Abstract
AIM
To determine how green tea and catechins can affect intraocular pressure (IOP) changes.
METHODS
Totally 43 young volunteers were included in the study. The experiment was held between noon and 2 p.m. Two extracts—green tea and epigallocatechin gallate (EGCG, 400 mg capsules) and placebo (400 mg capsules) were used in the study. Participants were divided into three groups. Green tea extract group (GT group) had 17 subjects, EGCG extract group 17 subjects, control (placebo) group 9 subjects. IOP was measured with the Icare tonometer before and 30min, 1, 1.5, 2h after the consumption of each extract and placebo. Results were analyzed using the IBM SPSS program. Statistical confidence level P<0.05.
RESULTS
The most significant reduction of IOP from the beginning of the experiment was measured after 2h in GT group (left 2.18±3.19 mm Hg, P=0.012; right 2.59±1.97 mm Hg, P<0.000) and after 1h in EGCG extract group (left 2.41±2.98 mm Hg, P<0.004; right 1.94±1.98 mm Hg, P<0.001). In control group no significant changes were measured.
CONCLUSION
People who have increased IOP or risk factors for glaucoma development, could benefit from drinking green tea or its concentrated extracts in moderate doses.
Keywords: intraocular pressure, glaucoma, ocular hypertension, tea, catechin
INTRODUCTION
Intraocular pressure (IOP) is a force exerted by the aqueous humor on the internal surface area of the anterior eye[1]. The circulation of aqueous humor is a physiologically important process for the normal IOP and normal function of the eye. The main eye structures related to aqueous humor circulation are the ciliary body (the ciliary processes are the sites of aqueous humor production), and the trabecular meshwork (TM) and the uveoscleral pathway (the main locations of aqueous humor outflow). The flow of aqueous humor against resistance generates an average IOP 15 mm Hg, which maintains the shape of the eye[2].
Impaired aqueous humor drainage and increased IOP harm the eye. Prolonged and very high increases in IOP usually involve optic nerve damage, which contributes to the development of primary open angle glaucoma[3]. Therefore, IOP reduction is the most important goal of disease treatment.
Glaucoma caused by high IOP is often treated with IOP lowering medication and sometimes even surgical treatment is needed. While medical and surgical treatment methods are required to reduce IOP, modifiable lifestyle activities could also help in fighting the disease, especially in the early stages.
One of the most popular daily drinks is green tea. But so far there is not much information on how green tea affects the human body, including IOP. It is considered to be the least processed type of tea and therefore contains the most antioxidants and useful polyphenols. One of the main groups of polyphenols found in green tea are catechins, of which epigallocatechin gallate (EGCG) is the most abundant (about 50% of all catechins) and the most active[4].
Recently, experiments have shown that catechins found in green tea can penetrate the tissue of the rat eye[5]. Considering that catechins can penetrate the tissue of the human eye in a similar way as the tissue of the rat eye, it can be speculated that catechins could have beneficial antioxidant effects in the human eye as well.
We report the results of a prospective, randomized double-blind placebo-controlled trial to evaluate the relationship between green tea consumption and direct IOP changes.
SUBJECTS AND METHODS
Ethical Approval
The experiment took place in Vilnius University, Faculty of Medicine located in Vilnius. Forty-three young adults (86 eyes) were investigated, no participants were lost during the experiment. All volunteers were healthy and had no clinical history of glaucoma, infectious eye diseases or ocular trauma. The mean age of participants was: 23.5y, median 23y (SD: 3.01). The study was conducted following the requirements of the Declaration of Helsinki. Participants signed a written informed consent form and they did not receive a stipend. The protocol, appendices and other relevant documentation were submitted to the Vilnius regional research ethics committee for reviewing and were approved. Clinical trial registration number 2021/01-1300-778.
In the experiment, we used two kinds of extracts (green tea extract and EGCG extract), as it would have been very difficult to measure the same amount of tea for each volunteer and placebo (400 mg empty capsule). Capsules were similar in color and size. Both extracts were made from the Camellia sinensis plant, amount of extract in each capsule was the same 400 mg. The main difference between the two extracts was the number of catechins. Green tea extract contained minimally 40% of total catechins (the exact amount of EGCG was not provided, we assume it to be around 80-100 mg). It is noted that up to 32 mg of naturally occurring caffeine and 60 mg of vitamin C was also included in the capsule. EGCG extract contained minimally 80% of total catechins and 50% of EGCG (200 mg). It is also noted that EGCG extract contained up to 4 mg naturally occurring caffeine and about 50 mg of decaffeinated green tea.
