Abstract
AIM
To evaluate the accuracy of segmented measurement of axial length (AL) in high myopia filled with silicone oil by immersion B-scan ultrasonography (immersion B-scan).
METHODS
From June 2016 to June 2020, a total of 67 ultra-high myopia inpatients (67 eyes) who underwent silicone oil removal combined with cataract extraction and intraocular lens (IOL) implantation were retrospectively enrolled. The preoperative axial length (AL) of 31 patients with severe cataract were segmented measured using immersion B-scan (B-scan group) and another 36 patients with mild or moderate cataract were measured using IOLMaster 500 (IOLMaster group). The post-operative ALs in two groups were both measured using IOLMaster 500. The IOL power was calculated with Haigis formula. The differences in ALs between pre- and post-surgery, as well as the postoperative refractive spherical equivalent, absolute refractive error, the prediction deviation of postoperative refraction and best corrected visual acuity (BCVA) were compared.
RESULTS
The pre- and post-operative ALs were 30.46±1.63 mm (range 28.09-33.51 mm) and 30.42±1.70 mm (range 28.03-33.90 mm) in B-scan group (t=0.644, P=0.542) and 30.51±1.21 mm (range 28.03-33.90 mm) and 30.43±1.27mm (range 28.54-33.50 mm) in IOLMaster group (t=1.843, P=0.074), respectively. Three months after surgery, BCVA were 0.45±0.13 (range 0.3-0.9) and 0.44±0.20 (range 0.2-1.0) in B-scan and IOLMaster group respectively (t=0.086, P=0.932). There was no significant difference of the postoperative spherical equivalent (-3.11±0.65 D vs -3.21±0.51 D, t=0.671, P=0.505) and the absolute refractive error (0.589±0.340 vs 0.470±0.245 D, t=1.615, P=0.112) between two groups. In B-scan group, absolute refractive error within ±0.50 D was found in 18 eyes (58.1%), within ±1.00 D in 26 eyes (83.9%), and within ±1.50 D in 31 eyes (100%). In IOLMaster group, absolute refractive error within ±0.50 D was found in 23 eyes (63.9%), within ±1.00 D in 34 eyes (94.4%), and within ±1.50 D in 36 eyes (Z=0.757, P=0.449).
CONCLUSION
The segmented measurement of ALs by immersion B-scan shows comparable measurement accuracy with that of IOLMaster 500 in ultra-high myopia patients with severe cataract secondary to silicone oil filling and can obtain an ideal postoperative refractive state.
Keywords: high myopia, axial length, immersion B-ultrasound, IOLMaster, silicone oil, cataract
INTRODUCTION
With the development of vitrectomy and the accurate mastery of the operative indications of silicone oil filling, a considerable number of patients with complex retinal detachment and vitreoretinal proliferative diseases can preserve good retinal function after vitrectomy combined with silicone oil filling[1]–[6]. Cataract is the most important complication after silicone oil filling[7]–[8]. At present, the surgery method combined silicone oil extraction with cataract extraction and intraocular lens implantation is widely used to treat this complication in China and other countries[9]–[16]. The accurate measurement of axial length (AL) is related to the intraocular lens (IOL) implantation and determines the postoperative visual function for patients with this kind of cataract. As a novel optical coherent biometric device, IOLMaster is easily-manipulated, non-contact, accurate and non-invasive[17]–[19]. However, due to the dependency of IOLMaster on optical principle, the ALs of patients with severe cataract cannot be accurately measured, and the traditional A-ultrasound measurement is still needed. Clinically, the ALs of most patients can be accurately measured by the traditional A-ultrasound. However, for patients with high myopia, especially those with ultra-high myopia before surgery, the pseudo prolongation of vitreous cavity caused by long vitreous cavity and silicone oil filling, intermingled with severe opacity of lens on ultrasonic propagation, retinal waves are often difficult or impossible to be measured in A-ultrasound. This leads to pseudo short AL or eye axis or large deviation and poor repeatability of AL.
To address the abovementioned clinical issues, based on our previous findings of immersion A/B ultrasound in accurately measuring the AL of high myopia[20], we evaluated the accuracy of immersion B-scan segmented measurement of ALs in ultra-high myopia patients with cataract secondary to silicone oil filling by comparing with IOLMaster 500, providing a theoretical basis for its clinical application.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Ethics Committee of PLA General Hospital, and all the patients had written the informed consents.
