Abstract
Neurite orientation dispersion and density imaging (NODDI) is a relatively new technique providing more detailed information on the microstructural bases of white matter. Given the previously reported white matter contributions to chronic pain, the aim of the present study is to investigate white matter tract by pain-specific differences in NODDI measures in a sample of community-dwelling older adults with and without chronic musculoskeletal pain while taking into consideration important confounders of this relationship. We further aimed to investigate associations between NODDI measures and clinical and experimental pain measures. As part of the Nepal study, a subset of older adults (>60 years old, n = 47), underwent multiple laboratory sessions providing self-reported and experimental pain measures and a diffusion weighted neuroimaging sequence. Older adults with chronic musculoskeletal pain (n = 29) had a lower neurite density with less geometric complexity across a number of white matter tracts compared to older pain-free controls (n = 18, corrected p’s < 0.05)). The neurite density measures were associated with greater self-reported pain intensity and anatomical pain sites, as well as greater experimental pain sensitivity (p’s < 0.05). There were also significant sex differences in neurite density and geometric complexity across multiple white matter tracts mainly around the hippocampus (corrected p’s < 0.05). Finally, there were no pain differences with respect to extra-cellular water diffusion (corrected p’s > 0.05). Our study demonstrates a reduction in neurite density with less geometric complexity in chronic musculoskeletal pain, with no evidence of neuroinflammatory processes, partly in a sex-dependent manner. An increased understanding of neurobiological mechanisms such as those measured by NODDI may contribute to the potential targeting of interventions in our older population suffering from chronic pain.
Introduction
Older adults often report chronic musculoskeletal pain that negatively impacts daily function and overall quality of life. Effective pain treatments for this growing segment of the population are limited and inadequate due to the lack of understanding of the neurobiological mechanisms underlying the multidimensional pain experience. A mechanistic understanding of pain within a given individual may ultimately provide personalized treatment targets. Given that the complex pain experience is sculpted by dynamic interactions in the brain, neuroimaging has significantly shed light into these mechanisms showing structural and functional alterations associated with chronic pain [1,2,31,32,42,43,49,52,57].
Of particular relevance to the field of pain and aging neurobiology is the ability to measure white matter (WM) microstructure using diffusion weighted imaging. A large number of studies suggest alterations in WM microstructure using a single tensor model with reported changes in fractional anisotropy (FA) measures across a number of tracts [28,29,33,35,36,38,51,54,58]. However, a recent systematic review reported inconclusive evidence in the direction of changes in white matter microstructure across various musculoskeletal pain conditions [25]. Contradictory findings across these studies may be due, in part, to the heterogeneity of individuals making up small sample sizes. Specifically, these samples often include a mixture of younger, middle- and older-age adults. A robust body of literature suggests that aging processes alone may significantly contribute to alterations in the brain’s WM microstructure, independent from pain-specific processes [23,27,34]. Thus, additional research is needed to examine WM microstructure within older adults with and without chronic pain.
The inability of FA to offer specificity with regards to the direction of changes in WM microstructure is a considerable source of variation that likely contributes to discrepancies in the pain imaging literature. The presence of crossing fibers and other complex geometric features in axonal projections hinders the straightforward interpretation of FA in relation to underlying microstructural changes. The widely applied single tensor model is insensitive to biophysical properties inherent to compartmentalized water that may otherwise inform on important aspects of tissue microstructure, such as the amount of axonal fibers, their orientation, and the relative proportion of free unhindered water. Decreases in FA may involve decreases in the density and/or increases in the dispersion orientation of neurites (i.e., dendrites and axons); and FA values are unable to differentiate between these possibilities [3]. Illustrating the importance of extending WM analyses beyond the diffusion tensor model and FA, a recent study indicated that chronic musculoskeletal pain is associated with lower complexity and density in several white matter tracts [6].
Neurite orientation dispersion and density imaging (NODDI) uses an alternative to the single tensor model to address some of the limitations of FA and provides relatively novel biomarkers that can better inform on WM microstructure [30,59]. NODDI provides estimates of intracellular volume fraction of water presumably in ‘neurites’ (neurite density index or NDI), extra-cellular unhindered water diffusion (isotropic volume fraction; ISO) and tract complexity or fanning (orientation dispersion index or OD) across individual tracts. While no study to date has examine NODDI measures in relation to pain, emerging evidence suggests age-specific alterations in NODDI measures. Overall, findings suggest that older age is significantly associated with lower tract complexity across the majority of tracts, reduced dendritic complexity or a regression of dendritic arborization, all indicative of less healthy white matter microstructure with older age [5,13,41,44]. Given the large age associations with widespread WM microstructure across tracts, NODDI may offer novel mechanistic insights into understanding white matter microstructure underlying pain and aging processes. Thus, the aim of the present study is to investigate specific tract-by-pain differences in NODDI measures in a sample of community-dwelling older adults with and without chronic musculoskeletal pain while taking into consideration important confounders of this relationship. We further aimed to investigate associations between NODDI measures and clinical and experimental pain measures in our older sample.
Materials and Methods
A. Participants
Community-dwelling older individuals (60 to 83 years old) who spoke and understood written English were recruited and screened for a larger study at the University of Florida (UF) focused on the neurobiology of age-related differences in pain modulation (Neuromodulatory Examination of Pain Across the Lifespan [NEPAL]). Potential participants were first screened over the phone and then again in person. We excluded individuals reporting any of the following conditions either over the phone screening or during the in-person baseline session: 1) Alzheimer’s, Parkinson’s or other neurological condition impacting the nervous system; 2) serious psychiatric conditions such as schizophrenia, major depression, bipolar disorder, 3) cardiovascular issues such as uncontrolled blood pressure over 150/95 mmHg, heart failure, or history of acute myocardial infarction; 4) systemic rheumatic conditions such as rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia; 5) chronic opioid use; 6) magnetic resonance imaging (MRI) contraindications such as any metal in the body; 7) excessive fear or anxiety regarding study procedures; 8) any recent hospitalization during the past 12 months for psychiatric illness; 9) self-reported HIV or AIDS; and 10) cognitive impairment as demonstrated by a score equal or less than 77 on the Modified Mini-Mental State Examination (3MS)[56]. Participants were recruited through posted fliers, newspaper ads, and word of mouth referrals. The NEPAL study aimed to recruit older adults representative of the healthy aging population with and without chronic pain, without targeting individuals with a specific chronic pain condition. Given the high prevalence of chronic pain in this population study advertisements were targeted to healthy older adults and were focused on studying brain aging processes. All procedures were reviewed and approved by the University of Florida’s Institutional Review Board and all participants provided verbal and written informed consent. We have previously reported on various aspects of the Nepal study participants and its detailed methodology [15,16,20,37]. To address the aims of the present study, we only included assessments of self-reported pain, depressive symptoms, a quantitative sensory testing battery and the diffusion weighted imaging sequence obtained from 3 separate experimental sessions.
A). Self-reported Pain Characteristics:
Older participants were categorized to the pain group if they reported pain on most days during the past 3 months as part of a standardized pain history interview. Using a body manikin part of the pain history, we calculated the number of anatomical painful sites reported by each participant. As part of the PainDETECT questionnaire, participants were asked:
How would you assess your pain now, at this moment?
How strong was the strongest pain during the past 4 weeks?
How strong was the pain during the past 4 weeks on average?
Responses are given using an 11-point numerical rating scale with anchors of none (0) and max. (10).
B). Depressive Symptoms:
The Center for Epidemiologic Studies Depression Scale (CES-D) questionnaire was used to measure the frequency of depressive symptoms during the past week on a 4-point Likert scale [50].
C). Experimental Pain
During the Quantitative Sensory Testing (QST) session, all participants were seated quietly in a temperature-controlled room. Research staff explained the study procedure to the participant prior to the start of testing. Although the Nepal study included assessment of non-painful QST measures, the present study was aimed at examining experimental pain profiles in older adults, thus, only the following QST painful measures were included in the present analysis.
