Key Points
Question
In a nonurban region in Germany, is the deployment of a flying intervention team to a local stroke center associated with shorter time to endovascular treatment compared with patient interhospital transfer to a referral center for patients with acute ischemic stroke?
Findings
In this nonrandomized controlled intervention study that included 157 patients, deployment of a flying intervention team, compared with patient interhospital transfer, was significantly associated with a shorter time to endovascular thrombectomy (median time from decision to pursue thrombectomy to start of procedure, 58 vs 148 minutes).
Meaning
The findings may support consideration of a flying intervention team for some stroke systems of care, although further research is needed to confirm long-term clinical outcomes and to understand applicability to other geographic settings.
Abstract
Importance
The benefit of endovascular thrombectomy (EVT) for acute ischemic stroke is highly time-dependent, and it is challenging to expedite treatment for patients in remote areas.
Objective
To determine whether deployment of a flying intervention team, compared with patient interhospital transfer, is associated with a shorter time to endovascular thrombectomy and improved clinical outcomes for patients with acute ischemic stroke.
Design, Setting, and Participants
This was a nonrandomized controlled intervention study comparing 2 systems of care in alternating weeks. The study was conducted in a nonurban region in Germany including 13 primary telemedicine-assisted stroke centers within a telestroke network. A total of 157 patients with acute ischemic stroke for whom decision to pursue thrombectomy had been made and deployment of flying intervention team or patient interhospital transfer was initiated were enrolled between February 1, 2018, and October 24, 2019. The date of final follow-up was January 31, 2020.
Exposures
Deployment of a flying intervention team for EVT in a primary stroke center vs patient interhospital transfer for EVT to a referral center.
Main Outcomes and Measures
The primary outcome was time delay from decision to pursue thrombectomy to start of the procedure in minutes. Secondary outcomes included functional outcome after 3 months, determined by the distribution of the modified Rankin Scale score (a disability score ranging from 0 [no deficit] to 6 [death]).
Results
Among the 157 patients included (median [IQR] age, 75 [66-80] y; 80 [51%] women), 72 received flying team care and 85 were transferred. EVT was performed in 60 patients (83%) in the flying team group vs 57 (67%) in the transfer group. Median (IQR) time from decision to pursue EVT to start of the procedure was 58 (51-71) minutes in the flying team group and 148 (124-177) minutes in the transfer group (difference, 90 minutes [95% CI, 75-103]; P < .001). There was no significant difference in modified Rankin Scale score after 3 months between patients in the flying team (n = 59) and transfer (n = 57) groups who received EVT (median [IQR] score, 3 [2-6] vs 3 [2-5]; adjusted common odds ratio for less disability, 1.91 [95% CI, 0.96-3.88]; P = .07).
Conclusions and Relevance
In a nonurban stroke network in Germany, deployment of a flying intervention team to local stroke centers, compared with patient interhospital transfer to referral centers, was significantly associated with shorter time to EVT for patients with acute ischemic stroke. The findings may support consideration of a flying intervention team for some stroke systems of care, although further research is needed to confirm long-term clinical outcomes and to understand applicability to other geographic settings.
This nonrandomized controlled intervention study compares the use of a flying intervention team with interhospital transfer of patients receiving endovascular thrombectomy in regard to time delay, feasibility of procedure, adverse events, and clinical outcome.
Introduction
Endovascular thrombectomy (EVT) for acute ischemic stroke is most effective when applied rapidly after stroke onset.1 Consequently, guidelines have recommended initiating treatment as soon as possible.2,3 However, implementation is challenging in rural areas. Lack of neurointerventional expertise is a major barrier in developing EVT-capable hospitals, and long distances to urban comprehensive stroke centers cause critical transfer delays.4,5
Local primary stroke centers are recommended to ensure area-wide coverage and rapid hyperacute stroke workup in rural areas.2,6 Telemedicine can help to reach this goal,7,8,9,10,11 and many “telestroke” (stroke telemedicine) networks have been developed over the past 20 years, spreading specialty knowledge from the comprehensive stroke centers to the primary stroke centers of their greater region.11 Because EVT is usually not offered in primary stroke centers, methods to expedite EVT for eligible patients were urgently needed to avoid time-consuming patient interhospital transfer.
Many primary stroke centers have longstanding experience in hyperacute stroke treatment as well as stroke unit care. A growing number of these hospitals are equipped with an angiography suite suitable for endovascular procedures (eg, for coronary or peripheral angiography), but lack neurointerventional expertise onsite. For EVT-eligible patients admitted to these primary stroke centers, a novel system of care has been proposed.12 Instead of transferring the patient to a referral center, a flying intervention team is transferred via helicopter from a network hub to the associated primary stroke center to perform EVT onsite.
The flying intervention team service was established for 13 primary stroke centers within the telestroke network of South-East Bavaria (TEMPiS) in 2018. The aim of this study was to compare the flying intervention team with interhospital transfer of patients receiving EVT regarding time delay, feasibility of procedure, adverse events, and clinical outcome.
Methods
The ethics committee of the Bavarian Chamber of Physicians, a corporation under public law and the mandatory committee for this project, approved the study (#17056). All patients received information about the inclusion in and the purpose of the registry. According to German legislative authorities, no informed consent was necessary for registry inclusion.
Study Design
This was a nonrandomized controlled intervention study comparing 2 systems of care in alternating weeks (Video). Patients with acute ischemic stroke admitted to participating primary stroke centers for whom the decision to pursue EVT had been made were either treated by a flying intervention team onsite at the primary stroke center or in a referral center after patient interhospital transfer. Flying team service was established in 26 weeks per year between 8 am and 10 pm, including weekends. In all other weeks, only patient interhospital transfer was available. Flying team and transfer weeks were predefined and evenly allocated in a 1:1 ratio. Patients for whom the flying team was deployed were compared with transfer patients of the control week. Recruitment started with initiation of the new system of care on February 1, 2018. Alternation of weeks was preplanned to continue until July 2021, with the aim to show whether the flying team was associated with shorter time to treatment and improved clinical outcome. A preplanned interim analysis of workflow and adverse events was performed after 12 months. Because of a large significant reduction in time to treatment, the ethical review board recommended termination of the study on October 24, 2019, because the time advantage was deemed too significant to further justify allocation of patients to flying team or transfer weeks for the purposes of scientific study. The flying team service continued to operate and subsequent patients were enrolled in an ad hoc ongoing observational registry.
Video. Study Design: Flying Intervention Team vs Patient Interhospital Transfer in Acute Ischemic Stroke.
