Abstract
Ethyl carbamate (EC) is a process contaminant that can be formed as a byproduct during fermentation and processing of foods and beverages. Elevated EC levels are primarily associated with distilled spirits, but this compound has also been found at lower levels in foods and beverages, including breads, soy sauce, and wine. Evidence from animal studies suggests that EC is a probable human carcinogen. Consequently, several governmental institutions have established allowable limits for EC in the food supply. This review will discuss EC formation mechanisms, occurrence of EC in the food supply, and EC dietary exposure assessments. Analytical methods currently used to detect EC, and advances in experimental technologies, such as nanosensors and surface-enhanced Raman spectroscopy (SERS) will also be discussed. Finally, application of mitigation methods to maintain levels of EC under allowable limits will be covered, including distillation practices, enzymatic treatments, and genetic engineering of yeast. Ongoing research in this field is needed to refine mitigation strategies and develop methods to rapidly detect EC in the food supply.
Keywords: Process contaminant, distilled spirits, GC-MS, thermal processing, urethane, exposure assessment
INTRODUCTION
Ethyl carbamate (also referred to as urethane; molecular weight, 89.09 g/mol; boiling point, 183 °C) occurrence in the food supply has been the subject of considerable research since this compound was classified as a probable human carcinogen in 1974 (90, 148). Because ethyl carbamate (EC) can form in foods and beverages during reactions that occur during the processing or fermentation of foods and beverages, it is considered a process contaminant (73, 237). Although EC formation occurs through several different pathways in food systems, a primary pathway involves select compounds reacting with ethanol (EtOH); therefore, the majority of the research has focused on the occurrence of EC in alcoholic beverages. The carcinogenic potential of EC has spurred efforts to develop methods to detect this compound, mitigate its formation during processing, advance remediation capabilities, and to establish regulations and recommendations for allowable limits for EC in foods and beverages (154, 233).
EC was postulated to have value as a chemotherapeutic agent until the 1940s, when research revealed that EC was, in fact, ineffective in treating cancer (65, 103). Subsequently, the United States (US) National Toxicology Program (NTP) designated EC as “reasonably anticipated to be a human carcinogen” based on data from animal models (148). NTP toxicology studies demonstrated that EC induces tumors in rodents at various tumors at various tissue sites, following different routes of exposure. Specifically, the NTP’s assessment found that oral exposure to EC in rodents resulted in lymphoma, leukemia, and cancer of multiple organ sites, including the lung, liver, mammary gland, skin, and stomach. Studies on co-exposure with EtOH suggest their interaction is relevant to EC tumorigenesis (13). Similarly, the World Health Organization’s International Agency for Research on Cancer (IARC) working group classified EC as a group 2A carcinogen, i.e., probably carcinogenic to humans (90, 159). EC is hypothesized to undergo metabolism in vivo to yield a highly reactive vinyl carbamate epoxide, which binds to nucleic acids and other biomacromolecules (198). DNA adducts formed from the reaction with the epoxide compound can increase the likelihood of mutations leading to carcinogenesis.
The Joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) estimated exposure to EC from foods and alcoholic beverages and determined that the risk posed by intake of EC from foods excluding alcoholic beverages would be of low concern. However, EC exposure from intake of food and alcoholic beverages combined warranted further attention, leading FAO/WHO to suggest that mitigation measures to reduce EC in some alcoholic beverages are needed (198). Other risk assessments, including by the European Food Safety Authority (EFSA), Environment and Climate Change Canada/Health Canada, and Schlatter et al. have drawn similar conclusions (53, 57, 176).
These risk assessments have encouraged the establishment of allowable limits for EC in beverages by various governmental agencies, summarized in Table 1. The highest EC allowable limits are for fruit brandy, which range from 400 µg/L for Canada and the Czech Republic to 1,000 µg/L for the European Union (56, 57, 85). Allowable limits for distilled spirits (e.g., tequila, whiskey, and vodka) range from 125 µg/L for the US to 210 µg/L for Brazil (17, 63). Canada specifically limits EC levels in sake (a type of rice wine) at 200 µg/L (85, 105). EC limits for table wine and fortified wines (wines with added distilled spirits, such as sherry and madeira wine) range from 15–30 µg/L for table wines to 60–100 µg/L for fortified wines.
Table 1.
Summary of allowable limits for ethyl carbamate in beverages.
| Beverage | Typical ethanol concentrationa | Allowable limit, µg/kg (Country/region) | References |
|---|---|---|---|
| Fruit brandyb | 40% | 400 (Canada and Czech Republic); 800 (Germany); 1,000 (France); 1,000 (European Unionc) | European Food Safety Authority (2007); European Commission (2016); Health Canada (2020) |
| Sake | 15–20% | 200 (Canada) | Health Canada (2020) |
| Distilled spiritsd | 40% | 125 (USe); 150 (Canada, Czech Republic, and France); 210 (Brazil) | Brazil Ministry of Agriculture (2014); Health Canada (2020); European Food Safety Authority (2007); US Federal Register (1990) |
| Fortified wines | 19–21% | 60 (USf); 100 (Canada) | US Federal Register (1990); Health Canada (2020) |
| Wine | 12% | 15 (USf); 30 (Canada and Czech Republic) | US Federal Register (1990); Health Canada (2020); European Food Safety Authority (2007) |
Bujake (1992); Tredoux and Ferreira (2012); Kwon et al. (2014); National Institutes of Health. “What Is A Standard Drink?” Available at: https://www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink. Accessed 26 MAR 2021.
Referred to in the European Commission document as spirits made with stone fruit or grapes. Referred to in other documents as liqueurs or distillates.
This value has been designated as a target recommendation by the European Commission.
Only whiskey is referred to in allowable limits for the US.
Voluntary limits made in an agreement with the FDA and the Distilled Spirits Council of the United States (DISCUS).
Voluntary limits made in an agreement with the FDA and the Wine Institute (WI) and American Association of Vintners (AAV).
