Abstract
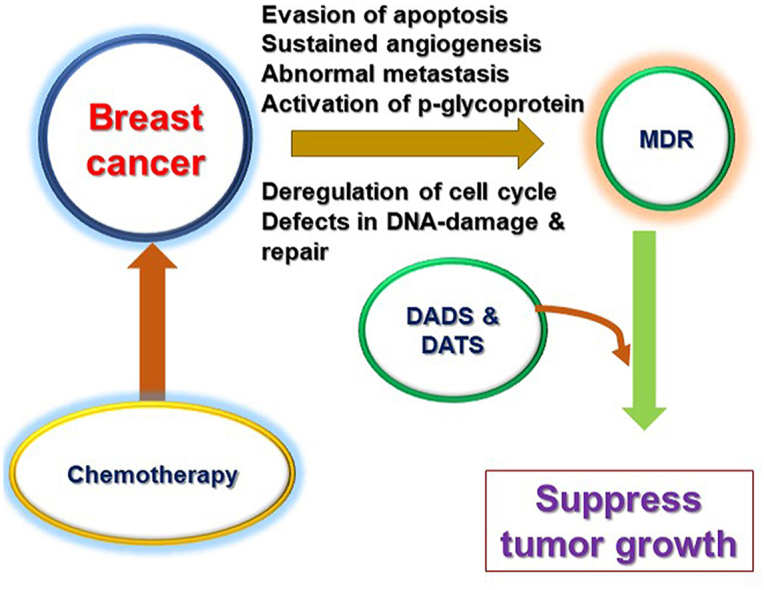
Breast cancer is one of the leading causes of cancer-related deaths in women worldwide. It is a cancer that originates from the mammary ducts and involves mutations in multiple genes. Recently, the treatment of breast cancer has become increasingly challenging owing to the increase in tumor heterogeneity and aggressiveness, which gives rise to therapeutic resistance. Epidemiological, population-based, and hospital-based case-control studies have demonstrated an association between high intake of certain Allium vegetables and a reduced risk in the development of breast cancer. Diallyl disulfide (DADS) and diallyl trisulfide (DATS) are the main allyl sulfur compounds present in garlic, and are known to exhibit anticancer activity as they interfere with breast cancer cell proliferation, tumor metastasis, and angiogenesis. The present review highlights multidrug resistance mechanisms and their signaling pathways in breast cancer. This review discusses the potential anticancer activities of DADS and DATS, with emphasis on drug resistance in triple-negative breast cancer (TNBC). Understanding the anticancer activities of DADS and DATS provides insights into their potential in targeting drug resistance mechanisms of TNBC, especially in clinical studies.
Keywords: Breast cancer, Diallyl disulfide, Diallyl trisulfide, Drug resistance, Metastasis
Graphical abstract
Highlights
-
•
The review describes the causes of drug resistance in TNBC.
-
•
The effects of DADS and DATS on drug resistance mechanisms in TNBC are presented.
-
•
The impacts of DADS and DATS on metastasis of TNBC are discussed.
-
•
Antitumor immune activities of DADS and DATS against TNBC are illustrated.
1. Introduction
Breast cancer (BC) is the most prevalent type of cancer globally, affecting approximately 2.1 million people every year and causing the most cancer-related deaths among women. The incidence of BC depends on several risk factors, including genetic predisposition, diet, age, family history, reproductive factors, lifestyle, and hormones. BC tumors are complex and exhibit a high-degree heterogeneity, with remarkably different alterations in the hormone receptor (HR) and the human epidermal growth factor receptor 2 (HER2) among tumors. The subtypes of BC include luminal cell-like BCs (luminal A and luminal B) which express receptors (estrogen receptors (ERs) and progesterone receptors), HER2+ BC, and basal-like/triple-negative BC (TNBC). Luminal A BC (HR+/HER2-) tumors are usually slow growing and are less aggressive than luminal B BC (HR+/HER2+) tumors. HER2+ SC is characterized by the increased levels of HER2+/Erb-B2 and Ki67 proliferation marker [1]. TNBC (HR−/HER2) is the most aggressive form of BC and has a poor prognosis due to the absence of HRs. TNBC patients with different molecular subtypes respond differently to treatment.
2. Drug-resistance mechanisms in TNBC
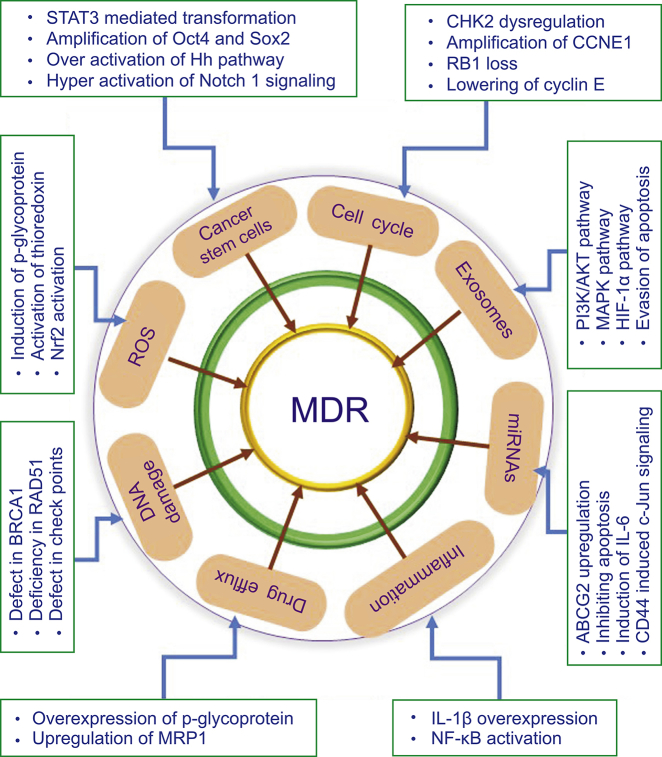
TNBC is curable, if localized at a primary site; however, the progression of primary tumors to distant organs is incurable and leads to death in patients. Chemotherapy is the most conventional approach to combating TNBC. Despite considerable developments, chemotherapy has become ineffective at combating advanced-stage BC due to multidrug resistance (MDR); therefore, treatment of TNBC patients is challenging for clinicians. In recent years, substantial efforts have been made to elucidate the molecular mechanisms of MDR in TNBC, especially to identify potential therapeutic targets [2]. These studies demonstrate that the mechanisms that confer MDR are complex and involve the tumor microenvironment (TME), drug efflux, cancer stem cells, and bulk tumor cells in multiple signaling pathways (Fig. 1). Luo et al. [3] demonstrated that the aberration in the checkpoint kinase 2 (CHK2) controls the cell cycle and imparts chemoresistance to chemotherapeutic drugs in TNBC cells. This study concluded that the Y390 mutation in CHK2 confers cisplatin (CIS) resistance by inducing apoptosis and cell cycle arrest through control of the p53/CHK2 pathway. Shu et al. [4] demonstrated that bromodomain and extra-terminal motif bromodomain inhibitors inhibit the oncogenic transcription programs by displacing the bromodomain and extra-terminal motif protein from chromatin, in order to compete with acetyl-lysine recognition modules in TNBC cells. Mani et al. [5] reported that enhanced DNA damage repair and checkpoint activation of the cell cycle are the major driving forces of drug resistance in TNBC. Prexasertib-treated TNBC cells had decreased levels of the DNA damage repair proteins, namely, BRCA1 and RAD51. Chen et al. [6] reported that the overexpression of cyclin E sensitizes TNBC cell lines and patient-derived xenograft models to Wee1 kinase inhibitors by inducing replication stress, indicating that drug resistance in TNBCs develops through decreased cyclin E expression.
Fig. 1.
Cellular and molecular mechanisms that are involved in driving drug resistance in breast cancer. ROS: reactive oxygen species; MDR: multidrug resistance; MAPK: mitogen-activated protein kinase; HIF: hypoxia inducible factor; IL: interleukin.
Thu et al. [7] reported that the interruption of the anaphase-promoting complex results in drug resistance to the monopolar spindle 1 inhibitor, CFI-402257, by increasing tolerance to genomic instability and inactivation of the spindle assembly checkpoints. EDD E3 ubiquitin ligase, which regulates the S-phase and G2/M DNA damage checkpoints, promotes drug resistance to CIS and doxorubicin (DOX) in TNBC by activating the target of rapamycin complex 1 pathway and enhancing the expression of antiapoptotic proteins [8]. Ozawa et al. [9] demonstrated the development of drug resistance to docetaxel and DOX in non-tumorigenic breast cells by culturing these cells with exosomes from drug-resistant TNBC cells, and that the reported drug resistance was mediated by changes in the genes that promote cell proliferation, especially in the PI3K/AKT, mitogen-activated protein kinases (MAPK), and hypoxia inducible factor-1 α (HIF-1α) pathways. TNBC cells adapt to 5-fluorouracil, DOX, or docetaxel-induced apoptosis by enhancing cytoprotective autophagy through the overexpression of Bcl2-associated athanogene 3, an antiapoptotic co-chaperone [10].