Study Population
Overall, 13 primary stroke centers of the telestroke network TEMPiS participated in the study (Figure 1). The TEMPiS network supports acute stroke treatment and further in-hospital stroke unit care onsite, including 24/7 telestroke service with bidirectional videoconference and imaging transfer, regular audit visits by multidisciplinary comprehensive stroke center staff to monitor process and treatment quality, standard operating procedures on stroke care, and regular benchmarking analyses.7,10 Five comprehensive stroke centers in the wider region of the network serve as endovascular referral centers for these hospitals. Emergency medical service brings patients with suspected acute stroke to the nearest stroke unit without prehospital triage for large vessel occlusion.
Figure 1. Map of Southeast Bavaria, Germany, Where the TEMPiS Network Operates.
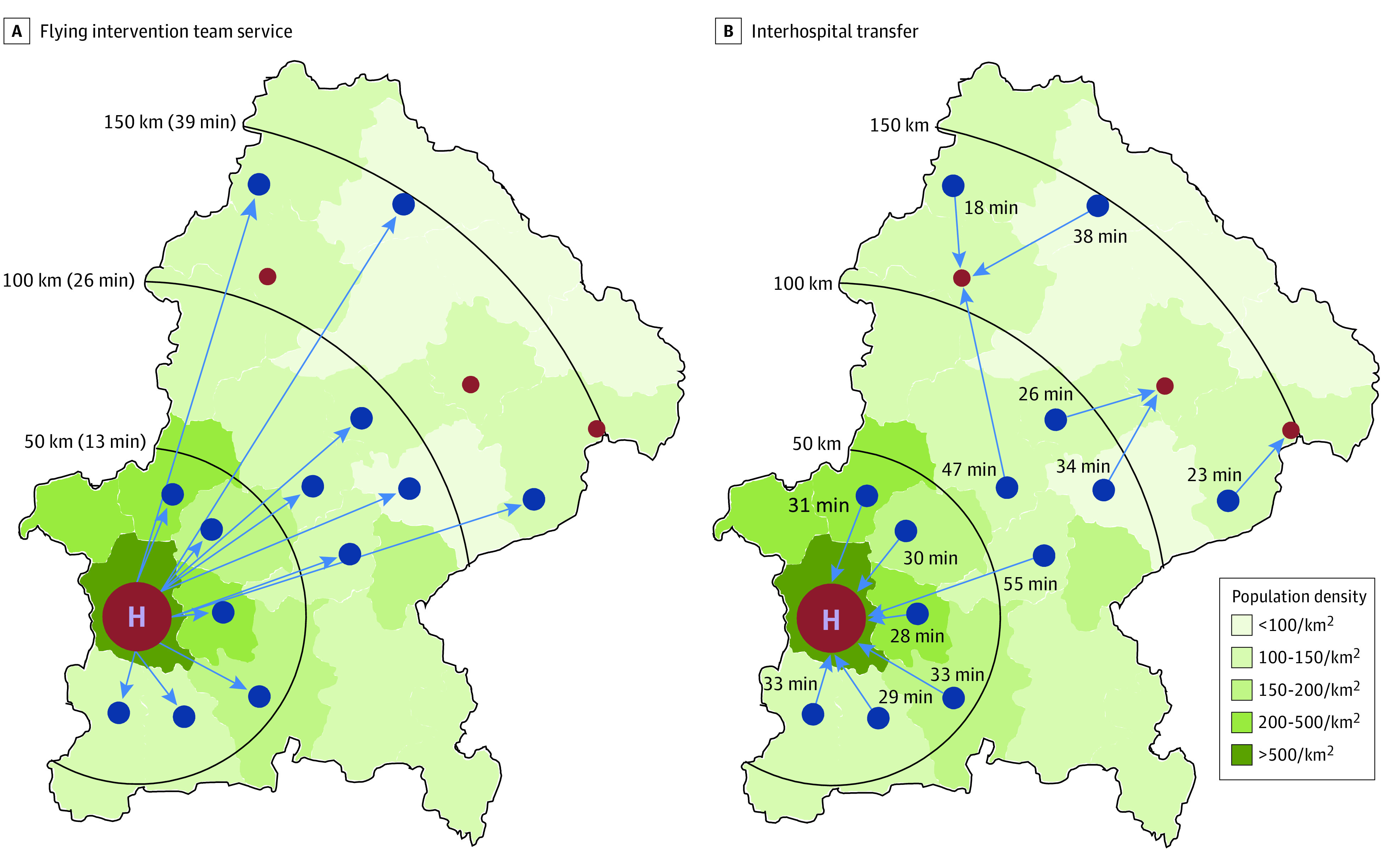
Blue dots indicate primary stroke centers taking part in the flying team project. Small red dots are referral centers for patient interhospital transfer. The large red dot indicates the helicopter site; it obscures the adjacent and another nearby referral center in the city of Munich. Blue arrows signify the usual pathway of flight or transfer. Additional hospitals that provide stroke care but did not participate in this project are not shown. A, All primary stroke centers receive flying intervention team service from Munich. Flight distance and flying time in minutes is given at each black half circle. B, Transfer directions and transfer time estimates from each primary stroke center to the preferred referral center are shown. Transfer time estimates were calculated taking into account general rates of airborne and ground transfer, a helicopter flying speed of 220 km/h, and estimates of driving times on optimal routes according to the route planner service of the General German Automobile Club (http://maps.adac.de).
Participating primary stroke centers were located in rural or intermediate populated areas,13 covered a region of 11 295 km2 with a population of 1.54 million,14 and treated approximately 5500 patients with acute stroke per year. All primary stroke centers had a neurology department or internal medicine department with a long-standing high-quality stroke unit, an intensive care unit, a helicopter pad, a monoplanar angiography suite, and onsite anesthesia support and provided further support by an angiography assistant. Requirements for participation are described in detail in Supplement 1.
Consecutive patients for whom a decision was made to pursue EVT and for whom the week-based predefined system of care (flying intervention team service or patient interhospital transfer) was deployed were included in the analysis (Figure 2). Patients who fulfilled the following eligibility criteria were included: 18 years or older and 85 years or younger; main target occlusion in intracranial internal carotid artery, middle cerebral artery (M1 or proximal M2), or basilar artery; decision to pursue EVT between 8 am and 10 pm; presentation within the defined time window from stroke onset (≤6 h for anterior circulation occlusion without possibility of advanced imaging in primary stroke center and ≤24 h for posterior circulation occlusion or anterior circulation with mismatch in advanced imaging in primary stroke center), Alberta Stroke Program Early CT Score greater than or equal to 6; no severely reduced life expectancy; and premorbid modified Rankin Scale (mRS) score less than or equal to 3 (see eMethods in Supplement 1 and the trial protocol in Supplement 2 for further details).
