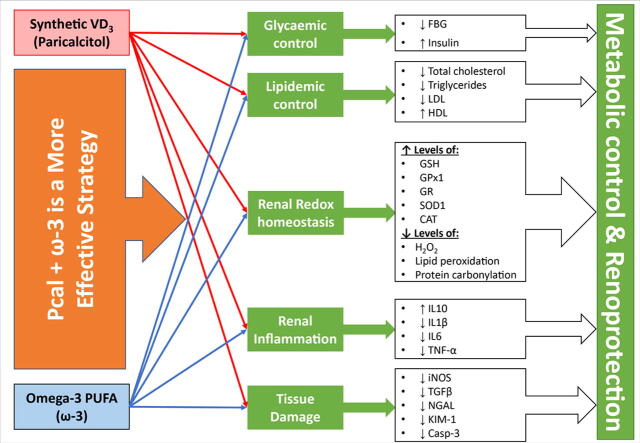
Graphical abstract
Keywords: INOS, KIM-1, NGAL, TGF-β, Vitamin D
Highlights
-
•
Pcal and ω-3 monotherapies moderately attenuated hyperglycaemia and dyslipidaemia.
-
•
Pcal and ω-3 monotherapies equally reduced renal oxidative stress and inflammation.
-
•
Pcal/ω-3 co-therapy showed enhanced anti-diabetic and renoprotection effects.
-
•
Co-therapy may induce boosted metabolic, anti-oxidative & anti-inflammatory actions.
Abstract
Introduction
Although the synthetic vitamin D analogue, Paricalcitol, and omega-3 Fatty acids (ω-3) alleviated diabetic nephropathy (DN), their combination was not previously explored.
Objectives
This study measured the potential ameliorative effects of single and dual therapies of Paricalcitol and/or ω-3 against DN.
Methods
Forty rats were assigned as follow: negative (NC) and positive (PC) controls, Paricalcitol, ω-3 and Paricalcitol + ω-3 groups. Diabetes was generated by high-fat/high-fructose diet and a single streptozotocin injection (40 mg/kg). DN was confirmed by raised fasting blood glucose (FBG), polyuria, proteinuria, and decreased urine creatinine levels. Paricalcitol intraperitoneal injections (0.25 µg/Kg/day; 5 times/week) and oral ω-3 (415 mg/kg/day; 5 times/week) started at week-9 and for eight weeks.
Results
The PC group showed hyperglycaemia, dyslipidaemia, abnormal renal biochemical parameters, elevated caspase-3 expression, and increased apoptosis by TUNEL technique. The mRNAs and proteins of the pathogenic molecules (TGF-β1/iNOS) and markers of tissue damage (NGAL/KIM-1) augmented substantially in the PC renal tissues relative to the NC group. The oxidative stress (MDA/H2O2/protein carbonyl groups) and pro-inflammatory (IL1β/IL6/TNF-α) markers increased, whereas the anti-inflammatory (IL10) and anti-oxidative (GSH/GPx1/GR/SOD1/CAT) declined, in the PC renal tissues. The monotherapy groups were associated with ameliorated FBG, lipid profile and renal functions, and diminished TGF-β1/iNOS/NGAL/KIM-1/Caspase-3 alongside the apoptotic index than the PC group. The oxidative stress and pro-inflammatory markers decreased, whilst the anti-oxidative and anti-inflammatory molecules escalated, in the monotherapy groups than the PC group. Although the Paricalcitol renoprotective actions were better than ω-3, all the biomarkers were abnormal than the NC group. Alternatively, the Paricalcitol + ω-3 protocol exhibited the best improvements in metabolic control, renal functions, oxidative stress, inflammation, and apoptosis. However, FBG and tissue damage were persistently higher in the co-therapy group than controls.
Conclusions
Both monotherapies showed modest efficacy against DN, whereas their combination displayed boosted renoprotection, possibly by enhancing renal anti-oxidant and anti-inflammatory pathways.
Introduction
Diabetic nephropathy (DN) is a prime predisposing factor for developing end stage kidney disease and chronic renal failure [1], [2]. While the clinical hallmark of DN is progressive albuminuria and declines in glomerular filtration rate (GFR), glomerulopathy also concurs with elevated amounts of neutrophil gelatinase-associated lipocalin (NGAL) and increased kidney injury molecule-1 (KIM-1) usually reflects tubular damage [3], [4]. DM incites nephropathy by increasing reactive oxygen (ROS) and nitrogen (RNS) species following mitochondrial damage and upregulation of inducible nitric oxide synthase (iNOS) enzyme, thus causing renal oxidative stress [5], [6], [7], [8]. Concurrently, hyperglycaemia and dyslipidaemia deter the renal anti-oxidant system by decreasing glutathione (GSH) and its associated peroxidase-1 (GPx1) and reductase (GR) enzymes alongside the catalase (CAT) and superoxide dismutase-1 (SOD1) enzymes [9], [10]. Incessant oxidative stress then triggers lipid and protein damages alongside renal chronic inflammation [5], [6], [7], [8]. Hyperglycaemia and dyslipidaemia could also provoke renal inflammation directly by increasing interleukin (IL)1β, IL6 and tumour necrosis factor (TNF)-α, and reducing the potent anti-inflammatory cytokine, IL10 [11], [12]. Persistent renal oxidative stress and inflammation then promote the expression of transforming growth factor-β (TGF-β) and caspase-3 (Casp-3), thus causing glomerular and tubular damage and apoptosis [13], [14].
The nephroprotection of the currently used anti-diabetic therapies is limited and, therefore, the search for novel more effective approaches against DN is warranted [1], [2]. In this context, vitamin D (VD) is a potent anti-oxidant and anti-inflammatory plethoric hormone that regulates a variety of cellular processes through its nuclear receptor (VDR) [15], [16]. However, hypercalcaemia and tissue calcification are common complications of using active VD continuously [15]. Hence, numerous non-calcaemic analogues have emerged, of which Paricalcitol (Pcal; 19-nor-1α-25–2(OH) D2) is an FDA-approved synthetic VDR-agonist utilised for managing secondary hyperparathyroidism associated with chronic renal diseases [15]. Pcal also showed VDR-mediated anti-oxidative and anti-inflammatory remedial activities against DN, but with substantially less calcaemic effects [17], [18], [19]. Alternatively, omega-3 fatty acids (ω-3), which are polyunsaturated fatty acids abundantly found in marine nutritional sources, enhanced glucose and lipid metabolism and reduced oxidative stress and inflammation, thus delayed the progression of DN in clinical and experimental studies [20], [21], [22], [23].
Despite the Pcal and ω-3 anti-diabetic and nephroprotective actions [17], [18], [19], [20], [21], [22], [23], their combination against DN was not previously explored. Hence, this study was conducted to measure the effects of single and dual Pcal and/or ω-3 therapies against pre-existing DN and its underlying molecular pathogenic mechanisms.
Materials and methods
Ethics statement
All animal experiments were performed according to the ethical policies and procedures approved by the Committee for the Care and Use of Laboratory Animals at Umm Al-Qura University, Saudi Arabia (Approval no. AMSEC 24/11-11-19).
Drugs and chemicals
Streptozotocin (STZ) ≥ 98% purity (Sigma-Aldrich Co., MO, USA), Paricalcitol (Zemplar™; AbbVie Inc., IL, USA) and fish oil from menhaden (Sigma-Aldrich Co.) rich in ω-3 (eicosapentaenoic acid [240 mg/ml], docosahexaenoic acid [480 mg/ml] and Docosapentaenoic Acid [60 mg/ml]) were used.