Additional governmental agencies and industry groups have established allowable limits or are involved in monitoring levels of EC. Australia and New Zealand do not have established allowable limits for EC based on a risk assessment, which showed levels in the food supply not to be of concern (67). The intergovernmental International Organization of Vine and Wine (OIV) also does not have allowable limits for EC in wine, but the OIV has adopted analytical methods for EC detection to encourage continued monitoring of this compound (91). In the US, EC allowable limits in alcoholic beverages are the result of a voluntary agreement with leading industry trade groups and the US Food and Drug Administration (FDA) (63).
OVERVIEW OF THE MECHANISMS AND KEY SUBSTRATES OF EC FORMATION IN FOODS AND BEVERAGES
The major contributors to EC formation in foods and beverages have been known for decades. Because EC formation mechanisms have been previously reviewed in detail by other authors, e.g., Jiao et al. (97), only key aspects will be described here. Broadly speaking, EC formation occurs when EtOH (typically produced by alcoholic fermentation) reacts with cyanate or compounds with a carbamoyl functional group, such as urea and citrulline (Figure 1) (152). The reaction between urea and EtOH is overall the most common pathway for EC formation in fermented foods and beverages, although one notable exception is that the high concentrations of EC in stone fruit spirits are driven by cyanate.
Figure 1.
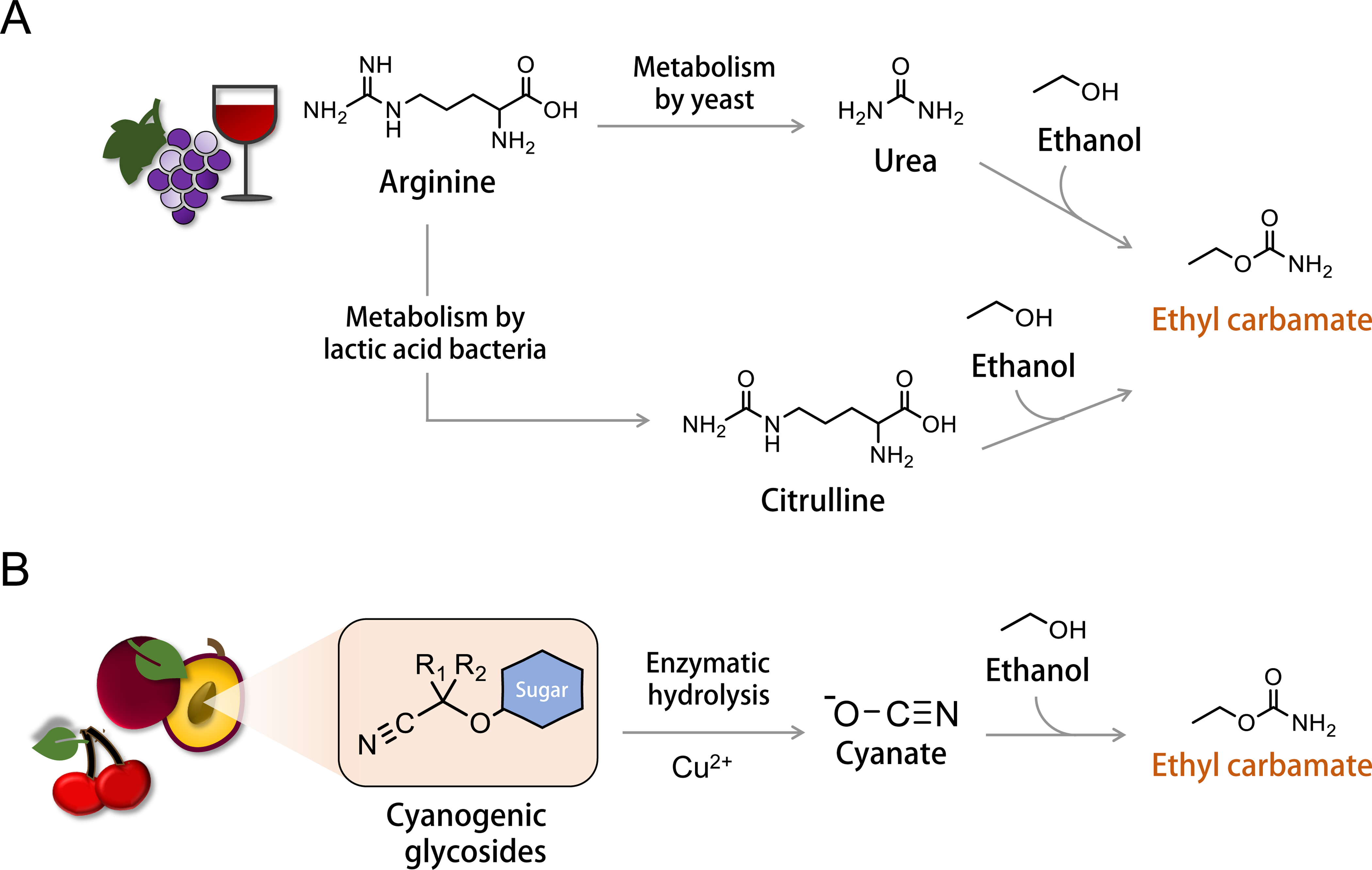
Simplified schematic of major ethyl carbamate formation mechanisms. (A) Arginine, rich in grapes, can be metabolized to urea (by yeast) and citrulline (by lactic acid bacteria) during fermentation. Urea can then react with ethanol produced during fermentation to yield ethyl carbamate. In addition, citrulline can react with ethanol to yield ethyl carbamate. (B) Cyanogenic glycosides from sources such as fruit stones (e.g., plum and cherry pits), grains, and sugar cane can undergo hydrolysis to yield cyanate. Copper ions (Cu2+) have the capability to enhance cyanate formation. In turn, cyanate can react with ethanol to form ethyl carbamate.