Figure 2. Flow of Patients in a Study of the Effect of a Flying Intervention Team vs Patient Interhospital Transfer on Time to Endovascular Thrombectomy.
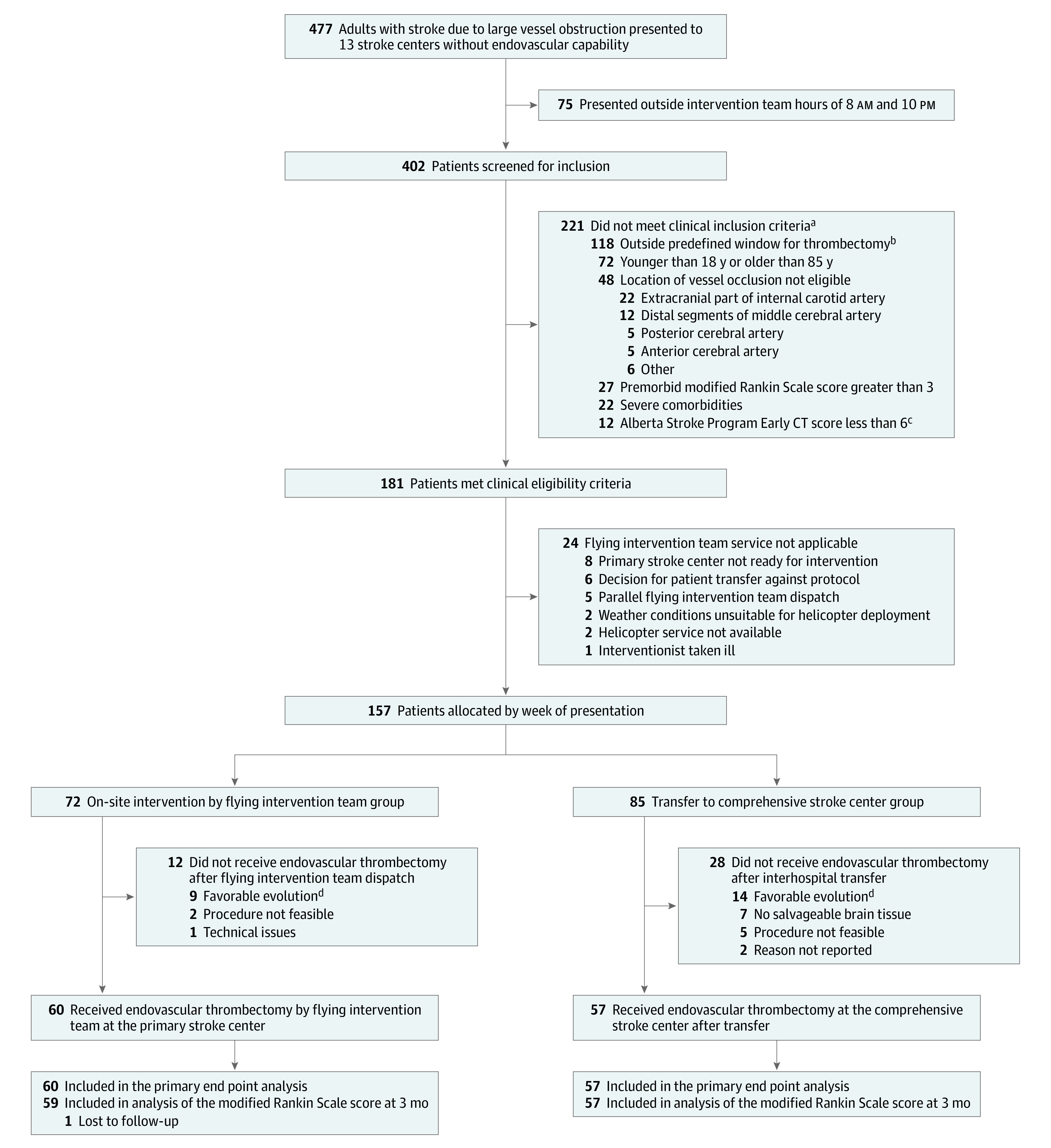
aMultiple clinical exclusion criteria were possible. Patients may be included in more than 1 category.
bGreater than 6 hours for anterior circulation occlusion without possibility of advanced imaging in primary stroke center and greater than 24 hours for posterior circulation occlusion or anterior circulation with mismatch in advanced imaging performed in primary stroke center.
cThe Alberta Stroke Program Early CT Score is a 10-point quantitative topographic score that measures the extent of early ischemic changes in patients with middle cerebral artery stroke. Starting with 10, a 1-point deduction is made for every region involved. Scores range from 0 (10 affected regions) to 10 (0 affected regions).
dClinical improvement and/or spontaneous recanalization.
Exposures
Intervention teams consisted of a neurointerventional radiologist and an angiography assistant and were set up by 2 comprehensive stroke centers (München Klinik Harlaching, Klinikum rechts der Isar). There was only 1 flying intervention team on call at a time. Overall, 5 neurointerventionists and 6 assistants participated. Interventionists had to have longstanding experience in neurointervention (for further details see eMethods in Supplement 1) and were trained on all local angiography suites beforehand. When on-call for flying team service, the team was not in charge of endovascular service in their comprehensive stroke center, but were able to accomplish nonurgent radiology tasks. During off hours, maximum permitted driving time to takeoff helipad was 20 minutes.
A helicopter service dedicated exclusively to the project covered the flying team service weeks. The deployments were approved as helicopter emergency medical service (EU regulation 956-2012) by the German Federation Aviation Office, but were not part of the emergency medical service of Bavaria. Pickup location of the intervention team was at either of the 2 centers. A helicopter was stationed within 5 minutes of flying distance to any of the 2 comprehensive stroke centers. Service included 2 pilots, maximum pickup time of 15 minutes, and qualification to fly after dawn (for further details see eMethods in Supplement 1).
Interventional material was brought by the intervention team in a special suitcase for each deployment. The suitcase comprised all intervention-specific items, including rescue material for common complications, and was sufficient for 3 subsequent endovascular procedures (see eMethods and eFigure 1 in Supplement 1). Basic material kits were stored in local hospitals and included items that were to be prepared before arrival of the intervention team, such as tubes, syringes, pads, heparin, and rinsing fluids (see eFigure 2 in Supplement 1).