Induction of diabetes mellitus and treatment protocols
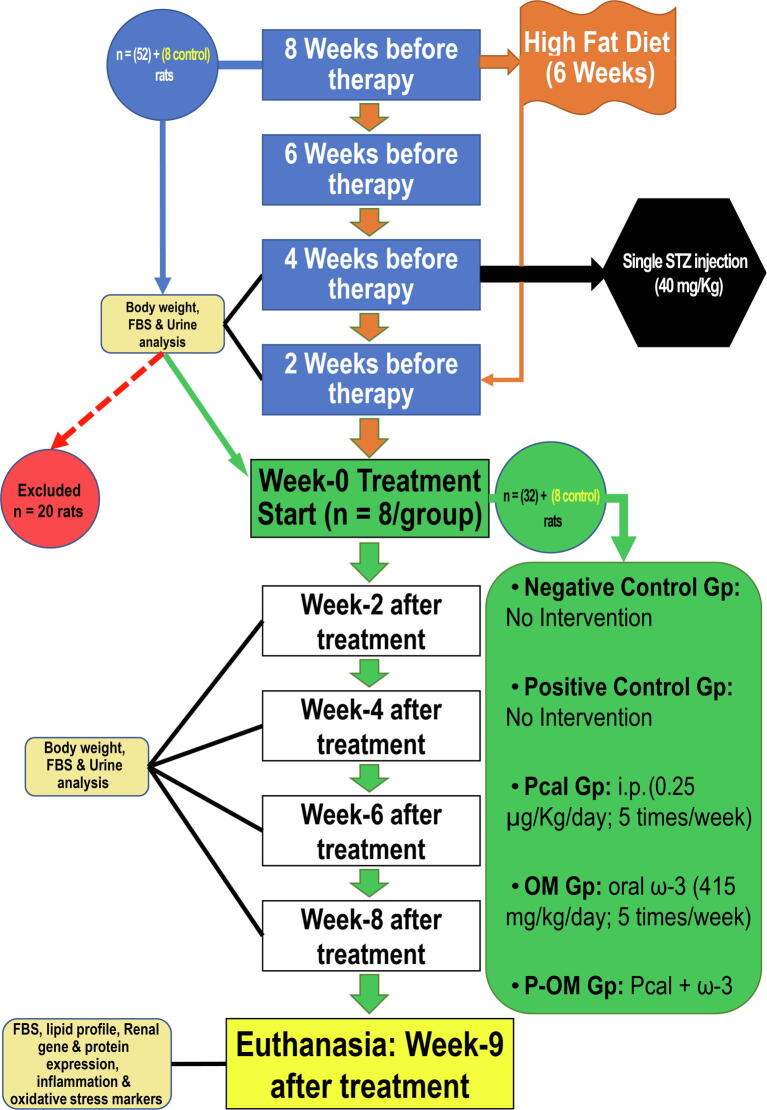
Sixty male Wistar rats weighing 160–180 g and of 7 weeks of age were used following one week of acclimatisation. All animals were maintained at room temperature (24 ± 1 °C) and 12hr light/dark cycle, and received standard laboratory diet (5% fat, 45% carbohydrate and 21% protein) with water ad libitum.
The study involved eight weeks for establishing DN followed by another eight weeks of treatment. Freshly made high-fructose/high-fat diet (HF/HFD), which included standard show with extra 10% fat alongside drinking water containing 20% fructose, was given to 52 rats for four consecutive weeks [24], [25]. Following overnight fasting, a single dose of freshly dissolved STZ (40 mg/kg) in 0.1 M citrate buffer (pH 4.5) was injected intraperitoneally in the animals that received HF/HFD (n = 52) to avoid the nephrotoxic effects of multiple STZ doses [26] as well as to imitate type 2 diabetes mellitus (DM) as previously reported [24], [25]. Additionally, the animals then received glucose solution (10% w/v) during the 24hr post-STZ to avoid hypoglycaemia. Three days later, fasting blood glucose (FBG) was measured in tail blood using an Accu-Chek glucometer (Roche Diabetes Care, Inc., IN, USA) and DM was confirmed by levels > 14 mmol/L (>250 mg/dL). Subsequently, the diabetic rats continued to receive HF/HFD for two weeks followed by two weeks of standard chow. Prior to treatment initiation, a 24hr urine (24hr-U) sample/rat was collected using metabolic cages (Braintree Scientific Inc.; MA, USA). DN was confirmed in 32 rats by polyuria, proteinuria, and decreased urine creatinine (Cr) concentrations than the negative control (NC) animals (n = 8).
The DN animals were then equally divided (8 rats/group) into: the positive (PC) control, Paricalcitol (Pcal) and ω-3 (OM) monotherapies, and the co-therapy group (P-OM) that concurrently received Pcal with ω-3. Freshly prepared Pcal intraperitoneal injections (0.25 µg/Kg/day; 5 times/week) and/or oral ω-3 (415 mg/kg/day; 5 times/week) therapies were given to the designated groups for eight successive weeks. In consonance with the dose conversion between human and rat [27], the Pcal and ω-3 used amounts matched the maximum recommended daily amounts for a 60 Kg body weight adult human (Pcal: 2.4 µg/day; 0.04 µg/Kg/day and ω-3: 4 g/day; 66.7 mg/kg/day) [28], [29]. Additionally, the applied doses and therapeutic durations are equivalent to those used by many prior studies that have shown no toxicological adverse events in their animal groups [18], [30]. The study workflow is summarised in Supplementary fig. 1.
Types of samples
Total body weight (TBW), FBG alongside 24hr-U physical (volume and flow) and biochemical parameters (total protein, Cr) were measured in all groups before therapy initiation (week-0) and for every two weeks throughout therapy. Following 12hr fasting, euthanasia was conducted during the first day of week-9 post-therapy with anaesthesia and cervical dislocation as previously described [16]. Two ml of blood/rat were obtained, and serum was kept in −20 °C. The two kidneys were also obtained from each rat and a portion was processed by conventional histopathology protocols before paraffin embedding. Another piece (0.5 gm) was lysed in RIPA buffer with protease inhibitors (Thermo Fisher Scientific; CA, USA), and the protein amounts were quantified by a BCA protein kit (Thermo Fisher Scientific). The samples were diluted with deionized water (1000 µg/mL) to be used for ELISA. The remaining kidney samples were preserved at −80 °C in RNALater (Thermo Fisher Scientific).
Metabolic and renal profiles
Insulin, FBG, kidney function parameters (Cr/urea/total protein/albumin) and calcium in blood together with the 24hr-U Cr and total protein levels were evaluated on Cobas e411 (Roche Diagnostics, Mannheim, Germany).
The amounts of 24hr-U flow and Cr clearance (Cr-Cl) were measured as follow:
Quantitative RT-PCR
A Paris kit (Thermo Fisher Scientific) was used to extract total RNA, and the cDNA was synthesised by utilising a high capacity Reverse Transcription Kit (Thermo Fisher Scientific). PCR was conducted on ABI® 7500 system using triplicate wells and 40 amplification cycles (95 °C/15 s and 60 °C/1min). Each well had SYBR Green (10 µl; Thermo Fisher Scientific), DNase/RNase free water (7 µl), 5 pmol (1 µl) of each set of primers (Supplementary Table 1) and 25 ng cDNA (1 µl). The negative controls included a minus-reverse transcription control from the prior RT step and a separate minus-template PCR, in which the cDNA was replaced by nuclease free. GAPDH gene was used for normalisation and the relative gene expression of rat Casp-3, TGF-β, NOS2, KIM-1 and NGAL was measured by the 2−ΔΔCt method.
Immunohistochemistry (IHC)
Polyclonal rabbit IgG antibodies were employed to detect iNOS, TGF-β and NGAL in renal tissues, whereas KIM-1 was localised by goat polyclonal IgG antibodies. The concentration for all primary antibodies (Thermo Fisher Scientific) was 1:200, and ImmPRESS® HRP Horse Anti-Rabbit or anti-Goat IgG Plus Polymer Peroxidase Kits (Vector Laboratories Inc., CA, USA) were utilised as per the supplier’s instructions. An identical method was applied for the negative control sections, but with substituting the primary antibodies with their equivalent primary isotype goat or rabbit IgG antibodies (Santa-Cruz Biotechnology Inc.; TX, USA). Examination was done with 20 × and 40 × objectives on a Leica DMi8 microscope (Leica Microsystems, Wetzlar, Germany) and the images were acquired from 10 different fields per section. The stain intensity of each molecule was quantified by the IHC tool in the ImageJ software (https://imagej.nih.gov/ij/) as previously reported [31].
TUNEL assay
Apoptosis/necrosis was evaluated in renal tissues with a Click-iT™ TUNEL Alexa Fluor™ 488 Imaging Assay (Thermo Fisher Scientific) as per the provided protocol. Cleaved Casp-3 was colocalised with the apoptotic bodies by using a sequential immunostain protocol. After completing the TUNEL protocol, anti-Casp-3 mouse IgG monoclonal antibodies (Thermo Fisher Scientific) were added at 1:100 concentration and the slides were incubated for 3 h. Tagged donkey anti-mouse (Alexa Fluor™ 555) IgG antibodies (Thermo Fisher Scientific) was then added for 30 min followed by counterstaining with DAPI (Thermo Fisher Scientific). The slides were observed on a Leica DMi8 microscope at 40 × magnification. The apoptosis index was calculated by counting the apoptotic/necrotic cells in 15 fields/section as previously described [31].