In BCs, drug resistance is developed through the transformation of normal cancer cells into cancer stem cells (CSCs). For instance, MDA-MB-231 cells develop drug resistance to DOX via STAT3-dependent expressions of octamer-binding transcription factor4 (Oct4) and superoxide dismutase 2 (Sox2) [11]. Poly ADP ribose polymerase (PARP) inhibitors are used for treatment of heritable cancers. Liu et al. [12] reported that the BRCA1 mutant TNBC stem cells are resistant to PARP inhibitors. However, the silencing of RAD51 with siRNA sensitizes BRCA1 mutant TNBC stem cells to PARP inhibitors and reduces the tumor growth in xenograft models. They also reported that drug resistance to PARP inhibitors in TNBC stem cells is mediated by enhanced cell proliferation via RAD51. Chen et al. [13] demonstrated that components in conditioned media from mesenchymal stem cells, mainly IL-8, induce resistance to DOX in TNBC cells by enhancing stemness through the alteration of biological functions of BC-resistant proteins. Further, Yeh et al. [14] reported that CXCL1 present in the conditioned media from mesenchymal stem cells mediates resistance to DOX via miR-106a-dependent upregulation of ABCG2, an ATP-binding cassette transporter in TNBC. Stem cells are also associated with drug resistance in TNBC [15]. This mechanistic study revealed that caveolin-1 mediates drug resistance in stem cells via the upregulation of β-catenin/ABCG2. Recently, Ryoo et al. [16] reported the relationship between drug resistance and the expression of nuclear factor E2-related factor 2 (Nrf2), which is a key regulator of antioxidant genes and CD44 markers in TNBC stem cells. Nrf2 activation promotes drug resistance via CD44-dependent p62 signaling. TNBC stem cells develop resistance to drug-induced apoptosis through a positive feedback loop, via neurotrophin nerve growth factor-mediated overexpression of its receptors, namely, p75 neurotrophin receptor and TrkA [17]. Additionally, drug resistance is promoted by developmental pathways, such as the hedgehog pathway [18]. For example, BC cells develop resistance to tamoxifen by increased stem cell proliferation, and by the dissemination of paracrine signaling to the TME via PI3K/AKT-dependent overexpression of GLI1 [19]. A recent study showed that a subpopulation of TNBC stem cells increases chemoresistance and metastasis via Notch1 signaling [20]. Darvishi et al. [21] described NF-κB as a critical mediator of the development of TNBC resistance to tyrosine kinase inhibitors. Hypoxia, inflammation, and reactive oxygen species (ROS) in the TME are highlighted as the hidden factors that exacerbate the NF-κB-mediated drug resistance in TNBC.
Recent studies have suggested that miRNAs contribute to the drug resistance in TNBCs. For example, a study found that miR-301b promotes drug resistance in TNBC by targeting cylindromatosis, thereby inhibiting drug-induced apoptosis [22]. This study showed that miR-301b targets cylindromatosis by interacting with the 3′-UTR of cylindromatosis mRNA via NF-κB activation. However, miR-181a mediates DOX resistance by increasing metastasis via the activation of signal transducer and activator of transcription 3 (STAT3) in TNBC cells, and activation of STAT3 is reliant on NF-κB-mediated IL-6 induction [23]. Another study showed that miRNA-21, activated by matrix hyaluronan/CD44-induced c-Jun signaling, mediates drug resistance in TNBC cells [24]. The binding of hyaluronan to CD44 enhances the expression of BCL2, an anti-apoptotic protein, and the expression of inhibitors of the apoptosis family of proteins via the c-Jun signaling pathway [24]. miRNA-21 also mediates the resistance to trastuzumab by modulating epithelial-mesenchymal transition (EMT) through the IL-6/STAT3/NF-κB-dependent signaling loop, and activation of the PI3K pathway, in HER2 BC cells [25], in addition to expanding the cancer stem cell population [26].
Zhang et al. [27] demonstrated that autophagy confers resistance of TNBC to epirubicin through overexpression of p-glycoprotein and through interference with NF-κB-mediated pro-apoptosis. Yin et al. [28] found that FZD8, a Wnt receptor, and FZD8 targets, LEF1 and TCF, mediate resistance to CIS by inducing stem cell characteristics in TNBCs. Wang et al. [29] conducted a study on IL-6 dependent resistance in MDA-MB-231 cells and found that IL-6 promotes drug resistance by upregulating HIF-α via activation of STAT3 and by reducing the sensitivity of TNBC cells to drug-induced apoptosis, through the downregulation of BAX expression and the overexpression of BCL2 and drug transporters, such as p-glycoprotein and MRP1. In TNBC, PDGFRβ mediates drug resistance to inhibitors of JAK or MEK by promoting the infiltration of CD8+ T cells [30]. In addition, inflammation is associated with TNBC drug resistance. IL-1β, one of the major inflammatory cytokines, mediates resistance to CIS by upregulating the expression of survival factors via ΔNP63α, an isoform of tumor protein 63, in BC cells [31]. Redox imbalance and reprogramming of cancer cell metabolism are also responsible for drug resistance in BC. BC cells develop resistance to tamoxifen by inducing ROS stress via thioredoxin (Trx) [32]. Wang et al. [33] demonstrated that the development of resistance to DOX, paclitaxel, or carboplatin in TNBC is associated with the overexpression of B7-H4, an immunoregulatory protein, through the PTEN/PI3K/AKT pathway.
3. Allyl sulfur compounds in garlic
Currently, phytochemicals are the most suitable lead candidates for anticancer drug development. Extensive research has been conducted on the development of novel therapeutic drugs from natural products [34]. Recent investigations have demonstrated that natural products work selectively and act specifically on tumor cells by targeting multiple pathways related to BC with minimal effect on normal cells [35]. Natural anticancer products that target drug resistance may be an effective treatment approach to mitigating TNBC. Examples of these natural anticancer products include taxanes derived from Taxus brevifolia [36], vinca alkaloids from Catharanthus roseus [37], and epipodophyllotoxins from Podophyllum hexandrum [38]. Dietary phytochemicals with anticancer properties against TNBC have therefore attracted the attention of researchers.
Allium sativum (garlic) was typically used in ancient Egypt, Greece, Rome, China, and India as a medicinal herb to treat wounds, digestive problems, and infections, in addition to being used as a seasoning for foods with religious significance. As an ancient Indian medicine, garlic was consumed as a tonic and rejuvenating agent to cure cough, loss of appetite, weakness, and hemorrhoids. Hippocrates used garlic to cure leprosy, whereas Aristophanes and Galena used it for the treatment of uterine tumors. The biological activity of garlic is attributed to the presence of water-soluble γ-glutamyl S-allyl cysteine and oil-soluble allyl sulfur compounds [39]. Water-soluble compounds include S-allyl cysteine and S-allyl mercaptocysteine, whereas oil-soluble allyl sulfur compounds include diallyl sulfide, diallyl disulfide (DADS), and diallyl trisulfide (DATS) [40]. Allicin is one of the main bioactive compounds found in raw garlic cloves [41]. Allicin decomposes to diallyl sulfide, DADS, and DATS derivatives during cooking; however, in the presence of organic oils, allicin forms ajoene and vinyl dithiones [42]. The presence of different bioactive sulfur compounds in raw, preserved, or cooked garlic has made it an essentially recommended commodity from centuries.
3.1. Pharmacological importance of DADS and DATS
Recent evidence has garnered considerable interest in allyl sulfur compounds (such as DADS and DATS) owing to their potential health benefits. The chemical structures of DADS and DATS were drawn using the online version of PubChem sketcher version 2.4 to represent compounds (Fig. 2). DADS is known to have various pharmacological effects, such as antioxidant and anti-inflammatory effects. The anti-inflammatory effects of DADS include reduction of cytokine secretion and suppression of tumor necrosis factor-α (TNF-α) activity, while its antioxidant effects include enhancement of the activity of glutathione peroxidase and superoxide dismutase (SOD) [43]. DADS also has cardiovascular protective effects as it ameliorates cardiac dysfunction induced by streptozotocin, by activating Nrf2 signaling [44]. DADS inhibits thromboxane formation; therefore, it displays antiplatelet activity [45]. Additionally, DADS shows hepatoprotective activity in CCL4-treated rats as it reduced CYP2E1 protein levels, prevents the depletion of cytosolic Nrf2, and reduces phase II detoxifying enzymes [46]. Furthermore, DADS protects human chondrocytes from IL-1β induced oxidative stress and apoptosis by reducing ROS levels, increases the nuclear translocation of Nrf2, and reduces the expression of BCL2 [47]. DADS has been reported to improve lipid metabolism by blocking the expression of PCSK9 and enhancing the uptake of low-density lipoprotein through the targeting of PI3K/AKT-SREBP2 signaling [48]. DADS has also been shown to have hypoglycemic activity as it regulates purine metabolism by reducing the activity of xanthine oxidase and adenosine deaminase in diabetic rats [49].
Fig. 2.
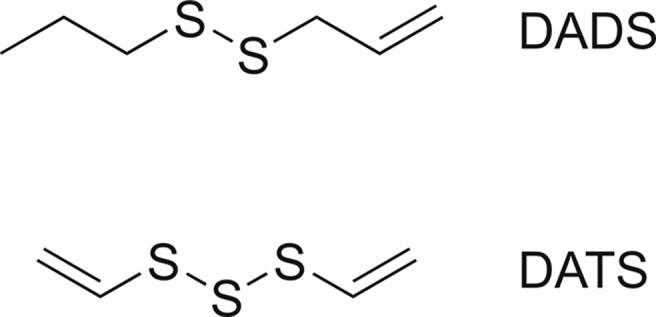
Chemical structures of diallyl disulfide (DADS) and diallyl trisulfide (DATS).