Pressure Pain Threshold:
A handheld digital pressure algometer (Algomed, Medoc) was applied with increasing pressure until the participant indicated the pressure changed to a sensation of pain by clicking a button. Pressure pain threshold was applied at a constant rate at two standard non-painful locations in a counterbalanced order: quadriceps and trapezius. Testing was stopped at a maximum pressure of 1000 kPa. Pressure pain threshold was Z-transformed with higher Z-scores indicating higher pressure pain thresholds.
Cold Pain Threshold:
A 30 × 30 thermode attached to the TSA-II Neurosensory Analyzer was applied to the thenar eminence and metatarsal. Starting at a temperature of 32 degrees Celsius, the temperature gradually decreased at a rate of 1 degree Celsius/second until pain was first perceived. A cutoff value of 0°C was used. Immediately following each trial, the participant rated the pain intensity using a 101-point visual analogue scale. Cold pain threshold was Z-transformed and reversed so that a higher Z-value indicated a lower cold detection threshold.
Heat Pain Threshold:
A 30 × 30 thermode attached to the TSA-II Neurosensory Analyzer was applied to the thenar eminence and metatarsal. Starting at a temperature of 32 degrees Celsius, the temperature gradually increased at a rate of 1 degree Celsius/second until pain was first perceived. A cutoff value of 50°C was used. Immediately following each trial, the participant rated the pain intensity using a 101-point visual analogue scale. Heat pain threshold was Z-transformed so that a higher Z-value indicated a lower cold detection threshold.
Pinprick Pain:
Sterile, single use Neurotips® were applied twice to the thenar eminence and metatarsal to assess pinprick pain sensations. Participants rated the pain experienced at each site using a 101-point visual analogue scale of each single stimulus. Pain intensity ratings from the two trials were averaged for each site and Z-transformed so that higher Z-values were reflective of a greater pinprick pain.
Punctate Temporal Summation:
A 300-gram calibrated nylon monofilament was applied to the thenar eminence and metatarsal site in a randomized order. First, participants rated the intensity using a 101-point visual analogue scale of a single punctate stimulus. Second, a series of 10 punctate stimuli were administered at a rate of one contact per second while the participant verbally rated the highest pain intensity. Two trials were conducted and pain intensity ratings were averaged by site. Single pain ratings were subtracted from the average at each anatomical site. Temporal summation magnitude was Z-transformed and a higher Z-score indicated a higher temporal summation magnitude.
D). Neuroimaging
Magnetic resonance imaging
MRI data were acquired using a 3T MRI unit (Achieva, Philips Medical Systems, Best, Netherlands) and a 32-channel head coil. The head was secured via cushions positioned inside the head coil to minimize head movement. For the diffusion scan, the data acquisition parameters included: TR=4,840ms, TE=86ms, FOV=112×112×70, voxel dimensions=2mm3, transverse slice orientation (SENSE factor p=2). The diffusion weighted image series included one b=0, 6 b=100 and 64 b=1000 (s/mm2) images. We also collected a high-resolution three-dimensional T1-weighted scan in the same MRI coordinate space as the diffusion images using an MP-RAGE sequence for detailed anatomic assessment (sagittal plane, TR/TE/TI= 7/3.2/2750ms, flip angle=8°; in-plane FOV=240mmx240mm; imaging matrix 240×240; 170 contiguous sagittal slices with 1mm slice thickness, 1×1×1mm3 isotropic voxels).
Diffusion tensor image (DTI) processing
Diffusion MRI scans were processed using FMRIB software library, FSL [53]. Eddy correction was applied to adjust any slight head displacements that may have occurred during image acquisition. Images were aligned with the B0 scan. Motion correction vectors were used to update gradient directions. Diffusion images were skull stripped using FSL BET and maps of FA and mean diffusivity (MD) were generated using a weighted least squares regression on DTIFIT in FSL [4] (Figure 1).
Figure 1.
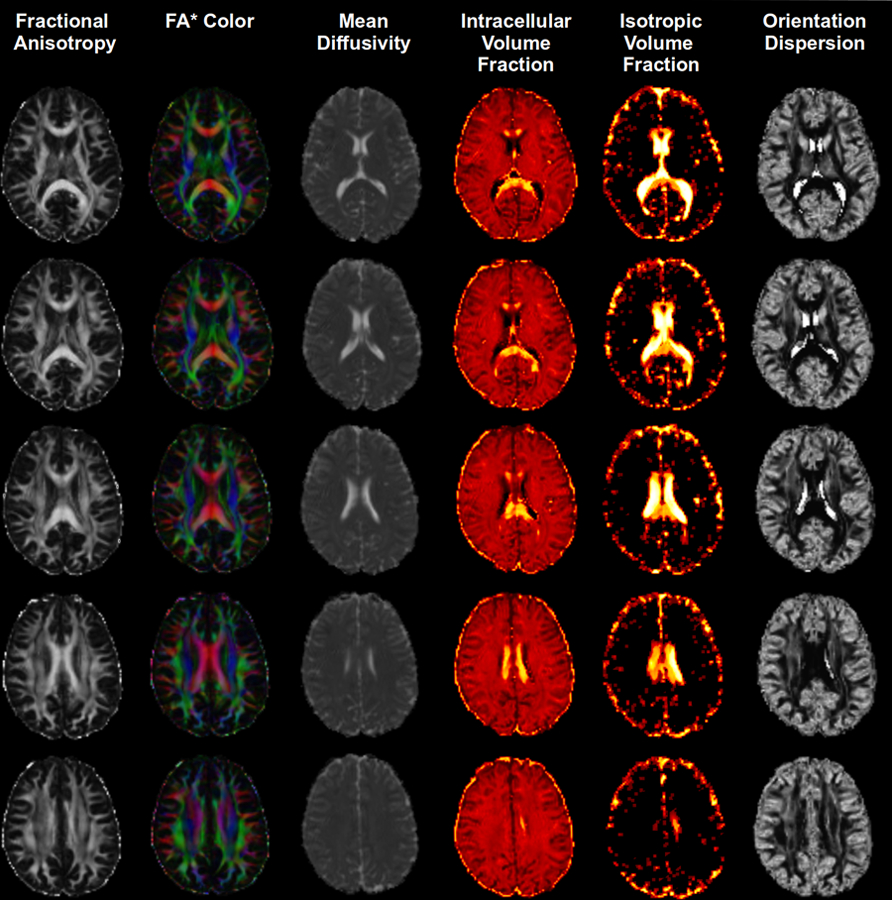
FA maps for the diffusion sequence for our sample.
NODDI processing
NODDI processing was applied to diffusion weighted images as implemented within the Accelerated Microstructure Imaging via Convex Optimization (AMICO) framework [21] and as previously described [11]. For the parameter fitting procedure, we assumed an intrinsic diffusivity of 1.7 µm2/ms and isotropic diffusivity of 3.0 µm2/ms [26]. The whole brain model-fitting generates maps of an intracellular volume fraction (ICVF: relative concentration of zero-radius cylinders modeling ‘neurites’), orientation dispersion (ODI: 0 for no dispersion as in highly organized parallel fiber bundles to a maximum of 1 for the highest degree of dispersion, as in cerebral cortical grey matter), and the isotropic free water fraction (ISO: 0 low isotropy to a maximum isotropy of 1) (Figure 1) [26]. FA maps were registered to the FMRIB58 FA template (2mm3 voxel resolution). Images were first linearly aligned to these templates using FSL linear registration tool FLIRT followed by non-linear warping using FNIRT. We used the Harvard-Oxford bilateral cortical and subcortical parcellation and the John Hopkins University white matter tract parcellation to measure OD, NDI, and ISO from a priori selected white matter regions known for their roles in various aspects of the pain experience. NODDI metrics were exported per ROI for statistical analyses and to determine the relationship with pain parameters (Figure 2).
Figure 2.