ELISA
The renal tissue levels of IL1β, IL6, IL10, and TNF-α were quantified by kits specific for rats (Cloud-Clone Corp.; TX, USA). GSH, SOD1, CAT, GPx1 and GR alongside malondialdehyde (MDA), protein carbonyl groups, and hydrogen peroxide (H2O2) were also measured in renal tissue by ELISA (Cell Biolabs, Inc.; CA, USA). The samples were processed in duplicate on an automated ELISA machine (Human Diagnostics; Wiesbaden, Germany) according the manufacturers’ protocols.
Statistical analysis
Statistical analysis was done by SPSS version 25, and each variable was analysed for normality and homogeneity using the Kolmogorov and Smirnov’s test and the Levene test, respectively. One-way ANOVA accompanied with Tukey’s HSD or Games-Howell post-hoc tests were performed to compare between the groups according to variance equality. Correlations were determined by Pearson’s test. Statistical significance was considered when P value was < 0.05.
Results
Metabolic profile and renal biochemical parameters
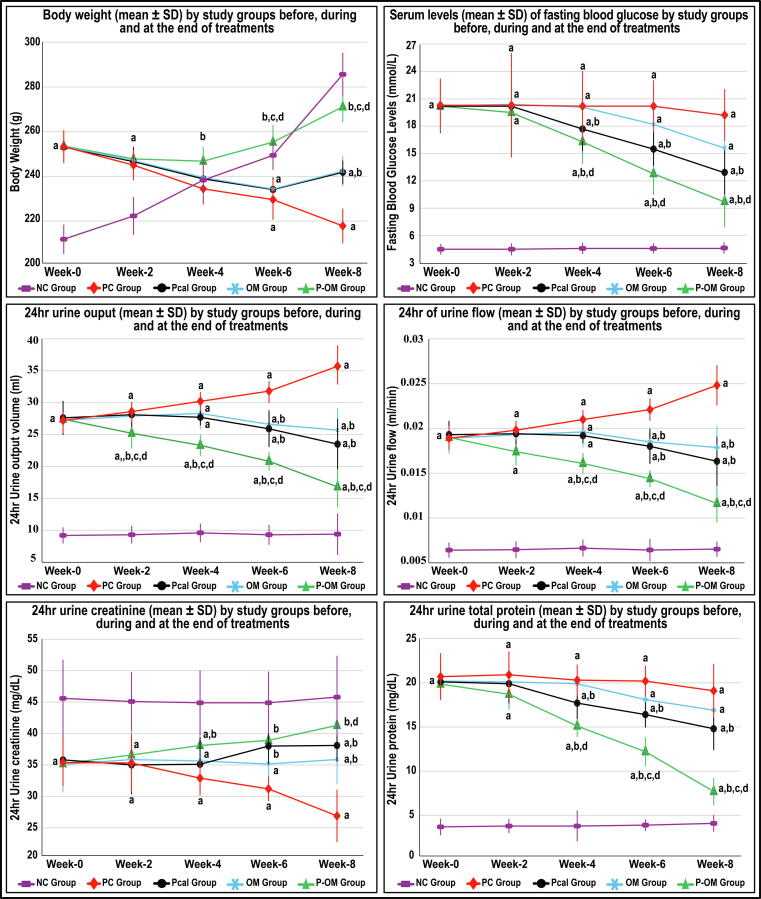
Before therapy (Week-0), all the DN groups were equal and had significantly higher TBW, FBG, 24hr-U volume and flow alongside proteinuria and decreased urine Cr relative to the NC group (Fig. 1; P < 0.0001 for all). The TBW and 24hr-U Cr levels declined, whilst the 24hr-U volume and flow increased, progressively in the PC group during the therapeutic period (P < 0.01 for all). However, the PC group FBG and 24hr-U protein levels were steady during the different weeks of therapy (Fig. 1). Moreover, FBG, total cholesterol, LDL, triglycerides, serum Cr and urea increased, whereas insulin, total protein, albumin, HDL, and Cr-Cl decreased, markedly in the PC group at euthanasia compared with the NC group (Table 1).
Fig. 1.
Dynamics (mean ± SD) of body weight, fasting blood glucose (FBG), 24hr urine output, 24hr urine flow, urine creatinine (Cr) and urine total proteins concentrations in the different study groups before, during and following therapy (data is shown as mean ± SD; a = P < 0.05 compared with the same week in the NC group; b = P < 0.05 compared with the same week in the PC group; c = P < 0.05 compared with the same week in the Pcal group and d = P < 0.05 compared with the same week in the OM group).
Table 1.
Concentrations (mean ± SD) of serum fasting blood glucose (FBG), insulin, lipid profile, renal biochemical parameters, and calcium in the study groups at euthanasia (week-9 post treatment).
| NC group | PC group | Pcal Group | OM Group | P-OM group | |
|---|---|---|---|---|---|
| FBG (mmol/L)* | 4.6 ± 0.4 | 19.2 ± 2.8b | 12.9 ± 2.4b,d | 16.6 ± 1.9b,c | 9.7 ± 2.8b,d,e,h |
| Insulin (μU/mL)* | 28.9 ± 6.6 | 6.5 ± 1.7b | 11.4 ± 3b,c | 10.2 ± 3.5b,c | 15.9 ± 4.1b,d |
| Total Cholesterol (mmol/L)** | 1.62 ± 0.12 | 2.41 ± 0.13b | 2.06 ± 0.15b,d | 2.05 ± 0.17b,d | 1.8 ± 0.08a,d,f,h |
| LDL (mmol/L)** | 0.63 ± 0.09 | 1.75 ± 0.15b | 1.31 ± 0.16b,d | 1.37 ± 0.16b,d | 0.99 ± 0.1b,d,e,h |
| HDL (mmol/L)** | 0.88 ± 0.06 | 0.56 ± 0.05b | 0.67 ± 0.06b,d | 0.68 ± 0.08b,d | 0.77 ± 0.04b,d,e,g |
| Triglycerides (mmol/L)** | 0.81 ± 0.07 | 1.62 ± 0.1b | 1.31 ± 0.1b,d | 1.26 ± 0.06b,d | 0.97 ± 0.07b,d,f,h |
| Total protein (g/dL)** | 7.2 ± 1.1 | 4.8 ± 0.5b | 5.2 ± 0.6b | 4.8 ± 0.4b | 6.4 ± 0.6d,f,h |
| Albumin (g/dL)** | 4.3 ± 0.6 | 2.9 ± 0.3b | 3.1 ± 0.3b | 2.9 ± 0.2b | 3.9 ± 0.4d.f.h |
| Creatinine (mg/dL)* | 0.48 ± 0.06 | 1.1 ± 0.17b | 0.73 ± .12b,d | 0.72 ± 0.15b,d | 0.46 ± 0.07d,f,h |
| Urea (mg/dL)** | 35.8 ± 6.9 | 73.1 ± 11.9b | 54.8 ± 7.1b,d | 58.1 ± 7.1b,d | 44.4 ± 7.2d,e,h |
| Creatinine clearance (mL/min)** | 0.55 ± 0.12 | 0.28 ± 0.03b | 0.36 ± 0.06b | 0.37 ± 0.08b | 0.51 ± 0.08d,e,g |
| Ca2+ (mg/dL)** | 9.6 ± 0.4 | 9.3 ± 0.4 | 9.7 ± 0.5 | 9.6 ± 0.5 | 9.8 ± 0.4 |
* = Games-Howell post-hoc test was used following ANOVA to compare between the groups.
** = Tukey’s HSD post-hoc test was used following ANOVA to compare between the groups.
a = P < 0.05 compared with NC group b = P < 0.01 compared with NC group.
c = P < 0.05 compared with PC group d = P < 0.01 compared with PC group.
e = P < 0.05 compared with Pcal group f = P < 0.01 compared with Pcal group.
g = P < 0.05 compared with OM group h = P < 0.01 compared with OM group.
