Abstract
Actinomycete isolates from indoor air and dust in water-damaged schools and children’s day care centers were tested for toxicity by using boar spermatozoa as an indicator. Toxicity was detected in extracts of four strains which caused a loss of sperm motility, and the 50% effective concentrations (EC50) were 10 to 63 ng (dry weight) ml of extended boar semen−1. The four strains were identified as Streptomyces griseus strains by 16S ribosomal DNA and chemotaxonomic methods. The four S. griseus strains had similar effects on sperm cells, including loss of motility and swelling of mitochondria, but we observed no loss of plasma membrane integrity or depletion of cellular ATP. None of the effects was observed with sperm cells exposed to extracts of other indoor actinomycete isolates at concentrations of ≥5,000 to 72,000 ng ml−1. The toxin was purified from all four strains and was identified as a dodecadepsipeptide, and the fragmentation pattern obtained by tandem mass spectrometry was identical to that of valinomycin. Commercial valinomycin had effects in sperm cells that were identical to the effects of the four indoor isolates of S. griseus. The EC50 of purified toxin from the S. griseus strains were 1 to 3 ng ml of extended boar semen−1, and the EC50 of commercial valinomycin was 2 ng ml of extended boar semen−1. To our knowledge, this is the first report of the presence of ionophoric toxin producers in an indoor environment and the first report of valinomycin-producing strains identified as S. griseus.
Building materials exposed to prolonged and/or repeated moisture damage are inhabited by complex microbial communities that include bacteria and fungi. Workers have searched for mycotoxins, particularly satratoxin, in indoor environments, because Stachybotrys chartarum has been linked to damage to health in houses with moisture problems (9, 10). Bacterial toxins have received little attention as hazardous agents in indoor environments. We searched for bacterial toxins in indoor environments by using boar spermatozoa as indicator cells (2). In this paper we describe Streptomyces griseus strains that emit a toxin in indoor air and in indoor dust. This toxin caused mitochondrial damage similar to the previously observed damage caused by extracts obtained from a water-damaged indoor wall in a children’s day care center (2). The toxin from Streptomyces isolates was purified and identified, and its biochemical effects were studied.
MATERIALS AND METHODS
Media and reagents.
Nodularin was purified from Nodularia sp. strain BY1 as described previously (4); synthetic anatoxin A was obtained from Calbiochem-Novabiochem Corp. (La Jolla, Calif.), and enniatin was obtained from Fluka (Buchs, Switzerland). The other commercially available reference toxins and chemicals used were obtained from the sources described elsewhere (3). Cereulide was purified from Bacillus cereus 4810 and F-5881 as described previously (3). Other chemicals were of analytical quality and were obtained from local sources.
Isolation of actinomycetes from air, dust, and building materials.
Actinomycetes were collected from air by using an Andersen sampler and tryptic soy agar. The plates were incubated at 22°C for 2 weeks. Strains were isolated from dust and building materials by using resuscitation media as described previously (2). Bacteria were cultivated for toxin production on tryptic soy agar at 28°C.
Identification.
Whole-cell fatty acids were analyzed as described by Nohynek et al. (17). The actinomycete isolates were identified by using procedures described by Rainey et al. (20) and Hain et al. (8). Genomic DNA extraction, PCR-mediated amplification of the 16S ribosomal DNA (rDNA), and purification of PCR products were carried out by using procedures described by Rainey et al. (20). Purified PCR products were sequenced with a Taq Dye-Deoxy terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) as recommended in the manufacturer’s protocol. An Applied Biosystems model 310 DNA genetic analyzer was used for electrophoresis of the sequence reaction products. Partial 16S rDNA sequences were determined by sequencing 16S rDNA PCR products with primer 27 F. The partial 16S rDNA sequences were aligned with sequences of members of the Actinomycetales by using the ae2 editor (12), and pairwise similarity values were determined.
Purification and analysis of the toxin from S. griseus strains.
Cells were harvested after 10 to 12 days and were extracted with methanol; the methanol was evaporated, and the residue was diluted in methanol (3). The extracts were tested for toxic effects (inhibition of spermatozoan motility, loss of plasma membrane integrity, decrease in cellular ATP level, swelling of mitochondria) by using protocols described previously (3). Methanol extracts of the S. griseus isolates were diluted 1:9 with water and injected into a Sep-pak C18 cartridge (Waters Co., Milford, Mass.). The cartridge was eluted with methanol-water (90:10) and with 100% methanol. The methanol extracts were evaporated to dryness, dissolved in acetonitrile-water (90:10) containing 0.075% trifluoroacetic acid (TFA), and fractionated by reverse-phase high-performance liquid chromatography (HPLC) (Smart; Pharmacia Biotech, Uppsala, Sweden) by using Sephasil C8 SC 5 μm columns (2.1 mm [inside diameter] by 100 mm). The eluents used were water containing 0.1% TFA (eluent A) and acetonitrile containing 0.075% TFA (eluent B). A 5-min gradient from 10% (vol/vol) eluent A–90% (vol/vol) eluent B to 100% eluent B was used. The flow rate was 100 μl/min, and detection was at 215 nm.
MS analyses.