When a patient presented to the primary stroke center and clinical triage revealed possibility of large-vessel occlusion, an alarm was given to the helicopter crew and flying intervention team to prepare for possible deployment. After decision to pursue EVT had been made and eligibility for flying team deployment had been determined via telemedicine by a neurologist and interventionist, final alarm was given to the pilots to start deployment. If patients were eligible, use of the flying team was mandatory in flying team service weeks. If flying team service was not applicable (eg, parallel flying team dispatch, onsite anesthesia support not available in primary stroke center, bad weather conditions), patients were transferred to a referral center. During helicopter transport of the intervention team, the patient in the primary stroke center was taken to the angiography suite. Basic angiography equipment was unpacked, general anesthesia was administered, and the patient was prepared on the theater table before arrival of the intervention team. After landing at the local helicopter pad, the intervention team was escorted by hospital staff directly to the angiography suite. The intervention team performed the endovascular procedure, supported by a local technical assistant familiar with local circumstances. After the procedure, handoff to the local team included a review of the intervention and immediate recommendations. The intervention team was then flown back to the comprehensive stroke center or to the next primary stroke center for a subsequent deployment. Postprocedural care was carried out by the primary stroke center local team on the stroke unit or intensive care unit.
Standard operating procedures were set in place for the entire chain of treatment (ie, prehospital, emergency department and teleconsultation management, decision to pursue EVT, processes for flying team/transfer alarm, patient in angiography suite, and interventional and postinterventional care in primary stroke center, including management of complications). Mandatory teleconsultation follow-up was performed after 24 hours. Quality treatment included management of serious adverse events, individual case discussions, and morbidity and mortality conferences.
Interhospital transfer of patients in the transfer weeks was executed by emergency medical services. Transfers for EVT were conducted with the same priority as primary stroke alerts. Acute stroke treatment in primary stroke centers until decision to pursue EVT followed the same standard operating procedures as in flying team service weeks. If indication for EVT was determined, high-priority transfer to the prenotified referral center was initiated immediately. Emergency medical services determined whether airborne transfer or ambulance was to be deployed. After arrival, patients received EVT and postprocedural care according to standards of the referral center.
Outcomes
The primary outcome was median time from decision to pursue EVT to initiation of procedure (groin puncture) in minutes. Prespecified secondary outcomes regarding time delays were rate of achieving time from decision to pursue EVT to groin puncture within 60 minutes, 120 minutes, 180 minutes, and 240 minutes; time from onset of symptoms or last time seen well to groin puncture; arrival in primary stroke center to groin puncture; first imaging to groin puncture; symptom onset or last time seen well to reperfusion; arrival in primary stroke center to reperfusion; first imaging to reperfusion; decision to pursue EVT to reperfusion; and groin puncture to reperfusion. Other prespecified secondary outcomes included successful reperfusion, periprocedural complications (such as intracranial vessel perforation, distal embolization, and arterial dissection), symptomatic and asymptomatic intracranial hemorrhage, malignant brain swelling, new ischemic stroke, extracranial hemorrhage, in-hospital death or palliative care decision within 7 days, and functional outcome at 3 months determined by the distribution of the mRS score (a disability scale with possible scores ranging from 0 [no deficit] to 6 [death], with scores of 5 and 6 considered together as the “worst” category) and by dichotomization of the mRS into scores of 0 to 2 vs 3 to 6 and by analyzing mRS scores of 6 (death) as a single variable. Clinical data at 3 months were collected via telephone interviews. Patients also had the option of completing a questionnaire that was returned by mail. Interviewers were not made aware of treatment allocation. mRS assessment was performed through a standardized, structured interview.15 Questions on the mRS were scheduled at the beginning of the interview to decrease the chance of unblinding by the patient.
Time of decision to pursue EVT was defined as time of final mutual decision of the teleconsultant and the interventionist at which the available system of care was initiated. Successful reperfusion of vessel was defined as antegrade reperfusion of more than half of the previously occluded target artery ischemic territory (≥2b on the modified thrombolysis in cerebral infarction scale). Time of successful reperfusion was defined as first evidence of successfully recanalized vessel. Symptomatic intracranial hemorrhage was defined as any postprocedural intracranial hemorrhage and worsening of the neurological deficit of greater than or equal to 4 points on the National Institute of Health Stroke Scale, irrespective of causality or location.
Statistical Analysis
Categorical data are presented as absolute and relative frequencies and continuous variables are presented as median (IQR).
The primary end point and other time intervals were compared between both groups using Mann-Whitney U tests. Comparison of categorical data regarding procedure and complications was based on Pearson χ2 tests. Time to treatment, complications, and successful reperfusion were analyzed only in patients in whom deployment of system of care was followed by EVT. No imputation methods were used for missing values in baseline data or outcomes. Functional outcome (mRS score) at 3 months of patients receiving EVT was analyzed by using nonadjusted and adjusted ordinal logistic regression models. The adjusted common odds ratio for a shift in the direction of a better outcome with the corresponding 95% CI was presented. Severe disability (mRS score of 5) and death (mRS score of 6) were combined in a single “worst” category.16 To assess proportionality of the odds ratios for cut-off values of the mRS (mRS score of 0-2 at 3 months, death within 3 months), unadjusted and adjusted odds ratios were calculated and interpreted by using binary logistic regression models. The multivariable models (ordinal and binary logistic regression) were adjusted for age, sex, and National Institute of Health Stroke Scale score. Patients who were lost to follow-up were excluded from the analyses of functional outcome. In a post hoc analysis, the multivariable models were extended to include occlusion sites to account for the imbalance in distribution seen in the baseline characteristics. In an additional post hoc analysis, functional outcomes at 3 months were compared between all patients allocated to receive on-site intervention by flying team vs transfer to referral center, including patients who did not receive EVT. These analyses were performed in the same manner as the prespecified analyses of the mRS score by using nonadjusted and adjusted regression models. All P values were 2-sided and a P value <.05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing). See the statistical analysis plan in Supplement 3 for further details.