The FBG and urine parameters were equal between the Pcal, OM and PC groups during the first four weeks of therapy (Fig. 1). At Week-6, the monotherapy groups showed marked declines in FBG, 24hr-U volume and flow, and 24hr-U protein amounts that coincided with an increase in urine Cr levels relative to the PC group. All the biomarkers were also comparable between the monotherapy groups, except for FBG that was significantly lower at Week-6 and 8 in the Pcal group (Fig. 1). Contrariwise, the co-therapy group showed marked ameliorations in FBG, TBW and urine parameters from Week-4 post-therapy, and the best improvements were seen at Week-8 relative to the PC, Pcal and OM groups (Fig. 1). At euthanasia, the P-OM group also showed the highest levels of serum insulin, total protein, albumin and HDL alongside the lowest serum Cr, urea, cholesterol, triglycerides and LDL concentrations relative to the PC, Pcal and OM groups (Table 1). Although the serum biochemical markers were equal in the P-OM and NC groups, the FBG, cholesterol, triglycerides and LDL were markedly higher, whilst insulin and HDL were lower, in the former group (Table 1).
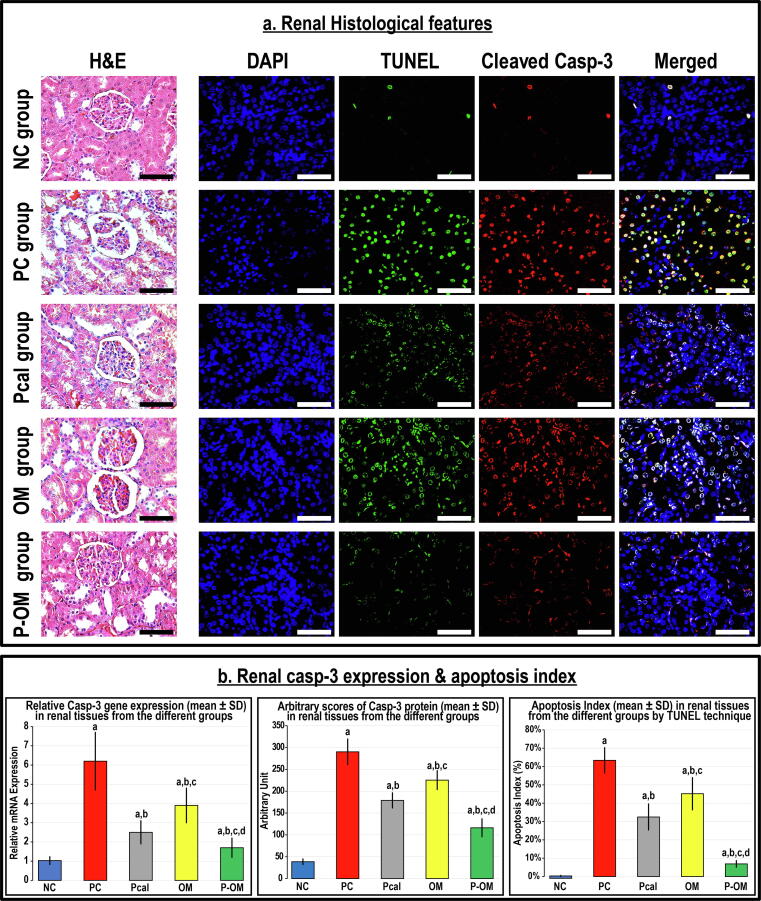
Renal histological features
The NC renal tissues showed normal histology by H&E and the numbers of apoptotic bodies and the expression of Casp-3 were low (Fig. 2). The renal tissues from the PC group exhibited extensive damage that was depicted via membrane disruption and cupping of Bowman's capsule, disintegration and shrinkage of glomerular capillaries, and tubular destruction and fragmentation (Fig. 2A). The Casp-3 mRNA and protein expression and numbers of apoptotic bodies were also significantly higher in the PC renal specimens than the controls (Fig. 2B).
Fig. 2.
(a) Renal histological features by H&E together with the immunofluorescence co-localisation of apoptotic bodies by TUNEL’s technique (green) with cleaved Casp-3 (red) and counterstaining with DAPI in the renal tissue specimens from all the study groups (40 × objective; scale bar = 10 µm). Moreover, (b) the relative expression of Casp-3 mRNA and protein alongside apoptosis index in the renal tissues from all groups are displayed as graph bars (data is shown as mean ± SD; a = P < 0.05 compared with the NC group; b = P < 0.05 compared with the PC group, c = P < 0.05 compared with the Pcal group and d = P < 0.05 compared with the OM group).
Both monotherapies lessened the histological features of glomerular and tubular damage, alongside decreased the Casp-3 mRNA and protein expression and the apoptosis index relative to the PC group (Fig. 2). However, the apoptosis index and the Casp-3 mRNA and protein were markedly elevated higher in the monotherapy groups than the NC group. Additionally, Pcal monotherapy showed better improvements than the OM group as indicated by the gene and protein expression of Casp-3 and apoptosis index. Alternatively, the co-therapy protocol revealed the utmost preservation of renal tissue morphology, the lowest apoptosis index, and markedly diminished the Casp-3 mRNA and protein expression in comparison with the PC, Pcal and OM groups. However, the apoptosis index and the mRNA and protein amounts of Casp-3 in the P-OM group were markedly elevated than the NC renal tissues (Fig. 2). Furthermore, the apoptosis index alongside the Casp-3 mRNA and protein showed strong positive and negative correlations with the serum and urine biochemical markers (Table 2).
Table 2.
Results of correlation analysis using Pearson’s test for renal apoptosis index in addition to the relative mRNA and protein expression of Casp-3 with the serum and urine concentrations of biochemical parameters at euthanasia (week-9 post treatment).
| Apoptosis index (%) | Relative Casp-3 mRNA expression | Relative Casp-3 protein expression | |
|---|---|---|---|
| Fasting blood glucose (mmol/L) | 0.857* | 0.849* | 0.883* |
| Serum insulin (μU/mL) | −0.760* | −0.663* | −0.822* |
| Total Cholesterol (mmol/L) | 0.856* | 0.800* | 0.878* |
| LDL (mmol/L) | 0.886* | 0.811* | 0.927* |
| HDL (mmol/L) | −0.797* | −0.681* | −0.847* |
| Triglycerides (mmol/L) | 0.915* | 0.797* | 0.917* |
| Serum creatinine (mg/dL) | 0.723* | 0.765* | 0.705* |
| Serum urea (mg/dL) | 0.805* | 0.695* | 0.824* |
| Serum total protein (g/dL) | −0.753* | −0.604* | −0.801* |
| 24hr Urine volume (ml) | 0.921* | 0.816* | 0.931* |
| 24hr urine creatinine (mg/dL) | −0.767* | −0.692* | −0.728* |
| 24hr Creatinine clearance (mL/min) | −0.739* | −0.602* | −0.724* |
| 24hr urine total protein (g/dL) | 0.937* | 0.786* | 0.923* |
* = P < 0.0001.