Electrospray (ESI) mass spectra were obtained with a model API300 triple quadrupole mass spectrometer (MS) (Perkin-Elmer Sciex Instruments, Thornhill, Ontario, Canada). The samples were dissolved in 50% methanol containing 5 mM ammonium acetate and were injected into the MS with a nanoelectrospray ion source (Protana A/S, Odense, Denmark) at a flow rate of about 30 nl/min. MS-MS spectra were obtained by colliding selected precursor ions with nitrogen collision gas with acceleration voltages of 45 to 55 V.
Nucleotide sequence accession numbers.
The partial 16S rDNA sequences determined in this study have been deposited in the EMBL data library under the following accession numbers: strain 8/ppi, Y17513; strain 10/ppi, Y17514; strain 123, Y17515; strain 157, Y17516; strain 703, Y17517; strain 147, Y17518; and strain 148, Y17519.
RESULTS
Identification of actinomycetes isolated from indoor environment.
Strains of actinomycetes were isolated from indoor building materials, from settled dust, and from air from water-damaged school buildings, children’s day care centers (in Helsinki, Finland), and animal sheds (in Uusimaa County, Finland). Twelve strains were identified by chemotaxonomic methods and by 16S rDNA sequencing as members of the genera Streptomyces, Nocardiopsis, and Dietzia (Table 1).
TABLE 1.
Toxicity to boar spermatozoa of methanol-soluble substances from actinomycetes isolated from indoor environments
| Strain | Toxicity to boar spermatozoaa
|
Source of strainb | |
|---|---|---|---|
| EC50 of methanol-soluble substances (ng ml−1) | EC50 of cell extract (mg ml−1) | ||
| Indoor isolates | |||
| S. griseus 2/ppi | 20 | 0.0005 (105)c | Children’s day care center (settled dust) |
| S. griseus 8/ppi | 50 | 0.001 (106) | Children’s day care center (settled dust) |
| S. griseus 10/ppi | 10 | 0.0009 (106) | Children’s day care center (settled dust) |
| S. griseus 1/k | 63 | 0.002 (106) | Elementary school (air) |
| Nocardiopsis albus 123 | 7,500 | 0.5 (108) | Children’s day care center (water-damaged building material) |
| S. griseus 157 | 59,000 | >9 (>109) | Children’s day care center (water-damaged building material) |
| Dietzia sp. strain 147 | >72,000 | >1 (>109) | Children’s day care center (water-damaged building material) |
| Dietzia sp. strain 148 | >86,000 | >1 (>109) | Children’s day care center (water-damaged building material) |
| Nocardiopsis dassonvillei 305 | >10,000 | >0.3 (>108) | Cattle barn (air) |
| Streptomyces albidoflavus 703 | 36,000 | 1 (108) | Cattle barn (settled dust) |
| Nocardiopsis dassonvillei 704 | >40,000 | >0.5 (>108) | Cattle barn (air) |
| Reference strains | |||
| S. griseus DSM 40236T | 26,000 | 1 (108) | DSMZ |
| Dietzia maris N 1009T | <36,000 | >1 (>108) | DSMZ |
| Bacillus mycoides ATCC 6462T | 67,000 | 2.5 (108) | ATCC |
| B. cereus ATCC 14569T | 16,000 | 1 (108) | ATCC |
| B. cereus 4810/72c | 28 | 0.002 (106) | Food poisoning (emetic) |
The EC50 is the concentration that paralyzed more than 50% of the sperm cells.
DSMZ, Deutsche Sammlung von Mikroorganism und Zellkulturen GmbH; ATCC, American Type Culture Collection.
The numbers in parentheses are the equivalent numbers of cells.
Data from reference 3.
Partial 16S rDNA sequences comprising around 400 nucleotides from the 5′ end of the 16S RNA gene were determined for strains 8/ppi, 1/k, 2/ppi, 10/ppi, 123, 157, 703, 704, 305, 147, and 148. The partial 16S rDNA sequences of strains 10/ppi, 8/ppi, 1/k, 10/ppi, and 157 exhibited showed the highest levels of similarity (>99%) to the 16S rDNA sequence of S. griseus (accession no. M76388). The chemotaxonomic properties of strains 2/ppi, 8/ppi, 10/ppi, and 1/k were diagnostic for all members of the genus Streptomyces, as follows: the whole-cell fatty acid composition is dominated by iso and anteiso fatty acids, and ll-diaminopimelic acid is the diamino acid of the peptidoglycan. The strains had all of the conventional markers for S. griseus, including straight chains of yellow spores and no melanin production. S. griseus was the species most frequently isolated from air in a water-damaged school (viable count, 60 CFU m−3) and from dust (viable count, 50 CFU mg−1) in water-damaged schools and children’s day care centers.
Indoor strains of S. griseus produce a mitochondrial toxin.