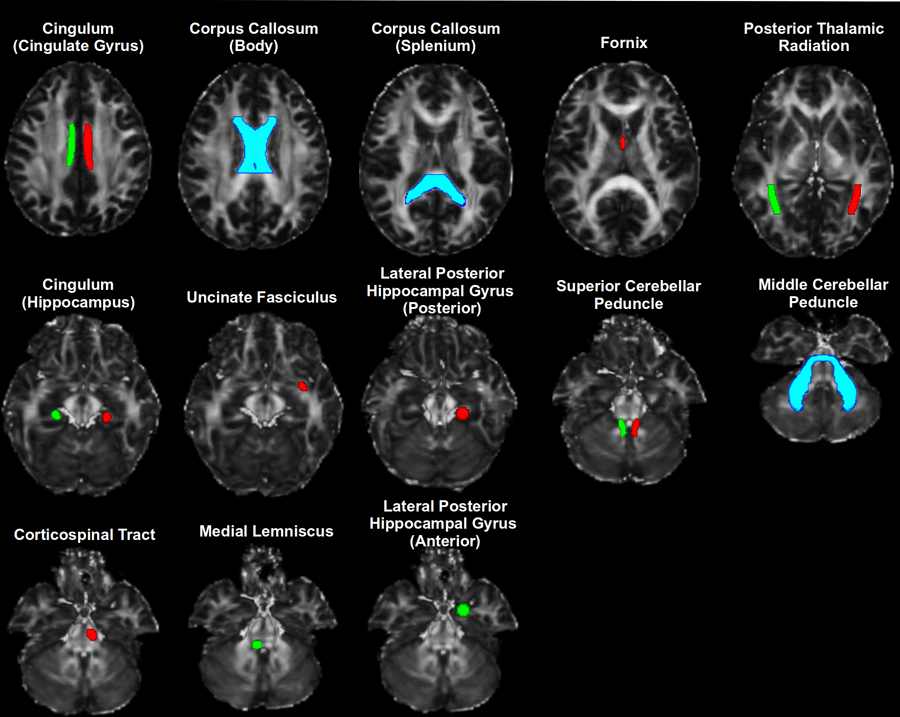
White matter tracts where NODDI measures survived Bonferroni correction.
Data and Statistical Analysis
To examine pain differences in NODDI measures, we employed a repeated measures generalized mixed model approach including 3-within (tracts, NODDI measures, and hemispheres) and 2-between (pain group (i.e., pain on most days during the past 3 months, sex) subject factors while accounting for age, EHI (i.e., handedness), MoCA (i.e., global cognitive function) and CES-D scores (i.e., depressive symptoms). We first ran a mixed-linear analysis of covariance (ANCOVA) model to control for the within-subject nature of the NODDI and brain hemisphere measures by including random effects for participant and participant X NODDI as well as participant X hemisphere interaction, with a variance components covariance structure and restricted maximum likelihood estimation. This model also allows for adjusting for relevant covariates. A global probability value was set at alpha = 0.05. Significant main and interaction effects were further explored by subsequent univariate analyses employing a General Linear Model approach with post-hoc Bonferroni corrections. Partial correlation coefficients were calculated to examine associations between individual NODDI measures with self-reported and experimental pain variables accounting for age, sex, handedness, cognitive function and depression. Results were considered significant only if they had a probability less than 0.05 that were supported by bias accelerated bootstrapped 95% confidence intervals.
Data Reduction of Experimental Pain Measures
Principal component analysis was employed to preserve global data structure while reducing the number of experimental pain variables and maximize the variance accounted for. The following Z-transformed QST variables at each test site were included in the analysis: cold pain threshold, heat pain threshold, cold pain ratings, heat pain ratings, pinprick pain detection, pressure pain threshold, and temporal summation. A principal component analysis with Varimax rotation identified components of QST measures. Per Kaiser’s rule, components with eigenvalues greater than 1 were retained. A scree plot was used to confirm the number of identified components. This approach is consistent with our previous work [8,14,17,18].
Results
A. Sample Characteristics
A total of 186 individuals were pre-screened over the telephone and in person for the Nepal study, with 85 individuals undergoing neuroimaging procedures. Of those participants, a subset (n = 47) underwent the last diffusion imaging sequence and comprise the present study sample. Most participants reported pain lasting at least 3 months on most days (61.7%), but the average pain duration was 10.1 years. The worst pain was most commonly located in the lower extremities (34.4%) and in the back regions (34.4%), and this was rated on average 4.8 out of 10 on a numerical rating scale. Table 1 summarizes demographic characteristics of our study sample.
Table 1.
Demographic characteristics of participants with and without chronic pain that underwent diffusion MRI as part of the Nepal study (n = 47).
| Older Controls (n = 18) |
Older Chronic Pain (n = 29) |
p-value | |
|---|---|---|---|
| Age, mean ± SD | 72.9 ± 6.8 | 71.1 ± 5.6 | 0.335 |
| Sex, no. (%) | 0.014 | ||
| Male | 10 (55.6) | 6 (20.7) | |
| Female | 8 (44.4) | 23 (79.3) | |
| Race, no. (%) | 0.022 | ||
| Caucasian | 18 (100) | 27 (93.1) | |
| African American | 0 (0) | 1 (3.4) | |
| Other | 0 (0) | 1 (3.4) | |
| MoCA, mean ± SD | 27.4 ± 2.0 | 26.2 ± 2.7 | 0.661 |
| CES-D, mean ± SD | 7.3 ± 5.4 | 8.0 ± 5.1 | 0.102 |
B. Whole Brain NODDI Measures by Pain Status
A repeated measure generalized mixed model was tested including 3-within (tracts, NODDI measures, and hemispheres) and 2-between (pain group (i.e., pain on most days during the past 3 months, sex) subject factors while accounting for age, EHI, MoCA and CES-D scores. There was a significant NODDI X hemisphere X pain X sex interaction (p = 0.001). These interactions were driven by pain-group main effects and pain by sex interaction effects in OD and NDI measures (p’s < 0.05) adjusting for age, EHI, MoCA and CES-D scores. Thus, post-hoc analyses were only examined within the OD and NDI measures.
B1. Pain and Sex Differences in OD
Univariate tests using a stringent post-hoc Bonferroni correction revealed that WM OD was significantly lower in those reporting pain compared to older pain-free controls in the right Posterior Thalamic Radiation (p = 0.015), the Fornix (p = 0.024), the Superior Cerebellar Peduncle (left: p = 0.016; right: p = 0.006), the Anterior Corona Radiata (p = 0.040), and the Uncinate Fasciculus (left: p = 0.026; right: p = 0.039). There were also significantly lower WM OD in older adults reporting pain compared to older pain-free controls in the Body (p = 0.024) and Splenium (p = 0.025) of the Corpus Callosum. Pain by sex interactions survived post-hoc Bonferroni correction where all male participants reporting pain had significantly lower WM OD values compared to control males in the Body (p = 0.011) and the Splenium of the Corpus Callosum (p = 0.016), the Hippocampus (Left: p = 0.024, Right: p = 0.011), the Posterior Thalamic Radiation (Left: p = 0.033, Right: p = 0.003), and the Superior Cerebellar Peduncle (left: p = 0.024; right: p = 0.036). Further, control males had significantly greater OD values compared to control females in the hippocampus (Left: p = 0.044, Right: p = 0.006). Results are summarized in Figure 3.
Figure 3.
