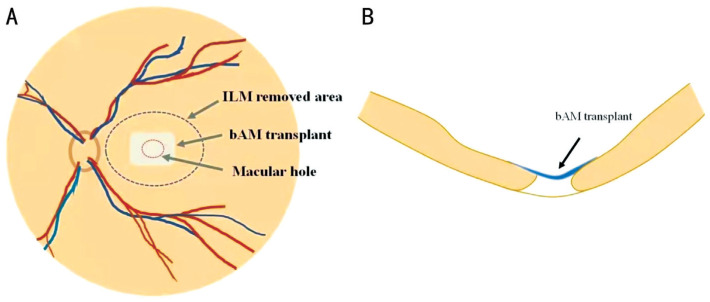
All subjects were treated with SO removal combined with biological amniotic membrane (bAM; Ruiji Co., Ltd., Jiangxi Province, China) covering on MH and C3F8 tamponade. The specific methods were as follows: 1) All patients underwent the SO removal firstly with a 3-port, 25-gauge surgical incision. 2) Phacoemulsification combined with intraocular lens (IOL) implantation was performed simultaneously in the case with cataracts. 3) The vitreous cavity was injected with triamcinolone acetonide (TA) to observe whether there was residual vitreous cortex, and if so, the residual vitreous cortex should be thoroughly removed. 4) After staining ILM by indocyanine green, we observed whether there was any residual ILM around MH and whether the peeling range of ILM was sufficient in the previous operation. And the peeling range of ILM would be extended if necessary. After the first two steps completed, the liquid under the retina was removed by fluid-air exchange. A 2×3 mm2 peeling range AM was pre-soaked in balanced saline use ILM tweezers, then passed the transplant through a 25-gauge valved trocar into the vitreous cavity and laid flat on the surface of MH. And adjusted the position of the transplant to the middle of the MH, make sure the edge of transplant was exceeded the MH (Figure 1). The flute needle was used to remove residual liquid around the transplant so that it was tightly attached retina and no longer moved. Last, 13% C3F8 was injected into vitreous cavity and the incision was sutured. After the surgery, the patients need to rest in face down or lateral position for 2 to 3wk, with anti-inflammatory and symptomatic treatment. All patients were reexamined OCT and scanning laser ophthalmosocopy (SLO) in 2wk, 1, 3, 6, and 12mo after surgery.
Figure 1. Operating procedure of bAM covering.
A: Peeling off the appropriate range of ILM (black dashed line) and spreading the bAM (gray rectangle) flat on the surface of MH (red dashed line); B: The structural relationship between bAM (blue tissue) and retina (yellow tissue) in transverse section. bAM: Biological amniotic membrane; ILM: Internal limiting membrane; MH: Macular hole.
Statistical Analysis
The SPSS 23.0 software was used for statistical analysis. The measurement data were expressed by mean±standard deviation. The independent sample t-test was used for comparison between groups, and matching sample t-test was used for comparison among groups, and the difference was statistically significant with P<0.05. The Chi-square and the exact probability method were used to compare the counting data between two groups.
RESULTS
A total of 17 eyes of 17 subjects met the inclusion criteria, and all subjects completed the treatment and one year follow-up without missing. The subjects included 6 males and 11 females with an average age of 49.18±5.42 (ranged 39 to 58)y. The mean preoperative BCVA was 1.66±0.22 (logMAR), and the mean ocular axial length was 30.28±1.04 (ranged 29.10 to 32.41) mm. Fourteen eyes had posterior sclera staphyloma, 12 eyes were combined with high IOP secondary to SO emulsification, 3 eyes combined with IOL eye, 14 eyes combined with cataracts. The basic clinical features before surgery are shown in Table 1.
Table 1. Demographic ophthalmologic characteristics and postoperative outcomes.