The experiment was held between noon and 2 p.m. Computer-generated randomization was used and participants were randomly divided into three groups. Green tea extract group (GT group) 17 subjects, EGCG extract group (EGCG group) 17 subjects, control (placebo capsule) group 9 subjects. Neither the researchers nor the volunteers knew who was assigned to which group.
IOP measurement process: the measurement was done without anaesthetic because an anaesthetic could reduce the tonometer reading. Each of the subjects was asked to relax and look straight ahead to a specific point. The tonometer was brought near to the subject's eye. The distance from the tip of the probe to the cornea of the eye was 4-8 mm. The measure took place by lightly pressing the measurement button. The tip of the probe hit the central cornea. Six measurements were made consecutively. After each successful measurement, there was a short beep. After the six measurements, the IOP was shown on the display after the P letter.
This procedure was repeated five times: before and 30min, 1, 1.5, and 2h after the consumption of each extract and placebo capsule.
Participants were also asked several questions related to epidemiological and demographic information: age, gender, weight, family history of glaucoma, physical activity, caffeine intake, smoking.
Statistical Analysis
Results were analyzed using the IBM SPSS statistics program created in 2018, software version v24.0. P<0.05 was statistically significant. Comparisons between the first and the next measurements were analyzed using paired t-tests.
RESULTS
Demographic information and ocular features of the subjects are provided in Table 1. We did not notice the relation between high IOP and glaucoma cases among family members. Smoking and physical activity did not correlate with IOP as well. The correlation between caffeine consumption and IOP was very weak and statistically not significant.
Table 1. Demographic information of participants.
| Parameters | All participants | EGCG group | Green tea group | Control group |
| Number | 43 (100) | 17 (39.53) | 17 (39.53) | 9 (20.93) |
| Age, mean±SD, y | 23.5±3.01 | 24.1±2.83 | 23.5±3.48 | 22.3±2.24 |
| Gender, female | 48.84 | 41.2 | 58.8 | 44.4 |
| Family history of glaucoma | 7 (16.28) | 4 (23.52) | 1 (0.06) | 2 (22.2) |
| Daily coffee consumption | 33 (76.7) | 12 (70.1) | 16 (94.1) | 5 (55.56) |
| Smoking | 12 (27.9) | 6 (35.3) | 5 (29.4) | 1 (11.1) |
| Moderate physical activity at least 2 times/wk | 26 (60.5) | 10 (58.8) | 10 (58.8) | 6 (66.67) |
EGCG: Epigallocatechin gallate; SD: Standard deviation.
n (%)
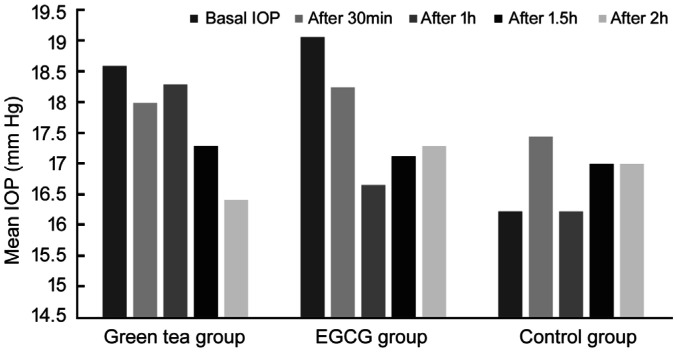
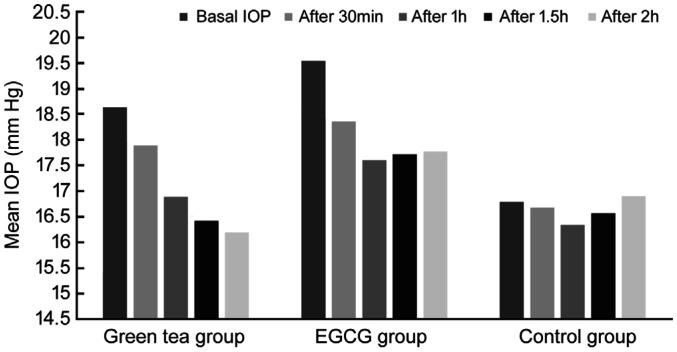
Mean IOP levels in the volunteers before and after consumption of each of the extracts are provided in Table 2. After 30min in GT and EGCG groups IOP decrease was not significant, in control group IOP increased in the left eye and lowered in the right eye, changes were not statistically significant. After 1h in GT group IOP lowered statistically significantly in the right, but not in the left eye (left eye 0.29±2.59 mm Hg, P=0.646; right eye 1.88±2.03 mm Hg, P=0.001); in EGCG