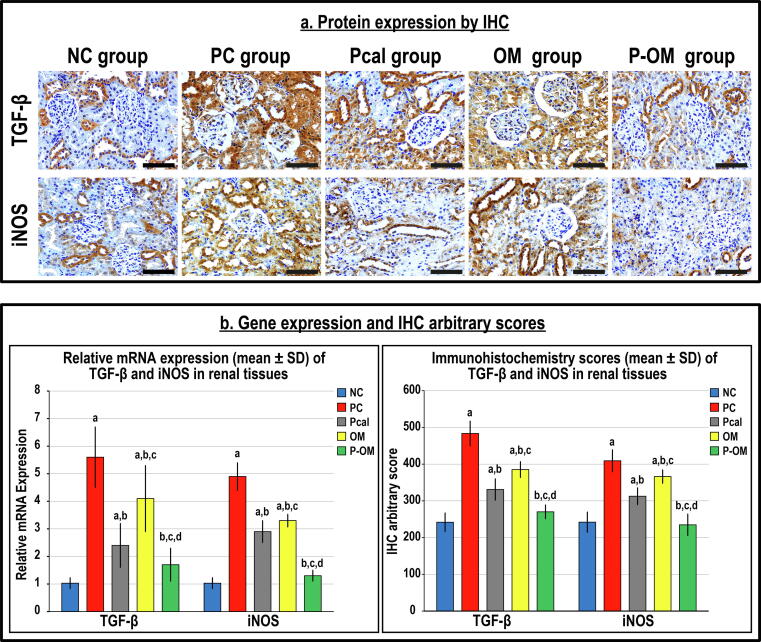
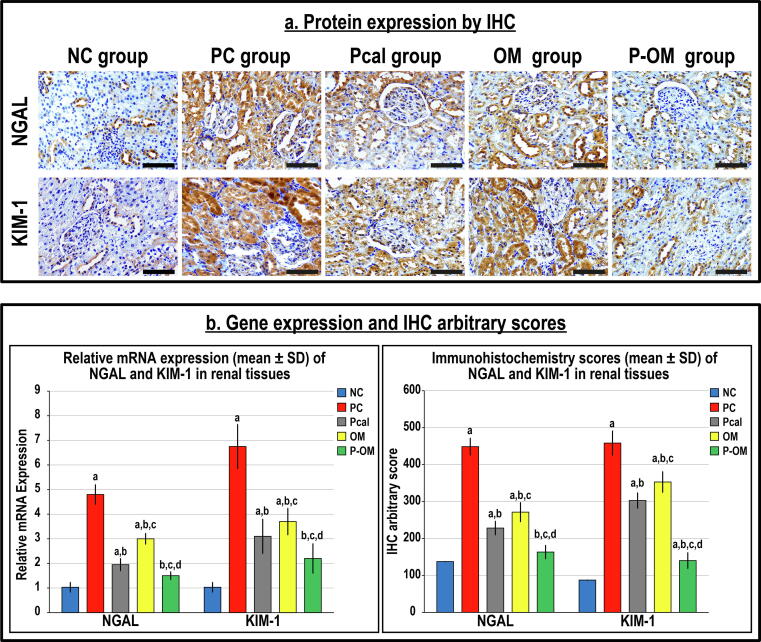
Renal tissue damage markers
The TGF-β1 with iNOS (Fig. 3) and NGAL with KIM-1 (Fig. 4) mRNAs were detected in the NC renal tissues, whilst their proteins were mainly localised in the cytoplasm of tubular epithelium by IHC. The PC group showed significant increases in the gene expression of TGF-β1 (5.5-fold), iNOS (5-fold), NGAL (4.5-fold) and KIM-1 (>6-fold) compared with the NC group (Fig. 3B & Fig. 4B). The TGF-β1 (1.8-fold), iNOS (1.6-fold), NGAL (4-fold) and KIM-1 (5-fold) proteins were also significantly elevated in the PC group than controls (Fig. 3B & Fig. 4B).
Fig. 3.
(a) Immunohistochemistry (IHC) localisation of TGF-β and iNOS in renal tissues (40 × objective; scale bar = 10 µm) alongside (b) their relative mRNA expression and IHC scores in the different study groups are displayed as graph bars (data is shown as mean ± SD; a = P < 0.05 compared with the NC group; b = P < 0.05 compared with the PC group, c = P < 0.05 compared with the Pcal group and d = P < 0.05 compared with the OM group).
Fig. 4.
(a) Immunohistochemistry (IHC) localisation of NGAL and KIM-1 in renal tissues (40 × objective; scale bar = 10 µm) alongside (b) their relative mRNA expression and IHC scores in the different study groups are displayed as graph bars (data is shown as mean ± SD; a = P < 0.05 compared with the NC group; b = P < 0.05 compared with the PC group, c = P < 0.05 compared with the Pcal group and d = P < 0.05 compared with the OM group).
While both monotherapy groups demonstrated major reductions in the genes and proteins of the targeted molecules compared with the PC group, the levels remained substantially higher than the NC group (Fig. 3 & Fig. 4). Moreover, the mRNAs and proteins of the targeted molecules were considerably lower in the Pcal group than the OM group. Although the P-OM group showed the lowest significant mRNA and protein amounts of TGF-β1, iNOS, NGAL and KIM-1 compared with the PC, OM and Pcal groups, the expression profiles were persistently higher than the NC group, except for iNOS and NGAL (Fig. 3 & Fig. 4).
Renal tissue concentrations of oxidative stress and inflammatory markers
The IL1β, IL6 and TNF-α concentrations escalated, whereas IL10 diminished, significantly in the PC tissue homogenates than the NC group (Table 3). Concurrently, the tissue amounts of MDA, H2O2 and protein carbonyl groups increased in the PC group and coincided with substantial reductions in GSH, SOD1, CAT, GPx1 and GR relative to the NC group (Table 3). While the levels of pro-inflammatory and pro-oxidative stress molecules declined, the amounts of anti-inflammatory and anti-oxidative markers increased, considerably in the Pcal and OM monotherapies in comparison with the PC group. However, all the markers, except GR, were markedly abnormal in the monotherapy groups relative to the controls (Table 3). Moreover, the levels of the targeted oxidative stress and inflammatory markers were comparable between the OM and Pcal groups. On the other hand, the dual therapy regimen resulted in the supreme marked declines of the targeted pro-inflammatory and oxidative stress markers alongside the maximal significant increases in the anti-inflammatory and anti-oxidant molecules compared with the PC and both single therapy groups. However, the levels of IL1β, H2O2 and protein carbonyls remained markedly higher in the P-OM than the NC groups (Table 3).
Table 3.
Renal tissue Concentrations (mean ± SD) of cytokines and oxidative stress markers in the study groups at euthanasia (week-9 post treatment).
| NC group | NC group | PC Group | PCal Group | OM group | |
|---|---|---|---|---|---|
| TNF-α (pg/mL)* | 34.2 ± 5.3 | 105.9 ± 14.7b | 62.4 ± 19.1a,d | 75.3 ± 22.9b,c | 35.2 ± 7.4d,e,g |
| IL1β (pg/mL)** | 35.8 ± 9.6 | 274.1 ± 32.7b | 176.4 ± 32.6b,d,f | 231.5 ± 24.1b,c,f | 97.7 ± 28.6b,d,f,g |
| IL6 (pg/mL)** | 74.2 ± 17.8 | 2267 ± 37b | 203.8 ± 27.3b,d | 234.7 ± 22.4b | 91.3 ± 23.7d,f,g |
| IL10 (pg/mL)* | 50.4 ± 14.6 | 11.6 ± 2.9b | 29.6 ± 8.8a,d | 21.2 ± 3.3b,d | 42 ± 7.9d,e,g |
| GSH (mg/g)** | 41.9 ± 6.6 | 18.7 ± 7.7b | 29.6 ± 4.4b,c | 25.3 ± 5.4b,c | 38.6 ± 6.3d,e,f |
| SOD1 (U/g)** | 48.3 ± 6.2 | 26.6 ± 4.3b | 33.9 ± 3.8b,c | 30.8 ± 3.7b,c | 42.5 ± 3.6d,f,h |
| CAT (U/mg)** | 288 ± 19.4 | 194.4 ± 24.7b | 229.4 ± 27.9b,c | 225.7 ± 20.7b,c | 267.9 ± 20.6d,e,g |
| GPx1 (µg/mg)** | 4.6 ± 1.2 | 2.7 ± 0.9a | 3.5 ± 0.5a,c | 3.3 ± 0.4a,c | 4.1 ± 0.9d |
| GR (µg/mg)** | 26.4 ± 4.4 | 16.5 ± 4.2 | 20.2 ± 3.4 | 21.1 ± 4.5 | 22.6 ± 6.7 |
| MDA (nmol/g)** | 29.9 ± 5.1 | 60.9 ± 6.8b | 40.6 ± 7.4a,d | 49.1 ± 8.8b,c | 35.7 ± 5.6d,g |
| H2O2 (μM/g)* | 1.1 ± 0.2 | 71.1 ± 10.2b | 52.5 ± 6b,d | 61.8 ± 5.2b,c | 17.3 ± 5.4b,d,f,g |
| Protein Carbonyl groups (nmol/g)* | 0.42 ± 0.08 | 6.9 ± 0.35b | 4.66 ± 0.55b,d | 5.22 ± 0.43b,d,e | 2.23 ± 0.83b,d,f,g |
* = Games-Howell post-hoc test was used following ANOVA to compare between the groups.
** = Tukey’s HSD post-hoc test was used following ANOVA to compare between the groups.
TNF-α = Tumour necrosis factor-α IL1β = Interleukin-1β IL6 = Interleukin-6.
IL10 = Interleukin-10 GSH = Glutathione SOD1 = Superoxide dismutase 1.
CAT = Catalase GPx1 = Glutathione peroxidase-1 GR = Glutathione reductase.