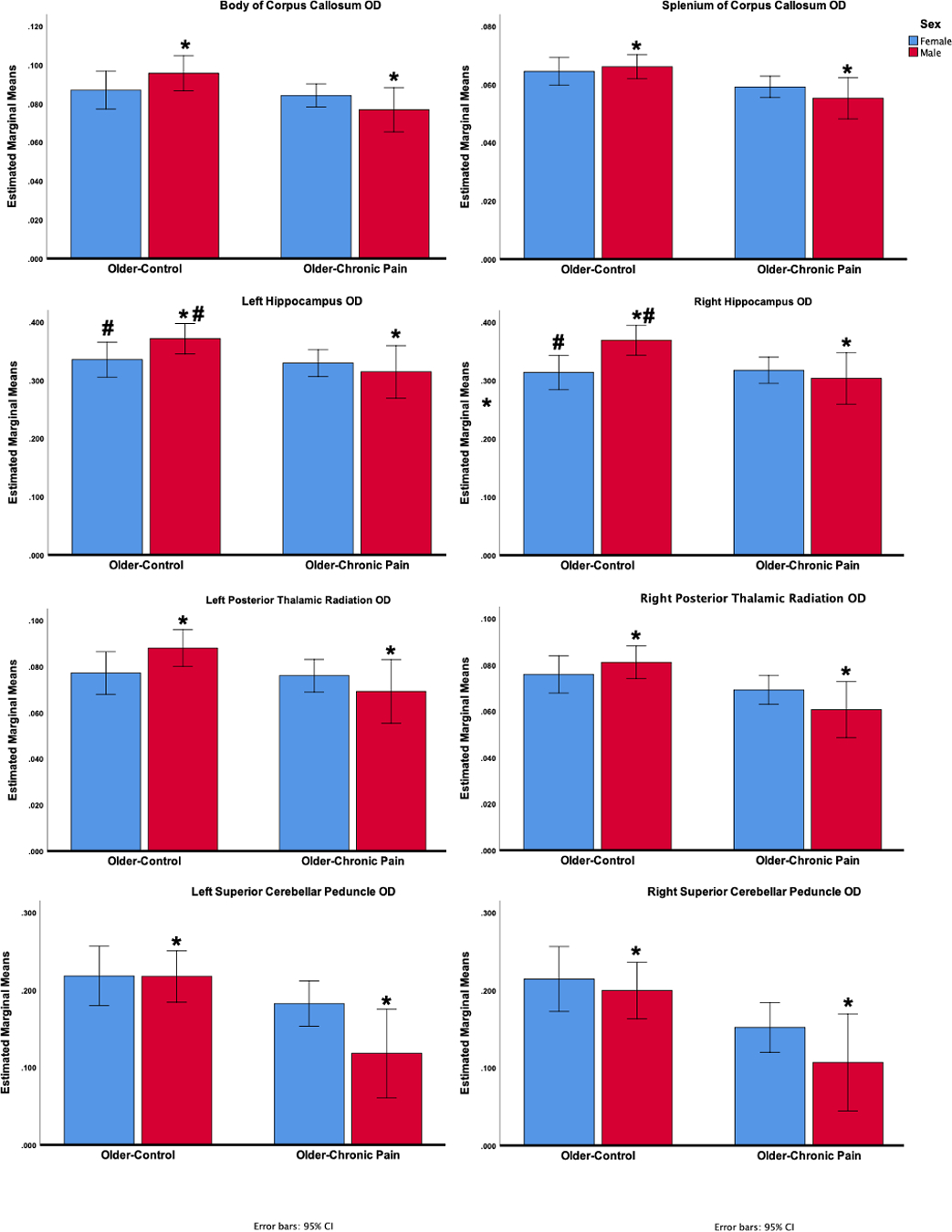
Pain by sex interactions in OD measures surviving Bonferroni corrections (mean estimates with 95% confidence intervals).
B2. Pain and Sex Differences in NDI
There were no significant main effects of pain on WM NDI across tracts, although there were significant pain by sex interactions. After adjusting for multiple comparisons using the Bonferroni procedure, older control males had significantly greater NDI values in the left and right cingulum-hippocampus (left: p = 0.040, right: p = 0.022), and the right medial lemniscus (p = 0.038) compared to older control females. In the older-pain, males had significantly lower NDI values in the left corticospinal tract (p = 0.039), the left anterior parahippocampal gyrus (p = 0.033), and the right anterior parahippocampal gyrus (p = 0.025) compared to females. Results are presented in Figure 4.
Figure 4.
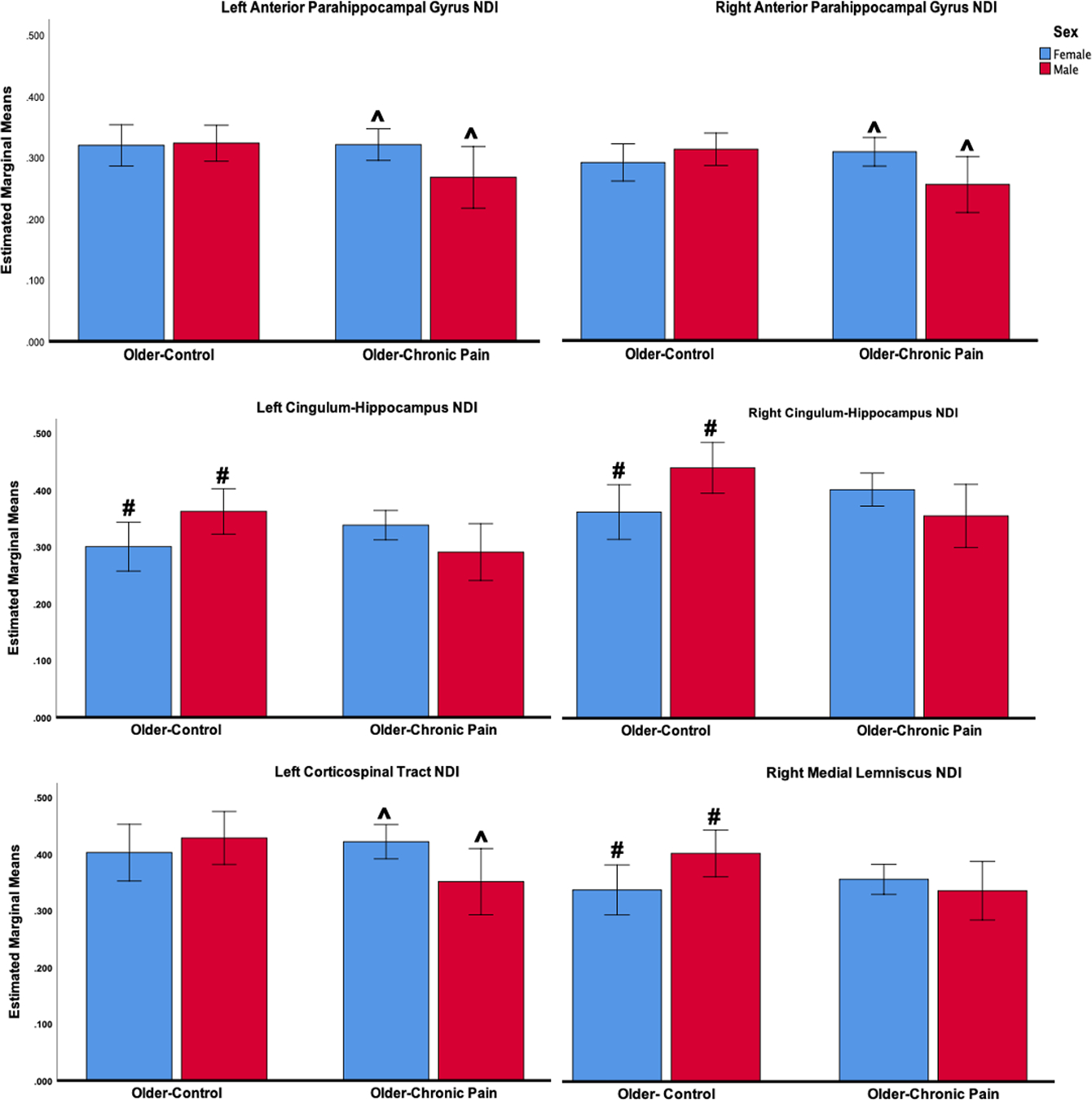
Pain by sex interactions in NDI measures surviving Bonferroni corrections (mean estimates with 95% confidence intervals).