EC formation can be accelerated by factors such as heat or thermal processing, transition metals, storage conditions, pH, and ultraviolet (UV) radiation. Several compounds can provide a carbamoyl functional group for EC formation. For example, arginine (an amino acid rich in grapes) can be catabolized by yeast during fermentation to yield urea, whereas cyanogenic glycosides in certain plant sources (e.g., plum and cherry pits) generate cyanate through the action of enzymes present in the plant tissues. To a lesser extent, bacterial metabolism of arginine produces citrulline, which can ultimately react with EtOH to form EC (99, 223). In wine production, arginine is a strong contributor to EC formation because arginine is abundant in grapes and the strains of yeast responsible for wine fermentation can metabolize arginine to either urea or citrulline. Formation of EC via citrulline is a less common synthesis route compared to urea, but still occurs in red and white table wine, yellow rice wine, and fermented soybean products (82, 195, 221, 232).
Distilled spirits containing stone fruits often have high levels of EC because their production provides ideal conditions for generating EC (22). The pits from stone fruits (e.g., apricot, cherry, and plum) are a rich source of cyanogenic glycosides, typically amygdalin. If these compounds are present during fermentation, the cyanate that results from the hydrolysis of cyanogenic glycosides can react with EtOH. Some manufacturers may not remove pits before fermentation due to its impact on the flavor profile of the spirits and because pit removal adds additional processing steps. Although the occurrence of cyanogenic glycosides does not always predict EC concentrations, elevated amounts of cyanogenic glycosides are often found in foods and beverages that have elevated EC levels.
Cyanogenic glycosides are present in other ingredients used to produce alcoholic beverages besides stone fruit. Notable sources of these glycosides are grains, sugar cane, and cassava, which are components of distilled spirits, such as whiskey, cachaça, and tiquira (41, 71, 110, 139, 150, 162). Besides ingredients, other factors that are associated with EC formation in spirits are higher temperatures and use of a copper vessel to collect condensed spirits post distillation (21, 71, 167, 168).
OCCURRENCE OF EC IN FOODS/BEVERAGES AND EXPOSURE ASSESSMENTS
Before allowable limits for EC levels in foods and beverages were considered, studies were conducted to identify the baseline level of EC present in these products (49, 153). Foods and condiments targeted for sampling included breads, fermented dairy products, pickled vegetables, soy sauce, and vinegar (Table 2). These foods, produced using fermentation reactions, are known to contain microorganisms that can generate urea through amino acid metabolism (73). The highest level of EC detected in the FDA’s initial survey of foods and condiments was 84 µg/kg (49). In comparison, alcoholic beverages such as beer, wine, and especially distilled spirits can contain substantially higher levels of EC (see Table 3) due to the high amounts of substrate available for EC formation—generally, EtOH, urea, and cyanate.
Table 2.
Summary of key data on the occurrence of ethyl carbamate in foods including condiments.a
| Food/condiment | Mean concentration (µg/kg)b | Concentration range (µg/kg)b | n | Analytical method | Country/region | References |
|---|---|---|---|---|---|---|
| Bread | 2.6c | ND–12 | 104 | GC/N-TEA; GC-MS | Canada; Denmark; US; Hong Kong | Diachenko et al. (1992); Haddon et al. (1994); Tang et al. (2011); Vahl et al. (1993) |
| Cheese | ND | ND | 17 | GC/N-TEA | US | Diachenko et al. (1992) |
| Fermented cabbage (kimchi) | 3.5 | ND–16 | 20 | GC-MS | Korea | Lee Kim et al. (2000) |
| Soy sauce | 16c | ND–130 | 20 | GC/N-TEA | China; Germany; Korea; Hong Kong; US |
Diachenko et al. (1992); Fauhl et al. (1993); Lee Kim et al. (2000); Koh et al. (2007); Tang et al. (2011); Mo et al. (2014); Choi et al. (2018) |
| Wine vinegar | 8.8 | 4–26 | 6 | GC/N-TEA | US | Diachenko et al. (1992) |
| Yogurt | 0.4 | ND–3 | 14 | GC/N-TEA | US | Diachenko et al. (1992) |
Abbreviations used: GC-MS, gas chromatography-mass spectrometry; GC/N-TEA, gas chromatography-thermal energy analyzer with nitrogen converter; ND, not detected.
Ethyl carbamate data rounded to two significant figures.
Mean of means from reference data shown for display purposes in table.
Table 3.
Summary of key data on the occurrence of ethyl carbamate in alcoholic beverages.a
| Beverage | Concentration mean (µg/kg)b | Concentration range (µg/kg)b | n | Analytical methods | Country/region | References |
|---|---|---|---|---|---|---|
| Beer | 1.7c | ND–20 | 232 | GC-MS | China; Hong Kong | Tang et al. (2011); Li et al. (2017) |
| Distilled spirits | 44c | ND–390 | 904 | GC/N-TEA; LC-FLD; GC-MS | China; Mexico; Germany; Guatemala; US | Diachenko et al. (1992); Madrera et al. (2009); Liu et al. (2013); Liu et al. (2011); Wu et al. (2012); Lachenmeier et al. (2009) |
| Fruit brandy | 1,000c | 3.5–5,100 | 103 | GC/N-TEA; GC-MS | Denmark; Korea; US | Diachenko et al. (1992); Vahl et al. (1993); Ha et al. (2006) |
| Stone fruit spirits | 1,400 | 10–18,000 | 631 | GC-MS | Germany | Lachenmeier (2005) |
| Sugar cane spirits/cachaça | 110c | 12–910 | 385 | GC-MS; HPLC-FLD | Brazil |
Nobrega et al. (2011); d’Avila et al. (2016); Bortoletto & Alcarde (2015); Masson et al. (2014) |
| Wine, fortified | 55c | ND–260 | 248 | GC/N-TEA; GC-MS; LC-MS | Denmark; US; Various from FAO submission; UK; Portugal; South Africa |
Diachenko et al. (1992); Vahl et al. (1993) FAO (2006); Hasnip et al. (2007); Perestreloa et al. (2010); Alberts et al. (2011) |
| Wine, table | 9.0c | ND–61 | 5,930 | GC/N-TEA; GC-MS; LC-MS | Denmark; China; Spain, South Africa; US; Various from FAO submission | Diachenko et al. (1992); Vahl et al. (1993); FAO (2006); Wu et al. (2012); Fu et al. (2016); Jagerdeo et al. (2002); Uthurry et al. (2004); Alberts et al. (2011) |
| Yellow rice wine | 150 | ND–580 | 221 | GC-MS; HPLC-FLD | China | Mo et al. (2014); Fu et al. (2010) |
Abbreviations used: FAO, Food and Agriculture Organization; GC-MS, gas chromatography-mass spectrometry; GC/N-TEA, gas chromatography-thermal energy analyzer with nitrogen converter; LC-MS, liquid chromatography-mass spectrometry; LC-FLD, liquid chromatography-fluorescence detection; ND, not detected.