group changes of IOP in both eyes were the most significant (left eye 2.41±2.98 mm Hg, P=0.004; right eye 1.94±1.98 mm Hg, P=0.001); in control group significant changes of IOP were not measured (left eye 0.0±2.0 mm Hg, P=1; right eye 0.44±2.79 mm Hg, P=0.645). After 1.5h in the groups of both extracts IOP reduction were statistically significant in both eyes, in control group IOP changes were not statistically significant. After 2h in GT group reduction of IOP was the most significant (left eye 2.18±3.19 mm Hg, P=0.012; right eye 2.59±1.97 mm Hg, P=0.000); in EGCG group reduction of IOP was smaller than in the earlier measurements, but still statistically significant (left eye 1.76±3.09 mm Hg, P=0.032; right eye 1.76±2.46 mm Hg, P=0.009); in control group IOP did not change significantly (left eye -0.78±2.64 mm Hg, P=0.402; right eye -0.11±4.28 mm Hg, P=0.940). We noticed that EGCG extract tended to react faster on IOP than green tea extract, but after 2h green tea extract tended to reduce IOP more than EGCG extract, which IOP lowering effects tended to diminish (Figures 1 and 2).
Table 2. Changes of IOP before and after the consumption of different extracts.
| Parameters | Green tea group |
EGCG group |
Control group |
|||
| Left eye | Right eye | Left eye | Right eye | Left eye | Right eye | |
| Basal IOP | 18.59±3.45 | 18.77±3.29 | 19.06±4.59 | 19.53±4.598 | 16.22±3.898 | 16.78±4.71 |
| After 30min | 18±4.11 | 17.88±3.69 | 18.24±3.72 | 18.35±3.79 | 17.44±2.88 | 16.67±4.39 |
| After 1h | 18.29±3.58 | 16.88±4.12 | 16.65±3.16 | 17.59±4.65 | 16.22±3.598 | 16.33±3.43 |
| After 1.5h | 17.29±3.51 | 16.41±3.43 | 17.12±3.94 | 17.71±3.74 | 17±4.39 | 16.56±4.64 |
| After 2h | 16.41±3.28 | 16.18±3.05 | 17.29±3.65 | 17.76±3.85 | 17±3.42 | 16.89±4.57 |
| Decrease of IOP | ||||||
| Δ after 30min, P | 0.59±2.59, 0.289 | 0.88±1.96, 0.083 | 0.82±3.43, 0.337 | 1.18±1.98, 0.026 | -1.22±3.23, 0.289 | 0.11±3.95, 0.935 |
| Δ after 1h, P | 0.29±2.59, 0.646 | 1.88±2.03, 0.001 | 2.41±2.98, 0.004 | 1.94±1.98, 0.001 | 0.0±2.0, 1.0 | 0.44±2.79, 0.645 |
| Δ after 1.5h, P | 1.29±2.28, 0.033 | 2.35±1.69, 0.000 | 1.94±2.99. 0,017 | 1.82±1.91, 0.001 | -0.78±3.83, 0.560 | 0.22±3.73, 0.863 |
| Δ after 2h, P | 2.18±3.19, 0.012 | 2.59±1.97, 0.000 | 1.76±3.09, 0.032 | 1.76±2.46, 0.009 | -0.78±2.64, 0.402 | -0.11±4.28, 0.940 |
IOP: Intraocular pressure; EGCG: Epigallocatechin gallate; Δ: Difference.
mean±SD, mm Hg, n=43
Figure 1. Left eye IOP changes.
Figure 2. Right eye IOP changes.
Although after consumption of each of the extracts higher IOP (18 mm Hg and higher) tended to reduce more than a lower IOP, a lower IOP has also reduced. In several cases, even a very low IOP has reduced (11 to 8 mm Hg).
In 1-hour time, IOP reduced more in the EGCG group than in the GT group. After 1.5h IOP decrease was greater in the GT group than in the EGCG group in the right, but not in the left eye. After 2h reduction of IOP in both eyes was greater in the GT group. The difference of IOP decrease between extracts was statistically significant only in the left eye and only after 1h. The other differences between the two extracts were not statistically significant (Table 3).