Cultures of indoor actinomycete isolates were extracted with methanol, and the extracts were tested by using boar spermatozoa. Table 1 shows that substances extracted from three dust-borne S. griseus strains from a children’s day care center (10/ppi, 2/ppi, and 8/ppi) and one airborne S. griseus strain (1/k) from an elementary school caused a loss of sperm motility at cell extract concentrations equivalent to 10 to 60 ng of methanol-soluble solids per ml of extended boar semen. This amount was extracted from 0.0005 to 0.002 mg of S. griseus cells (equivalent to 105 to 106 CFU). The toxicity thresholds for the indoor Streptomyces isolates were of the same order of magnitude as the toxicity threshold observed for the emetic toxin (cereulide)-producing strain B. cereus 4810/72 (Table 1).
When the type strain of S. griseus, strain DSM 40236, and S. griseus 157 were tested similarly, they had showed no effect on the motility of spermatozoa at concentrations that were up to 1,000-fold higher (corresponding to 109 CFU ml−1), indicating that toxin production was a strain-specific characteristic. Neither the type strain of B. cereus (ATCC 14579) nor any of the seven other strains of actinomycetes that were isolated from the same water-damaged buildings as the toxic strains and from animal sheds (Table 1) affected the motility of boar spermatozoa. The amounts of cell extract added to the semen had no effect on the osmolarity or pH of the extended boar semen.
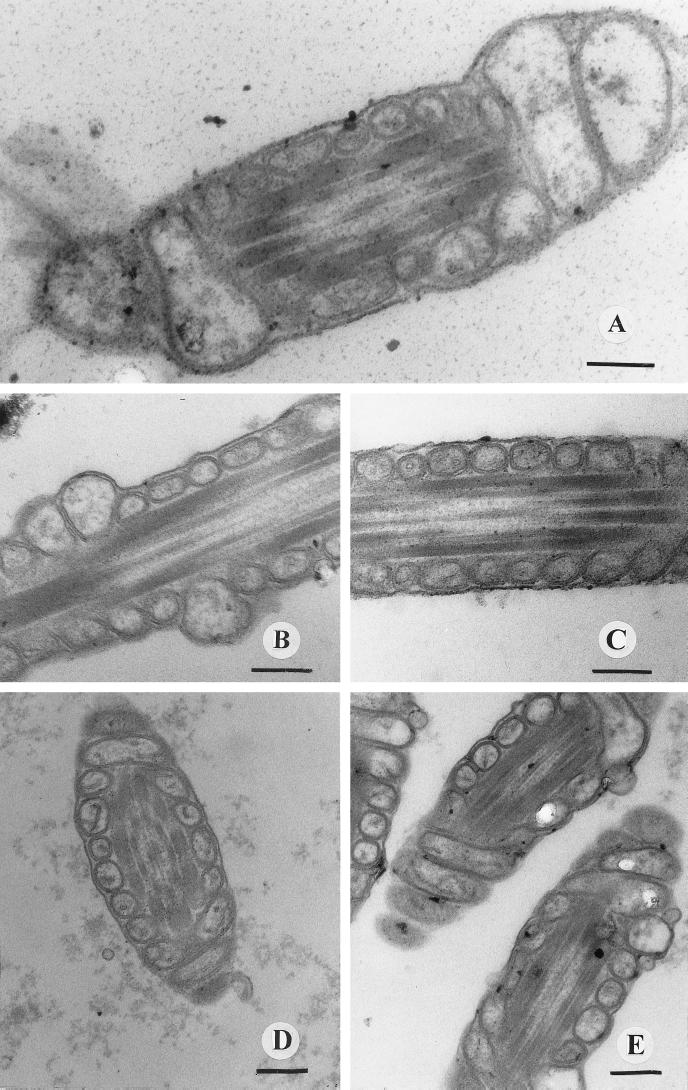
When the sperm cells exposed to extracts prepared from Streptomyces strains 10/ppi, 2/ppi, 8/ppi, and 1/k were examined with an electron microscope, we found that the extracts caused dose-dependent swelling of mitochondria (Fig. 1A). This indicates that the Streptomyces extracts contained a mitochondrial toxin. When an extract was fractionated by HPLC, the same HPLC fraction caused both swelling of mitochondria and loss of motility (Fig. 1B and C), indicating that the loss of motility of the sperm cells was linked to mitochondrial damage. No mitochondrial swelling (or loss of motility) was observed in sperm cells exposed to extracts of the type strain S. griseus DSM 40236 (Fig. 1D) or the nonemetic strains B. cereus ATCC 14579T and F-3453 (data not shown). A similar loss of motility and similar swelling of mitochondria were observed in sperm cells after they were exposed to commercial valinomycin (Fig. 1E) or to extracts prepared from the cereulide-producing emetic strains B. cereus 4810/72 and F-5881 (data not shown).
FIG. 1.
Thin sections of midpieces of boar spermatozoa exposed to extracts of S. griseus 8/ppi (A through C), extracts of S. griseus DSM 40236T (D), and commercial valinomycin (E) for 7 days. (A) Midpiece of a spermatozoon with mitochondrial damage. The frequency of swollen mitochondria in the spermatozoan midpiece was >60% after exposure to 20 μg (dry weight) of strain 8/ppi crude extract per ml. After exposure to 2 μg ml−1 the frequency of swollen mitochondria was <20% (data not shown). (B) Thin section of the midpiece of a boar spermatozoon exposed to a toxic strain 8/ppi HPLC fraction, showing swollen mitochondria with disrupted outer membranes. (C) Midpiece of a spermatozoon exposed to a nontoxic strain 8/ppi HPLC fraction. (D) Section of a midpiece exposed to a similarly prepared S. griseus DSM 40236T extract (20 μg [dry weight] per ml of extended boar semen). (E) Midpiece after exposure to 200 ng of commercial valinomycin ml−1. Bars = 200 nm.