DATS is another important pharmacologically active compound found in garlic. It has been shown to have antithrombotic, anticoagulant, and antiplatelet activities [50]. DATS exhibits cytoprotective activity against valproate-induced hepatotoxicity by maintaining the integrity of hepatocytes and by reducing hepatic steatosis and inflammation-induced necrosis through blocking the production of TNF-α, IL-6, and IL-1β, as well as by reducing COX2 expression and inhibiting NF-κB activity [51]. DATS has also been reported to have protective effects against alcohol-induced oxidative stress, as it enhances the generation of H2S through upregulation of cystathionine γ-lyase and cystathionine β-synthase expression, and reduces ROS. DATS also protects hepatocytes from ethanol-induced apoptosis by reducing the expression of BAX and enhancing the expression of BCL2 [52]. In rats with chemically-induced diabetes, DATS increases insulin secretion and improves oral glucose tolerance [53]. In CCL4-treated rats, DATS showed hepatoprotective activity as it improves the integrity of liver cell histology, and reduces the levels of hepatic enzymes. DATS also attenuates collagen deposition and inhibits hepatic stellate cells by reducing the expression of TGF1β, PDGF-β, and EGF receptors [54]. In addition, DATS has cardioprotective activity as it increases the bioavailability of NO and releases H2S [55]. These findings describe the pharmacological importance of DADS and DATS in ameliorating various stress-related diseases, including cancer.
3.2. Impact of DADS and DATS on BC
Epidemiological studies have shown that the intake of Allium vegetables is strongly associated with reduced BC risk. Population-based studies reveal that a high intake of garlic and onion may have preventive effects against BC [56]. Moreover, a hospital-based case-control study of 285 women confirmed to have an inverse association between consumption of Allium vegetables, particularly garlic and leek, and risk of BC [57]. Dietary guidelines for BC encourage increased intake of garlic and cruciferous vegetables, as a part of nutritional therapy, to improve the overall health of BC patients [58]. These vegetables contain highly reactive sulfur compounds that interact with cellular macromolecules, suggesting that these vegetables, especially garlic, are potent inhibitors of phospholipase A2 [59] and MAPKs [60], cytochrome P450 and glutathione-s-transferases [61] that may reduce cancer risk. DADS and DATS are major allyl sulfur compounds known to exhibit anticancer effects by interfering with cancer cell proliferation, tumor metastasis, and vascularization, which appears to be under redox control [62,63]. In addition, DADS and DATS interfere with cell signaling pathways that regulate cell apoptosis and cancer cell mitosis [64,65]. Furthermore, DADS antagonizes the effect of linoleic acid and synergizes the effect of eicosapentaenoic acid in BC [66]. These features suggest that allyl sulfur compounds should be considered as potential natural inhibitors.
3.3. Modulation of MDR by allyl sulfur compounds
The development of MDR is a considerable challenge in the treatment of TNBC. The cell cycle and apoptosis are regulated by various proteins (e.g., p53 and p21). The tumor suppressor gene, p53, is a master regulator of apoptosis. Many chemotherapeutics promote apoptosis in cancer cells by modulating pro-apoptotic and anti-apoptotic factors via the p53 pathway [67]. BAX is a pro-apoptotic protein that antagonizes BCL2, an anti-apoptotic protein, causing cytochrome leakage that subsequently activates APAF1. Activated APAF1 activates caspases, and the activated caspases induce cell-cycle arrest and subsequent cell death. Cells undergoing cell-cycle arrest can be protected from drug-induced apoptosis and ultimately develop drug resistance [68]. Migration and invasion are two distinctive characteristics of metastatic cancers. A recent study reported that metastasis-related genes promote drug resistance to chemotherapeutics by regulating various signaling pathways [69]. Preclinical studies have provided sufficient evidence indicating that angiogenesis is one of the hallmarks of cancer drug resistance [70]. Tumor cells protect themselves against oxidative damage by activating redox signaling and low oxygen conditions, thereby enhancing drug resistance. For example, the Trx system protects against oxidative damage, hypoxia, and oxidative stress. After rapid division, BC cells exceed Hayfleck's limit, and angiogenesis cannot supply required oxygen to the cells and develops hypoxia by increasing the expression of HIFs [71]. The changes in the epigenetic status of cancer are often linked to modifications in the function of miRNAs, the pattern of histone deacetylase activity, histone acetylation, hyperacetylation status, and histone deacetylation. This leads to gene silencing, which is progressively linked to cancer pathogenesis. Investigation of epigenetic mechanisms in drug-resistant cancers has revealed that drug resistance is induced by silencing or reactivating genes [72]. However, targeting the driving mechanisms of drug resistance sensitizes cancer cells to chemotherapeutics. The major proteins involved in the establishment of MDR are P-glycoprotein (ATP-binding cassette transporter) and multidrug resistant protein 2 (ATP-dependent transporter) [73]. Designing feasible therapeutic options against these drug transporters to kill more tumor cells is challenging. In line with this, the organosulfur compounds, DADS and DATS, may have different effects on drug transporter proteins. However, to date, there have been insufficient data on DADS and DATS in terms of the impact of these compounds on the mechanisms of MDR in BC. Therefore, the present review aims to highlight recent updates on the potential anticancer activities of DADS and DATS, with special attention to drug resistance in TNBC. The cellular and molecular mechanisms modulated by DADS and DATS, to combat drug resistance in BC, are summarized in Table 1 [43, [74], [105], [106], [127]].
Table 1.
Impact of diallyl disulfide (DADS) and diallyl trisulfide (DATS) on cellular and molecular mechanism mediating drug resistance in breast cancer (BC).
| Garlic compound | Cellular mechanism affected | Signaling pathway affected by | Refs. |
|---|---|---|---|
| Allyl sulfur compounds | Exert proapoptotic activity | Activating p53 | [74] |
| DADS and DATS | Exert an anti-proliferative effect on MDR-colorectal carcinoma cells | Changing the permeability of mitochondria | [75] |
| DADS | Reduce the viability and metastatic potential of TNBC cells | Downregulating MMP-9 and promoting the reversal of EMT through inhibition of the β-catenin signaling pathway | [76] |
| DADS | Reduce the proliferation, invasion, and migration of MCF-7 and MDA-MB-231 BC cells | Downregulating uPA and MMP-9 expression and upregulating TTP expression in vitro and in vivo | [77] |
| DADS | Inhibit the proliferation of BC cells | Inducing cell cycle arrest and apoptosis at the G0/G1 phase, via inhibition of the ERK1/2 pathway | [78] |
| DADS | Cause apoptosis in BC cells | Increasing the expressions of BAX, BAD, caspase-3, and caspase-9 | [79] |
| DADS | Induce cytotoxicity in BC cells | Downregulating BCL2 and survivin and upregulating caspase-9 | [80] |
| DATS | Ameliorate ER stress-induced apoptosis and reduce tumor growth of BC | Progressive activation of ER stress proteins, such as CHOP/GADD153 and GRP78/Bip, and upregulation of other proteins, such as ATF4 and eIF2 | [81] |
| DADS | Decrease FOXM1-dependent invasion of BC cells | Enhancing the expression of miR-134 | [82] |
| DATS | Reduce metastasis of BC cells | Suppressing MMP2/9 via blocking NF-κB and ERK/MAPK signaling pathways | [83] |
| DATS | Suppress both spontaneous and experimental metastasis of TNBC cells | Decreasing Trx-1, NF-κB, and MMP2/9 expressions in nude mice | [84] |
| DATS | Inhibit the proliferation, migration, and invasion of TNBC cells | Reversing the overexpression of lactate dehydrogenase A | [85] |
| DATS | Inhibit metastasis of TNBC cells | Blocking the activity and expressions of MMP2/9, via the NF-κB and ERK/MAPK pathways | [86] |
| DATS | Inhibit leptin-induced proliferation and metastasis of BC cells | Reducing the expression of BCL2, BCL-xL, cyclin D1, VEGF, and MMP-2 in BC cell lines and xenograft models targeting transcription factor STAT3 | [87] |
| DATS | Inhibit angiogenesis in BC | Inactivating Wnt/β-catenin signaling and inhibiting VEGF expression | [88,89] |
| DADS | Inhibit BC growth by inducing apoptosis | Enhancing the expression of caspase-3 and blocking degradation of p53 via upregulating NAD(P)H:quinone oxidoreductase 1 and superoxide dismutase and reducing glutathione | [90] |
| DADS and DATS | Inhibit BC growth | Reducing angiogenesis | [91] |
| DATS | Inhibit metastasis of BC cells | Attenuating activated transforming growth factor-β1 signaling | [92] |
| DADS | Inhibit metastasis of BC cells | Downregulating TNF-α-induced CCL2 release primarily via the reduction of IKKε and phosphorylated-ERK expression, as well as impairment of MAPK/ERK and NF-κB signaling pathways in TNBC cells | [93] |
| DATS | Inhibit BC growth | Targeting Notch ligands (Jagged-1 and Jagged-2) and α-secretases (ADAM 10 and ADAM 17) | [94] |
| DATS | Suppress breast tumorigenesis | Suppressing the Wnt/β-catenin pathway | [95] |
| DATS | Increase cell-cell adhesion and decrease the attachment of the extracellular matrix component (type 1 collagen) and MMPs in BC cells | Upregulating E-cadherin | [96,97] |