Ethyl carbamate data rounded to two significant figures.
Mean of means from reference data shown for display purposes in table.
Breads.
Fermentation that occurs during production of yeast breads can generate EC via EtOH reacting with cyanate derived from grains or with urea formed during amino acid metabolism by yeast. Dough conditioners—added to improve the texture and color of commercial bread—have also been associated with EC in breads (48). Specifically, researchers have linked use of azodicarbonamide (ADA) as a dough conditioner to increased levels of EC in the finished bread (24). ADA is a food additive approved for use as a dough conditioner by the FDA, but is not approved as a food additive in the EU (55, 186). Experiments were not able to directly ascertain if EC occurrence in bread made with ADA was the direct result of ADA breakdown products or whether ADA created chemical conditions that favored EC generation from other compounds. Although breads generally do not contain high levels of EC in comparison with other foods and beverages, they are of interest as a potential source of exposure due to high bread consumption levels.
Soy Sauce and other Fermented Soybean Products.
Collectively, studies on soy sauce indicate that traditionally fermented varieties generally contain higher EC levels compared to non-fermented soy sauce (34, 62). For example, several authors found that traditionally fermented soy sauce contained EC concentrations up to 130 µg/L, while non-fermented soy sauce contained low or non-detectable levels of EC (60, 101, 170). Other types of fermented soybean products such as miso, tempeh, and natto contained EC at levels ranging from non-detectable up to 5 µg/kg (79, 151). The differences in EC levels between liquid and solid fermented soybean products may be due to the higher concentrations of EtOH normally found in liquid products, but further work needs to be done to confirm this hypothesis (79, 151).
Alcoholic Beverages.
One of the earliest surveys of EC occurrence in alcoholic beverages was conducted by the FDA. Of the 89 wines and distilled spirits analyzed, a majority of samples (n=77) contained levels below 100 µg/L EC, although the fortified wines (i.e., sherry and port) contained levels greater than 500 µg/L EC (23, 183). Other studies found that Scotch and malt whisky had EC concentrations of 15–100 µg/L and fermented agave spirits (e.g., tequila) had a mean EC level of 50 µg/L, with no EC detected in gin and vodka (8, 109).
Surveys of beer and table wine samples conducted across Asia, Europe, North America, and South Africa have found a maximum level of ~60 µg/L EC, although most of the samples were found to have EC at lower or non-detectable levels (70, 83, 88, 98, 123, 189, 205). Yellow rice wine collected in surveys from China (typically made from fermented rice and other grains) showed considerable variation in EC levels (69, 146). Other surveys on alcoholic beverages have shown that distilled spirits typically contain higher EC concentrations compared to beer and wine (131, 132, 140).
In the distilled spirits category, liquor made with stone fruits has the highest levels of EC. In a large survey (n=631) of stone fruit distilled spirits collected in Germany over 18 years (1986–2004), the authors found that EC levels reached a high of 18,000 µg/L, with a mean of 1,400 µg/L (113). Over time, however, these authors observed a reduction in the number of samples exceeding Germany’s 800 µg/L EC limit, with large-scale distilleries generally more successful at reducing EC levels compared with smaller distilleries (113). In the US, an analysis of fruit brandy (n=89) that took place during the same timeframe found EC levels similar to those in the study done in Germany (mean = 1,197 µg/L) (49). More recent survey data still show relatively elevated levels of EC in stone fruit spirits in comparison to wine and beer, although levels tend to be lower than in the aforementioned studies. In samples from Korea, for example, the majority of fruit brandy and plum wine sample contained EC concentrations less than 350 µg/L (76, 121, 170).
Other types of distilled spirits besides those made with stone fruits contain elevated concentrations of EC. For example, the Brazilian distilled spirits cachaça and tiquira have attracted attention because data show EC concentrations can reach 1,000 µg/L and higher for cachaça and up to 3,500 µg/L for tiquira, exceeding the Brazilian EC allowable limit of 210 µg/L by ~5- to 17-fold (16, 110, 141, 165). Elevated EC levels found in these beverages have been related to cyanate precursors present in sugar cane and cassava, which are used in production of cachaça and tiquira, respectively. Processing steps taken at individual distilleries may have a large effect on EC values in these beverages because one analysis found that EC in sugar cane spirits varied widely between distilleries, ranging from below analytical detection up to 1,600 µg/L (20, 43, 173).
EC levels in distilled spirits produced in-home may be of concern due to the limited production controls that exist. Studies performed in Europe, including Hungary, Lithuania, Poland, Russia, and Ukraine, where there is a tradition of preparing in-home distilled spirits, indicate that in-home produced stone fruit spirits can contain EC levels in excess of 1,000 µg/L (108, 112, 114). This problem may be limited to stone fruit spirits, as many different types of in-home produced distilled spirits from Russia and Ukraine made without stone fruit do not have high levels of EC (111, 180).
EC Exposure Assessments.