Subjects
From June 2016 to June 2020, a total of 67 ultra-high myopia inpatients (67 eyes) who underwent silicone oil removal combined with cataract extraction and IOL implantation were retrospectively enrolled. The preoperative AL of 31 patients with severe cataract were segmented measured using immersion B-scan (B-scan group) and another 36 patients with mild or moderate cataract were measured using IOLMaster 500 (IOLMaster group). All patients of both groups were injected with 5500 silicone oil (Baush&Lomb Oxane, USA) during vitrectomy. Inclusive criteria: 1) ultra-high myopia (refractive diopter<-10.00 D or AL>28 mm); 2) all the eyes to be operated had a certain gazing ability (they could continuously gaze at IOLMaster or be fixed in the first eye position for more than 3s in the imaging process of immersion B-scan); 3) without other serious ocular diseases, other systemic or ocular diseases that affect the examination or surgery; 4) scheduled silicone oil removal when complete retinal reattachment was achieved within 6mo after silicone oil injection. Exclusion criteria were as follows: 1) silicone oil emulsification within 6mo after primary silicone oil injection; 2) silicone oil removal for various postoperative complications within 6mo after silicone oil injection; 3) other ophthalmic surgeries during the period between primary silicone oil injection and removal; 4) with uncomplete follow-up data.
Preoperative Examination
Before the surgery, the anterior parts (cornea, anterior chamber, iris and lens) and anterior vitreous body of all patients were examined using slit lamp, the intraocular pressure (IOP) was measured by using non-contact tonometer and the fundus was examined by A/B ultrasonic diagnosis apparatus.
Measurement Methods
The corneal curvature and anterior chamber depth (ACD) of both groups were measured with IOLMaster 500 (Zeiss, Germany). In B-scan group, the ALs were measured with immersion B-scan using Souer A/B ultrasonic diagnosis apparatus (A-scan probe frequency was 10 MHz, and the axial resolution of A, B-scan were 0.12 mm). While in IOLMaster group, the ALs were measured with IOLMaster 500 (Zeiss, Germany). The IOL power was calculated by the built-in Haigis formula in IOLMaster 500 for all patients. During the immersion B-scan, B-mode ultrasound gain was adjusted to 85 dB. A Hansen immersion shell was placed on the ocular surface and the B-ultrasound probe was inserted into the immersion shell 5-10 mm from the cornea. The center of the anterior and posterior lens capsule pointed to the macular, and the intersection point between the sampling line and retina was about 4.5 mm from the disc center or about 3 mm from the optic nerve edge (Figure 1A)[20]. The AL=Dab+986/1532×Dbc (Dab represents distance from the corneal vertex to the posterior pole of lens or central point of capsule; Dbc represents distance from the lens posterior pole or central point of capsule to the macular surface, respectively; Figure 1B)[21]. The final AL was calculated from the average value of three consecutive measurements of the distances. All examinations were performed by the same senior physician.
Figure 1. The image of a silicone oil-filled eye with segmented measurement using immersion B-scan.
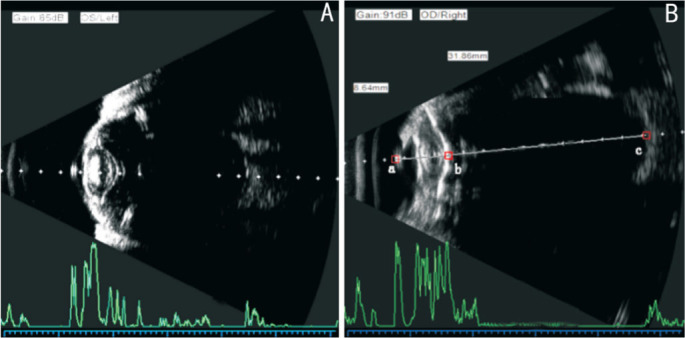
A: The sampling line of vector A ultrasound vertically points to the macular retina through the double light bands of the cornea and the center of the anterior and posterior capsule of the lens; B: AL=Dab+986/1532×Dbc. Dab represents the distance from the corneal vertex to the posterior pole of the lens or central point of the capsule, Dbc represents the distance from the posterior pole or central point of the capsule to the macular surface.