| DADS | Inhibit histone deacetylase activity and induces apoptosis | Hyperacetylated histone-dependent alterations in the expression in proapoptotic genes and pathways and the expression of certain proteins in the antiapoptotic BCL2 family of proteins | [98] |
| DATS | Exert anticancer activity against BC | Epigenetic-mediated upregulation of metallothionein 2A, thereby diminishing NF-κB signaling | [99,100] |
| DADS | Reduce BC cell proliferation and invasion | Overexpressing miR-34a via the inhibition of the ERK signaling pathway, which regulates cell proliferation, cell survival, cell adhesion, and motility | [101] |
| DATS | Inhibit invasion and angiogenesis in BC | Targeting Notch-1 signaling, thereby inducing the expression of tumor suppressor miRNAs | [102,103] |
| DATS | Inhibit migration and angiogenesis in BC | Suppressing HIF-1α transcriptional activity and expression of VEGF-A, and EMT-related protein in MDA MB-231 cells | [104] |
| DADS and DATS | Sensitize drug-resistant BC cells | Bringing about the accumulation of ROS | [105] |
| DADS | Reduce the proliferation, metastasis, stemness of BCSCs, and glucose metabolism | Targeting the CD44/PKM2/AMPK signaling pathways in BCSCs | [106] |
| DATS | Reduce invasion of BCSCs | Decreasing the expression of FOXQ1, which negatively controls DACH1 expression by interacting with the DACH1 promoter region | [107] |
| DATS | Reduce tumorsphere formation of BCSCs | Reducing the expressions of CD44, ALDH1A1, Nanog, and Oct4 | [108] |
| DATS | Inhibit the expression of EMT markers in BCSCs | Targeting Forkhead box Q1 | [107,109,110] |
| DADS | Suppress inflammation | Lowering the expressions of proinflammatory cytokines | [111] |
| DADS | Mediate anti-inflammatory activity in BC | Blocking NF-κB pathway | [43,112] |
| DADS | Suppress inflammation in BC | Diminishing ROS production and targeting IκBα phosphorylation | [113] |
| DATS | Reduce inflammation | Activating the nuclear factor E2-related factor 2-anti-oxidant response elements pathway | [114] |
| DATS | Suppress inflammation in BC | Inhibiting the NF-κB/TLR 4 and CXCL12/CXCR4 pathways | [115] |
| Autophagy promotes drug resistance in BC cells | Promoting cell survivalAutophagosome formation Activation of autophagy-related gene signaling cascade |
[116][117] [118] |
|
| DADS | Modulate autophagy in BC | Controlling mTOR pathway | [119] |
| DADS and DATS | Increase the activation of autophagy in BC cells | Preventing mTOR phosphorylation activity and decreasing apoptotic cell death in macrophages, thus promoting immunomodulatory effects | [120] |
| DADS | Induce autophagy-mediated cell death in BC cells | Reducing p-mTOR kinase activity and inhibiting the PI3K/Akt/mTOR pathway | [121] |
| Inflammatory microenvironment, with oxygen-deprived conditions was created | Infiltrating immune cells, cytokines, and numerous growth factors | [122,123] | |
| DADS and DATS | Induce cellular immune response, lymphocyte activation, immunoglobulin production, and macrophage phagocytosis in BC model in rats | Producing cytokines, such as IL-2, IL-12, TNF-α, and IFN-γ | [124] |
| DADS and DATS | Induce the immunomodulatory activity of neutrophils | Increasing calcium influx | [125] |
| DADS and DATS | Enhance immunomodulatory and anti-inflammatory responses in rats | Reducing the levels of lymphocytes and monocytes | [126] |
| Aged garlic | Provoke immunomodulatory activity in the BALB/c mouse model | Substantially increasing T-cell response and decreasing tumor size | [127] |
| DADS | Regulate CD4+ and CD8+ T cells | Suppressing pro-inflammation cytokine production including TNF-α, IL-1β, and IL-6 via inhibiting the NF-κB pathway | [43] |
MDR: multidrug resistance; MMP: matrix metalloproteinase; EMT: epithelial-mesenchymal transition; TNBC: triple-negative breast cancer; TTP: tristetraprolin; ERK: extracellular signal-regulated kinase; ER: endoplasmic reticulum; FOXM1: forkhead box M1; MAPK: mitogen-activated protein kinase; VEGF: vascular endothelial-derived growth factor; STAT: signal transducer and activator of transcription; ADAM: a disintegrin and metalloproteinase; HIF: hypoxia inducible factor; ROS: reactive oxygen species; PKM: pyruvate kinase M2; AMPK: AMP-activated protein kinase; BCSCs: BC stem cells; mTOR: mechanistic target of rapamycin; IL: interleukin.
3.4. Effects of allyl sulfur compounds on the cell cycle and apoptosis-mediated drug resistance
Previous studies have reported that allyl sulfur compounds exert proapoptotic activity by activating p53 [74]. These compounds influence cellular mechanisms in BC cell lines by downregulating anti-apoptotic factors and upregulating proapoptotic signal proteins and caspases. DADS has antioxidant, antiangiogenic, and anticancer activities. In light of this, recent evidence on garlic suggests that DADS and DATS exert an anti-proliferative effect on MDR-colorectal carcinoma cells by changing the permeability of mitochondria [75]. DADS treatment reduces the viability and metastatic potential of TNBC cells in an in vitro model by downregulating matrix metalloproteinase-9 (MMP-9) and promoting the reversal of EMT through inhibition of the β-catenin signaling pathway. DADS reduces tumor size by inducing apoptosis in the xenograft model, suggesting that DADS may be used as a potential therapeutic agent for treating or preventing BC [76]. Furthermore, DADS treatment remarkably reduces the proliferation, invasion, and migration of MDA-MB-231 BC cells in a xenograft tumor model and in an in vitro cell line model by downregulating uPA and MMP-9 expression and upregulating tristetraprolin expression [77]. A protein-nanoemulsion of DADS and α-linolenic acid substantially inhibits the proliferation of BC cells by inducing cell cycle arrest and apoptosis at the Go/G1 phase, via inhibition of the ERK1/2 pathway [78]. Palmitic acid containing solid-liquid nanoparticles with DADS causes apoptosis in BC cells by increasing the expressions of BAX, BAD, caspase-3, and caspase-9. However, negligible cytotoxicity to normal breast epithelial cells (MCF-12A) was reported in the pH-dependent release of DADS [79]. The receptor for advanced glycation end-products antibody-coated solid lipid NPs loads with DADS (DADS-SLN), specifically delivers DADS to TNBC cells, and induces cytotoxicity by downregulating BCL2 and survivin and upregulating caspase-9. This suggests that the receptor for advanced glycation end-products-mediated delivery improves the antitumor activity of DADS in TNBC patients [80]. A study showed that DATS treatment ameliorates endoplasmic reticulum (ER) stress-induced apoptosis and reduces tumor growth by progressive activation of ER stress proteins, such as CHOP/GADD153 and GRP78/Bip, and by upregulation of other proteins such as ATF4 and eIF2 [81]. The results of these studies suggest that DADS treatment sensitizes dysfunctional BC cells by targeting survival mechanisms.
3.5. Effects of allyl sulfur compounds on metastasis-dependent drug resistance
Targeting metastasis-related genes with natural compounds sensitizes BC cells to chemotherapeutics. Research has shown that oil-soluble allyl sulfur compounds of garlic effectively mediate the inhibition of these two crucial steps. DADS decreases forkhead box M1-dependent invasion by enhancing the expression of miR-134 [82]. An in vivo study using a zebrafish model showed the effectiveness of DATS in reducing metastasis by suppressing MMP2/9 via blocking NF-κB and extracellular-signal-regulated kinases (ERK)/MAPK signaling pathways [83]. Another study found that DATS treatment blocks the nuclear translocation of Trx-1 from the cytoplasm and diminishes production of the reduced form of Trx-1. In addition, DATS suppresses both spontaneous and experimental metastasis of TNBC cells by decreasing Trx-1, NF-κB, and MMP2/9 expressions in nude mice [84]. DATS has been shown to inhibit the proliferation, migration, and invasion of TNBC cells by reversing the overexpression of lactate dehydrogenase A [85]. DATS was also found to inhibit metastasis of TNBC cells by blocking the activity and expression of MMP2/9 via the NF-κB and ERK/MAPK pathways [86]. An additional study showed that DATS inhibits leptin-induced proliferation and metastasis, as well as expressions of BCL2, BCL-xL, cyclin D1, vascular endothelial-derived growth factor (VEGF), and MMP-2 in BC cell lines and xenograft models by targeting transcription factor STAT3 [87]. These studies suggest that DADS and DATS treatments eradicate metastasis-governed drug resistance in TNBC.
3.6. Impact of allyl sulfur compounds on angiogenesis-driven drug resistance
In the last decade, several antiangiogenic drugs, including drugs from phase I–III clinical trials, have been tested in preclinical studies. Recently, Almatroodi et al. [42] reviewed the potential impact of DADS and DATS on angiogenesis and related signaling pathways. Other studies have shown that DATS inhibits angiogenesis by inactivating Wnt/β-catenin signaling and inhibiting VEGF expression [88,89]. DADS also inhibits BC growth by inducing apoptosis via enhancing the expression of caspase-3 and blocking degradation of p53 via upregulating NAD(P)H:quinone oxidoreductase 1 and SOD and reducing glutathione [90]. Furthermore, the combination of garlic extract containing DADS and DATS with lemon extract was found to inhibit BC growth by reducing angiogenesis [91]. These studies suggest that DADS and DATS can be clinically beneficial for targeting tumor-induced angiogenesis in BC patients.