EC dietary exposure and risk assessments have been conducted in various regions, including Asia, Australia, Brazil, Europe, and North America (32, 36, 57, 97, 110, 181, 190, 198). Dietary intake estimates varied between studies, with differences in a population’s consumption of alcoholic beverages being a major determinant of the variance in EC exposure. Intake estimates from JECFA found that mean EC exposures from food alone were about 15 ng/kg body weight (bw) per day (198). For the six countries with sufficient data to estimate exposure from the total diet (i.e., including alcoholic beverages), the national estimates for mean intake for EC from food and alcoholic beverages ranged from 15 to 65 ng/kg bw per day. JECFA attributed the wide variability in EC levels among countries to the fact that mitigation measures have been effective in reducing EC concentrations in alcoholic beverages, and some of the data submitted for this analysis were relatively older, not reflecting more recent mitigation efforts (198).
Similarly, EFSA conducted an exposure assessment for EC from food and alcoholic beverages in 2007, relying on JECFA’s intake estimate from food of 15 ng/kg bw per day (57). Dietary exposure to EC from consumption of food and alcoholic beverages combined was estimated to be 65 ng/kg bw per day. The highest estimated EC exposure (558 ng/kg bw per day) was for a person consuming fruit brandy at the 95th percentile consumption level.
More recently in 2016, Environment and Climate Change Canada and Health Canada examined EC exposure using Monte Carlo simulation. At the 90th percentile for males and females aged 19 years and older, EC intake from food alone was estimated to be 20.3 and 20.0 ng/kg bw per day, respectively. EC intake from alcoholic beverages for male and females aged 19 years and older was estimated to be 106.0 and 59.0 ng/kg bw per day, respectively (53).
ANALYTICAL METHODS FOR DETERMINATION OF EC IN FOODS AND BEVERAGES
Analysis of food and beverages for EC can be accomplished via several different methods, with each having particular advantages and limitations. The major methods for EC quantitation use either gas chromatography (GC) or liquid chromatography (LC). The official AOAC method for alcoholic beverages and soy sauce uses gas chromatography-mass spectrometry (GC-MS) (27). Further details on the methods summarized in Table 4 will be discussed below. Alternative methods such as flow-injection mass spectrometry (FI-MS), enzyme-linked immunosorbent assay (ELISA), infrared (IR) spectroscopy, surface-enhanced Raman spectroscopy (SERS), and nanosensors will also be discussed. Interestingly, although there have been some efforts to produce a certified reference material for EC analysis, no such material appears to be available (192).
Table 4.
Summary of the main analytical methods used to detect ethyl carbamate in food and beverages.a
| Analytical Method | Advantages | Limitations | Limits of quantification (µg/L) for various matrices | References |
|---|---|---|---|---|
| GC-MS (official AOAC method) | • There is precedent in literature. • No analyte derivatization is necessary. • Method robustness. |
• Extraction can be lengthy and use large amounts of solvents. | 50, distilled spirits; 40, fortified wine; 10, table wine; 15, soy sauce | Canas et al. (1994) |
| GC-MS (with improved cleanup) | • Less solvent usage compared to the official AOAC method. • Increased analyte sensitivity. |
• Modifications to the official AOAC method may need to undergo validation. | 10, distilled spirits; 4.5, fortified wine; 1.2, table wine; 6, soy sauce | Leça et al. (2014); Mirzoian & Mabud (2006); Nóbrega et al. (2015); Wu, Zhang et al. (2014) |
| LC-FLD | • Low cost. • Detector is commonly found in analytical laboratories. |
• Often requires derivatization to enhance analyte signal. • The presence of interfering compounds can result in overstated analyte concentrations. |
16, distilled spirits; 5, fortified wine; 5, table wine; 13, soy sauce | Herbert et al. (2002); Zhang, Liu et al. (2014); Zhou, Liu et al. (2017) |
| LC-MS | • Capable of collecting information on non-volatile compounds. • Typically involves little sample preparation. |
• Requires extensive operator training. • Generally more expensive system compared to LC-FLD and GC-MS. |
2.1, distilled spirits; 0.5, fortified wine; 1.0, table wine; 0.1, soy sauce | Alberts et al. (2011); Leça et al. (2018); Park et al. (2007) |
Abbreviations used: GC-MS, gas chromatography-mass spectrometry; LC-MS, liquid chromatography-mass spectrometry; LC-FLD, liquid chromatography-fluorescence detection.
Gas Chromatography-Mass Spectrometry (GC-MS).
GC-MS methods were among the first used for detection of EC in foods and are still commonly used by analytical chemists for analysis of this compound (11, 29, 194, 212). The advantage of GC-MS is that is has the capability to quantify EC from a variety of foods and beverages, including wine, distilled spirits, breads, and pickled vegetables (18, 23, 25, 72, 93, 188, 206).
Governmental agencies and private industry primarily use GC-MS for EC analysis. Governmental agencies that have published literature using GC-MS for EC analysis include: Ontario Ministry of Agriculture and Food (Canada), National Research Centre for Certified Reference Materials (China), Chemical and Veterinary Investigation Laboratory (CVUA) of Karlsruhe (Germany), the FDA (US), National Institute of Hygienic Sciences (Japan), and the Norwich Food Science Laboratory (UK) (38, 47, 49, 79, 107, 116, 137). The robustness of GC-MS has allowed for successful multi-site validation, leading AOAC International to adopt an official GC-MS method for detection of EC in alcoholic beverages and soy sauce (27). The limits of quantitation (LOQ) for this method ranges from 10 µg/L for table wine up to 50 µg/L for distilled spirits.