Table 3. Difference of IOP changes between green tea and EGCG extracts.
| Parameters | Δ Green tea minus Δ EGCG (SD), P |
|
| Left eye | Right eye | |
| Δ basal IOP | -0.47 (6.63), 0.774 | -0.76 (6.87), 0.652 |
| Δ after 30min | -0.23 (4.29), 0.827 | -0.3 (2.8), 0.660 |
| Δ after 1h | -2.12 (3.95), 0.034 | -0.06 (2.84), 0.931 |
| Δ after 1.5h | -0.65 (3.76), 0.481 | 0.53 (2.55), 0.398 |
| Δ after 2h | 0.42 (4.41), 0.699 | 0.83 (3.15), 0.286 |
IOP: Intraocular pressure; EGCG: Epigallocatechin gallate; Δ: Difference.
DISCUSSION
Effects of catechins on the human body have not yet been fully investigated, but publications are showing the beneficial properties of these substances (especially of EGCG). It includes anticancer, anti-obesity, antidiabetic, anti-infectious, cardiovascular protective, hepatoprotective and neuroprotective effects[6]. The study of Bernatoniene and Kopustinskiene[7] reveals that phenolic hydroxyl groups of catechins can react with reactive oxygen and reactive nitrogen species and in that way, catechins can act as free radical scavengers.
Although the exact mechanisms of action of the most biologically active catechin–EGCG have not yet been fully understood, some certain proteins have been found to act directly on EGCG. One of such proteins is the membranes 67LR receptor, which has a high affinity for EGCG. 67LR is the major regulator of many cellular apoptosis or proliferation pathways. Additionally, it regulates the activity of cancer stem cells. EGCG is also thought to interact with the enzyme Pin1, the tumour suppressor gene TGFR-II, and metalloproteinases (mainly MMP2 and MMP9)[4].
While there is an increasing amount of evidence of the positive effects of catechins on human health, the information on how does it act on the eyes is limited. Chu et al[5] experiment on laboratory rats revealed that after oral consumption of green tea extract ocular structures of the rat eye absorbed a significant amount of catechins. The retina of the rat eye mainly absorbed gallocatechin while the aqueous humour tended to absorb EGCG. Time for catechins to reach maximum concentrations varied from 0.5h to 12.2h[5]. Her studies suggest that green tea extracts may protect the retinal ganglion cell layer in rats from ischemia[8], and may reduce the negative inflammatory effects of lipopolysaccharides on the rat retina[9]. Assuming that catechins in the human eye could act similarly to the eye of the laboratory rat, and keeping in mind that the most abundant catechin found in green tea, EGCG, is absorbed by the aqueous humour of the rat eye, it can be assumed that green tea and its extracts could affect IOP.
To understand better, how catechins can affect eye pressure, we need to know how IOP is regulated. In acute cases, the sympathetic nervous system is involved in the regulation of IOP (beta-2 receptors cause increased secretion of aqueous, while alfa-2 receptors cause decreased secretion)[1]. However, more often IOP is maintained by homeostatic mechanisms, which regulate the resistance of aqueous humour outflow through the TM into the Schlemm's canal (SC). Most of the resistance is thought to be from the extracellular matrix (ECM) of the juxtacanalicular region, the deepest portion of the TM, and from the inner wall of SC[1],[10]. Additionally, mechanical stretching of TM cells leads to increased activity of specific metalloproteinases (MMPs) that can initiate turnover of ECM cells. The turnover of ECM is required to maintain the outflow of aqueous humor[11]–[12]. However, on one hand MMP's act as ECM turnover enzymes, on the other hand, it is believed that they are important in keeping outflow pathways open[13]–[14]. In addition, the normal function of the ECM of the TM is also highly dependent on the activity of tissue inhibitors of metalloproteinases (TIMPs). When TIMP levels rise, MMP activity is inhibited, resulting in impaired ECM function, increased resistance to aqueous humour drainage, and increased IOP[15]. Prostaglandins and their analogues used to reduce IOP act by increasing ocular fluid outflow through the uveoscleral pathway[16], but they can also increase MMP expression and decrease TIMP activity, thereby improving ocular fluid drainage[17]. The described effect of metalloproteinases on aqueous humour outflow regulation can be linked to the EGCG ability to interact with MMP2 and MMP9. Assuming that EGCG can penetrate the human eye in a similar way to the eye of a laboratory rat in Chu et al[5] experiment, we think that catechins (especially EGCG) can act on metalloproteinases in the eye and in that way might help increase the outflow of the aqueous humor.