3.7. Effects of allyl sulfur compounds on EMT-mediated drug resistance
EMT has been recognized as a critical mechanism of drug resistance in BC. The potential activities of garlic extract and garlic-derived compounds on EMT and its signaling pathways have been explored in various cancer cell lines. Liu et al. [92] found that DATS inhibits metastasis of BC cells by attenuating activated transforming growth factor-β1 signaling. Bauer et al. [93] reported that DADS inhibits metastasis by downregulating TNF-α-induced CCL2 release primarily via the reduction of IKKε and phosphorylated-ERK expression, as well as by impairment of MAPK/ERK and NF-κB signaling pathways in TNBC cells. It is evident that the cytoskeleton, especially the tubulin component, is a direct target of DATS. In a study conducted by Kiesel and Stan [94], the effect of DATS on MDA-MB-231 cells was evaluated by targeting Notch ligands (Jagged-1 and Jagged-2) and α-secretases (a disintegrin and metalloproteinase (ADAM) 10 and ADAM 17). This study found that high responsiveness to garlic is exhibited during the initiation phase of breast tumorigenesis, rather than during the late stages. Notably, a study revealed that DATS can suppress the later stages of breast tumorigenesis by suppressing the Wnt/β-catenin pathway [95]. Accordingly, DATS is also shown to increase cell-cell adhesion and decrease the attachment of the extracellular matrix component (type 1 collagen) and MMPs by upregulating E-cadherin [96,97]. The findings of these studies suggest that DADS and DATS treatment may suppress drug resistance by preventing EMT in BC.
3.8. Effects of allyl sulfur compounds on epigenetic modulators of drug resistance
Allyl sulfur compounds in garlic function as epigenetic modulators and represent potential treatment options for BC. DADS inhibits histone deacetylase activity and induces apoptosis by histone-dependent alterations in the expressions of proapoptotic genes (under hyperacetylated state) and pathways and the expressions of certain proteins in the antiapoptotic BCL2 family of proteins [98]. DATS exerts anticancer activity by the epigenetic-mediated upregulation of metallothionein 2A, thereby diminishing NF-κB signaling [99,100]. Growing evidence indicates that miRNAs production is dysregulated in BCs. miR-34a is an important tumor suppressor in BCs. A study has reported that DADS reduces BC cell proliferation and invasion by overexpressing miR-34a via the inhibition of the ERK signaling pathway, which regulates cell proliferation, cell survival, cell adhesion, and motility [101]. In addition, DATS inhibits invasion and angiogenesis by targeting Notch-1 signaling, thereby inducing the expression of tumor suppressor miRNAs [102,103]. The findings of the above studies suggest that DADS and DATS can potentially inhibit BC growth by targeting epigenetic modulators of drug resistance.
3.9. Effects of allyl sulfur compounds on redox-mediated drug resistance
HIF-1α is highly expressed in tumors and is known to be critical to inducing drug resistance. DATS has been reported to suppress HIF-1α transcriptional activity and expression of Trx-1 in MDA MB-231 cells [104]. In addition, DADS and DATS sensitize drug-resistant BC cells by bringing about the accumulation of ROS [105]. These studies show that disturbance of the redox system promotes drug resistance in highly aggressive metastatic BCs, which can be targeted by DADS and DATS.
3.10. Effects of allyl sulfur compounds on BC stem cell-mediated drug resistance
BC stem cells (BCSCs) are a group of specific cells with the distinct characteristics of self-renewal, differentiation, and tumorigenic ability, which facilitates the progression of BC. BCSCs have been highlighted as potential targets for BC therapy because they impart drug resistance. Experiments on BCSCs in a xenograft model demonstrated that DADS reduces the proliferation, metastasis, and stemness of these cells, and reduces glucose metabolism, by targeting the CD44/pyruvate kinase M2/AMP-activated protein kinase signaling pathways [106]. Exposure of TNBC stem cells to DATS considerably reduces invasion by decreasing the expression of FOXQ1, which negatively controls Dachshund homolog 1 (DACH1) expression by interacting with the DACH1 promoter region [107]. DATS also reduces tumorsphere formation and the expressions of CSC markers (CD44, ALDH1A1, Nanog, and Oct4) [108]. Recent studies reveal that DATS inhibits the expression of EMT markers in BCSC by targeting Forkhead box Q1 [107,109,110]. DATS administration sensitizes BCSCs to chemotherapeutics by suppressing the expression of FOXQ1, which is partly responsible for the gain of stem cell-like properties and elevated EMT during cancer metastasis [107]. The combined results of these studies suggest that DADS and DATS can be used to target BCSC-driven drug resistance.
3.11. Effects of allyl sulfur compounds on inflammation-mediated drug resistance
DADS and DATS have been well-established as suppressors of carcinogenesis, but DADS and DATS have also been reported to suppress inflammation. DADS suppresses inflammation by lowering the expressions of proinflammatory cytokines [111]. The anti-inflammatory activity of DADS is mediated by the blockade of the NF-κB pathway [43,112]. DADS suppresses inflammation by diminishing ROS production and targeting IκBα phosphorylation [113]. DATS reduces inflammation by activating the Nrf2-anti-oxidant response elements pathway [114]. DATS also suppresses inflammation by inhibiting the NF-κB/TLR 4 and CXCL12/CXCR4 pathways [115]. Due to these effects, DADS and DATS appear to be feasible anti-inflammatory agents for future treatment of inflammatory BCs.
3.12. Effects of allyl sulfur compounds on autophagy-mediated drug resistance
Autophagy is a self-degenerative and physiologically stressful process, involving the removal of aggregated proteins and damaged cell organelles to sustain a balance between cell survival and apoptotic cell death [116]. Autophagy occurs through autophagosome formation, where degraded components are engulfed and transported into lysosomes for recycling [117]. Research on autophagy has demonstrated that it increases tumour growth by promoting tumour cell survival through activation of autophagy-related gene signaling cascade; therefore, autophagy promotes drug resistance [118]. DADS modulates autophagy via the mechanistic target of rapamycin (mTOR) pathway [119]. Similarly, treatment with DADS and DATS has been shown to increase the activation of autophagy by indirectly preventing mTOR phosphorylation activity and decreasing apoptotic cell death in macrophages, thus promoting immunomodulatory effects [120]. A study showed that DADS induces autophagy-mediated cell death reducing p-mTOR kinase activity and by inhibiting the PI3K/Akt/mTOR pathway [121].
3.13. Effects of allyl sulfur compounds on antitumor immunity-mediated drug resistance
Cancer immunotherapy combats MDR cancers by targeting various mechanisms that are involved in drug resistance. Immune infiltration of tumors creates a highly inflammatory microenvironment, with oxygen-deprived conditions supported by infiltrating immune cells, cytokines, and numerous growth factors [122,123]. Therefore, the modulation of immune responses may be a promising strategy to combat cancer. DADS and DATS were found to exhibit beneficial immune modulatory activities via the production of cytokines, such as IL-2, IL-12, TNF-α, and IFN-γ, suggesting that DADS and DATS induce cellular immune response, lymphocyte activation, immunoglobulin production, and macrophage phagocytosis [124]. DADS and DATS also induce the immunomodulatory activity of neutrophils by increasing calcium influx [125]. DADS and DATS were shown to enhance immunomodulatory and anti-inflammatory responses in rats by reducing the levels of lymphocytes and monocytes [126]. A study reported that the protein fraction of aged garlic provokes immunomodulatory activity in the BALB/c mouse model by substantially increasing T-cell response and decreasing tumor size [127]. DADS treatment was also found to regulate CD4+ and CD8+ T cells by suppressing the production of pro-inflammation cytokines including TNF-α, IL-1β, and IL-6 via inhibiting the NF-κB pathway [43]. These studies indicate that DADS and DATS appear to modulate immune responses in drug-resistant cancers.
4. Conclusion and future directions
Drug resistance is a challenge in combating BC and is driven by regulators of the cell cycle, apoptosis, metastasis, and angiogenesis in TME. Drug resistance is also induced by EMT, epigenetic modulators, redox regulation, cancer stem cells, inflammation, and autophagy. Presently, garlic continues to be of interest because of its chemistry and unique biological action. Epidemiological studies suggest the ability of garlic to reduce BC risk, while several experimental studies have indicated the potential impact of sulfur-containing garlic compounds in modifying the behavior of BC cells at each stage of BC carcinogenesis. Interaction studies on BC have revealed that DADS and DATS bind to BC targets. Further analysis is required to study the development of these compounds into suitable therapeutics against BC. Additionally, the consumption of garlic compounds DADS and DATS for BC prevention is also dependent on several dietary and environmental variables such as gut microbiota. Finally, DADS and DATS in garlic as therapeutic agents are beneficial for management of drug resistance in BC patients.