The spermatozoon-paralyzing agent in the extracts of cultures of S. griseus 2/ppi, 8/ppi 10/ppi, and 1/k was not sensitive to heating at 100°C (20 min), to treatment with acid (pH 2 HCl, 30 min) or alkali (pH 12, NaOH, 30 min), or to the action of pronase (Sigma) (100 μg ml−1, pH 7, 3 h, 37°C). This toxic agent could pass through microconcentrator membrane filters with nominal cutoffs of 100,000 and 10,000 g mol−1 as a methanol extract but not as an extract in water or dimethyl sulfoxide (DMSO). Thus, the sperm cell-paralyzing agent extracted from the indoor S. griseus strains was heat stable, nonpolar, and resistant to inactivation by heat, by extreme pH, or by protease and had an apparent molecular size of less than 10,000 g mol−1. In these respects it behaved like the extract prepared from the emetic strain B. cereus 4810/72.
Purification and identification of the toxin from S. griseus 2/ppi, 8/ppi, 10/ppi, and 1/k.
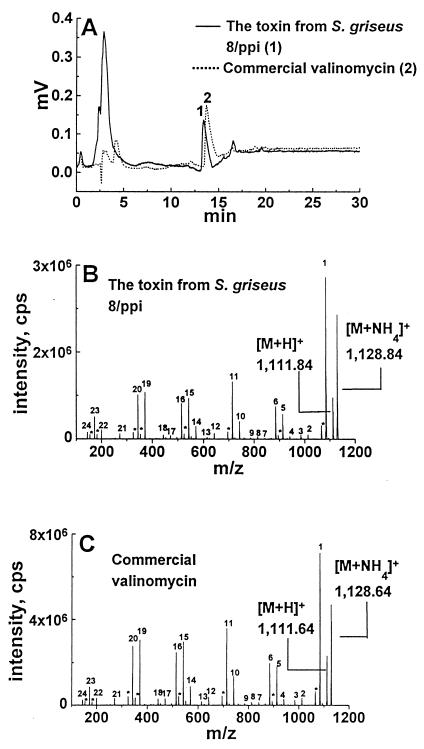
HPLC fractions which contained the agent toxic to sperm were collected (Fig. 2A). The fractions representing a single peak in strains 2/ppi, 8/ppi, 10/ppi, and 1/k were evaporated in a stream of N2, dissolved in methanol, and analyzed by ESI MS. Figure 2B shows the ESI MS-MS spectrum obtained for the ammonium adduct of the purified toxin from S. griseus 8/ppi (ion m/z 1,128.84). Figure 2C shows the spectrum obtained for the ammonium adduct valinomycin (ion m/z 1,128.64). The first fragment lost from the S. griseus 8/ppi toxin was ammonia, and the result was a protonated molecular ion of m/z 1,111.84 (Fig. 2B). This ion was similar to the protonated molecular ion m/z 1,111.64, which was obtained from commercial valinomycin (Sigma) (Fig. 2C). The assignments of the fragment ions observed are shown in Table 2. The mass values for all fragment losses observed with the toxin from S. griseus 8/ppi and commercial valinomycin (Sigma) were compared to the fragmentation pattern expected based on the structure of valinomycin, and they matched within 0.34 and 0.23 mass unit, respectively. The mass values for all fragment losses observed with the S. griseus 8/ppi toxin matched within 0.31 mass unit the mass values observed with the valinomycin standard. The ESI MS-MS spectra of the toxins purified from S. griseus 2/ppi, 10/ppi, and 1/k (data not shown) were identical to the spectra of the S. griseus 8/ppi toxin and to the spectra of the valinomycin standard. These data indicate that the methanol-extractable toxins of S. griseus 2/ppi, 8/ppi, 10/ppi, and 1/k were identical to valinomycin, a cyclic dodecadepsipeptide. The yields of valinomycin from 10- to 12-day-old cultures of the S. griseus strains were 600 to 1,400 ng mg (wet weight) of cells−1, as determined by HPLC in which commercial valinomycin was used for quantitation.
FIG. 2.
HPLC fractionation and ESI MS-MS fragmentation of the toxin from S. griseus 8/ppi and commercial valinomycin. (A) HPLC elution profiles of the extract from S. griseus 8/ppi and commercial valinomycin ESI-MS-MS fragmentation patterns of an ammonium adduct of the toxin purified from S. griseus 8/ppi (ion m/z 1,128.84) (B) and of an ammonium adduct of commercial valinomycin (ion m/z 1,128.64). (C) Samples were dissolved in 50% methanol containing ammonium acetate for the MS analysis. The peak numbers correspond to the fragment ions assigned in Table 2. The peaks marked with asterisks represent loss of water.
TABLE 2.