| ID | Age, y | Sex | Eye | Lens status | Axial length (mm) | Posterior sclera staphyloma | IOP (mm Hg) | Preop. BCVA (logMAR) | Postop. BCVA (logMAR) |
| 1 | 46 | M | OD | Opacity | 30.00 | Yes | 32.00 | 2.00 | 1.40 |
| 2 | 42 | M | OD | Opacity | 30.20 | Yes | 31.40 | 1.69 | 1.10 |
| 3 | 48 | F | OS | Opacity | 29.70 | Yes | 30.20 | 2.00 | 1.20 |
| 4 | 55 | F | OD | IOL | 31.20 | Yes | 24.00 | 1.60 | 0.90 |
| 5 | 51 | F | OD | Opacity | 30.19 | Yes | 22.00 | 1.50 | 1.00 |
| 6 | 39 | F | OD | Opacity | 29.92 | Yes | 25.00 | 1.69 | 1.10 |
| 7 | 40 | F | OS | Opacity | 29.51 | Yes | 31.30 | 2.00 | 1.20 |
| 8 | 49 | F | OD | Opacity | 29.80 | No | 30.70 | 1.60 | 0.90 |
| 9 | 51 | M | OS | IOL | 32.41 | Yes | 21.80 | 1.50 | 1.00 |
| 10 | 54 | M | OS | Opacity | 32.10 | Yes | 24.70 | 1.69 | 1.69 |
| 11 | 58 | F | OD | Opacity | 29.60 | No | 18.70 | 1.40 | 0.90 |
| 12 | 55 | F | OS | Opacity | 29.40 | Yes | 30.40 | 1.50 | 1.50 |
| 13 | 54 | M | OS | Opacity | 30.32 | Yes | 15.80 | 1.30 | 0.70 |
| 14 | 48 | F | OD | IOL | 32.10 | Yes | 16.90 | 2.00 | 1.20 |
| 15 | 46 | M | OS | Opacity | 29.10 | Yes | 29.50 | 1.69 | 0.90 |
| 16 | 52 | F | OD | Opacity | 29.30 | No | 14.30 | 1.50 | 0.80 |
| 17 | 48 | F | OD | Opacity | 29.86 | Yes | 17.70 | 1.60 | 0.82 |
M: Male; F: Female; IOL: Intraocular lens; IOP: Intraocular pressure; BCVA: Best-corrected visual acuity; Preop.: Preoperative; Postop.: Postoperative.
SO was removed successfully from all eyes, MH healed, and RD reattached. The recovery conditions of typical cases are shown in Figures 2 and 3. After surgery, one patient (5.89%, 1/17) was found AM translocation half a month, then performed a second operation to adjust AM and C3F8 tamponade. The VA improved in 15 eyes (82.35%, 15/17), no change in 2 eyes (11.76%, 2/17). The mean VA was raised from 1.66±0.22 before surgery to 1.08±0.27 (t=9.83, P<0.01). Comparison of the indicators of the patients before and after surgery are shown in Table 2. No serious complications occurred in all cases. The postoperative data in Tables 1, 2 and Figures 2, 3 were all 12mo after surgery.
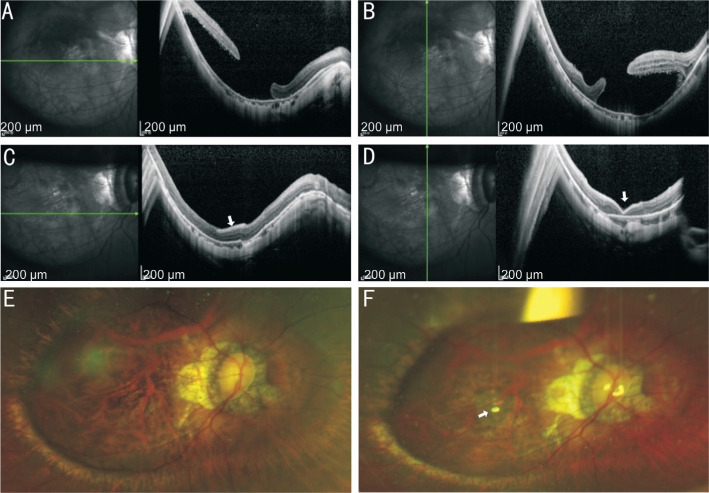
Figure 2. Information of the No.5 patient.
A, B: The OCT of macular area before operation showed the failure of MH recovery with macular regional RD; C, D: The OCT of macular area after operation showed that RD was reattached and MH was healed by covering AM (white arrow). E: The SLO before operation showed the failure of MH recovery with macular regional RD; F: The SLO after operation showed AM (white arrow) covered the MH. RD: Retinal detachment; MH: Macular hole; SLO: Scanning laser ophthalmosocopy; AM: Amniotic membrane.
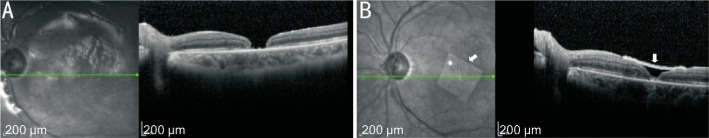
Figure 3. Information of the No.15 patient.