CRediT author statement
RamaRao Malla: Conceptualization, Supervision; Rakshmitha Marni: Writing - Original draft preparation; Anindita Chakraborty: Writing - Reviewing and Editing; Mohammad Amjad Kamal: Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work is supported by UGC-DAE-CSR, Kolkata (Grant No.: KC/CRS/19/RB-04/1047).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Malla R.R., Kumari S., Gavara M.M., et al. A perspective on the diagnostics, prognostics, and therapeutics of microRNAs of triple-negative breast cancer. Biophys. Rev. 2019;11:227–234. doi: 10.1007/s12551-019-00503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nedeljković M., Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8:957. doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo L., Gao W., Wang J., et al. Study on the mechanism of cell cycle checkpoint Kinase 2 (CHEK2) gene dysfunction in chemotherapeutic drug resistance of triple negative breast cancer cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:3176–3183. doi: 10.12659/MSM.907256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu S., Lin C.Y., He H.H., et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani C., Jonnalagadda S., Lingareddy J., et al. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2019;21 doi: 10.1186/s13058-019-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Low K.H., Alexander A., et al. Cyclin E overexpression sensitizes triple-negative breast cancer to wee1 kinase inhibition. Clin. Cancer Res. 2018;24:6594–6610. doi: 10.1158/1078-0432.CCR-18-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thu K.L., Silvester J., Elliott M.J., et al. Disruption of the anaphase-promoting complex confers resistance to TTK inhibitors in triple-negative breast cancer. Proc. Natl. Acad. Sci. U S A. 2018;115:E1570–E1577. doi: 10.1073/pnas.1719577115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald T.M., Thomas L.N., Daze E., et al. Prolactin-inducible EDD E3 ubiquitin ligase promotes TORC1 signalling, anti-apoptotic protein expression, and drug resistance in breast cancer cells. Am. J. Cancer Res. 2019;9:1484–1503. [PMC free article] [PubMed] [Google Scholar]
- 9.Ozawa P.M.M., Alkhilaiwi F., Cavalli I.J., et al. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018;172:713–723. doi: 10.1007/s10549-018-4925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das C.K., Linder B., Bonn F., et al. BAG3 overexpression and cytoprotective autophagy mediate apoptosis resistance in chemoresistant breast cancer cells. Neoplasia. 2018;20:263–279. doi: 10.1016/j.neo.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng C.C., Shi L.H., Wang X.J., et al. Stat3/Oct-4/c-Myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066. Int. J. Oncol. 2018;53:339–348. doi: 10.3892/ijo.2018.4399. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Burness M.L., Martin-Trevino R., et al. RAD51 mediates resistance of cancer stem cells to PARP inhibition in triple-negative breast cancer. Clin. Cancer Res. 2017;23:514–522. doi: 10.1158/1078-0432.CCR-15-1348. [DOI] [PubMed] [Google Scholar]
- 13.Chen D.R., Lu D.Y., Lin H.Y., et al. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh W.L., Tsai C.F., Chen D.R. Peri-foci adipose-derived stem cells promote chemoresistance in breast cancer. Stem Cell Res. Ther. 2017;8 doi: 10.1186/s13287-017-0630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Wang N., Li W., et al. Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35:2346–2356. doi: 10.1093/carcin/bgu155. [DOI] [PubMed] [Google Scholar]
- 16.Ryoo I.G., Choi B.H., Ku S.K., et al. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: implications for cancer stem cell resistance. Redox Biol. 2018;17:246–258. doi: 10.1016/j.redox.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy R., Mnich K., Gorman A.M. Nerve growth factor (NGF)-mediated regulation of p75NTR expression contributes to chemotherapeutic resistance in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2016;478:1541–1547. doi: 10.1016/j.bbrc.2016.08.149. [DOI] [PubMed] [Google Scholar]
- 18.Sari I.N., Phi L.T.H., Jun N., et al. Hedgehog signaling in cancer: a prospective therapeutic target for eradicating cancer stem cells. Cells. 2018;7 doi: 10.3390/cells7110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhateja P., Cherian M., Majumder S., et al. The hedgehog signaling pathway: a viable target in breast cancer? Cancers (Basel) 2019;11 doi: 10.3390/cancers11081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzam D.J., Zhao D., Sun J., et al. Triple negative breast cancer initiating cell subsets differ in functional and molecular characteristics and in γ-secretase inhibitor drug responses. EMBO Mol. Med. 2013;5:1502–1522. doi: 10.1002/emmm.201302558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darvishi B., Farahmand L., Eslami S.Z., et al. NF-κB as the main node of resistance to receptor tyrosine kinase inhibitors in triple-negative breast cancer. Tumour Biol. 2017;39 doi: 10.1177/1010428317706919. 1010428317706919. [DOI] [PubMed] [Google Scholar]
- 22.Song H., Li D., Wu T., et al. MicroRNA-301b promotes cell proliferation and apoptosis resistance in triple-negative breast cancer by targeting CYLD. BMB Rep. 2018;51:602–607. doi: 10.5483/BMBRep.2018.51.11.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu J., Xue A., Chi Y., et al. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene. 2016;35:1302–1313. doi: 10.1038/onc.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L., Bourguignon L.Y. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol. Cancer. 2014;13 doi: 10.1186/1476-4598-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Mattos-Arruda L., Bottai G., Nuciforo P.G., et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 2015;6:37269–37280. doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korkaya H., Kim G.I., Davis A., et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L.H., Yang A.J., Wang M., et al. Enhanced autophagy reveals vulnerability of P-gp mediated epirubicin resistance in triple negative breast cancer cells. Apoptosis. 2016;21:473–488. doi: 10.1007/s10495-016-1214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin S., Xu L., Bonfil R.D., et al. Tumor-initiating cells and FZD8 play a major role in drug resistance in triple-negative breast cancer. Mol. Cancer Ther. 2013;12:491–498. doi: 10.1158/1535-7163.MCT-12-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K., Zhu X., Zhang K., et al. Interleukin-6 contributes to chemoresistance in MDA-MB-231 cells via targeting HIF-1α. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22039. [DOI] [PubMed] [Google Scholar]
- 30.Kalimutho M., Sinha D., Mittal D., et al. Blockade of PDGFRβ circumvents resistance to MEK-JAK inhibition via intratumoral CD8(+) T-cells infiltration in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2019;38 doi: 10.1186/s13046-019-1075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendoza-Rodríguez M.G., Ayala-Sumuano J.T., García-Morales L., et al. IL-1β inflammatory cytokine-induced TP63 isoform ΔNP63α signaling cascade contributes to cisplatin resistance in human breast cancer cells. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penney R.B., Roy D. Thioredoxin-mediated redox regulation of resistance to endocrine therapy in breast cancer. Biochim. Biophys. Acta. 2013;1836:60–79. doi: 10.1016/j.bbcan.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Yang C., Liu X.B., et al. B7-H4 overexpression contributes to poor prognosis and drug-resistance in triple-negative breast cancer. Cancer Cell Int. 2018;18 doi: 10.1186/s12935-018-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 35.Khan A.Q., Uddin S. Anticancer activity of natural compounds. Asian Pac. J. Cancer Prev. 2021;22:1–2. doi: 10.31557/APJCP.2021.22.S1.1. [DOI] [PubMed] [Google Scholar]
- 36.Mamadalieva N.Z., Mamedov N.A. Vol. 6, Springer; Switzerland AG, 2020. Taxus Brevifolia a high-value medicinal plant, as a source of Taxol. Medicinal and Aromatic Plants of North America; pp. 201–218. [Google Scholar]
- 37.Taher M.A., Nyeem M.A.B., Billah M.M., et al. Vinca alkaloid-the second most used alkaloid for cancer treatment-A review. Inter. J. Physiol. Nutr. Phys. Educ. 2017;2:723–727. [Google Scholar]
- 38.Kalam M.A., Malik A.H., Ganie A.H., et al. Medicinal importance of papra (royle) in unani system of medicine. J. Complement. Integr. Med. 2021;18:485–490. doi: 10.1515/jcim-2020-0178. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian M.S., Nandagopal Ms G., Amin Nordin S., et al. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from alliums: a potential drug candidate. Molecules. 2020;25 doi: 10.3390/molecules25184111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omar S.H., Al-Wabel N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson L.D., Hunsaker S.M. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients. 2018;10 doi: 10.3390/nu10070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almatroodi S.A., Alsahli M.A., Almatroudi A., et al. Garlic and its active compounds: a potential candidate in the prevention of cancer by modulating various cell signalling pathways. Anticancer Agents Med. Chem. 2019;19:1314–1324. doi: 10.2174/1871520619666190409100955. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Li A., Feng X., et al. Pharmacological investigation of the anti-inflammation and anti-oxidation activities of diallyl disulfide in a rat emphysema model induced by cigarette smoke extract. Nutrients. 2018;10 doi: 10.3390/nu10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He H., Ma Y., Huang H., et al. A comprehensive understanding about the pharmacological effect of diallyl disulfide other than its anti-carcinogenic activities. Eur. J. Pharmacol. 2021;893 doi: 10.1016/j.ejphar.2020.173803. [DOI] [PubMed] [Google Scholar]