C. NODDI Associations with Self-Reported Pain
Only statistically significant correlations that are supported by bias accelerated bootstrapped 95% confidence intervals are reported. Anatomical pain sites were significantly correlated with OD values in the superior cerebellar peduncle (left: r = −0.360, p = 0.019, Bootstrapped CI −0.612, −0.052; right: r = −0.453, p = 0.003, Bootstrapped CI −0.690, −0.173). Momentary pain ratings were significantly associated with OD values in the right Anterior Corona Radiata (r = −0.328, p = 0.034, Bootstrapped CI −0.607, −0.007).
Ratings of the strongest pain over the past 4 weeks were significantly associated with OD values in the right Anterior Corona Radiata (r = −0.486, p = 0.001, Bootstrapped CI −0.728, −0.182), the fornix (r = −0.318, p = 0.040, Bootstrapped CI −0.586, −0.042), the left posterior thalamic radiation (r = −0.325, p = 0.036, Bootstrapped CI −0.547, −0.034), the right posterior thalamic radiation (r = −0.449, p = 0.003, Bootstrapped CI −0.658, −0.162), and the right Superior Cerebellar Peduncle (r = −0.352, p = 0.022, Bootstrapped CI −0.619, −0.080). Average pain ratings during the past 4 weeks were significantly associated with OD values in the right Anterior Corona Radiata (r = −0.369, p = 0.016, Bootstrapped CI −0.642, −0.091), the right posterior thalamic radiation (r = −0.327, p = 0.034, Bootstrapped CI −0.553, −0.076), and the right superior cerebellar peduncle (r = −0.307, p = 0.048, Bootstrapped CI −0.541, −0.038). Although pain duration was significantly associated with multiple OD measures, these statistically significant associations did not survive bootstrapping procedures. None of the self-reported pain measures were significantly associated with NDI values across tracts. Results are presented in Figure 5.
Figure 5.
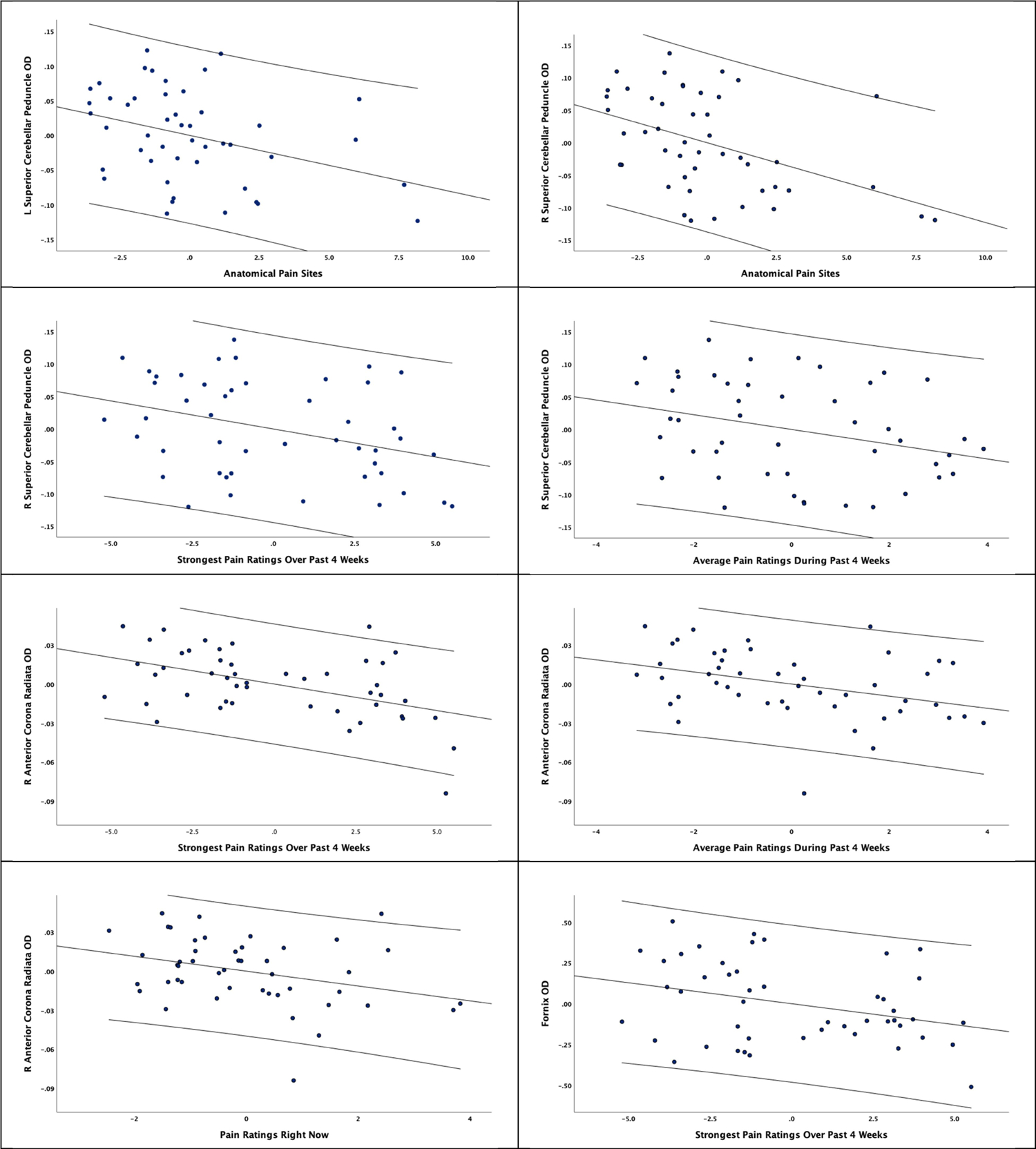
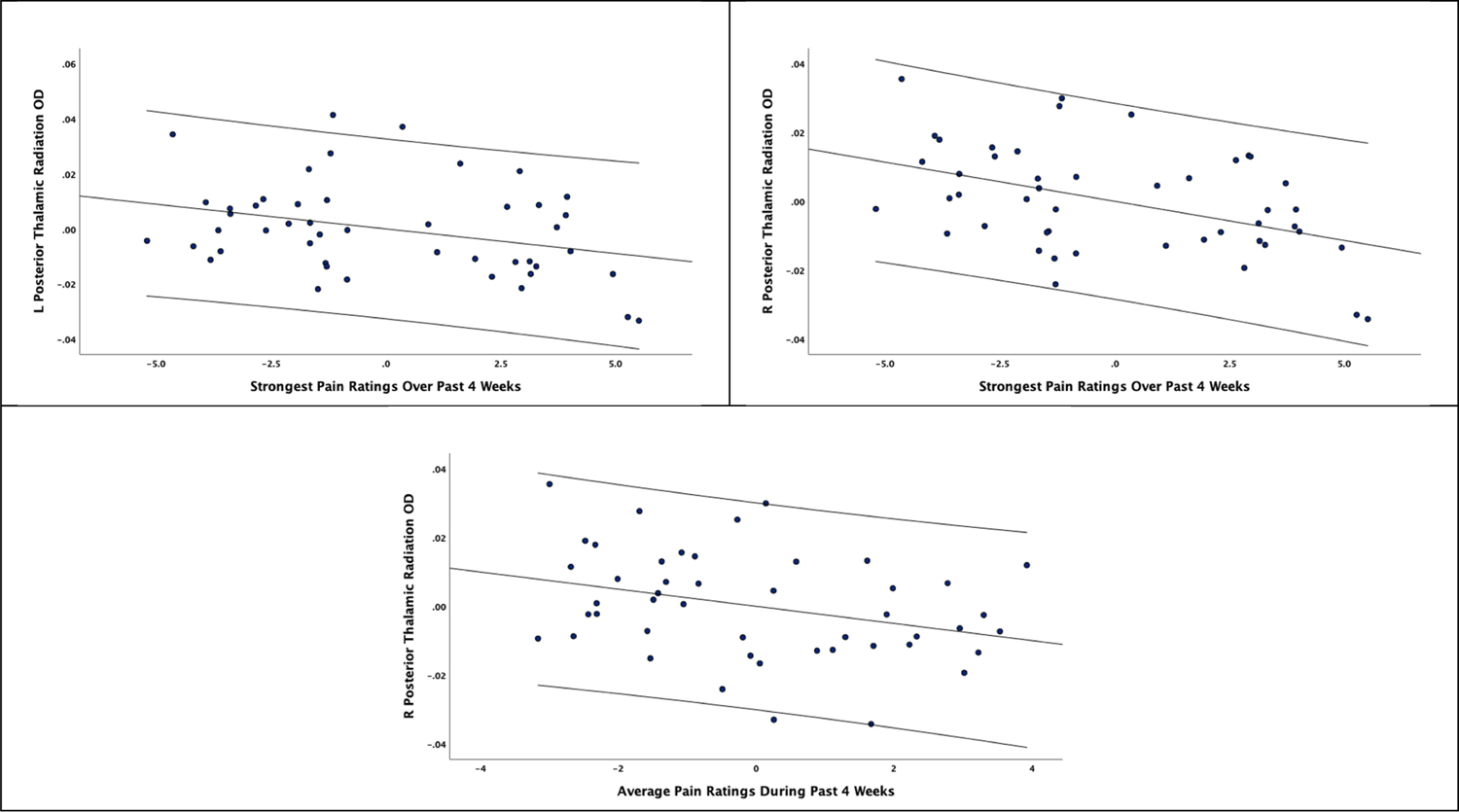
Partial correlation coefficients of OD associations with self-reported pain measures along with 95% confidence intervals.