Surgical Methods
After retrobulbar anesthesia, the surgery was performed with Alcon Legacy 20000 phacoemulsification apparatus. A 3 mm incision (I-shape) parallel to the lamellar sclera was made (11 o'clock, 2 mm behind limbus). A 3.2 mm triangular knife was used and viscoelastic agent (AVI) was injected into anterior chamber. Continuous circular capsulorhexis was performed with a diameter of 5.5-6 mm. A corneal auxiliary incision was made at 9 o'clock (left eye) or 3 o'clock (right eye). Lens cortex was aspirated by auto-I/A system. The silicone oil was extracted with cannula needle and syringe under negative pressure with an incision at the upper pars plana. After the silicone oil was completely removed, the retina was observed by pan-retinoscopy. Then, a single focus foldable IOL (Rayner 620H, UK) was implanted into the capsule.
Postoperative Measurement
The refractive state of the patients and the best corrected visual acuity (BCVA) recorded by logMAR were obtained. The diopter was calculated by spherical equivalent (SE). The absolute refractive error of the two methods was calculated, that is, the difference between the reserved diopter and the actual diopter 3mo after surgery.
All the surgeries in this study were performed by the same experienced surgeon and all the patients were implanted with the same type of IOL. In the selection of formulas for calculating the IOL power, previous studies have shown that the Haigis formula has a high accuracy for highly myopic patients with AL>28 mm[22]–[26].
Statistical Analysis
Statistical analyses were performed using SPSS12.0 software. Chi-square test was used to compare the gender difference of both groups. The difference of ALs before and after surgery of both groups, and postoperative absolute refractive error and BCVA between both groups were compared using t-test. The rank sum test was used to compare the distribution of absolute refractive error between the two groups in the range ±0.50, ±1.00, and ±1.50 D. The statistical level was set to α=0.05.
RESULTS
A total of 31 (31 eyes) with severe cataract were enrolled as the B-scan group, including 21 males and 10 females, aged from 25 to 59y (mean 42.8±9.8y). The preoperative visual acuity was light perception (LP) or hand move (HM) before eye. And 36 patients (36 eyes) were enrolled as IOLMater group, including 23 males and 13 females, aged from 24 to 61y (mean: 41.9±9.5y). The preoperative visual acuity was finger counting (FC) to 0.25, as shown in Table 1.
Table 1. Characteristics of study population.
| Characteristics | B-scan group n=31 | IOLMaster group n=36 | P |
| Age, y | 42.8±9.8 | 41.9±9.5 | 0.822 |
| Gender (M/F) | 21/10 | 23/13 | 0.740 |
| Preop. AL | 30.46±1.63 | 30.51±1.21 | 0.542 |
| Preop. UCVA range | LP or HM | FC to 0.25 | 0.046 |
AL: Axial length; UCVA: Uncorrected visual acuity; LP: Light perception; HM: Hand move; FC: Finger counting.
The patients' main details at the time of treatment were summarized in Table 1.
In B-scan group, the baseline ALs measured by immersion B-ultrasound was 30.46±1.63 (range 28.09-33.51) mm, while the postoperative ALs measured by IOLMaster 500 was 30.51±1.21 (range 28.03-33.90) mm (t=0.644, P=0.542). In IOLMaster group, the baseline ALs measured by IOLMaster 500 was 30.51±1.21 (range 28.43-33.69) mm, while the ALs measured by IOLMaster 500 at 3mo after surgery was 30.43±1.27 (range 28.54-33.50) mm (t=1.843, P=0.074).
At 3mo after surgery, the BCVA was not significant difference between two groups (t=0.086, P=0.932), as well as the absolute refractive error (t=1.615, P=0.112) and mean postoperative refractive spherical equivalent between two groups (t=0.671, P=0.505). In B-scan group, absolute refractive error within ±0.50, ±1.00, and ±1.50 D was found in 18 (58.1%), 26 (83.9%), and 31 (100%) cases, respectively. In IOLMaster group, it was 23 (63.9%), 34 (94.4%), and 36 (100%) cases (Z=0.757, P=0.449; Table 2).
Table 2. The postoperative refractive status in the two groups.
| Parameters | B-scan group, n=31 | IOLMaster group, n=36 | Statistic | P |
| Postop. BCVA (logMAR) | 0.45±0.13 (0.3 to 0.9) | 0.44±0.20 (0.2 to 1.0) | 0.086 | 0.932 |
| Postop. SE (D) | -3.11±0.65 (-1.75 to -4.25) | -3.21±0.51 (-2.25 to -4.25) | 0.671 | 0.505 |
| Mean absolute error (D) | 0.589±0.340 (-1.37 to 1.21) | 0.470±0.245 (1.06 to 1.02) | 1.615 | 0.112 |
| Prediction error, n (%) | 0.757 | 0.449 | ||
| Within ±0.50 D | 18 (58.1) | 23 (63.9) | ||
| Within ±1.00 D | 26 (83.9) | 34 (94.4) | ||
| Within ±1.50 D | 31 (100.0) | 36 (100.0) |
BCVA: Best corrected visual acuity; logMAR: Logarithm of the minimum angle of resolution; SE: Spherical equivalent; Predictive error: Predicted postoperative error minus postoperative spherical equivalent of the refractive error.