- 45.Bordia A., Verma S.K., Srivastava K.C. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fatty Acids. 1998;58:257–263. doi: 10.1016/s0952-3278(98)90034-5. [DOI] [PubMed] [Google Scholar]
- 46.Lee I.C., Kim S.H., Baek H.S., et al. Protective effects of diallyl disulfide on carbon tetrachloride-induced hepatotoxicity through activation of Nrf2. Environ. Toxicol. 2015;30:538–548. doi: 10.1002/tox.21930. [DOI] [PubMed] [Google Scholar]
- 47.Hosseinzadeh A., Jafari D., Kamarul T., et al. Evaluating the protective effects and mechanisms of diallyl disulfide on interlukin-1β-induced oxidative stress and mitochondrial apoptotic signaling pathways in cultured chondrocytes. J. Cell. Biochem. 2017;118:1879–1888. doi: 10.1002/jcb.25907. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y.-R., Li L., Sun X.-C., et al. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metabol. Cardiovasc. Dis. 2021;31:322–332. doi: 10.1016/j.numecd.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Goudappala P., Gowda C.Y., Kashinath R.T. Diallyl disulfide regulates purine metabolism and their metabolites in diabetes mellitus. Indian J. Physiol. Pharmacol. 2021;65:28–34. [Google Scholar]
- 50.Mikaili P., Maadirad S., Moloudizargari M., et al. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds, Iran. J. Basic Med. Sci. 2013;16:1031–1048. [PMC free article] [PubMed] [Google Scholar]
- 51.Shaaban A.A., El-Agamy D.S. Cytoprotective effects of diallyl trisulfide against valproate-induced hepatotoxicity: new anticonvulsant strategy. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390:919–928. doi: 10.1007/s00210-017-1393-0. [DOI] [PubMed] [Google Scholar]
- 52.Chen L.-Y., Chen Q., Zhu X.-J., et al. Diallyl trisulfide protects against ethanol-induced oxidative stress and apoptosis via a hydrogen sulfide-mediated mechanism. Int. Immunopharmacol. 2016;36:23–30. doi: 10.1016/j.intimp.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Liu C.-T., Hse H., Lii C.-K., et al. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005;516:165–173. doi: 10.1016/j.ejphar.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Zhu X., Zhang F., Zhou L., et al. Diallyl trisulfide attenuates carbon tetrachloride-caused liver injury and fibrogenesis and reduces hepatic oxidative stress in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387:445–455. doi: 10.1007/s00210-014-0959-3. [DOI] [PubMed] [Google Scholar]
- 55.Predmore B.L., Kondo K., Bhushan S., et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desai G., Schelske-Santos M., Nazario C.M., et al. Onion and garlic intake and breast cancer, a case-control study in Puerto Rico. Nutr. Cancer. 2020;72:791–800. doi: 10.1080/01635581.2019.1651349. [DOI] [PubMed] [Google Scholar]
- 57.Pourzand A., Tajaddini A., Pirouzpanah S., et al. Associations between dietary Allium vegetables and risk of breast cancer: a hospital-based matched case-control study. J. Breast Cancer. 2016;19:292–300. doi: 10.4048/jbc.2016.19.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Li S., Meng X., et al. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9 doi: 10.3390/nu9070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henao Castañeda I., Pereañez J.A., Preciado L.M., et al. Sulfur compounds as inhibitors of enzymatic activity of a snake venom phospholipase A2: benzyl 4-nitrobenzenecarbodithioate as a case of study. Molecules. 2020;25 doi: 10.3390/molecules25061373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X., Kassie F., Mersch-Sundermann V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat. Res. 2005;589:81–102. doi: 10.1016/j.mrrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Lii C.-K., Tsai C.-W., Wu C.-C. Garlic allyl sulfides display differential modulation of rat cytochrome P450 2B1 and the placental form glutathione S-transferase in various organs. J. Agric. Food Chem. 2006;54(14):5191–5196. doi: 10.1021/jf052484u. [DOI] [PubMed] [Google Scholar]
- 62.Thejass P., Kuttan G. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by diallyl disulfide (DADS) Life Sci. 2007;80:515–521. doi: 10.1016/j.lfs.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 63.Singh S.V., Powolny A.A., Stan S.D., et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–9511. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malki A., El-Saadani M., Sultan A.S. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol. Ther. 2009;8:2175–2185. doi: 10.4161/cbt.8.22.9882. [DOI] [PubMed] [Google Scholar]
- 65.Yin X., Zhang R., Feng C. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol. Rep. 2014;32:1748–1756. doi: 10.3892/or.2014.3361. [DOI] [PubMed] [Google Scholar]
- 66.Nakagawa H., Tsuta K., Kiuchi K., et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22:891–897. doi: 10.1093/carcin/22.6.891. [DOI] [PubMed] [Google Scholar]
- 67.Jan R., Chaudhry G.E. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019;9:205–218. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feeney G.P., Errington R.J., Wiltshire M., et al. Tracking the cell cycle origins for escape from topotecan action by breast cancer cells. Br. J. Cancer. 2003;88:1310–1317. doi: 10.1038/sj.bjc.6600889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishiguchi Y., Oue N., Fujiwara-Tani R., et al. Role of metastasis-related genes in cisplatin chemoresistance in gastric cancer. Int. J. Mol. Sci. 2019;21 doi: 10.3390/ijms21010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puccinelli M.T., D Stan S. Dietary bioactive diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18081645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hahm E.R., Singh S.V. Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res. Treat. 2014;144:47–57. doi: 10.1007/s10549-014-2841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdel-Hafiz H.A. Epigenetic mechanisms of tamoxifen resistance in luminal breast cancer. Diseases. 2017;5 doi: 10.3390/diseases5030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee C.H. Reversing agents for ATP-binding cassette drug transporters. Methods Mol. Biol. 2010;596:325–340. doi: 10.1007/978-1-60761-416-6_14. [DOI] [PubMed] [Google Scholar]
- 74.Hong Y.S., Ham Y.A., Choi J.H., et al. Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp. Mol. Med. 2000;32:127–134. doi: 10.1038/emm.2000.22. [DOI] [PubMed] [Google Scholar]
- 75.Ohkubo S., Dalla Via L., Grancara S., et al. The antioxidant, aged garlic extract, exerts cytotoxic effects on wild-type and multidrug-resistant human cancer cells by altering mitochondrial permeability. Int. J. Oncol. 2018;53:1257–1268. doi: 10.3892/ijo.2018.4452. [DOI] [PubMed] [Google Scholar]
- 76.Huang J., Yang B., Xiang T., et al. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the β-catenin signaling pathway. Mol. Nutr. Food Res. 2015;59:1063–1075. doi: 10.1002/mnfr.201400668. [DOI] [PubMed] [Google Scholar]
- 77.Xiong T., Liu X.W., Huang X.L., et al. Tristetraprolin: a novel target of diallyl disulfide that inhibits the progression of breast cancer. Oncol. Lett. 2018;15:7817–7827. doi: 10.3892/ol.2018.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciocci M., Iorio E., Carotenuto F., et al. H2S-releasing nanoemulsions: a new formulation to inhibit tumor cells proliferation and improve tissue repair. Oncotarget. 2016;7:84338–84358. doi: 10.18632/oncotarget.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Talluri S.V., Kuppusamy G., Karri V.V., et al. Application of quality-by-design approach to optimize diallyl disulfide-loaded solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017;45:474–488. doi: 10.3109/21691401.2016.1173046. [DOI] [PubMed] [Google Scholar]
- 80.Siddhartha V.T., Pindiprolu S.K.S.S., Chintamaneni P.K., et al. RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: in vitro studies. Artif. Cells Nanomed. Biotechnol. 2018;46:387–397. doi: 10.1080/21691401.2017.1313267. [DOI] [PubMed] [Google Scholar]
- 81.Yu L.M., Li S., Tang X.L., et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: role of SIRT1 activation. Apoptosis. 2017;22:942–954. doi: 10.1007/s10495-017-1378-y. [DOI] [PubMed] [Google Scholar]
- 82.Li Y.G., Wang Z.Y., Li J.M., et al. Diallyl disulfide suppresses FOXM1-mediated proliferation and invasion in osteosarcoma by upregulating miR-134. J. Cell. Biochem. 2018;120:7286–7296. doi: 10.1002/jcb.28003. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y.P., Zhu P.T., Wang Y.Y., et al. Antimetastatic therapies of the polysulfide diallyl tisulfide against triple-negative breast bancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y., Zhao Y., Wei Z., et al. Targeting thioredoxin system with an organosulfur compound, diallyl trisulfide (DATS), attenuates progression and metastasis of triple-negative breast cancer (TNBC) Cell. Physiol. Biochem. 2018;50:1945–1963. doi: 10.1159/000494874. [DOI] [PubMed] [Google Scholar]