MDA = Malondialdehyde H2O2 = Hydrogen peroxide.
a = P < 0.05 compared with NC group b = P < 0.01 compared with NC group c = P < 0.05 compared with PC group.
d = P < 0.01 compared with PC group e = P < 0.05 compared with Pcal group f = P < 0.01 compared with Pcal group.
g = P < 0.01 compared with OM group.
Discussion
This study explored the remedial effects of Pcal and ω-3 single and dual therapies against DN in respect to metabolic control, oxidative stress, and inflammation. DN was confirmed before therapy initiation by elevated FBG, polyuria, proteinuria and decreased urine Cr levels in all groups compared with the NC group. At euthanasia, the PC group showed hyperglycaemia, dyslipidaemia, low insulin, and abnormal renal biochemical parameters. Moreover, substantial tissue damage, profuse apoptosis and elevated TGF-β, iNOS, NGAL, KIM-1 and Casp-3 mRNAs and proteins were detected in the PC renal tissues than the NC group. The apoptosis index and the Casp-3 mRNA and protein also correlated directly with FBG, cholesterol, triglycerides, LDL, Cr, and urea, whilst they linked inversely with insulin, HDL, total protein, and 24hr-U Cr. Concurrently, the oxidative stress (MDA, H2O2 & protein carbonyl groups) and inflammatory (TNF-α, IL1β & IL6) markers increased, whereas the anti-oxidant (GSH, SOD1, CAT, GPx1 & GR) and anti-inflammatory (IL10) molecules decreased, in the PC renal tissues than the NC group.
Consistent with our data, DN is characterised by albuminuria and decreased GFR as well as is associated with increased NGAL and KIM-1 levels that highly correlate with the progression of renal damage, even during the early normoalbuminuric stage [1], [2], [3], [4]. At the molecular level, chronic hyperglycaemia and lipotoxicity induce renal mitochondrial damage and upregulate iNOS, thus increasing the levels of ROS and RNS with successive lipid peroxidation and protein carbonylation [5], [6], [7], [8]. DM-induced renal redox dyshomeostasis also involves declines in GPx1, SOD1, GR and CAT alongside GSH levels [9], [10]. Hyperglycaemia and dyslipidaemia likewise promote chronic inflammation by increasing TNF-α, IL1β and IL6 combined with a marked decrease in the anti-inflammatory cytokine, IL10 [11], [12]. Persistent oxidative stress and inflammation then contribute to glomerulopathy and tubulointerstitial damage by increasing TGF-β that activates the main executor of apoptosis, Casp-3 [13], [14]. Our data agrees with many previous reports and sustains the notion that the development and progression of DN occur by interlaced pathogenic processes involving chronic hyperglycaemia, dyslipidaemia, oxidative stress and inflammation [9], [10], [11], [12].
The standard anti-diabetic therapies have limited efficacy against DN progression and a better strategy would include drugs that could simultaneously enhance glycaemic control, reduce lipotoxicity and directly hinder renal oxidative stress and inflammation [1], [2]. Pcal, which is a synthetic active VD-analogue, inhibited β-cells apoptosis and increased insulin production in rat by attenuating STZ-induced pancreatic oxidative stress and inflammation [32]. Pcal also improved renal damage by impeding ROS production and decreasing TNF-α and Casp-3, whilst promoting several renal antioxidant and anti-inflammatory pathways [18]. Congruently, Pcal exhibited similar ameliorative actions against DN in mice [33] and clinical studies [17], [18], [19] as well as lowered NGAL levels following the modulation of pro-inflammatory molecules in patients with chronic kidney disease [34]. Similarly, many clinical trials revealed favourable metabolic and nephroprotective effects for ω-3 that were associated with increased total anti-oxidant capacity in diabetic patients with coexisting coronary artery disease [22] or nephropathy [21]. Equally, ω-3 also showed anti-diabetic, anti-oxidative stress and anti-inflammatory actions, thus decreased albuminuria and delayed DN progression in rats [20], [23].
Herein, FBG, cholesterol, LDL, triglycerides, Cr, urea, and proteinuria declined significantly with both monotherapies compared with the PC group. Pcal and ω-3 were also associated with higher serum insulin and HDL alongside increased urine Cr levels relative to the PC group. Furthermore, both monotherapies displayed moderately preserved renal tissue morphology, markedly decreased Casp-3 mRNA and protein, and lowered the apoptosis index than the PC group. The levels of renal anti-oxidant and anti-inflammatory molecules in the monotherapy groups also increased significantly and coincided with marked decreases in the oxidative stress and inflammatory markers, TGF-β, iNOS, NGAL and KIM-1 than the PC group. Our results correlate with many studies and accentuates the potential values of Pcal [17], [18], [19] and ω-3 [20], [21], [22], [23] in the treatment of DM, which could involve enhanced metabolic control besides anti-oxidative and anti-inflammatory renoprotective effects.
However, the Pcal group showed better renal morphology, less apoptotic cells, and lower TGF-β, iNOS, Casp-3, NGAL and KIM-1 expression than the OM group. Our data suggests that Pcal could exert extra renoprotective actions than ω-3, which might involve the regulation of renal haemodynamic by inhibiting renin activity [35], improving endothelial function [36] and/or reducing atherosclerosis [37]. Although there is no report in the literature related to the Pcal effects on dyslipidaemia as well as the renal expression of TGF-β and iNOS in relation to DN, Pcal attenuated renal fibrosis by decreasing TGF-β [38]. Moreover, the Pcal anti-inflammatory and anti-oxidative renoprotective actions in uremic rats occurred with significant declines in iNOS [39]. Interestingly, Pcal treatment in mice deficient in apolipoprotein-E markedly increased the cardiac adiponectin levels [40], a key regulator of glucose and lipid metabolism that improves insulin resistance, triglycerides catabolism and uptake of free fatty acids [41]. Moreover, adiponectin inhibited lipotoxicity-induced pancreatic β-cells apoptosis [42], alleviated nitrative stress in aorta by inhibiting iNOS [43], and attenuated oxidative stress and decreased renal TGF-β in diabetic rats [44]. Hence, we speculate that Pcal could surpass ω-3 in delaying DN progression by promoting renal adiponectin [40] alongside decreasing renal iNOS [39], thus reducing renal lipotoxicity, oxidative stress and inflammation that consequently inhibit TGF-β and Casp-3 [38], [39]. However, additional studies are needed for exploring the Pcal and ω-3 effects on renal haemodynamic and the expression of adiponectin with its receptors in relation to glucose and lipid metabolism to validate our suggestions.
While both monotherapies were moderate and the biomarkers were persistently abnormal than the NC group, the co-therapy protocol showed better efficacies in alleviating hyperglycaemia, hyperlipidaemia, proteinuria, oxidative stress, inflammation, tissue damage and apoptosis. Moreover, the serum biomarkers of renal functions alongside the tissue oxidative stress and inflammatory markers were equal between the P-OM and NC groups. At present, none of the previous studies investigated the potential effects of Pcal and ω-3 dual therapy against major chronic diseases, including DM. In contrast, the VITAL-DKD clinical trial is the only study that explored the renoprotective effects of VD (cholecalciferol) and/or ω-3 supplements in diabetic patients, and the outcomes of all protocols were equal to placebo [45]. An explication for the differences between our data and the VITAL-DKD study could be related to the efficacy of the used VD analogues in triggering VDR-mediated renoprotection since cholecalciferol is a non-active VD, whereas Pcal is an active analogue that potently stimulates VDR [46]. The doses in the VITAL-DKD trial were also markedly lower than those used by many of the clinical studies that showed favourable renoprotective actions for cholecalciferol (2000 IU/day vs. ≥ 7000 IU/day) [47], [48] or ω-3 (1 g/day vs. 4 g/day) [22], [29]. Hence, we speculate that adding high daily doses of ω-3 (4 g/day) to natural or synthetic VDR-agonists (e.g. calcitriol or Pcal), could provide a propitious approach against DN than combining low doses of cholecalciferol and ω-3. Moreover, Pcal could be a better option than calcitriol for avoiding hypercalcaemia and tissue calcification [15].