Fragment ions and fragment losses in the MS-MS spectra of the toxin from S. griseus 8/ppi and commercial valinomycin
| Fragment ion m/z (no.)
|
Fragment loss(es)a |
m/z
|
|||||
|---|---|---|---|---|---|---|---|
| Observed
|
Calculated (valinomycin) | Differenceb
|
|||||
| S. griseus toxin | Commercial valinomycin | S. griseus toxin | Commercial valinomycin | S. griseus toxin | Commercial valinomycin | ||
| 1,083.83 (1) | 1,083.64 (1) | CO | 28.01 | 28.00 | 27.99 | −0.02 | −0.01 |
| 1,012.83 (2) | 1,012.63 (2) | Val | 99.01 | 99.07 | 0.06 | 0.06 | |
| 984.72 (3) | 984.43 (3) | CO-Val | 127.12 | 127.21 | 127.06 | −0.06 | −0.15 |
| 940.83 (4) | 940.63 (4) | Val-OAla | 171.01 | 171.01 | 171.09 | 0.08 | 0.08 |
| 912.82 (5) | 912.63 (5) | Val-OVal and | 199.02 | 199.01 | 199.12 | 0.10 | 0.11 |
| CO-OAla-Val | 199.02 | 199.01 | 199.08 | 0.06 | 0.07 | ||
| 884.72 (6) | 884.63 (6) | CO-Val OVal | 227.12 | 227.02 | 227.11 | −0.01 | 0.10 |
| 841.62 (7) | 841.62 (7) | Val-OAla-Val | 270.22 | 270.02 | 20.16 | −0.06 | 0.14 |
| 813.82 (8) | 813.62 (8) | Val-OVal-Val and | 298.02 | 298.02 | 298.19 | 0.17 | 0.17 |
| CO-Val-OAla-Val | 298.02 | 298.02 | 298.15 | 0.13 | 0.13 | ||
| 785.72 (9) | 785.62 (9) | CO-Val-OVal-Val | 326.12 | 326.02 | 326.18 | 0.06 | 0.16 |
| 741.42 (10) | 741.42 (10) | OAla-Val-OVal-Val and OVal-Val-OAla-Val | 370.42 | 370.22 | 370.21 | −0.21 | −0.01 |
| 713.71 (11) | 713.42 (11) | CO-OAla-Val-OVal-Val and CO-OVal-Val-OAla-Val | 398.13 | 398.22 | 398.20 | 0.07 | −0.02 |
| 642.52 (12) | 642.42 (12) | Val-OAla-Val-OVal-Val | 469.32 | 469.22 | 469.28 | −0.04 | 0.06 |
| 614.31 (13) | 614.42 (13) | CO-Val-OAla-Val-OVal-Val | 497.53 | 497.22 | 497.27 | −0.27 | 0.05 |
| 570.52 (14) | 570.42 (14) | OAla-Val-OVal-Val-OAla-Val | 541.32 | 541.22 | 541.30 | −0.02 | 0.08 |
| 542.52 (15) | 542.42 (15) | CO-OAla-Val-OVal-Val-OAla-Val | 569.32 | 569.22 | 569.29 | −0.03 | 0.07 |
| OVal-Val-OAla-Val-OVal-Val | 569.32 | 569.22 | 569.33 | 0.01 | 0.11 | ||
| 514.52 (160 | 514.42 (16) | CO-OVal-Val-OAla-Val-OVal-Val | 597.32 | 597.22 | 597.32 | 0.00 | 0.10 |
| 471.42 (17) | 471.22 (17) | Val-OAla-Val-OVal-Val-OAla-Val | 640.42 | 640.42 | 640.34 | −0.08 | −0.08 |
| 443.32 (18) | 443.22 (18) | CO-VAl-OAla-Val-OVal-Val-OAla-Val | 668.52 | 668.42 | 668.33 | −0.19 | −0.09 |
| 371.23 (19) | 371.03 (19) | (-Val- OAla-Val-OVal-)2 and (-OAla-VAl- OVal-Val-)2 | 740.61 | 740.61 | 740.39 | −0.22 | −0.22 |
| 343.23 (20) | 343.03 (20) | CO(-Val- OAla-Val-OVal-)2 and CO(-OAla-Val- OVal-Val-)2 | 768.61 | 768.61 | 768.38 | −0.23 | −0.23 |
| 272.23 (21) | 272.03 (21) | (-Val- OAla-Val-OVal-)2Val | 839.61 | 839.61 | 839.46 | −0.15 | −0.15 |
| 200.03 (22) | 200.23 (22) | (-Val- OAla-Val-OVal-)2 Val-OAla | 911.61 | 911.41 | 911.48 | −0.13 | 0.07 |
| 172.03 (23) | 172.03 (23) | (-Val-OVal-Val-OAla-)2 Val-OVal and | 939.81 | 939.61 | 939.51 | 0.03 | −0.10 |
| CO(-Val- OAla-VAl-OVal-)2 Val-OAla | 939.81 | 939.61 | 939.477 | −0.34 | −0.14 | ||
| 144.12 (24) | 144.02 (24) | CO(-Val-OVal-Val-OAla-)2 Val-OVal | 967.72 | 967.62 | 967.50 | −0.22 | −0.12 |
Val is a valine residue. OAla and OVal are lactic acid and 2-hydroxyisovaleric acid residues, respectively. The calculated monoisotopic masses (m/z) are 99.07 (Val), 72.02 (OAla), 100.05 (OVal), and 27.99 (CO). Fragment ions represent the mass values for MS-MS spectra obtained from the precursor ions m/z 1,128.84 (toxin from S. griseus 8/ppi) and m/z 1,128.64 (commercial valinomycin). Fragment loss is protonated molecular ion m/z 1,111.84 (toxin from S. griseus 8/ppi) and m/z 1,111.64 (commercial valinomycin) minus fragment ion. The peptide sequence of fragment loss was determined by using the known structure (7) (-Val-OAla-Val-OVal-)3 of valinomycin.