Results
Between February 1, 2018, and October 24, 2019, a total of 157 patients fulfilled the inclusion criteria for this study, of whom 72 received flying team care and 85 were transferred. EVT was performed in 60 patients (83%) in the flying team group vs 57 (67%) in the transfer group. Flying team thrombectomies were performed by 5 interventionists, while transfer thrombectomies were performed by 19 interventionists. EVT was not performed after deployment of flying team or after patient transfer because of clinical improvement or spontaneous recanalization (9 patients [13%] in the flying team group and 14 [16%] in the transfer group), lack of salvageable brain tissue (7 patients [8%] in the transfer group), the procedure was not deemed feasible (2 patients [3%] in the flying team group and 5 [6%] in the transfer group), and technical issues (1 patient [1%] in the flying team group) (Figure 2). Table 1 outlines the baseline characteristics by treatment group. Median (IQR) age of patients was 77 (67-82) years in the flying team group and 75 (66-79) years in the transfer group. There were more women in the flying team group compared with the transfer group (45 [62%] vs 35 [41%]) and the median (IQR) National Institute of Health Stroke Scale score was higher (15 [10-18] vs 13 [8-18]). M2 segment occlusions were more frequent in the flying team than the transfer group (28% vs 20%), while ICA (8% vs 12%) and basilar artery (10% vs 16%) were more frequent in the transfer group. Baseline characteristics for patients who ultimately received EVT are shown in eTable 1 in Supplement 1.
Table 1. Demographics and Baseline Characteristics of Patients in a Study of the Effect of a Flying Intervention Team vs Patient Interhospital Transfer on Time to Endovascular Thrombectomy (EVT).
| Characteristic | No. (%) | |
|---|---|---|
| Flying team (n = 72) | Transfer (n = 85) | |
| Demographics | ||
| Age, median (IQR), y | 77 (67-82) | 75 (66-79) |
| Sex | ||
| Women | 45 (62) | 35 (41) |
| Men | 27 (38) | 50 (59) |
| Medical historya | ||
| Hypertension | 47 (66) | 58 (68) |
| Atrial fibrillation | 23 (33) | 23 (27) |
| History of stroke | 18 (25) | 17 (21) |
| Diabetes mellitus | 17 (24) | 14 (17) |
| History of myocardial infarction | 5 (7) | 12 (14) |
| Index stroke | ||
| NIHSS, median (IQR)b | 15 (10-18) | 13 (8-18) |
| NIHSS groupsb | ||
| Mild stroke (NIHSS ≤5) | 7 (10) | 10 (12) |
| Moderate stroke (NIHSS 6-15) | 33 (46) | 45 (53) |
| Severe stroke (NIHSS >15) | 32 (44) | 30 (35) |
| tPA treatment in primary stroke center | 50 (69) | 58 (68) |
| Occlusion sitec | ||
| MCA, M1 segment | 37 (51) | 43 (51) |
| MCA, M2 segment | 20 (28) | 17 (20) |
| Basilar artery | 7 (10) | 14 (16) |
| ICA | 6 (8) | 10 (12) |
| ICA with M1 involvement (“carotid T”) | 6 (8) | 8 (9) |
| Etiologyd | n = 71 | n = 85 |
| Macroangiopathy | 42 (59) | 42 (49) |
| Cardiac embolism | 16 (23) | 16 (19) |
| Unknown source | 11 (15) | 24 (28) |
| Other known source | 2 (3) | 3 (4) |
| Time before decision to pursue EVT, median (IQR), min | ||
| Symptom onset to arrival in primary stroke center | 85 (62-157) | 91 (60-150) |
| Arrival in primary stroke center to first imaging | 11 (6-15) | 13 (8-19) |
| First imaging to decision to pursue EVT | 40 (27-54) | 44 (29-63) |
| Straight-line distance, median (IQR), km | ||
| Between flying team base and primary stroke center | 50 (42-54) | |
| Between primary stroke center and referral center | 42 (34-50) | |
| Mode of patient transfer | ||
| Airborne | 40 (48) | |
| Ground | 43 (52) | |
Abbreviations: ICA, internal carotid artery; MCA, middle cerebral artery; tPA, tissue plasminogen activator.
Medical history was assessed by medical personnel during hospital stay.
The National Institutes of Health Stroke Scale (NIHSS) is used for the evaluation of acute stroke (score range, 0-42; greater values indicate greater severity).
Because multiple occlusion sites are possible, patients may be included in more than 1 category.
Etiology was determined by medical personnel during hospital stay; 1 patient in the flying team group was not documented.
Primary Outcome
The median (IQR) time from decision to pursue EVT to groin puncture was 58 (51-71) minutes in the flying team group vs 148 (124-177) minutes in the transfer group (difference, 90 min [95% CI, 75-103]; P < .001) (Table 2). All patients in the flying team group who underwent EVT, except for 2, received treatment earlier compared with any patient in the transfer group (see eFigure 3 in Supplement 1). In the flying team group, groin puncture was completed within 2 hours in all patients who underwent EVT vs 23% of patients who underwent EVT in the transfer group.
Table 2. Procedural Outcomes and Complications in Patients Who Underwent Endovascular Thrombectomy .
| Outcome | No. (%) | Absolute difference (95% CI) | P valuea | |
|---|---|---|---|---|
| Flying team (n = 60) |
Transfer (n = 57) | |||
| Primary | ||||
| Time from decision to pursue EVT to groin puncture, median (IQR), min | 58 (51 to 71) | 148 (124 to 177) | 90 (75 to 103) | <.001 |
| Secondary | ||||
| Time from decision to pursue EVT to groin puncture, min | ||||
| <60 | 33 (55) | 0 | 55.0 (41.0 to 66.9) | |
| <120 | 60 (100) | 13 (23) | 77.2 (63.4 to 86.2) | |
| <180 | 60 (100) | 42 (75) | 26.3 (14.9 to 39.0) | |
| <240 | 60 (100) | 54 (96) | 5.3 (−1.7 to 14.4) | |
| Time to groin puncture, median (IQR), min | ||||
| From symptom onset | 190 (155 to 258) | 296 (240 to 372) | 106 (60 to 132) | <.001 |
| From arrival in primary stroke center | 112 (96 to 132) | 212 (185 to 252) | 100 (82 to 126) | <.001 |
| From first imaging | 101 (86 to 115) | 194 (167 to 226) | 93 (79 to 113) | <.001 |
| Time to reperfusion, median (IQR), minb | ||||
| From symptom onset | 243 (194 to 301) | 340 (279 to 438) | 97 (59 to 154) | <.001 |
| From arrival in primary stroke center | 149 (126 to 182) | 260 (225 to 314) | 111 (78 to 149) | <.001 |
| From first imaging | 135 (118 to 158) | 244 (205 to 297) | 109 (77 to 134) | <.001 |
| From decision to pursue EVT | 95 (82 to 108) | 193 (158 to 241) | 98 (84 to 129) | <.001 |
| From groin puncture | 36 (26 to 46) | 45 (28 to 59) | 9 (−3 to 21) | .07 |
| Outcome of EVT | ||||
| Successful reperfusionc | 55 (92) | 48 (84) | 7.5 (−4.7 to 20.0) | .21 |
| Periprocedural complications | ||||
| Distal embolization | 4 (7) | 1 (2) | 4.9 (−3.6 to 14.3) | |
| Intracranial perforation | 1 (2) | 5 (9) | −7.1 (−17.4 to 1.6) | |
| Arterial dissection | 1 (2) | 0 | 1.7 (−4.8 to 8.9) | |
| In-hospital complications | ||||
| Death or palliative care within 7 d | 12 (20) | 10 (18) | 2.5 (−11.9 to 16.5) | |
| Asymptomatic ICH | 8 (14) | 9 (16) | −2.5 (−15.7 to 10.6) | |
| Malignant brain swelling | 7 (12) | 6 (11) | 1.1 (−11.0 to 13.1) | |
| New ischemic stroke | 6 (10) | 2 (4) | 6.5 (−3.5 to 17.0) | |
| Symptomatic ICH | 4 (7) | 9 (16) | −9.1 (−21.4 to 2.6) | |
| Extracranial hemorrhage | 3 (5) | 1 (2) | 3.3 (−5.0 to 12.1) | |
Abbreviations: EVT, endovascular thrombectomy; ICH, intracranial hemorrhage.