mean±SD (range)
All the surgeries were successfully completed with retinal reattachment, and no obvious silicone oil droplets were found in the vitreous cavity. The anterior capsule and posterior capsule of the lens were intact in all patients, and the IOLs were implanted into the capsule.
DISCUSSION
High myopia is very common and one of the major causes of social blindness. It is characterized by AL elongation, and induces various specific complications, including retinal detachment from peripheral retinal tears, foveoschisis, cataract formation, macular hole with or without retinal detachment, peripapillary deformation, choroidal/scleral thinning, dome-shaped macula, myopic choroidal neovascularization, and glaucoma[27]–[28].
With the development of vitreous retinal surgery, vitrectomy combined with intravitreal filling of silicone oil is a predominant surgical approach for complex vitreoretinal disease, but it could make the lens affected, leading to the development or aggravation of cataracts whose incidence was reported up to be 100%[7]–[8]. To avoid the increased trauma and economic burden of two surgeries (extraction of silicone oil was performed, lens extraction combined with IOL implantation were performed at stage I), all patients in this study underwent silicone oil removal combined with lens removal and IOL implantation.
Clinically, for patients with cataract after vitrectomy combined with silicone oil filling due to retinal detachment, the ultimate goal of silicone oil extraction combined with cataract extraction and IOL implantation is to preserve the residual visual function as much as possible. The factors affecting visual function include patient- and surgery-related factors[19]. In this study, no significant intergroup differences in age, gender, and ALs were found, and all the causes of retinal detachment were relatively uniform. The difference in preoperative uncorrected visual acuity between the two groups was mainly due to the various severity of lens opacity, and the opaque lens would be removed, so the influence of this factor on our results could be excluded. Surgery-related factors include surgical proficiency, surgical incision, IOL selection, and IOL implantation accuracy[22],[29]–[31].
This formula was utilized to calculate the required IOL power in our present study. In view of the comparative uniformity of patient, surgeon, surgery method, IOL type and calculation formula, we deemed that the biometric measurements were the main reason influencing the differences between the methods in this study. Studies have proposed that 54% deviation between actual diopter and prediction of diopter after cataract surgery comes from AL measurement, whereas 38% predicted from postoperative ACD and another 8% came from the evaluation of corneal curvature. Therefore, the accuracy of preoperative eye AL measurement is of particular importance[32]–[34]. In this study, the corneal curvature and ACD were measured using IOLMaster. AL is an indicator avoid refractive errors. Therefore, the main goal of this study is to investigate how to minimize the ALs measurement error.
Silicone oil is a colorless transparent liquid with good light transmittance and refractive index of 1.40. Therefore, it is feasible to carry out optical biological measurement of silicone oil filling eyes theoretically. IOLMaster, as a novel non-contact optical coherent biological biometric instrument, is easy to operate and non-contact measurement. IOLMaster can be used to accurately measure the ALs of silicone oil-filled eyes[35]–[36]. However, the ALs of patients with severe cataract cannot be measured by IOLMaster, which depends on optical principle, but with the help of traditional A-ultrasound. Clinically, traditional A-ultrasound can be used to accurately measure the ALs of most patients, but for patients with high myopia, especially those with ultra-high myopia before surgery, the pseudo prolongation of vitreous cavity caused by long vitreous cavity and silicone oil filling in patients with axial myopia, coupled with the effect of severe lens opacity on ultrasonic transmission, makes it difficult or impossible to measure retina echo during A-ultrasound measurement, leading to pseudo-short ALs or large deviation and poor repeatability of ALs. The basic principle of segmented measurement of AL by immersion B-scan ultrasonography is to use corresponding sound velocity values for segmented sound velocity measurement of media with different sound velocity on the measurement path in the eyeball, so it is particularly critical to determine the sound velocity of silicone oil. The ultrasonic speed of silicone oil is related to its viscosity, and there are differences in the sound speed of different brands and models of silicone oil. In our study, all patients filled with the same silicone oil and undergone scheduled silicone oil removal when complete retinal reattachment was achieved within 6mo after silicone oil injection without silicone oil emulsification. Compared with IOLMaster 500, this measurement method has the possibility of corneal injury, and has higher requirements for the technique and experience of the examiner. Based on our measurement skills of accurately measuring the AL of high myopia by immersion A/B ultrasound in previous studies[20], we evaluated the accuracy of immersion B-scan segmented measurement of ALs in ultra-high myopia patients with cataract secondary to silicone oil filling by comparing with IOLMaster 500, providing a theoretical basis for its clinical application. To our knowledge, there is no study showing the reliability of our device for the measurement of AL in ultra-high myopia patients with cataract secondary to silicone oil filling in the current literature.