Since then, there have been efforts to extend the AOAC official method to additional matrices, as the presence of simple sugars, lipids, and proteins in a food or beverage matrix may adversely affect EC quantitation by GC-MS (171). In the AOAC method, a solid phase extraction (SPE) procedure is employed to extract EC from liquid samples. SPE has also been used for extraction of EC from solid foods, including pickled vegetables, fish, bread, and cheese (122, 128). SPE can significantly reduce matrix interferences, with some authors reporting the ability to detect EC at the ng/L (~parts per trillion) level when samples are analyzed using GC-MS (37). However, the lengthy extraction procedure for EC analysis by GC-MS has encouraged researchers to develop methods that reduce sample preparation times (145, 149, 207).
Sample preparation can be virtually eliminated by using headspace solid phase microextraction (SPME), and SPME has been used to analyze both alcoholic beverages and solid foods (89, 138, 158, 218). Additional approaches with potential to increase sample throughput include microextraction by packed sorbent (MEPS), ultrasound-assisted microextraction, pressurized liquid extraction, and aqueous two-phase extraction systems (118, 126, 127, 136).
Over the past several years, there have been continued efforts to improve the sensitivity and specificity of the GC-MS method. Several analytical laboratories have moved from using single quadrupole GC-MS instrumentation to triple quadrupole tandem mass spectrometry (GC-MS/MS) systems. Tandem mass spectrometers have increased analyte sensitivity and provide higher confidence in analyte identification through compound fragmentation (213). One application of GC-MS/MS for analysis of EC in breads has been reported by Hamlet & Jayaratne (78), where the use of legacy methods could have resulted in inaccurate data due to analytical interferences. GC-MS/MS methods can also collect data on a large number of analytes in a single analysis, including multiple chemical contaminants and flavor compounds present in trace amounts (64, 66). GC-MS/MS also allows for potentially simplified sample preparation and reduced analytical separation times without decreased confidence in compound identification (226).
Liquid chromatography-fluorescence detection (LC-FLD) and liquid chromatography-mass spectrometry (LC-MS).
The use of LC methods to assess EC in foods and beverages is a recent development compared to analysis by GC-MS. LC methods generally require less sample preparation time than GC-MS methods and can simultaneously collect data on non-volatile compounds. Detection in LC is typically accomplished using FLD or MS. FLD does not provide as high a degree of specificity as MS in detecting EC, resulting in potential interferences from pigmented compounds and other components of a food matrix. However, the low cost of FLD allows it to be available in most laboratories. Consequently, LC-FLD has been used to detect EC in a variety of foods and beverages, particularly distilled spirits and wine (69, 87, 191, 196, 230). Methods using LC-FLD for EC analysis typically incorporate chemical derivatization to increase analyte sensitivity and reduce matrix effects. Several authors have reported that analysis of derivatized EC (most commonly derivatized with xanthydrol) using LC-FLD was able to achieve sensitivity comparable to GC-MS (1, 222).
LC-MS methods for EC quantitation have been developed that achieve a high degree of analyte sensitivity and specificity (155). An electrospray ionization (ESI) probe is commonly used in LC-MS analysis, but use of ESI for EC analysis can result in a low signal because EC is difficult to ionize. Instead of ESI, atmospheric pressure chemical ionization (APCI) has been used to produce methods with greater sensitivity (120). For example, Alberts et al. used APCI to develop a LC-MS/MS method with detection limits comparable to GC-MS (~0.6 µg/L EC) (2). Other researchers have derivatized EC before analysis using ESI to increase EC ionization efficiency (46). Some researchers have been able to avoid EC derivatization by using state-of-the-art ESI probes to produce methods with detection limits only moderately higher (~2 µg/L EC) than APCI (~0.6 µg/L EC) (119, 227). The tradeoff of using an ESI probe with lower sensitivity versus an APCI probe may be worthwhile because ESI probes are already commonly used for LC-MS methods.
Flow-Injection Mass Spectrometry (FI-MS).
The increased mass resolution of recent MS technology has led to the use of FI-MS, which does not use front-end chromatography before detection. Omitting chromatographic separations precludes obtaining compound retention time characteristics and typically results in decreased sensitivity, but these downsides are often outweighed by the advantages. Although preliminary, research conducted using FI-MS for EC analysis reported detection of EC levels in samples as low as 7.5 µg/L with an analysis time of only two minutes (166). A rapid FI-MS method could have potential as a high-throughput screening method for food and beverages, replacing GC-MS methods that can have analysis times of 24 minutes or longer. However, a rapid FI-MS method would require extensive validation, as FI-MS methods are susceptible to matrix effects, and detection of compounds with identical nominal mass (i.e., isobaric compounds) could artificially inflate EC concentrations.
Enzyme-linked immunosorbent assay (ELISA).
ELISA is most commonly used to detect and quantify proteins, but it can also be used to detect small molecules. Use of ELISA for detection of EC would allow for quantification without the need for expensive analytical equipment (133, 203). Luo et al. generated a prototype ELISA to quantify EC standards with a detection limit of 16 µg/L (134). However, this assay was not able to detect EC in wine samples at ~40 µg/L because of a necessary 10-fold dilution step during sample preparation. Thus, further research is needed to refine this assay to detect low levels in food or beverage matrices. Researchers have taken different approaches to increase ELISA sensitivity, including ratiometric fluorescence ELISA (RF-ELISA) or derivatization of EC with xanthydrol (68, 135). If these assays are shown to be effective across a range of EC concentrations in various food matrices—and if they become more economical—they show potential for use in routine screening of samples without the need for complex analytical instrumentation.
Infrared (IR) Spectroscopy.
IR spectroscopy was originally explored as a method to quantitate EC in foods and beverages, although sensitivity limitations prevented its widespread use (147). However, increases in IR instrument sensitivity over time have led to some exploratory work regarding its use in EC detection. One study used Fourier-transform IR (FT-IR) to screen stone-fruit distilled spirits for elevated EC, and results showed that FT-IR correctly classified 85% of the test beverages when an 800 µg/L threshold was applied (106). Near-infrared spectroscopy (NIR) can quantify pure EC standards as low as 100 µg/L, but work is needed to validate this assay in a food matrix (197). FT-IR and NIR show promise as relatively inexpensive, rapid, and non-destructive techniques; however, these preliminary studies suggest that the current technology may only be useful for screening food or beverage samples with relatively high EC levels.