P values were derived using Mann-Whitney U tests for time metrics and Pearson χ2 tests for categorical data.
Reperfusion was defined as modified thrombolysis in cerebral infarction (mTICI) grade ≥2b.
The mTICI is a score used to rate reperfusion after endovascular treatment and consists of 5 grades (0, 1, 2a, 2b, and 3). Scores ≥2b indicate successful reperfusion.
Secondary Outcomes
Overall median (IQR) time delay from onset to reperfusion was 243 (194-301) minutes in the flying team group and 340 (279-438) minutes in the transfer group (difference, 97 min [95% CI, 59 to 154]; P < .001). In patients who underwent EVT, no significant differences were seen between the flying team and the transfer group regarding rate of successful reperfusion (modified thrombolysis in cerebral infarction scale grade ≥2b) (92% vs 84%; absolute difference, 7.5 [95% CI, −4.7 to 20.0]) and median (IQR) duration of procedure (from groin puncture to reperfusion, 36 [26-46] vs 45 [28-59] min; difference, 9 min [95% CI, −3 to 21]). For additional secondary outcomes, including periprocedural and in-hospital complications, see Table 2.
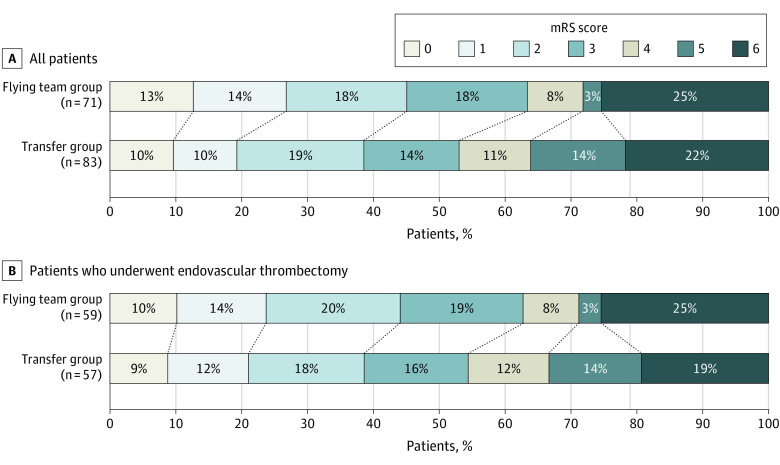
There was no significant difference in median (IQR) mRS score after 3 months between patients in the flying team and transfer groups who received EVT (3 [2-6] vs 3 [2-5]), with an adjusted common odds ratio for improved functional outcome in the flying team group of 1.91 (95% CI, 0.96-3.88; P = .07) (Figure 3; eFigure 4 in Supplement 1).
Figure 3. Functional Outcome at 3 Months in a Study of the Effect of a Flying Intervention Team vs Patient Interhospital Transfer on Time to Endovascular Thrombectomy.
Scores on the modified Rankin Scale (mRS) range from 0 (no symptoms) to 6 (death). Severe disability (score of 5) and death (score of 6) were combined in a single category in the ordinal logistic regression model. Of all patients included, follow-up data were missing in 3 patients (2 in the transfer group and 1 in flying team group). In patients who underwent EVT, follow-up data was missing in 1 patient in the flying team group. In patients who received EVT, the adjusted common odds ratio of the flying team group was 1.91 (95% CI, 0.96-3.88; P = .07) for an improved mRS score, 1.64 (95% CI, 0.72-3.72; P = .24) for good clinical outcome, and 1.14 (95% CI, 0.44-2.94; P = .79) for death within 3 months. In the whole study population, including patients who eventually did not receive EVT, the flying team group was significantly associated with an improved mRS score after 3 months (adjusted common odds ratio, 1.91 [95% CI, 1.05-3.50]; P = .04). Multivariable logistic regression models were adjusted for age, sex, and National Institute of Health Stroke Scale score. No missing values were present in the baseline data used in this model.
Post Hoc Outcomes
Considering all patients, including patients who eventually did not receive EVT, the flying team group was significantly associated with an improved median (IQR) mRS score after 3 months (3 [1-6] vs 3 [2-5]; adjusted common odds ratio, 1.91 [95% CI, 1.05-3.50]; P = .04) (Figure 3). After adding occlusion sites to the multivariable model, the adjusted common odds ratio for a better mRS score at 3 months in the flying team group was 2.27 (95% CI, 1.10-4.79; P = .03) in patients who received EVT and 2.21 (95% CI, 1.19-4.17; P = .01) in all patients (see eTable 2 in Supplement 1).
Discussion
In a nonurban stroke network in Germany, deployment of a flying intervention team to local stroke centers, compared with patient interhospital transfer to referral centers, was significantly associated with shorter time to endovascular thrombectomy for patients with acute ischemic stroke.
The underlying idea behind the flying intervention team is reduction of time to treatment by parallelization of processes. Patient interhospital transfer comprises several consecutive processes, such as preparation for transfer, transportation, reevaluation (ie, clinical and, at times, imaging), and preparation in the angiography suite of the referral center. This process results in long transfer delays. The time from onset to groin puncture reported in the transfer group was in line with other studies that have reported time delays (median of 239-350 min).17 With the flying team service, transportation of the medical team is performed in parallel to basic supply preparation in the angiography room, initiation of general anesthesia, draping of patient, and puncture site preparation. Therefore, groin puncture can be performed directly after arrival of the team.