To explore ALs measurement accuracy of the immersion B-scan segmented measuring method, ALs of IOLMaster group were measured by using the silicone oil-filled lens eye mode of the IOLMaster's AL menu. The ALs measurement accuracy was illustrated by the comparison of ALs of IOLMaster group before and after surgery and the prediction deviation in 3mo after surgery, and our results were in line with previous studies[37]. In the present study, after failing to measure the ALs of 31 patients in B-scan group with IOLMaster, the contact A-ultrasound was used to measure in the sitting position, showing that retina echo of 13 patients could not be even detected. We have solid clinical research experience in the accurate measurement of AL of high myopia by using immersion B-scan[20]. To solve the above-mentioned problem, after full-eye imaging with immersion B-scan in this study, segmented measurement with A-ultrasound was performed. Its basic mechanism is to measure (or correct) each segment of media with distinct sound velocity on the measurement path using the corresponding sound velocity. In addition, although 20 mHz has a higher resolution than 10 mHz B-type ultrasound, its sound attenuation is large and the detection depth is small, which cannot meet the measurement of this study. Therefore, 10 mHz B-type ultrasound was adopted in this study. Compared with IOLMaster, this measurement method has the possibility of corneal injury, and has higher requirements for the technique and experience of the examiner.
In this study, there was no statistical difference between the preoperative ALs (segmented measurement using immersion-B scan) and the postoperative ALs (IOLMater) in B-scan group, indicating the high accuracy of segmented measurement method using immersion B-scan as IOLMaster in measuring ALs and the prediction of postoperative refraction. The immersion B-scan image is of two-dimensional brightness modulation type, can display various biometric reference interfaces intuitively, especially the vitreous cavity in pseudo-dilated state after silicone oil filled. In addition, we can guide patients to rotate eyes to adjust eye position at any time through the intuitive real-time image. The sampling lines of vector A-ultrasound could provide accurate guide information for placing electronic measuring ruler, avoiding the possible mal-alignment. This is the basis of accurate AL measurement by immersion B-scan ultrasound method. After the precise measurement of each segment length, all patients were injected with the same type of silicone oil, and the correction coefficient of vitreous cavity length was the same, which could explain the accurate measurement of ALs with segmented measurement method by using immersion B-scan in this study.
We proposed that the improved accuracy of immersion B-ultrasound biometry benefits from several quality control methods as following: 1) by adjusting the patient's eye position and the infiltration depth of B-ultrasound probe, the double light band of cornea, the light band of anterior and posterior capsule of lens and the light band of retina with clear and intact optic nerve were clearly displayed on B-ultrasound echogram, and the tangent lines of light band of cornea, anterior and posterior capsule of lens were parallel to each other; 2) obtaining the widest light band of the posterior capsule; 3) the sampling line of vector A-ultrasound is perpendicular to all corneal-retinal interfaces from and passes through the pupil center; 4) Bergès et al[38] suggested that the intersection between the sampling line of vector A-ultrasound and the retina should be approximately 4.5 mm from the optic disc center, or the line from the corneal vertex to the optic disc center should be bias to the temporal side by 15°, where the macular central foveola located. However, it is worth noting that, based on PCI technology, although the agreement between the two devices was excellent, the IOLMaster 700 was more effective in obtaining biometric measurements in eyes with posterior subcapsular and dense nuclear cataracts[39]–[43]. It means that the ALs of a few patients in B-scan group may be accurately measured by IOLMaster 700. The limited number of cases is another limiting factor in our study. In addition, the retrospective nature of this study limits our comparisons. A larger patient series is needed for clearer information.