Our data, however, revealed that FBG, lipid profile, proteinuria, apoptosis index and the tissue damage biomarkers were markedly higher in the P-OM group than controls. Our observations infer that adding other anti-diabetic drugs to the Pcal/ω-3 co-therapy could be compulsory to efficiently control hyperglycaemia and might produce a superlative strategy against DN. Recently, the American Diabetes Association and the European Association for the Study of Diabetes have endorsed the use of glucagon-like peptide 1 (GLP-1) receptor agonists or sodium-glucose cotransporter-2 (SGLT2) inhibitors for glycaemic control in diabetic patients with signs of progressive nephropathy [2]. Therefore, future studies should explore the anti-diabetic and renoprotective effects of adding Pcal and/or ω-3 with GLP-1 receptor agonists and SGLT2 inhibitors for treating DM and its complications.
In conclusion, both Pcal and ω-3 single therapies exhibited limited glycaemic and lipidemic control and were associated with moderate nephroprotection by attenuating renal tissue oxidative stress and inflammation. In contrast, Pcal and ω-3 fatty acids co-therapy appears to be a more effective strategy against DN progression, and the enhanced renoprotective effects may include improved regulations of glucose and lipid metabolism alongside boosted renal anti-oxidative and anti-inflammatory mechanisms. Nevertheless, this study did not measure the effects of the drugs on renal haemodynamic, GFR as well as the renal molecular pathways involved in glucose and lipid homeostasis. Further studies are, therefore, still needed to measure the effects of Pcal and/or ω-3 fatty acids on renal blood flow, glomerular and tubular functions in addition to the regulation of renal metabolic pathways. Moreover, future studies should also explore the potential beneficial effects of combining Pcal and/or ω-3 with clinically approved anti-diabetic drugs for averting DN development and progression.
Compliance with Ethics Requirement
Approval (AMSEC 24/11-11-19) was obtained from the Committee for the Care and Use of Laboratory Animals at Umm Al-Qura University prior to study initiation.
CRediT authorship contribution statement
Mohamed El-Boshy: Conceptualization, Funding acquisition, Resources, Project administration, Data curation, Writing – original draft, Supervision. Aiman Alsaegh: Methodology, Formal analysis, Investigation. Ahmed H. Qasem: Methodology, Formal analysis, Investigation. Ramya A. Sindi: Methodology, Formal analysis, Investigation. Abdelghany H. Abdelghany: Methodology, Formal analysis, Investigation. Hossam Gadalla: Formal analysis, Investigation. Doha Reda: Formal analysis, Investigation. Firas Azzeh: Conceptualization, Methodology, Funding acquisition, Resources, Project administration, Data curation. Shakir Idris: Methodology. Jawwad Ahmad: Methodology. Bassem Refaat: Conceptualization, Writing – original and revised manuscript, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank the Deanship of Scientific Research in Umm Al-Qura University for supporting this work by Grant Code: 19-MED-1-01-0021.
Funding
This work was supported by the Deanship of Scientific Research in Umm Al-Qura University [Grant Code: 19-MED-1-01-0021]. The funding organisation was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.08.010.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Hua F. New insights into diabetes mellitus and its complications: a narrative review. Ann Transl Med. 2020;8(24):1689. doi: 10.21037/atm-20-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C., D’Alessio D.A., Davies M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Carvalho J.A.M., Tatsch E., Hausen B.S., Bollick Y.S., Moretto M.B., Duarte T., et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem. 2016;49(3):232–236. doi: 10.1016/j.clinbiochem.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Quang T.H., Nguyet M.P., Thao D.P., Thi M.H., Dam L., Thi H.H., Van A.P., Luong T.C., Tuyet M.N.T., Duy Q.D., Nhu B.D., Duc T.N. Evaluation of urinary neutrophil gelatinase associated lipocalin and kidney injury molecule-1 as diagnostic markers for early nephropathy in patients with type 2 diabetes mellitus. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2020;13:2199–2207. doi: 10.2147/DMSO.S258678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton R.C. Role of glucose metabolism and mitochondrial function in diabetic kidney disease. Curr DiabRep. 2021;21(2):6. doi: 10.1007/s11892-020-01372-2. [DOI] [PubMed] [Google Scholar]
- 6.Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 7.Taslimi Parham, Gulçin İlhami. Antioxidant and anticholinergic properties of olivetol. J Food Biochem. 2018;42(3):e12516. doi: 10.1111/jfbc.2018.42.issue-310.1111/jfbc.12516. [DOI] [Google Scholar]
- 8.Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 9.Sagoo M.K., Gnudi L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radical Biol Med. 2018;116:50–63. doi: 10.1016/j.freeradbiomed.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Wei Pascal Zhongping, Fung Winston Wing-Shing, Ng Jack Kit-Chung, Lai Ka-Bik, Luk Cathy Choi-Wan, Chow Kai Ming, et al. Metabolomic Changes of Human Proximal Tubular Cell Line in High Glucose Environment. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-53214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangoi Manuela, Carvalho José, Guarda Naiara, Duarte Thiago, Duarte Marta, Premaor Melissa, et al. Association between Urinary Levels of Interleukin-6, Interleukin-10 and Tumor Necrosis Factor-Alpha with Glomerular and Tubular Damage Indicators in Patients with Type 2 Diabetes. Clin Lab. 2019;65(11/2019) doi: 10.7754/Clin.Lab.2019.190410. [DOI] [PubMed] [Google Scholar]
- 12.Araújo Liliane Silvano, Torquato Bianca Gonçalves Silva, da Silva Crislaine Aparecida, dos Reis Monteiro Maria Luíza Gonçalves, dos Santos Martins Ana Luisa Monteiro, da Silva Marcos Vinícius, et al. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020;21(1) doi: 10.1186/s12882-020-01960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.B., Zhu H., Song W., Su J.H. Gremlin Regulates Podocyte Apoptosis via Transforming Growth Factor-β (TGF-β) Pathway in Diabetic Nephropathy. Med Sci Monitor: Int Med J Exp Clin Res. 2018;24:183–189. doi: 10.12659/MSM.905758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L.S., Li J., Jia-Ping L. Rhein-8-O-β-D-glucopyranoside inhibited high glucose-induced apoptosis of human mesangial cells by regulating the lincRNA ANRIL/let-7a/TGF-β1/Smad signaling pathway. Exp Therap Med. 2020;19(4):2871–2878. doi: 10.3892/etm.2020.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslam Akhmed, Ahmad Jawwad, Baghdadi Mohammed A., Idris Shakir, Almaimani Riyad, Alsaegh Aiman, et al. Chemopreventive effects of vitamin D(3) and its analogue, paricalcitol, in combination with 5-fluorouracil against colorectal cancer: The role of calcium signalling molecules. Biochim Biophys Acta, Mol Basis Dis. 2021;1867(3):166040. doi: 10.1016/j.bbadis.2020.166040. [DOI] [PubMed] [Google Scholar]
- 16.El‐Boshy Mohamed, Refaat Bassem, Almaimani Riyad A., Abdelghany Abdelghany H., Ahmad Jawwad, Idris Shakir, et al. Vitamin D(3) and calcium cosupplementation alleviates cadmium hepatotoxicity in the rat: Enhanced antioxidative and anti-inflammatory actions by remodeling cellular calcium pathways. J Biochem Mol Toxicol. 2020;34(3) doi: 10.1002/jbt.v34.310.1002/jbt.22440. [DOI] [PubMed] [Google Scholar]
- 17.Parvanova A., Trillini M., Podestà M.A., Iliev I.P., Ruggiero B., Abbate M., et al. Moderate salt restriction with or without paricalcitol in type 2 diabetes and losartan-resistant macroalbuminuria (PROCEED): a randomised, double-blind, placebo-controlled, crossover trial. The lancet Diab Endocrinol. 2018;6(1):27–40. doi: 10.1016/S2213-8587(17)30359-5. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed Osama M., Ali Tarek M., Abdel Gaid Mohamed A., Elberry Ahmed A., Mukhopadhyay Partha. Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, p53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. PLoS ONE. 2019;14(9):e0214349. doi: 10.1371/journal.pone.021434910.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster A., Al-Makki A., Shepler B. Use of paricalcitol as adjunctive therapy to renin-angiotensin-aldosterone system inhibition for diabetic nephropathy: a systematic review of the literature. Clin Ther. 2019;41(11):2416–2423. doi: 10.1016/j.clinthera.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 20.De Assis A.M., Rech A., Longoni A., Da Silva Morrone M., De Bittencourt Pasquali M.A., Perry M.L., Souza D.O., Moreira J.C. Dietary n-3 polyunsaturated fatty acids revert renal responses induced by a combination of 2 protocols that increase the amounts of advanced glycation end product in rats. Nutr. Res. (New York, NY) 2015;35:512–522. doi: 10.1016/j.nutres.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Soleimani A., Taghizadeh M., Bahmani F., Badroj N., Asemi Z. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. (Edinburgh, Scotland) 2017;36(1):79–84. doi: 10.1016/j.clnu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Elajami Tarec K., Alfaddagh Abdulhamied, Lakshminarayan Dharshan, Soliman Michael, Chandnani Madhuri, Welty Francine K. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. Journal of the American Heart Association. 2017;6(7) doi: 10.1161/JAHA.116.004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitlov Uljević Marija, Starčević Kristina, Mašek Tomislav, Bočina Ivana, Restović Ivana, Kević Nives, et al. Dietary DHA/EPA supplementation ameliorates diabetic nephropathy by protecting from distal tubular cell damage. Cell Tissue Res. 2019;378(2):301–317. doi: 10.1007/s00441-019-03058-y. [DOI] [PubMed] [Google Scholar]