Many efforts have been made to reduce time to recanalizing therapies in stroke.18 Mobile stroke units are powerful means to reduce time delays in urban areas.19,20 Improvement of in-hospital processes for EVT are adequate tools for comprehensive stroke centers.18 But, for rural or even remote areas, measures to reduce time delays until EVT have had moderate effect thus far.21,22 The flying team approach reported in this study reversed the usual system of care in rural areas: the neurointerventional expert was transferred, not the patient.
Reperfusion rate, duration of procedure, and periprocedural complication rates of the flying team demonstrate that EVT was feasible in the local primary stroke centers. Flying team care was highly standardized regarding process workflows, staff resources, technical equipment, and surrounding working conditions to lessen the chance for complications. The general quality of telemedicine-assisted stroke unit care has been shown previously7,9,10 and was achieved by extensive training programs, quality management, and standardization of care.
Results on functional outcomes in this study were in line with a meta-analysis of randomized clinical trials, which showed that in those with successful reperfusion, each hour delay was associated with a less favorable degree of disability.1 However, the difference between the groups in this study was statistically significant only in post hoc analyses. Furthermore, no significant difference was seen when dichotomizing mRS scores in the groups of 0 to 2 vs 3 to 6. Therefore, the results must be interpreted with care.
The overall mortality rate at 3 months in this study was higher than in the abovementioned meta-analysis (15.3%).23 This difference may be partly due to the wider inclusion criteria used in this study, resulting in a higher median age of participants.23
Initial reports of other teams that used cars to perform endovascular therapy in a connected hospital without onsite neurointerventionists have been published and underline the feasibility of transferring the interventionist instead of the patient.24,25,26 In the urban surrounding of New York City, a mobile team was associated with significant reductions in door-to-recanalization times compared with secondary transfer.26 The feasibility of transferring a neurointerventionist via helicopter has also been shown in a proof-of-concept case.27 Other groups have shown that in-hospital delays in primary stroke centers can be improved with structured measures such as an awaiting ambulance during primary stroke center processes, which is an approach that may also be viable in certain settings.28,29,30
The large time reduction associated with the flying team model in this study meets the demand of the acute stroke guidelines.2,3 By effectively turning primary stroke centers into EVT-capable stroke centers, the flying team model may help to compensate for inequalities of stroke care between urban and rural settings.
Limitations
This study has several limitations. First, findings are limited by the nonrandomized study design. The flying team service was set up as a provisional, part-time service in Southeast Bavaria for 26 weeks per year, therefore randomization on the patient level was not possible. Second, practitioners were not blinded to the current available system of care. This may have caused selection bias. Third, the new system of care under investigation was evaluated in a single region, established within an existing network with almost 2 decades of comprehensive quality management, and set up considering the shortages (and available resources) within the local health system. Therefore, it may not be generalizable to other rural areas with different standards of care. Fourth, interventionists were not identical in the 2 groups. The flying team service consisted of 5 interventionists, whereas the transfer group was treated in 5 different referral centers in and outside the TEMPiS network, with many more interventionists involved. Skills, experience, and treatment approaches might have varied between the groups and might have influenced outcomes. However, the flying team service was initiated to improve stroke outcome results compared with current clinical practice in these remote areas, acknowledging a wide scale of patient ages and stroke severities and differences in the treatment modalities in the existing referral centers. Although these limitations may have had a limited effect on the primary end point, the improvement in clinical outcome has to be interpreted with care, and the results do not allow to conclude an effect of the system of care, but rather to acknowledge an association. Fifth, although overall delays from onset to puncture in the transfer group were similar to those of national registries, regional specifics may have influenced delays, such as obligation for medical crew to accompany patients with ongoing intravenous thrombolysis infusion or possibility of repeated imaging at referral center. Other health care systems may enable faster transfer delays, thus possibly reducing the direct advantage of a flying intervention team. Sixth, this model of care is resource intensive, especially when the helicopter is exclusively dedicated to flying team remote service. Analyses for cost-effectiveness are warranted.
Conclusions
In a nonurban stroke network in Germany, deployment of a flying intervention team to local stroke centers, compared with patient interhospital transfer to referral centers, was significantly associated with shorter time to endovascular thrombectomy for patients with acute ischemic stroke. The findings may support consideration of a flying intervention team for some stroke systems of care, although further research is needed to confirm long-term clinical outcomes and to understand applicability to other geographic settings.
eMethods
eFigure 1. Suitcase and traveling bags with interventional material brought along by the Flying Intervention Team with each deployment
eFigure 2. Angiography kit stored locally to be prepared by onsite staff before arrival of Flying Intervention Team
eTable 1. Demographics and baseline characteristics for patients with EVT
eTable 2. Clinical outcomes at 3 months
eFigure 3. Time from decision for EVT to groin puncture in ascending order
eFigure 4. Functional Outcome at 3 months in flying team and transfer patients (mRS 0-2 vs. 3-6)
Statistical analysis plan
Trial protocol
References
- 1.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez de la Ossa N, Abilleira S, Dorado L, et al. ; Catalan Stroke Code and Reperfusion Consortium . Access to endovascular treatment in remote areas: analysis of the reperfusion treatment registry of Catalonia. Stroke. 2016;47(5):1381-1384. doi: 10.1161/STROKEAHA.116.013069 [DOI] [PubMed] [Google Scholar]