In conclusion, our study demonstrated that the immersion B-scan imaging is not affected by the cataract severity, and its biometry accuracy is generally consistent with that of IOLMaster 500 in ultra-high myopia patients with silicone oil filling concurrent severe cataract. It can be used as an accurate and reliable biological measurement method to solve clinical problems. In addition, immersion B-scan ultrasound method has advantages in acquiring the whole eye ultrasound images from anterior to posterior simultaneously and biological measurement under visualization, its more clinical practical value deserves further exploration.
Acknowledgments
The authors thank Mao-Nian Zhang, MD and Shou-Zhi He, MD, for their careful reading of the manuscript and their useful comments and suggestions.
Foundation: Supported by National Natural Science Foundation of China (No.82070921).
Conflicts of Interest: Yang QH, None; Zhang HT, None; Li XQ, None; Chen B, None; Li ZH, None; Huang YF, None; Jin X, None; Zhang Y, None; Wang LQ, None.
REFERENCES
- 1.Antoun J, Azar G, Jabbour E, Kourie HR, Slim E, Schakal A, Jalkh A. Vitreoretinal surgery with silicone oil tamponade in primary uncomplicated rhegmatogenous retinal detachment: clinical outcomes and complications. Retina. 2016;36(10):1906–1912. doi: 10.1097/IAE.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio V, Reibaldi M, Macchi I, Fallico M, Pizzo C, Patane C, Russo A, Longo A, Pizzo A, Cillino G, Cillino S, Vadalà M, Rinaldi M, Rejdak R, Nowomiejska K, Toro MD, Avitabile T, Ortisi E. Preoperative, intraoperative and postoperative corticosteroid use as an adjunctive treatment for rhegmatogenous retinal detachment. J Clin Med. 2020;9(5):1556. doi: 10.3390/jcm9051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves MGR, Afshar AR, Pershing S. Need for retinal detachment reoperation based on primary repair method among commercially insured patients, 2003-2016. Am J Ophthalmol. 2021;229:71–81. doi: 10.1016/j.ajo.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao L, Zhu XH. Advances in the treatment of rhegmatogenous retinal detachment. Int J Ophthalmol. 2019;12(4):660–667. doi: 10.18240/ijo.2019.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu I, Ishikawa H, Nishikawa H, Morikawa S, Okamoto F, Sakamoto T, Sugimoto M, Kondo M, Iwasaki M, Kinoshita T, Toibana T, Mitamura Y, Takamura Y, Motohashi R, Shimura M, Sakurai Y, Takeuchi M, Gomi F. Scleral buckling versus vitrectomy for young Japanese patients with rhegmatogenous retinal detachment in the era of microincision surgery: real-world evidence from a multicentre study in Japan. Acta Ophthalmol. 2019;97(5):e736–e741. doi: 10.1111/aos.14050. [DOI] [PubMed] [Google Scholar]
- 6.Eibenberger K, Georgopoulos M, Rezar-Dreindl S, Schmidt-Erfurth U, Sacu S. Development of surgical management in primary rhegmatogenous retinal detachment treatment from 2009 to 2015. Curr Eye Res. 2018;43(4):517–525. doi: 10.1080/02713683.2018.1428996. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Chen B, Yang HJ, Song YW, Zhang D, Soetikno BT, Sun XF. The correlation of pars Plana incision and transient hypotony after silicone oil removal. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):e44–e51. doi: 10.3928/23258160-20180907-06. [DOI] [PubMed] [Google Scholar]
- 8.Al-Wadani SF, Abouammoh MA, Abu El-Asrar AM. Visual and anatomical outcomes after silicone oil removal in patients with complex retinal detachment. Int Ophthalmol. 2014;34(3):549–556. doi: 10.1007/s10792-013-9857-9. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Cheng WJ, Zhuang H, Guo J, Xu GX. Safety and efficacy of transpupillary silicone oil removal in combination with micro-incision phacoemulsification cataract surgery: comparison with 23-gauge approach. BMC Ophthalmol. 2018;18(1):200. doi: 10.1186/s12886-018-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sizmaz S, Esen E, Isik P, Cam B, Demircan N. Outcome and complications of combined phacoemulsification and 23-gauge pars Plana vitrectomy. J Ophthalmol. 