Surface-enhanced Raman Spectroscopy (SERS).
The emerging spectroscopy technology SERS has been utilized in research to produce assays for EC detection. A gold nanoparticle substrate has been used by several authors as a SERS amplifier to detect EC levels as low as 0.1 µg/L in alcoholic beverages (124, 214). Silver nanoparticles also have the capability to serve as SERS amplifiers, and have been used in assays to probe alcoholic beverages with a large range of EC concentrations (161). Although SERS shows promise as a rapid method of EC detection, continued work is needed to optimize the SERS amplifier to ensure that analytical results are reproducible.
Active Packaging and Nanosensor Systems.
EC sensor systems are in development that can be incorporated into polymers, potentially leading to creation of an easy-to-use test material that produces a visible color change upon contact with a target compound (216, 217). One potential application for this sensor system is in portable test kits for food inspectors where EC can be detected by mixing sample (e.g., distilled spirits or wine) with sensor-enabled test material for rapid analysis. Another possible application is “active packaging” where a section of a bottle of distilled spirits could be embedded with nanosensors to produce a visible color change if elevated amounts of EC are present. Several authors have fabricated prototype molecularly imprinted polymers that can detect EC in rice wine and brandy, and data produced from this nanosensor were in agreement with results from GC-MS analysis (74, 211).
Electrochemical impedance sensor systems are under development as a rapid and portable means for EC analysis in foods and beverages. Currently, these systems have been able to detect pure EC standards at ng/L concentrations (96, 229). However, because the sensitivity of these sensor systems is much lower in an alcoholic beverage versus a pure standard, further research will need to be done to produce a sensor that is more robust in a beverage matrix.
EFFECT OF PROCESSING ON EC CONCENTRATIONS IN FOODS AND BEVERAGES
As mentioned previously, EC formation is dependent on multiple chemical reactions occurring in the food or beverage matrix during processing (Figure 1). Broadly speaking, EC formation routes incorporate both the metabolic activity of microorganisms that occurs during fermentation (e.g., arginine metabolism to urea) and the chemical reaction of substrates (e.g., EtOH reacting with cyanate). Both formation mechanisms need to be considered when forming an approach to limit EC levels in the food supply, especially because certain products, such as stone fruit sprits, can contain high levels of EC due to contributions from both routes. Research on the effects of processing on EC formation has largely focused on the fermentation microorganisms that form EC precursors, the impact of distillation process, and the product storage conditions that promote or inhibit EC formation.
Fermentation.
Changes in chemical composition that occur to a food or beverage during fermentation can influence formation of EC (5, 35, 169). Certain strains of yeast and bacteria used for fermentation can alter the levels of EC precursor compounds (209). Several authors reported that EC levels in cachaça are ~20–50% lower if a starter culture is used instead of allowing native microbiota to induce fermentation (143). The presence of the yeast strain Wickerhamomyces anomalus has been associated with elevated levels of urea in foods, in contrast to Schizosaccharomyces pombe, which is able to reduce EC precursors via metabolism (14, 15, 208). The impact of certain yeast strains on EC levels during fermentation can be significant, as one author found that EC levels in yellow rice wine were 90% lower using Saccharomyces cerevisiae ZJU instead of a traditional fermentation starter (61). It is important to note that the focus of these studies was on levels of EC, and the influence of fermentation organisms on flavor profiles or other critical aspects of the products were not considered.
The ability of bacteria to influence EC levels in fermented food and beverages is mixed, but generally data show that certain bacterial strains are capable of modifying EC formation. Bacterial fermentation is especially relevant to red wine production, which typically undergoes malolactic fermentation by lactic acid bacteria. Although laboratory-scale experiments in model systems show that lactic acid bacteria have the capability to metabolize EC precursors (such as arginine), some studies have indicated that malolactic fermentation did not significantly affect EC levels in red wine (28, 144, 157). Use of Lactobacillus brevis and other lactic acid bacteria in a fermentation starter culture for production of distilled spirits led to EC levels that were at least 40% lower compared to a control fermented without the starter culture (51, 219, 231). These reductions were postulated to be from uptake of EC precursors by the bacteria. An additional aspect of fermentation to consider is salinity because it may affect how certain bacteria culture strains accumulate EC precursors (221). The diversity of microbiota in fermented foods and beverages presents a challenge when attempting to isolate the major microorganisms that may affect EC formation. Yet, characterizing such microorganisms is important because they can potentially have a large role in modifying levels of EC precursors.
Distillation.
Distilled spirits often have higher EC concentrations compared to other alcoholic beverages, leading researchers to assess whether certain features of the distillation process affect EC formation. The higher percentage of EtOH present in distilled spirits can itself result in elevated EC by serving as a substrate in EC formation. However, certain aspects of distillation—including high temperatures—can accelerate EC formation (10, 142). Studies that compare EC levels in distilled spirits samples from various distilleries have shown that the type of distillation process appears to be a major source of variation in EC concentrations (150, 163).
An underlying factor for the particularly high levels of EC often found in sugar cane spirits is hypothesized to be use of a copper metal distillation apparatus (also called a still), commonly used for distillation (19, 21, 178). A positive correlation between copper ions (Cu2+) and EC levels in distilled spirits has been found, and mechanistic experiments have elucidated a pathway where EC formation is driven by copper catalyzing both cyanate formation and cyanate reacting with EtOH (6). Copper metal (Cu0), like that found in the copper still, can ionize to Cu2+ at high temperatures in an acidic environment (6, 19, 102). In light of this, a still fabricated of stainless steel and noble metals instead of copper has been developed to limit these reactions (150). Some experiments show that use of copper versus stainless steel stills during distillation can have a significant effect on EC levels, but it is difficult to draw a definitive conclusion because differences in the placement of copper metal within the still may affect EC formation (6). Indeed, while use of a copper vessel to collect distillate (the finished distilled spirits) appears to result in elevated EC levels, use of copper column tubing and heating kettles to boil the fermented mash may instead reduce cyanate levels (56, 150).