Difference between the observed fragment loss and the calculated monoisotopic mass.
Biological properties of purified sperm-toxic agent from S. griseus 2/ppi, 8/ppi, 10/ppi, and 1/k compared to the biological properties of other toxins and chemicals.
Table 3 shows the toxicity thresholds for the S. griseus sperm toxin, valinomycin, extracted from strains 2/ppi, 8/ppi, 10/ppi, and 1/k with boar spermatozoa, and for selected microbial toxins and chemicals. The toxicity thresholds for valinomycin purified from four strains of S. griseus were between 1 and 3.2 ng ml of extended boar semen−1. The following seven preparations were toxic to sperm cells: purified toxins from S. griseus 10/ppi, 8/ppi, 2ppi, and 1/k, cereulide purified from B. cereus 4810/72 and F5881, and commercially obtained valinomycin and gramicidin. Valinomycin and cereulide caused a loss of sperm motility at concentrations of ≤3 ng ml−1 and caused mitochondria to swell at concentrations of <400 ng ml−1 but did not deplete ATP in the cells at concentrations up to 12,500 ng ml−1. Gramicidin (<3 ng ml−1) caused a loss of motility. Calcimycin A 23187 caused a loss of motility at a concentration of 32 ng ml−1 and depleted ATP at a concentration of 125 ng ml−1 but caused no visible morphological damage to sperm cells even at a concentration of 2,000 ng ml−1. Enniatin inhibited motility at a concentration of 300 ng ml−1 but did not cause mitochondria to swell at concentrations up to 5,000 ng ml−1. The sperm cells were not sensitive to nodularin and to commercial preparations of anatoxin a, ionomycin, surfactin, polymyxin B, and 2,4-dinitrophenol; the 50% effective concentrations (EC50) of these compounds for the spermatozoan vitality parameters ranged from 100 to >50,000 ng ml−1 (Table 3). N,N-Dihexylcarbodiimide caused mitochondria to swell at a concentration of 1,000 ng ml−1. In conclusion, the extracts prepared from S. griseus 10/ppi, 8/ppi, 2/ppi, and 1/k eliminated motility and caused mitochondria of sperm cells to swell, as did cereulide from emetic B. cereus strains and valinomycin, while the other compounds tested had no effect or had an effect only at dosages that were 1,000- to 10,000-fold higher.
TABLE 3.
Toxicity thresholds of selected toxins and chemicals for vitality parameters of boar spermatozoa
| Compound | EC50 (ng ml−1) that resulted ina:
|
|||
|---|---|---|---|---|
| Swelling of mito-chondria | Depletion of ATP | Loss of motility | Damage to plasma membrane | |
| Purified toxin from indoor S. griseus strainsb | ||||
| 8/ppi | <500 | >10,000 | 1 | >1,000 |
| 2/ppi | <500 | >10,000 | 3.2 | >1,000 |
| 10/ppi | >10,000 | 2 | >1,000 | |
| 1/k | >10,000 | 2.9 | >1,000 | |
| Cereulide | <400 | >1,000 | 0.5 | >1,000 |
| Valinomycin | <400 | >12,500 | 2 | >1,000 |
| Calcimycin A 23187 | >2,000 | 125 | 32 | |
| Gramicidin (mixture of gramicidins A, B, C, and D) | <3 | |||
| Ionomycin | >1,000 | 10,000 | 1,000 | |
| Polymyxin B sulfate | >50,000 | >50,000 | >50,000 | |
| Surfactin | >5,000 | 5,000–10,000 | 5,000 | |
| Enniatin | >5,000 | NDc | 300 | |
| Nodularin | ND | >40,000 | >40,000 | |
| Anatoxin A | ND | >40,000 | >40,000 | |
| N,N-Dihexylcarbodi-imide | 1,000 | 10,000 | >100, <1,000 | |
| 2,4-Dinitrophenol | >10,000 | >10,000 | <10,000 | |
Expressed as the endpoint dilution that resulted in a >50% change in the vitality parameter for extended boar semen compared to that of spermatozoa exposed to diluent only. Nodularin, anatoxin A, N,N-dihexylcarbodiimide, and 2,4-dinitrophenol were diluted in DMSO, and the other chemicals were diluted in methanol. Cereulide and valinomycin were diluted in parallel in methanol and in DMSO, but no difference in the endpoint dilutions was observed.