2019;2019:7918237. doi: 10.1155/2019/7918237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman R, Kolb S, Bong CX, Stephenson J. Accuracy of user-adjusted axial length measurements with optical biometry in eyes having combined phacovitrectomy for macular-off rhegmatogenous retinal detachment. J Cataract Refract Surg. 2016;42(7):1009–1014. doi: 10.1016/j.jcrs.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Shiraki N, Wakabayashi T, Sakaguchi H, Nishida K. Optical biometry-based intraocular lens calculation and refractive outcomes after phacovitrectomy for rhegmatogenous retinal detachment and epiretinal membrane. Sci Rep. 2018;8(1):11319. doi: 10.1038/s41598-018-29553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savastano A, Lenzetti C, Finocchio L, Bacherini D, Giansanti F, Tartaro R, Piccirillo V, Savastano MC, Virgili G, Rizzo S. Combining cataract surgery with 25-gauge high-speed pars Plana vitrectomy: a prospective study. Eur J Ophthalmol. 2021;31(2):673–678. doi: 10.1177/1120672120902030. [DOI] [PubMed] [Google Scholar]
- 14.Port AD, Nolan JG, Siegel NH, Chen XJ, Ness SD, Subramanian ML. Combined phaco-vitrectomy provides lower costs and greater area under the curve vision gains than sequential vitrectomy and phacoemulsification. Graefes Arch Clin Exp Ophthalmol. 2021;259(1):45–52. doi: 10.1007/s00417-020-04877-4. [DOI] [PubMed] [Google Scholar]
- 15.Antaki F, Milad D, Javidi S, Dirani A. Vitreoretinal surgery in the post-lockdown era: making the case for combined phacovitrectomy. Clin Ophthalmol. 2020;14:2307–2309. doi: 10.2147/OPTH.S270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozal SA, Garip R, Ozal E, Kupeli A. Evaluation of axial length changes after combined phacovitrectomy for macula-off rhegmatogenous retinal detachment. Beyoglu Eye J. 2019;4(3):136–140. doi: 10.14744/bej.2019.84856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parravano M, Oddone F, Sampalmieri M, Gazzaniga D. Reliability of the IOLMaster in axial length evaluation in silicone oil-filled eyes. Eye (Lond) 2007;21(7):909–911. doi: 10.1038/sj.eye.6702452. [DOI] [PubMed] [Google Scholar]
- 18.Shajari M, Cremonese C, Petermann K, Singh P, Müller M, Kohnen T. Comparison of axial length, corneal curvature, and anterior chamber depth measurements of 2 recently introduced devices to a known biometer. Am J Ophthalmol. 2017;178:58–64. doi: 10.1016/j.ajo.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Li HR, Li QC. Differences in axial length and IOL power based on alternative A-scan or fellow-eye biometry in macula-off rhegmatogenous retinal detachment eyes. Ophthalmol Ther. 2022;11(1):347–354. doi: 10.1007/s40123-021-00439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang QH, Chen B, Peng GH, Li ZH, Huang YF. Accuracy of axial length measurements from immersion B-scan ultrasonography in highly myopic eyes. Int J Ophthalmol. 2014;7(3):441–445. doi: 10.3980/j.issn.2222-3959.2014.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suk KK, Smiddy WE, Shi W. Refractive outcomes after silicone oil removal and intraocular lens implantation. Retina. 2013;33(3):634–641. doi: 10.1097/IAE.0b013e31826d37e4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Liang XY, Liu S, Lee JW, Bhaskar S, Lam DS. Accuracy of intraocular lens power calculation formulas for highly myopic eyes. J Ophthalmol. 2016;2016:1917268. doi: 10.1155/2016/1917268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffer KJ, Savini G. IOL power calculation in short and long eyes. Asia Pac J Ophthalmol (Phila) 2017;6(4):330–331. doi: 10.22608/APO.2017338. [DOI] [PubMed] [Google Scholar]
- 24.Tan X, Zhang J, Zhu Y, Xu J, Qiu X, Yang G, Liu Z, Luo L, Liu Y. Accuracy of new generation intraocular lens calculation formulas in vitrectomized eyes. Am J Ophthalmol. 2020;217:81–90. doi: 10.1016/j.ajo.2020.04.035. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Wang W, Liu Z, Yang G, Qiu X, Xu J, Jin G, Li Y, Zhang S, Tan X, Luo L, Liu Y. Accuracy of new-generation intraocular lens calculation formulas in eyes undergoing combined silicone oil removal and cataract surgery. J Cataract Refract Surg. 2021;47(5):593–598. doi: 10.1097/j.jcrs.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 26.Kane JX, van Heerden A, Atik A, Petsoglou C. Intraocular lens power formula accuracy: comparison of 7 formulas. J Cataract Refract Surg. 2016;42(10):1490–1500. doi: 10.1016/j.jcrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X, Matsui KO, Wu PC, Sankaridurg P, Chia A, Rosman M, Lamoureux EL, Man R, He M. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 29.Kang SI, Moon K, Jun JH. Accuracy of three intraocular lens-power formulas in predicting refractive outcomes in different intraocular lenses. J Korean Ophthalmol Soc. 2016;57(12):1891–1896. [Google Scholar]