When the fluid of the eye does not drain as well as it should, eye pressure gets too high and ocular hypertension may develop. The increased IOP is the primary risk factor for glaucomatous optic nerve damage and reduction of the eye pressure is the only treatable component of the disease progression[11],[18]. Drugs that can slow down the production of aqueous humor or improve the drainage of aqueous humor are used for glaucoma treatment. With the help of these drugs, an attempt is made to maintain the target IOP, which is individual to each person and depends on the severity of glaucoma. Maintaining the target IOP is thought to significantly slow down the progression of optic nerve damage[19]. In addition, previous studies have shown that each millimetre of mercury of IOP reduction can reduce the risk for glaucoma development by 10%[20]. However, there are situations where the patient needs to take medications that are contraindicated for him or cause unpleasant side effects. For example, beta-2 receptor blocker timolol is contraindicated for people with asthma due to adverse effects on lung function[21], and prostaglandin analogues, when used only in one eye, can cause a visual difference between eyes. The treated eye would be more hyperemic than the other, the number and length of the eyelashes would increase, and the pigmentation of the iris and eyelashes would also be more pronounced in the eye treated with prostaglandin analogues[22]. Therefore, when some drugs for the treatment of glaucoma are contraindicated and when existing treatment is not sufficient enough to reduce IOP, green tea extracts may be helpful as an additional therapy.
It is also important to note that IOP tends to vary depending on the time of the day. Although it is still difficult to perform a 24-hour IOP test, mainly due to the difficulty of measuring IOP at night, as well as the different IOP values when a person is lying down and sitting, the prevailing approach is that IOP is highest at night and lowest at noon[23]. These fluctuations are not important for a person with a normally functioning aqueous humor circulation system. However, fluctuations of IOP during the day are important for people with glaucoma. When a person has glaucoma, an increase in IOP interferes with its recovery to a normal state and thus provokes even greater optic nerve damage[24]. For this reason, at the time when IOP is at the highest level and when this increase is likely to do the most of the damage, it would be beneficial to use additional therapy, maybe even using green tea and its extracts.
Talking about the therapeutic effects of green tea extracts, it is necessary to mention the side effects. According to a systematic review of the safety of green tea and its extracts, the main adverse effect of concentrated green tea extracts, especially EGCG, are hepatotoxicity. Liver damage depends on certain factors. If a person is starving, has gastrointestinal disorders, or already has liver problems, the negative effects of concentrated green tea extracts may be even more pronounced. It is also very important to pay attention to the dose. The systematic review states that for a person with a healthy liver, 338 mg/d EGCG in the form of concentrated extracts (capsules or tablets) and 704 mg/d EGCG taken with beverages such as green tea (50 to 200 mg EGCG per cup) are considered harmless[25]. Therefore, by taking 1 capsule of EGCG extract used in the study (200 mg EGCG) and 2-3 capsules of green tea extract used in the study (about 100 mg of EGCG per capsule) or drinking 3-6 cups of green tea per day (catechin content depends on the type and preparation of tea)[26] is likely that we will not develop side effects.
To recommend green tea or its extracts as a means of reducing IOP, it is important to know how to get the most out of it. The bioavailability of catechins in humans is low. In the body, catechins are very poorly absorbed and the absorption becomes unstable and can be very rapidly degraded in the first-pass metabolism. In addition, to increase the concentration of EGCG in the human body, a relatively large amount should be consumed[27], which can harm the liver. Therefore, scientists search how to increase the bioavailability of catechins without increasing catechin intake. Experiments suggest that various carriers can be used to improve the bioavailability of catechins. One example would be nanocarriers based on milk proteins, namely casein. One study has shown that sodium caseinate can adsorb large amounts of EGCG and thus improve the bioavailability of this substance. The experiment also states that the binding of catechins to casein does not reduce their antioxidant effects[28]. However, it is also important to pay attention to the substances that are available for everyone and can have a synergistic effect with catechins. In this perspective, the focus is on ascorbic acid, otherwise known as vitamin C and sucrose. Studies suggest that vitamin C, when used in combination with EGCG, may improve its absorption and increase its biological efficacy[29]–[30]. Another important aspect in making recommendations for the use of green tea extracts, is the changes in their absorption depending on the food eaten. An experiment was performed to investigate the bioavailability of EGCG by consuming an extract capsule with a light breakfast, on an empty stomach and in combination with strawberry sherbet. Although the increased stability of EGCG in strawberry-flavoured sherbet (due to low temperature and low pH) was expected to increase its absorption, the results were not slightly different from the absorption of EGCG when consumed with a light breakfast. However, the absorption of oral EGCG extract without breakfast was much higher than in the above cases and this difference was statistically significant[31]. Therefore, to get the most benefit from green tea extracts, you need to take them with vitamin C, on an empty stomach, with a glass of water. If EGCG is obtained from green tea, you can squeeze the lemon juice into the tea or add a slice of lemon to enrich the tea with vitamin C.