D. NODDI Associations with Experimental Pain
D.1. Principal Component Analyses of Experimental Pain Measures
As shown in Table 3, five principal components were identified with a total of 79.6% variance explained. Component 1 was made up of Cold Pain Ratings and Heat Pain Ratings at the hand and foot. Component 2 was composed of Cold Pain Thresholds and Heat Pain Thresholds at the hand and foot. Component 3 was made up of Pinprick pain ratings at the hand and foot. Component 4 consisted of Punctate Temporal Summation at the hand and foot and Component 5 included Pressure Pain Thresholds at the quadriceps and trapezius muscles.
D.2. NODDI Associations with Experimental Pain Measures
Thermal pain thresholds were significantly associated with OD values in the posterior thalamic radiation (left: r = −0.375, p = 0.029, Bootstrapped CI −0.617, −0.033; right: r = −0.357, p = 0.038, Bootstrapped CI −0.626, −0.031), while pressure pain thresholds were significantly correlated with OD values in the left Uncinate Fasciculus (r = 0.346, p = 0.045, Bootstrapped CI 0.005, 0.612). No other OD measures were correlated with thermal pain ratings, pinprick pain and temporal summation after bootstrapping procedures. Thermal pain thresholds were only significantly correlated with the left corticospinal tract NDI value (r = −0.413, p = 0.015, Bootstrapped CI −0.630, −0.076).
Discussion
The aim of the present study was to investigate pain-specific differences in white matter microstructure employing various NODDI measures across multiple tracts in a sample of community-dwelling older adults. We further examined associations of significant NODDI measures with self-reported and experimental pain sensitivity measures. Several key findings emerged. First, older adults with chronic pain had a lower neurite density with less geometric complexity across a number of white matter tracts compared to older pain-free controls. Second, less geometric complexity in neurite density was associated with greater self-reported pain intensity and anatomical pain sites, as well as greater experimental pain sensitivity. Third, there were sex differences in neurite density and geometric complexity across multiple white matter tracts mainly around the hippocampus. Finally, there were no pain differences in our sample with respect to extra-cellular water diffusion or isotropic volume fraction.
To our knowledge, this is the first study employing a multi-shell diffusion approach to describe fiber orientation dispersion as well as densities within the same imaging voxel in older individuals with and without chronic musculoskeletal pain. NODDI relies on a biophysical model that separates the diffusion of water in brain tissue into three types of microstructural compartments: intra-neurite, extra-neurite, and cerebrospinal fluid (CSF) [59]. Our findings are consistent with the some of the existing literature where lower fractional anisotropy has been reported in persons with various chronic pain conditions [6,7,12,25,38], including older individuals with musculoskeletal pain [19]. However, it is not clear whether lower FA values are due to changes in the density and/or the dispersion orientation of neurites [3]. Our findings suggest that chronic pain in older adults is associated with a reduced geometrical complexity of neurite architecture across several white matter tracts compared to older pain-free controls surviving a stringent correction for multiple comparisons. The lower geometrical complexity in neurite architecture in those reporting chronic pain was observed across projection (i.e., corticospinal, anterior corona radiata, posterior thalamic radiation), association (i.e., uncinate fasciculus) and commissural fibers (i.e., corpus callosum) as well as fibers in the cerebellum (i.e., superior cerebellar peduncle) and the limbic system (i.e., cingulum, hippocampus, fornix). Further, the reduced geometrical complexity in neurite architecture was also significantly associated with a greater number of anatomical pain sites, greater self-reported momentary and average pain intensity as well as experimental thermal and pressure pain sensitivity. These multiple white matter tracts are involved in the sensory-motor and cognitive-emotional aspects of the complex, multidimensional pain experience. Similar findings in the white matter of the limbic and sensorimotor networks, the cerebellum and the corpus callosum have been previously reported in chronic musculoskeletal pain [6,7,12,38]. In a prospective one-year longitudinal study [38], baseline differences in white matter microstructure strongly predicted transition to chronic pain with minimal changes over the study period. Thus, differences across these tracts may represent pre-existing susceptibility that predisposes individuals to pain chronification and not changes specifically due to chronic pain. Future studies are needed to determine the temporal pattern of these changes in older individuals.
In addition, our control male participants had a greater geometrical complexity of neurite architecture compared to the control females and the male participants reporting chronic pain. Further, the male participants reporting chronic pain also had a reduced geometrical neurite architecture complexity compared to the female participants reporting chronic pain. Interestingly, most of these sex differences were observed across hippocampal tracts. Our findings are consistent with previously reported sex differences in white matter microstructure pathways to and from the hippocampus, as reflected by higher fractional anisotropy in males compared to females [9]. Further, women show a greater prevalence of chronic pain [24] and are also more likely to experience greater cognitive decline in Alzheimer’s disease [40], but to date there is no literature examining pain by sex interaction in white matter microstructure. Thus, some of these differences are likely a combination of basal sex differences in hippocampal structure and function as well as sex differences in response to stressors such as pain that are known to impact hippocampal plasticity [10,22,39,45]. Future studies focused on pain and sex interactions in brain microstructure are needed to gain a better understanding of underlying neurobiological mechanisms that may be useful for targeting pain treatments.
Finally, there were no pain differences in our sample with respect to extra-cellular water diffusion or isotropic volume fraction. While older age has been previously associated with greater extracellular free-water volume [55], to our knowledge, pain differences in these measures have not been reported to date. Extracellular free-water volume is similar to the isotropic volume fraction metric obtained from NODDI, and is thought to increase with neuroinflammatory processes [47,48], although it may also increase because of other factors such as neurodegeneration [46]. Therefore, our study suggests that neuroinflammatory or neurodegenerative processes may not contribute to chronic musculoskeletal pain in our sample of cognitively healthy community-dwelling older adults.