The toxic agent was purified from methanol extracts of S. griseus cultures, and the amounts of toxic agent were determined by reverse-phase HPLC by using commercial valinomycin as the standard.
ND, not determined.
DISCUSSION
We found that S. griseus isolates obtained from dust and air in water-damaged buildings produced valinomycin. We showed previously that extracts from water-damaged indoor building materials paralyzed sperm and caused mitochondria to swell (2). Identical effects were observed with pure cultures of S. griseus strains, as well as commercially obtained valinomycin. Valinomycin-producing cultures were readily isolated from dust and air from water-damaged buildings.
The cultures of S. griseus 8/ppi, 2/ppi, 10/ppi, and 1/k contained about 1 μg of valinomycin per mg (wet weight) of cells. The airborne S. griseus viable count in the building was 60 CFU m−3, and the dust viable count was 50 CFU mg−1. Viable counting of airborne and dust-borne bacteria is known to underestimate the cell count by factors of 1,000 to 10,000 (14, 15, 18). Viable as well as nonviable Streptomycetes spores may remain airborne due to their small size and great hydrophobicity (16). Therefore, the actual airborne load of S. griseus biomass in the water-damaged school and children’s day care centers may have reached a level of 105 cells m−3, which is equivalent to 0.1 ng of valinomycin m−3.
Valinomycin is a potassium ionophore (7). It eliminated progressive and rapid motility in exposed boar spermatozoa but did not affect plasma membrane integrity or the intracellular levels of ATP, indicating that ATP production by glycolysis continued to be active. Swelling of the inner mitochondrial membrane was observed in spermatozoa paralyzed by valinomycin; this is similar to effects observed with another toxic dodecadepsipeptide, cereulide, isolated from emetic food-poisoning outbreaks (3). No mitochondrial swelling was observed in sperm cells paralyzed by gramicidin, a membrane channel-forming linear homopeptide protonophore (7).
Depsipeptide toxins acting as ionophores and creating ion channels across bacterial or mitochondrial membranes (5, 7) are known to be produced by many bacteria and fungi. The significance of such toxins in the environment is not known due to the lack of a suitable bioassay for detection in environmental samples. Boar spermatozoa proved to be sensitive indicator cells as they lost motility when they were exposed to extremely low doses (≤1 ng ml−1) of valinomycin, gramicidin, and cereulide. The low sterol content of the boar spermatozoan plasma membrane makes these cells permeable, whereas the spermatozoa of other domestic animals and humans, which have higher amounts of sterols in the plasma membrane, are less sensitive to ionophores (5, 19). Boar spermatozoa are ineffective under anoxic conditions and exhibit only flickering motility in the absence of oxidative phosphorylation (13). Boar spermatozoan motility, therefore, is a sensitive indicator for agents that affect oxidative phosphorylation.
Disrupted mitochondrial physiology and swelling and ongoing cytosolic ATP synthesis have been shown to trigger both apoptotic and necrotic processes (23), indicating that exposure to mitochondrial toxins may be a severe health hazard. Cereulide, a depsipeptide ionophore produced by emetic strains of B. cereus, has been shown to cause fatal food poisoning and mitochondrial damage in inner organs when it is ingested (1, 11, 22). Members of the genus Streptomyces have been isolated frequently from water-damaged buildings (21). It is known that valinomycin is produced by Streptomyces fulvissimus, a species that is not related to S. griseus as determined by 16S rDNA sequence comparisons (20a). Valinomycin production is thus a strain-specific characteristic, not a species-specific characteristic, that may be exhibited by many other Streptomyces species found in indoor air and dust. Exposure to microbially generated mitochondrial toxins that are inhaled may pose a risk to organs that are rich in mitochondria and depend on oxidative phosphorylation (e.g., the brain, heart, and kidneys). Acute renal failure due to inhalation of mycotoxins has been reported (6).
The S. griseus toxin, which is identical to valinomycin, was extremely stable under extreme environmental conditions. This toxin may accumulate for long periods of time when building materials are exposed to repeated water damage. To our knowledge, this is the first report of isolation of an ionophoric toxin from indoor air or dust in water-damaged buildings. This is also the first report of valinomycin-producing strains that are identified as S. griseus.
ACKNOWLEDGMENTS
This work was financially supported by grants from the Foundation of Work Environment (Finland), the Centre of Excellence Fund of the University of Helsinki, the Technology Development Center of Finland, and the Academy of Finland.
We thank the Artificial Insemination Center (AI Cooperative, Kaarina, Finland) and Magnus Andersson (Department of Animal Reproduction, Helsinki University) for providing the boar semen. We thank Tuire Koro and Mervi Lindman for preparing thin sections. Equipment at the laboratory for electron microscopy of the Helsinki University Biocenter was at our disposal.