- 24.Barrière David André, Noll Christophe, Roussy Geneviève, Lizotte Farah, Kessai Anissa, Kirby Karyn, et al. Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep. 2018;8(1) doi: 10.1038/s41598-017-18896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oraby Mamdouh A., El-Yamany Mohammed F., Safar Marwa M., Assaf Naglaa, Ghoneim Hamdy A. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother. 2019;109:910–920. doi: 10.1016/j.biopha.2018.10.100. [DOI] [PubMed] [Google Scholar]
- 26.Nørgaard Sisse A, Søndergaard Henrik, Sørensen Dorte B, Galsgaard Elisabeth D, Hess Constanze, Sand Fredrik W. Optimising streptozotocin dosing to minimise renal toxicity and impairment of stomach emptying in male 129/Sv mice. Lab Anim. 2020;54(4):341–352. doi: 10.1177/0023677219872224. [DOI] [PubMed] [Google Scholar]
- 27.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Yucheng, Qian Jiaqi, Chen Nan, Huang Zhaoxing, Jiang Gengru, Li Xuewang, et al. Efficacy and initial dose determination of paricalcitol for treatment of secondary hyperparathyroidism in Chinese subjects. Clin Nephrol. 2014;81(01):20–29. doi: 10.5414/CN107762. [DOI] [PubMed] [Google Scholar]
- 29.Han Eugene, Yun Yujung, Kim Gyuri, Lee Yong-ho, Wang Hye Jin, Lee Byung-Wan, et al. Effects of omega-3 fatty acid supplementation on diabetic nephropathy progression in patients with diabetes and hypertriglyceridemia. PLoS ONE. 2016;11(5):e0154683. doi: 10.1371/journal.pone.015468310.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre C., Buesen R., Riffle B., Wandelt C., Sottosanto J.B., Marxfeld H., et al. Safety assessment of EPA+DHA canola oil by fatty acid profile comparison to various edible oils and fat-containing foods and a 28-day repeated dose toxicity study in rats. Food Chem Toxicol: Int J Published British Industrial Biol Res Assoc. 2019;124:168–181. doi: 10.1016/j.fct.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Refaat B., Abdelghany A.H., BaSalamah M.A., El-Boshy M., Ahmad J., Idris S. Acute and chronic iron overloading differentially modulates the expression of cellular iron-homeostatic molecules in normal rat kidney. J Histochemistry Cytochemistry: Off J Histochemistry Soc. 2018 doi: 10.1369/0022155418782696. 22155418782696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali T.M., El Esawy B., Elaskary A. Effect of paricalcitol on pancreatic oxidative stress, inflammatory markers, and glycemic status in diabetic rats. Irish J Med Sci. 2018;187(1):75–84. doi: 10.1007/s11845-017-1635-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakhoul N., Thawko T., Farber E., Dahan I., Tadmor H., Nakhoul R., et al. The therapeutic effect of active vitamin D supplementation in preventing the progression of diabetic nephropathy in a diabetic mouse model. J Diab Res. 2020;2020:7907605. doi: 10.1155/2020/7907605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucisano S., Arena A., Stassi G., Iannello D., Montalto G., Romeo A., et al. Role of paricalcitol in modulating the immune response in patients with renal disease. Int J Endocrinol. 2015;2015 doi: 10.1155/2015/765364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung Sungjin, Kim Soojeong, Kim Minyoung, Koh Eun Sil, Shin Seok Joon, Park Cheol Whee, et al. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS ONE. 2017;12(7):e0181757. doi: 10.1371/journal.pone.018175710.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundwall K., Jacobson S.H., Jörneskog G., Spaak J. Treating endothelial dysfunction with vitamin D in chronic kidney disease: a meta-analysis. BMC Nephrol. 2018;19(1):247. doi: 10.1186/s12882-018-1042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giakoumis M., Tsioufis C., Dimitriadis K., Sonikian M., Kasiakogias A., Andrikou E., et al. Effects of oral paricalcitol therapy on arterial stiffness and osteopontin in hypertensive patients with chronic kidney disease and secondary hyperparathyroidism. Hellenic J Cardiol: HJC = Hellenike kardiologike epitheorese. 2019;60(2):108–113. doi: 10.1016/j.hjc.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Salanova Villanueva L., Gil Giraldo Y., Santos Sánchez-Rey B., Aguilera Peralta A. Paricalcitol regulatory effect on inflammatory, fibrotic and anticalcificating parameters in renal patiente. Far beyond mineral bone disease regulation. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2020;40(2):171–179. doi: 10.1016/j.nefro.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Finch Jane L., Suarez Edu B., Husain Kazim, Ferder Leon, Cardema Michelle C., Glenn Denis J., et al. Effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria, and renal oxidative stress in uremic rats. American journal of physiology. Renal physiology. 2012;302(1):F141–F149. doi: 10.1152/ajprenal.00293.2011. [DOI] [PubMed] [Google Scholar]
- 40.Suarez-Martinez Edu, Husain Kazim, Ferder Leon. Adiponectin expression and the cardioprotective role of the vitamin D receptor activator paricalcitol and the angiotensin converting enzyme inhibitor enalapril in ApoE-deficient mice. Therap Adv Cardiovasc Dis. 2014;8(6):224–236. doi: 10.1177/1753944714542593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanai Hidekatsu, Yoshida Hiroshi. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019;20(5):1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye R., Wang M., Wang Q.A., Scherer P.E. Adiponectin-mediated antilipotoxic effects in regenerating pancreatic islets. Endocrinology. 2015;156(6):2019–2028. doi: 10.1210/en.2015-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai X., Li X., Li L., Huang X.Z., Liu Y.S., Chen L., et al. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE-/- mice: roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol Med Rep. 2015;11(3):1715–1721. doi: 10.3892/mmr.2014.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan F., Liu Y.H., Liu F.Y., Peng Y.M., Tian J.W. Intraperitoneal administration of the globular adiponectin gene ameliorates diabetic nephropathy in Wistar rats. Mol Med Rep. 2014;9(6):2293–2300. doi: 10.3892/mmr.2014.2133. [DOI] [PubMed] [Google Scholar]
- 45.de Boer I.H., Zelnick L.R., Ruzinski J., Friedenberg G., Duszlak J., Bubes V.Y., et al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2019;322(19):1899–1909. doi: 10.1001/jama.2019.17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stancu S., Chiriac C., Maria D.T., Mota E., Mircescu G., Capusa C. Nutritional or active vitamin D for the correction of mineral metabolism abnormalities in non-dialysis chronic kidney disease patients? Acta Endocrinol (Bucharest, Romania: 2005) 2018;14(4):505–513. doi: 10.4183/aeb.2018.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felício João Soares, Oliveira Alana Ferreira de, Peixoto Amanda Soares, Souza Ana Carolina Contente Braga de, Abrahão Neto João Felício, de Melo Franciane Trindade Cunha, et al. Albuminuria reduction after high dose of vitamin D in patients with type 1 diabetes mellitus: a pilot study. Front Endocrinol. 2017;8 doi: 10.3389/fendo.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esfandiari A., Pourghassem Gargari B., Noshad H., Sarbakhsh P., Mobasseri M., Barzegari M., et al. The effects of vitamin D(3) supplementation on some metabolic and inflammatory markers in diabetic nephropathy patients with marginal status of vitamin D: A randomized double blind placebo controlled clinical trial. Diab Metabolic Syndr. 2019;13(1):278–283. doi: 10.1016/j.dsx.2018.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.