A: The patient with PMMH associated with RD underwent underwent the failed primary surgery. The edges of MH were reattached, but the central tissue defected; B: The same patient underwent second surgery of AM covering. The central defect tissue of MH reached complete healing after covering AM (white arrow). PMMH: Pathological myopia macular hole; RD: Retinal detachment; MH: Macular hole; AM: Amniotic membrane.
Table 2. Indicators of the patients before and after surgery.
| Parameters | Macular hole healing | Retinal detachment | IOL | IOP (mm Hg) | Axial length (mm) | Need to use IOP-lowering drugs | BCVA (logMAR) |
| Preop. | 0 | 13 | 3 | 24.49±6.19 | 30.28±1.04 | 12 | 1.66±0.22 |
| Postop. | 17 | 0 | 17 | 17.83±3.64 | 30.26±1.02 | 3 | 1.08±0.27 |
| t, P | P<0.01 | P<0.01 | P<0.01 | t=5.63, P<0.01 | t=1.82, P=0.09 | P<0.01 | t=9.83, P<0.01 |
Preop.: Preoperative; Postop.: Postoperative; IOL: Intraocular lens; IOP: Intraocular pressure; BCVA: Best-corrected visual acuity.
n
DISCUSSION
The PMMH and its related complication (MH associated with RD) seriously affect the visual function of patients. If the treatment is not timely, the risk of secondary blindness and eye atrophy is extremely high. The treatment of PMMH mainly depends on surgery. Although there are many surgical techniques in clinical treatment, the curative effect is unsatisfactory or the surgery is difficult to be popularized due to technical difficulties.
Based on the low success rate of routine surgery, the scholars from all over the world have made a lot of improvements and innovations in surgical techniques. The technique mainly focused on the treatment method of ILM and the healing of MH, including autologous transplantation of ILM[12], inverted ILM flap[13]–[15], MH edge massage[16]–[17], autologous blood covering[18]–[19], lens capsule transplantation[20], autologous transplantation of retinal free flap[21], the hAM plug[11],[22], etc. These innovative techniques have made some achievements in improving the healing rate of MH.
However, the above techniques still have some drawbacks. The techniques related to ILM require excellent surgical ability of operator and need long time to learn these techniques[23]–[24]. In addition, for those patients in this study who already had the ILM peeling but failed to achieve MH healing and retinal reattaching, there is no surplus ILM in their macular region. Therefore, the above techniques are useless for these patients.
The techniques such as MH edge massage and autologous blood covering only assist MH healing, but have no essential improvement in the overall clinical effect.
The lens capsule transplantation, autologous transplantation of retinal free flap, and other innovative techniques can be applied in patients with recurrent and refractory MH in our study. In particularly, autologous transplantation of retinal free flap requires cutting a piece of retinal tissue in the peripheral retina. This operation itself may cause a new harm to retina, and is prone to occur complications such as choroidal and retinal hemorrhage. The two kinds of surgical techniques also require extremely high skill of the operator and are not suitable for wide application.
In recent two years, the technique of amniotic membrane (AM) plug has been paid more and more attention and favor because of its low cost and high healing rate of MH. The so-called AM plug is a kind of technique which transplant the AM, as embolus, under the MH and between the retinal neuroepithelium and pigment epithelium to plug hole, and promote MH healing. In fact, AM as an adjunctive therapy is nothing new, which has been applied in various clinical specialties, and play a good role in promoting the treatments of many diseases[25]–[26]. For example, the AM is used to assist the treatment of otitis media with tympanic membrane perforation in otolaryngology[27], and used to assist ocular surface reconstruction and prevent symblepharon in operation of severe ocular surface diseases in the specialty of ophthalmic keratopathy[28]–[29], and so on. But the application of AM in the treatment of MH is an innovation. The representative researches were from the team of Caporossi et al[7]. These researches verified good curative effect of AM plug in treatment of patients with PMMH and MH associated with RD[22],[30].
However, the technique of AM plug still needs to be discussed and improved in clinical application. The disadvantages of using AM plug invented by Caporossi et al[7] can be summarized as follows: the AM is filled between the retinal nerve epithelium and retinal pigment epithelium (RPE) during operation, which may touch or damage the RPE under the MH and damage visual function. Because the AM was plugged in the retinal interlayer, the structure of macular area is disordered after the MH healing, and the influence to macular function cannot be ignored. And bimanualness is required during the operation, so that an extra surgical incision will be added when the ceiling lamp is used. Furthermore, after MH filled by AM, subretinal fluid needs to be removed from a drainage hole artificially cut from the posterior retina. These two operating steps increase the harm to retina. Compared with autologous transplantation of retinal free flap, AM plug has significantly reduced iatrogenic injuries and improved clinical efficacy. But based on the above analysis, this technique has space for further upgrading and improvement.