Before the study, we assumed that catechins (mainly EGCG) of green tea can lower IOP by acting on metalloproteinases (MMP2 and MMP9) in the eye. For this reason, in the experiment, we used two Camellia sinensis extracts with different amounts of catechins. About twice higher amount of catechins in EGCG extract (80% total catechins ∼320 mg, 50% of EGCG ∼200 mg) than in green tea extract (40% of total catechins ∼160 mg, around 80-100 mg of EGCG) was probably the reason for the more rapid reduction of IOP. However, after two hours green tea extract reduced IOP more than EGCG extract, which IOP lowering effects tended to diminish. We should notice that green tea extract also contained 60 mg of vitamin C, which is thought to enhance the bioavailability of catechins in humans[32]. It is known that catechins have poor bioavailability in the human body[27], for this reason, even a small amount of vitamin C in green tea extract could have improved its IOP reducing effects.
In addition, the amount of catechins (mainly EGCG) both in the green tea extract capsule and in one cup of green tea is almost the same ∼50-100 mg of EGCG (depends on the composition of green tea)[26]. For this reason, we assume that consumption of green tea should similarly reduce IOP as green tea extract.
The limitation of the study is that we do not know if EGCG action on metalloproteinases in the eye is a key factor for IOP reduction. In addition, we cannot tell if green tea and its extracts can have a cumulative IOP reducing effect in the eye and if it can reduce IOP in people with glaucoma. Therefore, another research covering aspects mentioned above should be made in the future. Our study demonstrates that green tea and EGCG extracts can decrease IOP statistically significantly, but the clinical significance is yet not clear. However, drinking green tea or its extracts in moderate doses might have beneficial effects, especially for people with high IOP, or for those who have risk factors for glaucoma development.
Acknowledgments
Authors' contributions: Gasiunas K wrote the initial draft of the paper; Galgauskas S measured IOP for volunteers, revised the manuscript; Access to data: data was received directly from volunteers (after measuring IOP and after asking individual questions), no other sources of data collection were used in the study; Access to materials: green tea and EGCG extracts were bought in an online pharmacy store.
Conflicts of Interest: Gasiunas K, None; Galgauskas S, None.
REFERENCES
- 1.Machiele R, Motlagh M, Patel BC. StatPearls. Treasure Island (FL): StatPearls Publishing; Aug 28, 2020. Intraocular pressure. [PubMed] [Google Scholar]
- 2.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prum BE, Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, Herndon LW, Jr, Lim MC, Williams RD. Primary open-angle glaucoma preferred practice pattern(®) guidelines. Ophthalmology. 2016;123(1):P41–P111. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Negri A, Naponelli V, Rizzi F, Bettuzzi S. Molecular targets of epigallocatechin-gallate (EGCG): a special focus on signal transduction and cancer. Nutrients. 2018;10(12):E1936. doi: 10.3390/nu10121936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu KO, Chan KP, Wang CC, Chu CY, Li WY, Choy KW, Rogers MS, Pang CP. Green tea catechins and their oxidative protection in the rat eye. J Agric Food Chem. 2010;58(3):1523–1534. doi: 10.1021/jf9032602. [DOI] [PubMed] [Google Scholar]
- 6.Isemura M. Catechin in human health and disease. Molecules. 2019;24(3):E528. doi: 10.3390/molecules24030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(4):E965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YP, Xu CY, Chen YH, Liang JJ, Xu YX, Chen SL, Huang SF, Yang QC, Cen LP, Pang CP, Sun XH, Ng TK. Green tea extract ameliorates ischemia-induced retinal ganglion cell degeneration in rats. Oxid Med Cell Longev. 2019;2019:8407206. doi: 10.1155/2019/8407206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren JL, Yu QX, Liang WC, Leung PY, Ng TK, Chu WK, Pang CP, Chan SO. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci Rep. 2018;8(1):429. doi: 10.1038/s41598-017-18888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li XB, Aga MN, Bradley JM. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014;30(2-3):94–101. doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Tyagi SC. Metalloproteinases as mediators of inflammation and the eyes: molecular genetic underpinnings governing ocular pathophysiology. Int J Ophthalmol. 2017;10(8):1308–1318. doi: 10.18240/ijo.2017.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: a tissue with a unique extracellular matrix and specialized function. J Ocul Biol. 2013;1(1):3. [PMC free article] [PubMed] [Google Scholar]