Our findings must be interpreted with caution. First, our cross-sectional study design cannot determine whether the white matter differences are pre-existing or are due to pain-specific processes. Second, our participants are cognitively intact, older individuals recruited from the community, reporting mild to moderate levels of pain, thus, they may not be representative of the older adults that experience more severe levels of musculoskeletal pain or older individuals with lower cognitive function. Third, most of our participants were non-Hispanic white individuals, thus, it is not clear whether these findings would be similar in persons of other race and ethnicities. Future longitudinal studies with larger samples of males and females, including individuals from diverse backgrounds, with a broader range of cognitive function and more severe pain levels are needed to replicate and extend our findings.
In conclusion, our study further demonstrates that white matter abnormalities are present in older individuals with chronic musculoskeletal pain; either as a cause, predisposing factor, consequence, or compensatory mechanism. Our results suggest a reduction in neurite density with less geometric complexity in chronic musculoskeletal pain, with no evidence of neuroinflammatory processes, partly in a sex-dependent manner. An increased understanding of neurobiological mechanisms such as those measured by NODDI may contribute to the potential targeting of interventions in our older population suffering from chronic pain.
Figure 6.
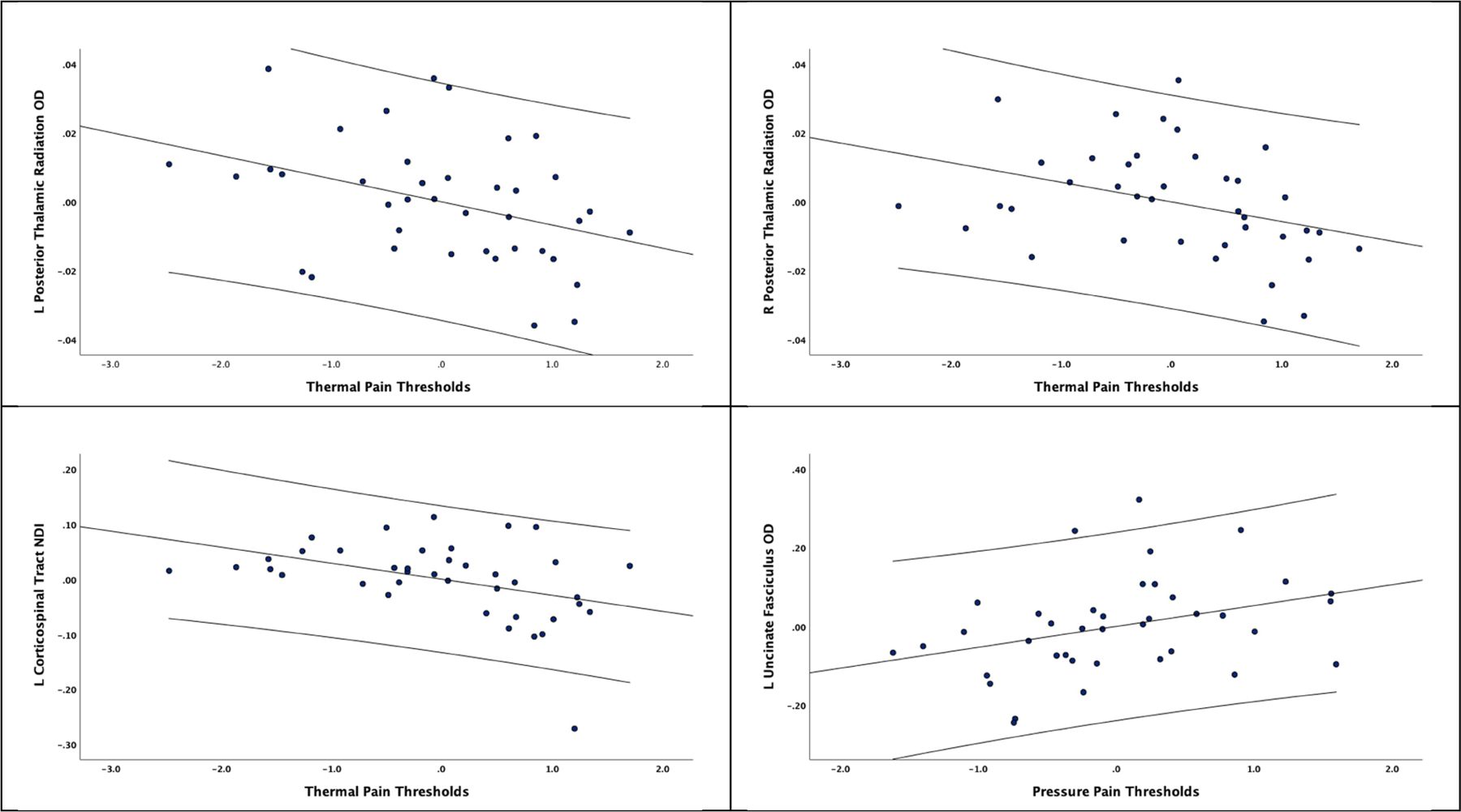
Partial correlation coefficients of OD and NDI associations with experimental pain measures along with 95% confidence intervals.
Table 2:
Principal components analysis: loadings and eigenvalues of experimental pain measures.
| Thermal Pain Ratings | Thermal Pain Thresholds | Pinprick Pain Ratings | Punctate Temporal Summation | Pressure Pain Thresholds | |
|---|---|---|---|---|---|
| CPR Hand | 0.861 | ||||
| HPR Hand | 0.849 | ||||
| HPR Foot | 0.847 | ||||
| CPR Foot | 0.824 | ||||
| CPT Hand | −0.809 | ||||
| HPT Foot | 0.778 | ||||
| CPT Foot | −0.773 | ||||
| HPT Hand | 0.743 | ||||
| Pinprick Hand | 0.927 | ||||
| Pinprick Foot | 0.919 | ||||
| TS Hand | 0.910 | ||||
| TS Foot | 0.834 | ||||
| PPT quadriceps | 0.909 | ||||
| PPT Trapezius | 0.804 | ||||
|
| |||||
| % Variance | 22.954 | 18.423 | 13.797 | 12.546 | 11.906 |
| % Cumulative Variance | 22.954 | 41.378 | 55.175 | 67.121 | 79.629 |
Acknowledgements:
We are grateful to our volunteers for their participation and the NEPAL study team (Paige Lysne, Lorraine Hoyos, Darlin Ramirez, Brandon Apagueno and Rachna Sannegowda). This work was supported by the National Institutes of Health (NIA awards K01AG048259, R01AG059809, R01AG067757), the University of Florida Clinical Translational Sciences Institute (NCATSUL1TR001427) the Cognitive Aging & Memory Clinical Translational Program, McKnight Brain Foundation, the University of Florida Claude D. Pepper Older Americans Independence Center (P30 AG028740).