Based on the previous researches, our team proposed a new technique named AM covering. The difference between this new technique and AM plug is that the AM only covers on the surface of MH and will not damage the RPE below the MH. And MH still heals according to its own anatomical structure. And there is no harmful effect on the outer structure of retina, which maximally protects the visual function of patients theoretically. During the operation, the subretinal fluid was removed through MH, and there is no need to add extra iatrogenic retinal hole as internal drainage, so as to reduce iatrogenic injury. The operation is easy and can be performed with only one hand, and there is no need of the ceiling light and fourth incision. As shown in Figure 2B and 2D, the AM was located on the surface of macular area, the macular structure was clear, and the MH was completely healed. The Figure 3A showed the macular structure of a patient with PMMH associated with RD who underwent the surgery of PPV with ILM peeling and SO tamponade. As shown in the Figure 3A, the edge of MH was attached but the central tissue lost after the previous surgery. The Figure 3B showed the macular structure of this patient after second surgery of AM covering. The central lost tissue of foveal reached complete healing. In this study, the removal of SO, reattached of RD and healing of MH were all achieved in these 17 patients who underwent failed primary surgery. This result showed that the technique of AM covering can achieve the same therapeutic effect as AM plug.
The material used in this study was bAM rather than fresh hAM mentioned in the research[22]. The advantage of bAM is that this material is easy to obtain and can be used directly, while fresh hAM requires temporary processing of grafts, and there is a potential transmission risk of infectious disease. Although the main disadvantage of bAM is that it provides less somatomedin than fresh hAM during HM healing, bAM has played a good therapeutic effect in promoting MH healing in our research.
In summary, the technique of AM covering uses the materials which are easy to obtain, and has potential advantages such as no damage and short learning curve. The design of this technique avoids the defects of previous surgical techniques, improves the success rate of surgery for MH and MH associated with RD, and protects the visual function of patient to the maximum extent. This technique is especially suitable for the patients who have unhealed MH after multiple operations and have no ILM in macular area. Limited to the small number of cases in this study, more randomized controlled studies with a longer follow-up period are needed to corroborate our results.
Acknowledgments
Foundation: Supported by Medical Research Project of Sichuan Province (No.S20018).
Conflicts of Interest: Qiao G, None; Zhang XJ, None; Tang ZY, None; Zou QX, None; He CM, None; Lei XM, None; Zhao L, None; Quan Y, None; Yang HQ, None; Cao K, None; Dong WJ, None.
REFERENCES
- 1.Wei RH, Lu DQ, Jin N, Du B. Interpretation of the International Myopia Institute white papers focusing on myopia prevention and control. Rec Adv Ophthalmol. 2019;39(8):701–713. [Google Scholar]
- 2.Wang WD, Yao YN, Tang LN, Hu YS. Prevalence and influencing factors of myopia for the Chinese junior high school students. Chin J Dis Control Prev. 2019;23(9):1057–1106. [Google Scholar]
- 3.Ku PW, Steptoe A, Lai YJ, Hu HY, Chu DC, Yen YF, Liao Y, Chen LJ. The associations between near visual activity and incident myopia in children: a nationwide 4-year follow-up study. Ophthalmology. 2019;126(2):214–220. doi: 10.1016/j.ophtha.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Wong YL, Saw SM. Epidemiology of pathologic myopia in Asia and worldwide. Asia Pac J Ophthalmol. 2016;5(6):394–402. doi: 10.1097/APO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, He XG. Strengthening the understanding of the pathological evolution of myopia. Zhonghua Yan Ke Za Zhi. 2019;55(10):721–725. doi: 10.3760/cma.j.issn.0412-4081.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Caporossi T, Pacini B, de Angelis L, Barca F, Peiretti E, Rizzo S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of ≥30 mm. Retina. 2020;40(10):1946–1954. doi: 10.1097/IAE.0000000000002699. [DOI] [PubMed] [Google Scholar]
- 8.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 9.Haritoglou C, Wolf A, Wachtlin J. Surgery of large and persistent macular holes. Ophthalmologe. 2019;116(11):1011–1019. doi: 10.1007/s00347-019-00949-x. [DOI] [PubMed] [Google Scholar]