- 30.Lenkova GA. Specific features of measuring the optical power of artificial refractive and diffractive–refractive eye lenses. Geometrical and Applied Optics. 2016;121:310–321. [Google Scholar]
- 31.El-Khayat AR, Brent AJ, Peart SAM, Chaudhuri PR. Accuracy of intraocular lens calculations based on fellow-eye biometry for phacovitrectomy for macula-off rhegmatogenous retinal detachments. Eye (Lond) 2019;33(11):1756–1761. doi: 10.1038/s41433-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo S, Lee CE, Kim YK, Lee SY, Jeoung JW, Park KH. Factors affecting refractive outcome after cataract surgery in primary angle-closure glaucoma. Clin Exp Ophthalmol. 2016;44(8):693–700. doi: 10.1111/ceo.12762. [DOI] [PubMed] [Google Scholar]
- 33.Kang TS, Park HJ, Jo YJ, Kim JY. Long-term reproducibility of axial length after combined phacovitrectomy in macula-sparing rhegmatogenous retinal detachment. Sci Rep. 2018;8(1):15856. doi: 10.1038/s41598-018-34266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto M, Yoshida I, Sodeno T, Sakai A, Masahara H, Maeno T. Postoperative refractive prediction error measured by optical and acoustic biometry after phacovitrectomy for rhegmatogenous retinal detachment without macular involvement. J Ophthalmol. 2019;2019:5964127. doi: 10.1155/2019/5964127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pongsachareonnont P, Tangjanyatam S. Accuracy of axial length measurements obtained by optical biometry and acoustic biometry in rhegmatogenous retinal detachment: a prospective study. Clin Ophthalmol. 2018;12:973–980. doi: 10.2147/OPTH.S165875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tranos PG, Allan B, Balidis M, Vakalis A, Asteriades S, Anogeianakis G, Triantafilla M, Kozeis N, Stavrakas P. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):987–993. doi: 10.1007/s00417-019-04583-w. [DOI] [PubMed] [Google Scholar]
- 37.Wu B, Chen S, Liu YC, Li Y, Gao JM, Li JH, He GH. The comparison of immersion B-scan guided with respective sonic velocity and Lenstar LS900 onmeasurement of axial length in silicone oil-filled eyes. Chinese Journal of Ocular Fundus Diseases. 2017;33(6):605–608. [Google Scholar]
- 38.Bergès O, Puech M, Assouline M, Letenneur L, Gastellu-Etchegorry M. B-mode-guided vector-A-mode versus A-mode biometry to determine axial length and intraocular lens power. J Cataract Refract Surg. 1998;24(4):529–535. doi: 10.1016/s0886-3350(98)80297-6. [DOI] [PubMed] [Google Scholar]
- 39.Bullimore MA, Slade S, Yoo P, Otani T. An evaluation of the IOLMaster 700. Eye Contact Lens. 2019;45(2):117–123. doi: 10.1097/ICL.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 40.Savini G, Taroni L, Hoffer KJ. Recent developments in intraocular lens power calculation methods-update 2020. Ann Transl Med. 2020;8(22):1553. doi: 10.21037/atm-20-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozal SA, Kupelı A, Ozal E, Gurlu V. Optical biometry-based axial length alterations after intravitreal dexamethasone implant. Arq Bras Oftalmol. 2019;82(3):195–199. doi: 10.5935/0004-2749.20190040. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZY, Yang WL, Li DJ, Chen W, Zhao Q, Li YF, Cui R, Shen L, Xian JF. Comparison of biometry with the Pentacam AXL, IOLMaster 700 and IOLMaster 500 in cataract patients. Zhonghua Yan Ke Za Zhi. 2019;55(7):515–521. doi: 10.3760/cma.j.issn.0412-4081.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Cho YJ, Lim TH, Choi KY, Cho BJ. Comparison of ocular biometry using new swept-source optical coherence tomography-based optical biometer with other devices. Korean J Ophthalmol. 2018;32(4):257. doi: 10.3341/kjo.2017.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]