- 5.Hammond G, Luke AA, Elson L, Towfighi A, Joynt Maddox KE. Urban-rural inequities in acute stroke care and in-hospital mortality. Stroke. 2020;51(7):2131-2138. doi: 10.1161/STROKEAHA.120.029318 [DOI] [PubMed] [Google Scholar]
- 6.Acute medical and surgical management. In Clinical Guidelines for Stroke Management. Stroke Foundation; 2022. Accessed April 11, 2022. https://app.magicapp.org/#/guideline/QnoKGn/section/jNVp7j
- 7.Audebert HJ, Schenkel J, Heuschmann PU, Bogdahn U, Haberl RL; Telemedic Pilot Project for Integrative Stroke Care Group . Effects of the implementation of a telemedical stroke network: the telemedic pilot project for integrative stroke care (TEMPiS) in Bavaria, Germany. Lancet Neurol. 2006;5(9):742-748. doi: 10.1016/S1474-4422(06)70527-0 [DOI] [PubMed] [Google Scholar]
- 8.Hubert GJ, Santo G, Vanhooren G, et al. Recommendations on telestroke in Europe. Eur Stroke J. 2019;4(2):101-109. doi: 10.1177/2396987318806718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubert GJ, Meretoja A, Audebert HJ, et al. Stroke thrombolysis in a centralized and a decentralized system (Helsinki and Telemedical Project for Integrative Stroke Care Network). Stroke. 2016;47(12):2999-3004. doi: 10.1161/STROKEAHA.116.014258 [DOI] [PubMed] [Google Scholar]
- 10.Müller-Barna P, Hubert GJ, Boy S, et al. TeleStroke units serving as a model of care in rural areas: 10-year experience of the TeleMedical project for integrative stroke care. Stroke. 2014;45(9):2739-2744. doi: 10.1161/STROKEAHA.114.006141 [DOI] [PubMed] [Google Scholar]
- 11.Wilcock AD, Schwamm LH, Zubizarreta JR, et al. Reperfusion treatment and stroke outcomes in hospitals with telestroke capacity. JAMA Neurol. 2021;78(5):527-535. doi: 10.1001/jamaneurol.2021.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubert GJ, Kraus F, Maegerlein C, et al. The “flying intervention team”: a novel stroke care concept for rural areas. Cerebrovasc Dis. 2021;50(4):375-382. doi: 10.1159/000514845 [DOI] [PubMed] [Google Scholar]
- 13.Eurostat regional yearbook 2018. Eurostat. Accessed September 9, 2020. https://ec.europa.eu/statistical-atlas/viewer/?config=RYB-2018.json&mids=BKGCNT,BKGCRL,C11M01,CNTOVL&o=1,0.35,1,0.7&ch=BKG,C04,TRT¢er=53.91306,40.94368,7&lcis=BKGCRL&lf=VALUE&#
- 14.Kreisfreie Städte und Landkreise nach Fläche, Bevölkerung und Bevölkerungsdichte am 31.12.2019. Statistisches Bundesamt. Accessed September 9, 2020. https://www.destatis.de/DE/Themen/Laender-Regionen/Regionales/Gemeindeverzeichnis/Administrativ/04-kreise.html
- 15.Saver JL, Filip B, Hamilton S, et al. ; FAST-MAG Investigators and Coordinators . Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke. 2010;41(5):992-995. doi: 10.1161/STROKEAHA.109.571364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 17.Maas WJ, Lahr MMH, Buskens E, van der Zee DJ, Uyttenboogaart M; CONTRAST Investigators . Pathway design for acute stroke care in the era of endovascular thrombectomy: a critical overview of optimization efforts. Stroke. 2020;51(11):3452-3460. doi: 10.1161/STROKEAHA.120.030392 [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Jadhav AP, Bonafe A, et al. ; SWIFT PRIME investigators . Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology. 2016;279(3):888-897. doi: 10.1148/radiol.2016160204 [DOI] [PubMed] [Google Scholar]
- 19.Ebinger M, Winter B, Wendt M, et al. ; STEMO Consortium . Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622-1631. doi: 10.1001/jama.2014.2850 [DOI] [PubMed] [Google Scholar]
- 20.Ebinger M, Siegerink B, Kunz A, et al. ; Berlin_PRehospital Or Usual Delivery in stroke care (B_PROUD) study group . Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. 2021;325(5):454-466. doi: 10.1001/jama.2020.26345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangadharan S, Lillicrap T, Miteff F, et al. Air vs road decision for endovascular clot retrieval in a rural telestroke network. Front Neurol. Published online December 4, 2020. doi: 10.3389/fneur.2020.00628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanou MI, Stadler V, Baku D, et al. Optimizing patient selection for interhospital transfer and endovascular therapy in acute ischemic stroke: real-world data from a supraregional, hub-and-spoke neurovascular network in Germany. Front Neurol. Published online December 4, 2020. doi: 10.3389/fneur.2020.600917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 24.Seker F, Fiehler J, Möhlenbruch MA, et al. Time metrics to endovascular thrombectomy in 3 triage concepts: a prospective, observational study (NEUROSQUAD). Stroke. 2020;51(1):335-337. doi: 10.1161/STROKEAHA.119.027050 [DOI] [PubMed] [Google Scholar]
- 25.Wei D, Oxley TJ, Nistal DA, et al. Mobile interventional stroke teams lead to faster treatment times for thrombectomy in large vessel occlusion. Stroke. 2017;48(12):3295-3300. doi: 10.1161/STROKEAHA.117.018149 [DOI] [PubMed] [Google Scholar]
- 26.Morey JR, Oxley TJ, Wei D, et al. ; Mount Sinai Stroke Investigators . Mobile interventional stroke team model improves early outcomes in large vessel occlusion stroke: the NYC MIST trial. Stroke. 2020;51(12):3495-3503. doi: 10.1161/STROKEAHA.120.030248 [DOI] [PubMed] [Google Scholar]
- 27.Hui FK, El Mekabaty A, Schultz J, et al. Helistroke: neurointerventionalist helicopter transport for interventional stroke treatment: proof of concept and rationale. J Neurointerv Surg. 2018;10(3):225-228. doi: 10.1136/neurintsurg-2017-013050 [DOI] [PubMed] [Google Scholar]
- 28.Gaynor E, Griffin E, Thornton J, et al. Ambulance waiting and associated work flow improvement strategies: a pilot study to improve door-in-door-out time for thrombectomy patients in a primary stroke center. J Neurointerv Surg. Published online July 13, 2021. doi: 10.1136/neurintsurg-2021-017653 [DOI] [PubMed] [Google Scholar]
- 29.McTaggart RA, Moldovan K, Oliver LA, et al. Door-in-door-out time at primary stroke centers may predict outcome for emergent large vessel occlusion patients. Stroke. 2018;49(12):2969-2974. doi: 10.1161/STROKEAHA.118.021936 [DOI] [PubMed] [Google Scholar]
- 30.Choi PMC, Tsoi AH, Pope AL, et al. Door-in-door-out time of 60 minutes for stroke with emergent large vessel occlusion at a primary stroke center. Stroke. 2019;50(10):2829-2834. doi: 10.1161/STROKEAHA.119.025838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Suitcase and traveling bags with interventional material brought along by the Flying Intervention Team with each deployment
eFigure 2. Angiography kit stored locally to be prepared by onsite staff before arrival of Flying Intervention Team
eTable 1. Demographics and baseline characteristics for patients with EVT
eTable 2. Clinical outcomes at 3 months
eFigure 3. Time from decision for EVT to groin puncture in ascending order
eFigure 4. Functional Outcome at 3 months in flying team and transfer patients (mRS 0-2 vs. 3-6)
Statistical analysis plan
Trial protocol