References
- [1].Beaulieu C The basis of anisotropic water diffusion in the nervous system - A technical review. NMR Biomed 2002;15. [DOI] [PubMed] [Google Scholar]
- [2].Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging. Magn Reson Med 2003;50. [DOI] [PubMed] [Google Scholar]
- [3].Billiet T, Vandenbulcke M, Mädler B, Peeters R, Dhollander T, Zhang H, Deprez S, Van den Bergh BRH, Sunaert S, Emsell L. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 2015;36. [DOI] [PubMed] [Google Scholar]
- [4].Bishop JH, Shpaner M, Kubicki A, Clements S, Watts R, Naylor MR. Structural network differences in chronic muskuloskeletal pain: Beyond fractional anisotropy. Neuroimage 2018. [DOI] [PubMed] [Google Scholar]
- [5].Buckalew N, Haut MW, Aizenstein H, Rosano C, Edelman KD, Perera S, Marrow L, Tadic S, Venkatraman V, Weiner D. No Title 2013;5. doi: 10.1016/j.pmrj.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cardoso JS, Riley JL, Glover T, Sibille KT, Bartley EJ, Goodin BR, Bulls HW, Herbert M, Addison AS, Staud R, Redden DT, Bradley LA, Fillingim RB, Cruz-Almeida Y. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain 2016;157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou KH, Cheng Y, Chen IY, Lin CP, Chu WC. Sex-linked white matter microstructure of the social and analytic brain. Neuroimage 2011. [DOI] [PubMed] [Google Scholar]
- [8].Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus 2006. [DOI] [PubMed] [Google Scholar]
- [9].Colon-Perez LM, Ibanez KR, Suarez M, Torroella K, Acuna K, Ofori E, Levites Y, Vaillancourt DE, Golde TE, Chakrabarty P, Febo M. Neurite orientation dispersion and density imaging reveals white matter and hippocampal microstructure changes produced by Interleukin-6 in the TgCRND8 mouse model of amyloidosis. Neuroimage 2019;202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Coppieters I, Meeus M, Kregel J, Caeyenberghs K, De Pauw R, Goubert D, Cagnie B. Relations Between Brain Alterations and Clinical Pain Measures in Chronic Musculoskeletal Pain: A Systematic Review. J Pain 2016;17:949–962. doi: 10.1016/j.jpain.2016.04.005. [DOI] [PubMed] [Google Scholar]
- [11].Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cruz-Almeida Y, Cardoso J, Riley JL, Goodin B, King CD, Petrov M, Bartley EJ, Sibille KT, Glover TL, Herbert MS, Bulls HW, Addison A, Staud R, Redden D, Bradley LA, Fillingim RB. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol 2017;98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cruz-Almeida Y, Cole J. Pain, aging and the brain: new pieces to a complex puzzle 2019;00:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cruz-Almeida Y, Fillingim RB, Riley JL, Woods AJ, Porges E, Cohen R, Cole JH, Riley J III, Adam W, Porges E, Cohen R, Cole JH. Chronic Pain is Associated with a Brain Aging Biomarker in Community-Dwelling Older Adults. Pain 2019;160:1119–1130. doi: 10.1097/j.pain.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res 2013;65:1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cruz-Almeida Y, Riley JL, Fillingim RB. Experimental pain phenotype profiles in a racially and ethnically diverse sample of healthy adults. Pain Med (United States) 2013;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cruz-Almeida Y, Rosso A, Marcum Z, Harris T, Newman AB, Nevitt M, Satterfield S, Yaffe K, Rosano C. Associations of Musculoskeletal Pain With Mobility in Older Adults: Potential Cerebral Mechanisms. J Gerontol A Biol Sci Med Sci 2017;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cruz-Almeida Y, Sinha P, Rani A, Huo Z, Fillingim RBR, Foster T. Epigenetic Aging is Associated with Clinical and Experimental Pain in Community-Dwelling Older Adults. Mol Pain 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 2015;105. [DOI] [PubMed] [Google Scholar]
- [20].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH, Fagan M, Andrew RD. - Neurogenesis in the adult human hippocampus - Intracellular study of calcium-related events in cat magnocellular neuroendocrine cells. Nat Med 1998. [Google Scholar]
- [21].Farokhian F, Yang C, Beheshti I, Matsuda H, Wu S. Age-related gray and white matter changes in normal adult brains. Aging Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fillingim RB. Biopsychosocial contributions to sex differences in pain. BJOG An Int J Obstet Gynaecol 2015;122. [DOI] [PubMed] [Google Scholar]
- [23].Gronemann DC, Koch K, Bantel C, Soros P. Diffusion tensor imaging of white matter microstructure in chronic pain: a tract-based spatial statistics study and a systematic review 2020. [Google Scholar]
- [24].Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianuş A, Yiannakas MC, Newcombe J, Zhang H, Alexander DC, DeLuca GC, Gandini Wheeler-Kingshott CAM. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol 2017;4:663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. NIH Public Access, 2009. 109–17 pp. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex 2010. [DOI] [PubMed] [Google Scholar]
- [27].Kim DJ, Lim M, Kim JS, Son KM, Kim HA, Chung CK. Altered white matter integrity in the corpus callosum in fibromyalgia patients identified by tract-based spatial statistical analysis. Arthritis Rheumatol 2014. [DOI] [PubMed] [Google Scholar]
- [28].Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu YC. Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI, and q-space study. Neuroimage 2016;128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lieberman G, Shpaner M, Watts R, Andrews T, Filippi CG, Davis M, Naylor MR. White matter involvement in chronic musculoskeletal pain 2014;15:1110–1119. doi: 10.1016/j.jpain.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu P, Wang G, Liu Y, Yu Q, Yang F, Jin L, Sun J, Yang X, Qin W, Calhoun VD. White matter microstructure alterations in primary dysmenorrhea assessed by diffusion tensor imaging. Sci Rep 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lutz J, Jäger L, De Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion-tensor and volumetric imaging study. Arthritis Rheum 2008;58:3960–3969. [DOI] [PubMed] [Google Scholar]
- [33].Lysne P, Cohen R, Hoyos L, RBRB Fillingim, Riley JLJL, Cruz-Almeida Y. Age and pain differences in non-verbal fluency performance: Associations with cortical thickness and subcortical volumes. Exp Gerontol 2019;126:110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Vania Apkarian A. Brain white matter structural properties predict transition to chronic pain. Pain 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McEwen BS. Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Front Neuroendocrinol 2018. [DOI] [PubMed] [Google Scholar]
- [36].McPherson S, Back C, Buckwalter JG, Cummings JL. Gender-related cognitive deficits in Alzheimer’s disease. Int Psychogeriatrics 1999. [DOI] [PubMed] [Google Scholar]
- [37].McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: The role of GABA signaling. Trends Mol Med 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Merluzzi AP, Dean DC, Adluru N, Suryawanshi GS, Okonkwo OC, Oh JM, Hermann BP, Sager MA, Asthana S, Zhang H, Johnson SC, Alexander AL, Bendlin BB. Age-dependent differences in brain tissue microstructure assessed with neurite orientation dispersion and density imaging. Neurobiol Aging 2016;43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014;111:1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012;32:5747–56. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nazeri X, Chakravart M, Rotenberg DJ, Rajji TK, Rathi X, Michailovich OV., Voineskos AN. Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. J Neurosci 2015;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci 2008. [DOI] [PubMed] [Google Scholar]
- [43].Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, Lai S, Okun MS, Vaillancourt DE. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain 2015;138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62. [DOI] [PubMed] [Google Scholar]
- [45].Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 2012;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Radloff LS. The CES-D Scale. Appl Psychol Meas 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- [47].Van Riper SM, Alexander AL, Koltyn KF, Stegner AJ, Ellingson LD, Destiche DJ, Dougherty RJ, Lindheimer JB, Cook DB. Cerebral white matter structure is disrupted in Gulf War Veterans with chronic musculoskeletal pain. Pain 2017. [DOI] [PubMed] [Google Scholar]
- [48].Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006;123. [DOI] [PubMed] [Google Scholar]
- [49].Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- [50].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, Vol. 23. [DOI] [PubMed] [Google Scholar]
- [51].Sundgren PC, Petrou M, Harris RE, Fan X, Foerster B, Mehrotra N, Sen A, Clauw DJ, Welsh RC. Diffusion-Weighted and Diffusion Tensor Imaging in Fibromyalgia Patients: A Prospective Study of Whole Brain Diffusivity, Apparent Diffusion Coefficient, and Fraction Anisotropy in Different Regions of the Brain and Correlation With Symptom Severity. Acad Radiol 2007. [DOI] [PubMed] [Google Scholar]
- [52].Tanner JJ, Amin M, Hardcastle C, Parvataneni H, Vaillancourt DE, Mareci TH, Price CC. Better brain and cognition prior to surgery is associated with elevated postoperative brain extracellular free-water in older adults. Front Aging Neurosci 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Teng EL, Chui HC. The Modified Mini-Mental State (MMS) examination. J Clin Psychiatry 1987. [PubMed] [Google Scholar]
- [54].Yoon EJ, Kim YK, Shin HI, Lee Y, Kim SE. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res 2013. [DOI] [PubMed] [Google Scholar]
- [55].Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61. [DOI] [PubMed] [Google Scholar]