REFERENCES
- 1.Agata N, Ohta M, Masashi M, Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 1995;129:17–20. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- 2.Andersson M A, Nikulin M, Köljalg U, Andersson M C, Rainey F A, Reijula K, Hintikka E-L, Salkinoja-Salonen M. Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–397. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson M A, Mikkola R, Helin J, Andersson M C, Salkinoja-Salonen M. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl Environ Microbiol. 1998;64:1338–1343. doi: 10.1128/aem.64.4.1338-1343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annila A, Lehtimäki J, Mattila K, Eriksson J, Sivonen K, Rantala T, Drakenberg T. Solution structure of nodularin, an inhibitor of serine/threonine-specific protein phosphatases. J Biol Chem. 1996;271:16695–16702. doi: 10.1074/jbc.271.28.16695. [DOI] [PubMed] [Google Scholar]
- 5.Booth I. Bacterial transport energetics and mechanisms. In: Anthony C, editor. Bacterial energy transduction. London, United Kingdom: Academic Press; 1988. pp. 377–428. [Google Scholar]
- 6.Di Paolo N, Guarnieri A, Loi F, Sacchi G, Mangiarotti A, Di Paolo M. Acute renal failure from inhalation of mycotoxins. Nephron. 1993;64:621–625. doi: 10.1159/000187411. [DOI] [PubMed] [Google Scholar]
- 7.Gräfe U. Biochemie der Antibiotika: Struktur-Biosynthese-Wirkmechanismus. 1992. p. 167. and 219–318. Spectrum Akademischer Verlag GmbH, Heidelberg, Germany. [Google Scholar]
- 8.Hain T, Ward-Rainey N, Kroppenstedt R, Stackebrandt E, Rainey F. Discrimination of Streptomyces albidoflavus strains based on the size and number of 16S-23S ribosomal DNA intergenic spacers. Int J Syst Bacteriol. 1997;47:202–206. doi: 10.1099/00207713-47-1-202. [DOI] [PubMed] [Google Scholar]
- 9.Hendry K, Cole E. A review of mycotoxins in indoor air. J Toxicol Environ Health. 1993;38:183–189. doi: 10.1080/15287399309531711. [DOI] [PubMed] [Google Scholar]
- 10.Johanning E, Biagini D, Hull D, Morey P, Jarvis P, Landsbergis P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in water-damaged office environment. Int Arch Occup Environ Health. 1996;68:207–218. doi: 10.1007/BF00381430. [DOI] [PubMed] [Google Scholar]
- 11.Mahler H, Pasi A, Kramer J, Schulte P, Scoging A, Baer W, Kraehenbuehl S. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336:1143–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 12.Maidak B, Larsen N, McCaughey M, Overbeek R, Olsen G, Fogel K, Blandy J, Woese C. The Ribosomal Database Project. Nucleic Acid Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann T, Lutwak-Mann C. Male reproductive function and semen. Heidelberg, Germany: Springer Verlag; 1982. pp. 196–197. [Google Scholar]
- 14.Marthi B. Resuscitation of microbial aerosols. In: Lightheart B, Mohr A, editors. Atmospheric microbial aerosols. New York, N.Y: Chapman & Hall; 1994. pp. 192–225. [Google Scholar]
- 15.McFeters G, Yu F, Pyle B, Stewart P. Physiological assessment of bacteria using fluorochromes. J Microbiol Methods. 1995;21:1–13. doi: 10.1016/0167-7012(94)00027-5. [DOI] [PubMed] [Google Scholar]
- 16.Mozer N, Rouxhet P. Methods for measuring hydrophobicity of microorganisms. J Microbiol Methods. 1987;6:99–112. [Google Scholar]
- 17.Nohynek L, Häggblom M, Palleroni N, Kronquist K, Nurmiaho-Lassila E-L, Salkinoja-Salonen M. Characterization of Mycobacterium fortuitum strain capable of degrading polychlorinated phenolic compounds. Syst Appl Microbiol. 1993;16:126–134. [Google Scholar]
- 18.Palmgren U, Ström G, Blomquist G, Malmberg P. Collection of airborne microorganisms on Nuclepore filters, estimation and analysis—CAMNEA method. J Appl Microbiol. 1986;61:401–406. doi: 10.1111/j.1365-2672.1986.tb04303.x. [DOI] [PubMed] [Google Scholar]
- 19.Paulenz H. Spermiemembranens struktur og funksjon I relasjon til kuldesjokk. Nor Veterinaertidskr. 1993;105:1135–1142. [Google Scholar]
- 20.Rainey F, Ward-Rainey N, Kroppenstedt R, Stackebrandt E. The genus Nocardiopsis represent a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Norcardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 20a.Rainey, F. A. Unpublished data.
- 21.Räty K, Raatikainen O, Holmalahti J, von Wright A, Joki S, Pitkänen A, Saano V, Hyvärinen A, Nevalainen A, Buti I. Biological activities of actinomycetes and fungi isolated from the indoor air of problem houses. Int Biodeter Biodeter. 1996;1996:134–154. [Google Scholar]
- 22.Sakurai N, Koike K, Irie Y, Hayashi H. The rice culture filtrate of Bacillus cereus isolated from emetic type food poisoning causes mitochondrial swelling in a HEp-2 cell. Microbiol Immunol. 1994;38:337–343. doi: 10.1111/j.1348-0421.1994.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 23.Vander Heiden M, Chandel N, Williamson E, Schumacker P, Thompson C. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]