- 85.Cheng S.Y., Yang Y.C., Ting K.L., et al. Lactate dehydrogenase downregulation mediates the inhibitory effect of diallyl trisulfide on proliferation, metastasis, and invasion in triple-negative breast cancer. Environ. Toxicol. 2017;32:1390–1398. doi: 10.1002/tox.22333. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y., Zhu P., Wang Y., et al. Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S.H., Hahm E.R., Singh K.B., et al. Diallyl trisulfide inhibits leptin-induced oncogenic signaling in human breast cancer cells but fails to prevent chemically-induced luminal-type cancer in rats. J. Cancer Prev. 2020;25:1–12. doi: 10.15430/JCP.2020.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai K.C., Hsu S.C., Yang J.S., et al. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J. Cell Mol. Med. 2015;19:474–484. doi: 10.1111/jcmm.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tao Q., Wu C., Xu R., et al. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of glioma cells by inactivating Wnt/β-catenin signaling. Cell Tissue Res. 2017;370:379–390. doi: 10.1007/s00441-017-2678-9. [DOI] [PubMed] [Google Scholar]
- 90.Sujatha P., Anantharaju P.G., Veeresh P.M., et al. Diallyl disulfide (DADS) retards the growth of breast cancer cells in vitro and in vivo through apoptosis induction. Biomed. Pharmacol. J. 2017;10:1619–1630. [Google Scholar]
- 91.Talib W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition. 2017;43–44:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y.P., Zhao Y., Wang Y.Y., et al. Suppressive role of diallyl trisulfide in the activated platelet-mediated hematogenous metastasis of MDA-MB-231 human breast cancer cells. Int. J. Mol. Med. 2017;39:1516–1524. doi: 10.3892/ijmm.2017.2953. [DOI] [PubMed] [Google Scholar]
- 93.Bauer D., Redmon N., Mazzio E., et al. Diallyl disulfide inhibits TNFα induced CCL2 release through MAPK/ERK and NF-Kappa-B signaling. Cytokine. 2015;75:117–126. doi: 10.1016/j.cyto.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiesel V.A., Stan S.D. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem. Biophys. Res. Commun. 2017;484:833–838. doi: 10.1016/j.bbrc.2017.01.184. [DOI] [PubMed] [Google Scholar]
- 95.Li X., Meng Y., Xie C., et al. Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J. Cell. Biochem. 2018;119:4134–4141. doi: 10.1002/jcb.26613. [DOI] [PubMed] [Google Scholar]
- 96.Jiang X., Zhu X., Liu N., et al. Diallyl trisulfide inhibits growth of NCI-H460 in vitro and in vivo, and ameliorates cisplatin-induced oxidative injury in the treatment of lung carcinoma in xenograft mice. Int. J. Biol. Sci. 2017;13:167–178. doi: 10.7150/ijbs.16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H.-C., Chu Y.-L., Hsieh S.-C., et al. Diallyl trisulfide inhibits cell migration and invasion of human melanoma a375 cells via inhibiting integrin/facal adhesion kinase pathway. Environ. Toxicol. 2017;32:2352–2359. doi: 10.1002/tox.22445. [DOI] [PubMed] [Google Scholar]
- 98.Singh A.K., Bishayee A., Pandey A.K. Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients. 2018;10 doi: 10.3390/nu10060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan Y.M., Lin S.Y., Xing R., et al. Epigenetic upregulation of metallothionein 2A by diallyl trisulfide enhances chemosensitivity of human gastric cancer cells to docetaxel through attenuating NF-κB activation. Antioxid. Redox Signal. 2016;24:839–854. doi: 10.1089/ars.2014.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin S., Wang X., Pan Y., et al. Transcription factor myeloid zinc-finger 1 suppresses human gastric carcinogenesis by interacting with metallothionein 2A. Clin. Cancer Res. 2019;25:1050–1062. doi: 10.1158/1078-0432.CCR-18-1281. [DOI] [PubMed] [Google Scholar]
- 101.Xiao X., Chen B., Liu X., et al. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112720. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Li Y., Zhang J., Zhang L., et al. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis. 2013;34:1601–1610. doi: 10.1093/carcin/bgt065. [DOI] [PubMed] [Google Scholar]
- 103.Chiang E.P., Chiu S.C., Pai M.H., et al. Organosulfur garlic compounds induce neovasculogenesis in human endothelial progenitor cells through a modulation of MicroRNA 221 and the PI3-K/Akt signaling pathways. J. Agric. Food Chem. 2013;61:4839–4849. doi: 10.1021/jf304951p. [DOI] [PubMed] [Google Scholar]
- 104.Wei Z.H., Shan Y.L., Tao L., et al. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol. Carcinog. 2017;56:2317–2331. doi: 10.1002/mc.22686. [DOI] [PubMed] [Google Scholar]
- 105.Na H.K., Kim E.H., Choi M.A. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem. Pharmacol. 2012;84:1241–1250. doi: 10.1016/j.bcp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 106.Xie X., Huang X., Tang H., et al. Diallyl disulfide inhibits breast cancer stem cell progression and glucose metabolism by targeting CD44/PKM2/AMPK signaling, Curr. Cancer Drug Targets. 2018;18:592–599. doi: 10.2174/1568009617666171024165657. [DOI] [PubMed] [Google Scholar]
- 107.Kim S.H., Kaschula C.H., Priedigkeit N., et al. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J. Biol. Chem. 2016;291:13495–13508. doi: 10.1074/jbc.M116.715219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liskova A., Kubatka P., Samec M., et al. Dietary phytochemicals targeting cancer stem cells. Molecules. 2019;24:899. doi: 10.3390/molecules24050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiao Y., Jiang X., Lee S.T., et al. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res. 2011;71:3076–3086. doi: 10.1158/0008-5472.CAN-10-2787. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H., Meng F., Liu G., et al. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;71:1292–1301. doi: 10.1158/0008-5472.CAN-10-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y.Q., Xue R., Jin X.C. Antiarthritic activity of diallyl disulfide against Freund’s adjuvant-induced arthritic rat model. J. Environ. Pathol. Toxicol. Oncol. 2018;37:291–303. doi: 10.1615/JEnvironPatholToxicolOncol.2018027078. [DOI] [PubMed] [Google Scholar]
- 112.Zhang N., Wang Y.L., Zhang J.L., et al. Diallyl disulfide attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, lipid peroxidation and inflammation in mice. Mol. Med. Rep. 2019;20:1363–1372. doi: 10.3892/mmr.2019.10316. [DOI] [PubMed] [Google Scholar]
- 113.Feng C., Luo Y., Nian Y., et al. Diallyl disulfide suppresses the inflammation and apoptosis resistance induced by DCA through ROS and the NF-κB signaling pathway in human barrett’s epithelial cells. Inflammation. 2017;40:818–831. doi: 10.1007/s10753-017-0526-4. [DOI] [PubMed] [Google Scholar]
- 114.Miltonprabu S., Sumedha N.C., Senthilraja P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017;50:107–120. doi: 10.1016/j.intimp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 115.Lee H.H., Jeong J.W., Hong S.H., et al. Diallyl trisulfide suppresses the production of lipopolysaccharide-induced inflammatory mediators in BV2 microglia by decreasing the NF-κB pathway activity associated with toll-like receptor 4 and CXCL12/CXCR4 pathway blockade. J. Cancer Prev. 2018;23:134–140. doi: 10.15430/JCP.2018.23.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J., Liu W., Yang H. Balancing apoptosis and autophagy for Parkinson's disease therapy: targeting BCL-2. ACS Chem. Neurosci. 2019;10:792–802. doi: 10.1021/acschemneuro.8b00356. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura S., Izumi M. Chlorophagy is ATG gene-dependent microautophagy process. Plant Signal. Behav. 2019;14 doi: 10.1080/15592324.2018.1558679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li L., Liu W.L., Su L. The role of autophagy in cancer radiotherapy. Curr. Mol. Pharmacol. 2020;13:31–40. doi: 10.2174/1874467212666190809154518. [DOI] [PubMed] [Google Scholar]
- 119.Suangtamai T., Tanyong D.I. Diallyl disulfide induces apoptosis and autophagy via mTOR pathway in myeloid leukemic cell line. Tumour Biol. 2016;37:10993–10999. doi: 10.1007/s13277-016-4989-y. [DOI] [PubMed] [Google Scholar]
- 120.Wu Y.Y., Hu Y.Q., Zhou H.Y. Organosulfur compounds induce cytoprotective autophagy against apoptosis by inhibiting mTOR phosphorylation activity in macrophages. Acta Biochim. Biophys. Sin. 2018;50:1085–1093. doi: 10.1093/abbs/gmy114. [DOI] [PubMed] [Google Scholar]
- 121.Yue Z.Q., Guan X., Chao R., et al. Diallyl disulfide induces apoptosis and autophagy in human osteosarcoma MG-63 cells through the PI3K/Akt/mTOR pathway. Molecules. 2019;24 doi: 10.3390/molecules24142665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deepak K.G.K., Vempati R., Nagaraju G.P. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020;153:104683. doi: 10.1016/j.phrs.2020.104683. [DOI] [PubMed] [Google Scholar]
- 123.Malla RR., Deepak K., Merchant N. Breast tumor microenvironment: emerging target of therapeutic phytochemicals. Phytomedicine. 2020;70:153227. doi: 10.1016/j.phymed.2020.153227. [DOI] [PubMed] [Google Scholar]
- 124.Horn N., Miller G., Ajuwon K.M., et al. Garlic diallyl disulfide and diallyl trisulfide mitigates effects of pro-oxidant induced cellular stress and has immune modulatory function in LPS-stimulated porcine epithelial cells. J. Anim. Sci. 2017;95:4045–4051. doi: 10.2527/jas2017.1546. [DOI] [PubMed] [Google Scholar]
- 125.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I. Neutrophil immunomodulatory activity of natural organosulfur compounds. Molecules. 2019;24:1809. doi: 10.3390/molecules24091809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hashizume Y., Shirato K., Abe I. Diallyl disulfide reduced dose-dependently the number of lymphocyte subsets and monocytes in rats. J. Nutr. Sci. Vitaminol. 2012;58:292–296. doi: 10.3177/jnsv.58.292. [DOI] [PubMed] [Google Scholar]
- 127.Ebrahimi M., Mohammad Hassan Z., Mostafaie A. Purified protein fraction of garlic extract modulates cellular immune response against breast transplanted tumors in BALB/c mice model. Cell J. 2013;15:65–75. [PMC free article] [PubMed] [Google Scholar]