- 10.Fotis K, Alexander P, Sax J, Reddie I, Kang CY, Chandra A. Macular detachment for the treatment of persistent full-thickness macular holes. Retina. 2019;39(Suppl 1):S104–S107. doi: 10.1097/IAE.0000000000002370. [DOI] [PubMed] [Google Scholar]
- 11.Wu TT, Kung YH, Chang CY, Chang SP. Surgical outcomes in eyes with extremely high myopia for macular hole without retinal detachment. Retina. 2018;38(10):2051–2055. doi: 10.1097/IAE.0000000000001806. [DOI] [PubMed] [Google Scholar]
- 12.Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, Hosogi M, Shirakata Y, Okanouchi T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861–869.e1. doi: 10.1016/j.ajo.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Mete M, Alfano A, Guerriero M, Prigione G, Sartore M, Polito A, Pertile G. Inverted internal limiting membrane flap technique versus complete internal limiting membrane removal in myopic macular hole surgery: a comparative study. Retina. 2017;37(10):1923–1930. doi: 10.1097/IAE.0000000000001446. [DOI] [PubMed] [Google Scholar]
- 14.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014;34(4):664–669. doi: 10.1097/IAE.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 15.Baba R, Wakabayashi Y, Umazume K, Ishikawa T, Yagi H, Muramatsu D, Goto H. Efficacy of the inverted internal limiting membrane flap technique with vitrectomy for retinal detachment associated with myopic macular holes. Retina. 2017;37(3):466–471. doi: 10.1097/IAE.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol (Phila) 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Ji M, Di R, Qi Y, Pei C, Gao S, Liu SW, Xie AM, Cheng YH. Parafoveal retinal massage combined with autologous blood cover in the management of giant, persistent or recurrent macular holes. Int J Ophthalmol. 2020;13(11):1773–1779. doi: 10.18240/ijo.2020.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, Hwang YS, Wu WC, Chen TL. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–1898. doi: 10.1016/j.ophtha.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Wu AL, Chuang LH, Wang NK, Chen KJ, Liu L, Yeung L, Chen TL, Hwang YS, Wu WC, Lai CC. Refractory macular hole repaired by autologous retinal graft and blood clot. BMC Ophthalmol. 2018;18(1):213. doi: 10.1186/s12886-018-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–170. doi: 10.1097/IAE.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 21.Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134(2):229–230. doi: 10.1001/jamaophthalmol.2015.5237. [DOI] [PubMed] [Google Scholar]
- 22.Caporossi T, de Angelis L, Pacini B, Tartaro R, Finocchio L, Barca F, Rizzo S. A human amniotic membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2020;98(2):e252–e256. doi: 10.1111/aos.14174. [DOI] [PubMed] [Google Scholar]
- 23.Chatziralli IP, Theodossiadis PG, Steel DHW. Internal limiting membrane peeling in macular hole surgery; why, when, and how? Retina. 2018;38(5):870–882. doi: 10.1097/IAE.0000000000001959. [DOI] [PubMed] [Google Scholar]
- 24.Faria MY, Ferreira NP, Cristóvao DM, Mano S, Sousa DC, Monteiro-Grillo M. Tomographic structural changes of retinal layers after internal limiting membrane peeling for macular hole surgery. Ophthalmic Res. 2018;59(1):24–29. doi: 10.1159/000480243. [DOI] [PubMed] [Google Scholar]
- 25.Dekaris I, Gabrić N. Preparation and preservation of amniotic membrane. Dev Ophthalmol. 2009;43:97–104. doi: 10.1159/000223842. [DOI] [PubMed] [Google Scholar]
- 26.Zheng SQ, Chen TS, Ji SZ, Luo PF, Xiao SC. Advances in preparation and clinical application of amniotic membrane graft. Zhonghua Shao Shang Za Zhi. 2017;33(8):514–516. doi: 10.3760/cma.j.issn.1009-2587.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhu BL, Xu B, Huang YE, Zhou SY, Xu M, Hou J. Clinical observation on endoscopic myringoplasty with biological amniotic membrane for the treatment of tympanic membrane perforation. Chinese Journal of Otorhinolaryngology-Skull Base Surgery. 2018;24(5):468–471. [Google Scholar]
- 28.Nie AQ, Li Q, Li W. Analysis on the efficacy of biological amniotic membrane transplantation for complicated ocular surface diseases. Chin J Ocul Traum Occupat Eye Dis. 2018;40(5):348–351. [Google Scholar]
- 29.Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108(14):243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, Giansanti F. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–S103. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]