- 14.Keller KE, Aga MN, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88(4):676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashworth Briggs EL, Toh T, Eri R, Hewitt AW, Cook AL. TIMP1, TIMP2, and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients. Mol Vis. 2015;21:1162–1172. [PMC free article] [PubMed] [Google Scholar]
- 16.Tejwani S, Machiraju P, Nair AP, Ghosh A, Das RK, Ghosh A, Sethu S. Treatment of glaucoma by prostaglandin agonists and beta-blockers in combination directly reduces pro-fibrotic gene expression in trabecular meshwork. J Cell Mol Med. 2020;24(9):5195–5204. doi: 10.1111/jcmm.15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Yoneda M, Gosho M, Kato T, Zako M. Bimatoprost, latanoprost, and tafluprost induce differential expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases. BMC Ophthalmol. 2016;16:26. doi: 10.1186/s12886-016-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quigley HA. Glaucoma. The Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 19.Sihota R, Angmo DW, Ramaswamy D, Dada T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66(4):495–505. doi: 10.4103/ijo.IJO_1130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152(3):340–344.e2. doi: 10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Morales DR, Dreischulte T, Lipworth BJ, Donnan PT, Jackson C, Guthrie B. Respiratory effect of beta-blocker eye drops in asthma: population-based study and meta-analysis of clinical trials. Br J Clin Pharmacol. 2016;82(3):814–822. doi: 10.1111/bcp.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen WC, Huang BQ, Yang J. Ocular surface changes in prostaglandin analogue-treated patients. J Ophthalmol. 2019;2019:9798272. doi: 10.1155/2019/9798272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YR, Kook MS, Joe SG, Na JH, Han S, Kim S, Shin CJ. Circadian (24-hour) pattern of intraocular pressure and visual field damage in eyes with normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2012;53(2):881–887. doi: 10.1167/iovs.11-7846. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Caprioli J. Intraocular pressure fluctuation: is it important? J Ophthalmic Vis Res. 2018;13(2):170–174. doi: 10.4103/jovr.jovr_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu J, Webster D, Cao J, Shao A. The safety of green tea and green tea extract consumption in adults - results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Jówko E. Green Tea Catechins and Sport Performance. In: Lamprecht M, editor. Antioxidants in Sport Nutrition. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. [PubMed] [Google Scholar]
- 27.Cai ZY, Li XM, Liang JP, Xiang LP, Wang KR, Shi YL, Yang R, Shi M, Ye JH, Lu JL, Zheng XQ, Liang YR. Bioavailability of tea catechins and its improvement. Molecules. 2018;23(9):2346. doi: 10.3390/molecules23092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabouri S, Wright AJ, Corredig M. In vitro digestion of sodium caseinate emulsions loaded with EGCG. Food Hydrocoll. 2017;69:350–358. [Google Scholar]
- 29.Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43(1):95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son YR, Chung JH, Ko S, Shim SM. Combinational enhancing effects of formulation and encapsulation on digestive stability and intestinal transport of green tea catechins. J Microencapsul. 2016;33(2):183–190. doi: 10.3109/02652048.2016.1144816. [DOI] [PubMed] [Google Scholar]
- 31.Naumovski N, Blades BL, Roach PD. Food inhibits the oral bioavailability of the major green tea antioxidant EGCG in humans. Antioxidants (Basel) 2015;4(2):373–393. doi: 10.3390/antiox4020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung JH, Kim S, Lee SJ, Chung JO, Oh YJ, Shim SM. Green tea formulations with vitamin C and xylitol on enhanced intestinal transport of green tea catechins. J Food Sci. 2013;78(5):C685–C690. doi: 10.1111/1750-3841.12112. [DOI] [PubMed] [Google Scholar]