Toasting and Thermal Application.
In-home thermal processing including toasting and other methods of heating breads can increase EC levels by two- to three-fold, with levels reaching up to ~30 µg/kg. Interestingly, toasting and baking can also increase levels of other process-induced contaminants, such as acrylamide and furan (26, 30, 77, 236). Although the exact formation mechanisms for EC in breads are unknown, this effect has been replicated by at least two other authors using various types of bread (78, 177).
Storage Conditions and Bottle Type.
EC levels can increase significantly during the storage of finished beverages, especially when temperature is elevated (59, 117). One study found that EC levels in red and white wine increased by as much as 30-fold over 12 months of storage at 43 °C (182). Several authors have demonstrated the effect of storage temperature on EC levels in rice wine (130, 204). For instance, experiments by Wu et al. found that EC concentrations in yellow rice wine increased from 74 µg/L to 509 µg/L when stored at 37 °C for 400 days, while sample kept at 4 °C had an increase of 10 µg/L (204). Even subjecting rice wine to elevated temperatures from hot-filling during bottling can increase EC levels, with levels increasing from 30 µg/L (control) to 180 µg/L after sample was subjected to treatment at 95 °C (125).
Studies have shown that light/UV exposure during the storage of distilled spirits can affect EC formation. Lachenmeier et al. found that EC levels in a sample of stone fruit spirits had an average increase of 1,300 µg/L after 4 h of direct UV exposure (113). A more modest increase in EC levels (~20%) was found in sugar cane spirits exposed to ambient light in glass bottles over six months (220).
Related to storage, the barrel aging process used for distilled spirits can cause increases in EC. The EC levels in grain spirits, plum wine, and cachaça have all been shown to increase by as much as five- to six-fold above their initial levels during barrel aging (7, 81, 172). The increases in EC levels is dependent on the particular beverage; Hashiguchi et al. found that mean EC levels in non-barrel-aged plum wine were 80 µg/L versus 300 µg/L in barrel-aged, whereas Santiago et al. found low EC levels even after cachaça was barrel aged (~10 µg/L in barrel-aged product) (81, 172). Some have pointed to lignin from the wooden barrel as having a role in EC formation because controlled experiments have found that barrel-aged distilled spirits contain higher levels of this compound compared to distilled spirits aged in glass (172).
CURRENT PRACTICES FOR MITIGATION OF EC IN FOODS AND BEVERAGES
There are a number of methods to limit the formation of EC in the food supply, and ongoing research is refining best practices associated with EC mitigation. The established EC mitigation strategies detailed in Table 5 will be discussed in the following sections.
Table 5.
Summary of current practices for mitigation of ethyl carbamate in food and beverages.
| Mitigation method | Comments | References |
|---|---|---|
| Input ingredients | • Grapes with excessive nitrogen levels can contribute to ethyl carbamate formation. | Butzke & Bisson (1997) |
| Genetically engineered yeast | • Yeast with enhanced capability to reduce urea has been commercialized. • Bread and red wine made with this modified yeast are reported to have reduced ethyl carbamate formation. |
Heller et al. (2006) |
| Removal of stone fruit pits | • Reduction in the number of stone fruit pits in the fermentation mash can reduce ethyl carbamate formation. | Codex Committee (2009) |
| Distillation | • Efficient separation of the “heads” and “tails” fractions from the “hearts” fraction during distillation reduces ethyl carbamate levels in the distillate. | Codex Committee (2009) |
| Distillation apparatus (a still) | • A still with copper boiling kettle can reduce levels of cyanate. • Use of a stainless-steel collection vessel compared to a copper vessel can prevent ethyl carbamate formation post-distillation. |
Codex Committee (2009) |
| Enzymatic Treatment (urease) | • Treatment reduces levels of the ethyl carbamate precursor urea. • Generally recognized as safe (GRAS) by the FDA. • Permitted for use in wine by the International Association of Vine and Wine (OIV). |
FDA (1993); OIV (2017) |
| Storage conditions | • The finished product should be stored in tinted bottles and away from excessive heat. | Codex Committee (2009) |
Documents related to EC mitigation in alcoholic beverages, principally in stone fruit spirits, have been published by governmental agencies and academic institutions. Noteworthy publications include a preventative action manual for wine authored by the University of California, Davis, in conjunction with the FDA; a code of practice for stone fruit distillates developed by the Codex Committee on Contaminants in Foods; and a recommendation document related to production of stone fruit spirits and marc spirits published by the European Commission (22, 39, 56).
Enzymatic Degradation.
The addition of certain enzymes to foods and beverages to degrade EC and EC precursors mimics specific metabolic processes that occur during fermentation. Urease has been designated by the OIV as one of several processing aids (164) permitted for use in wine production, and has been successfully added to certain wines, reducing urea by greater than 95% (54, 92, 95). Urease is generally recognized as safe (GRAS) in the US to reduce urea in wine (184). Because urease activity is reduced by components commonly found in wine, such as malic acid, fluoride, and phenolic compounds, pilot trials need to be conducted to ensure the enzyme’s effectiveness (22, 31).
Genetically Engineered Yeast.
Metabolic pathways in yeast have been optimized using both modern genetic engineering and traditional breeding techniques to reduce the formation of EC or its precursors (Figure 2). Many of these experiments have been performed to better understand the biochemical pathways in yeast and their corresponding genetic drivers, but some have been conducted to engineer strains for commercial purposes (94). Use of a commercialized strain of genetically engineered yeast with enhanced capability to degrade urea (ECMo01) was able to reduce EC levels by ~50% and ~90% in bread and red wine, respectively (86). Both the FDA and Health Canada did not have any objections to the safety assessment done by the firm that produces this engineered yeast strain (84, 185).
Figure 2.
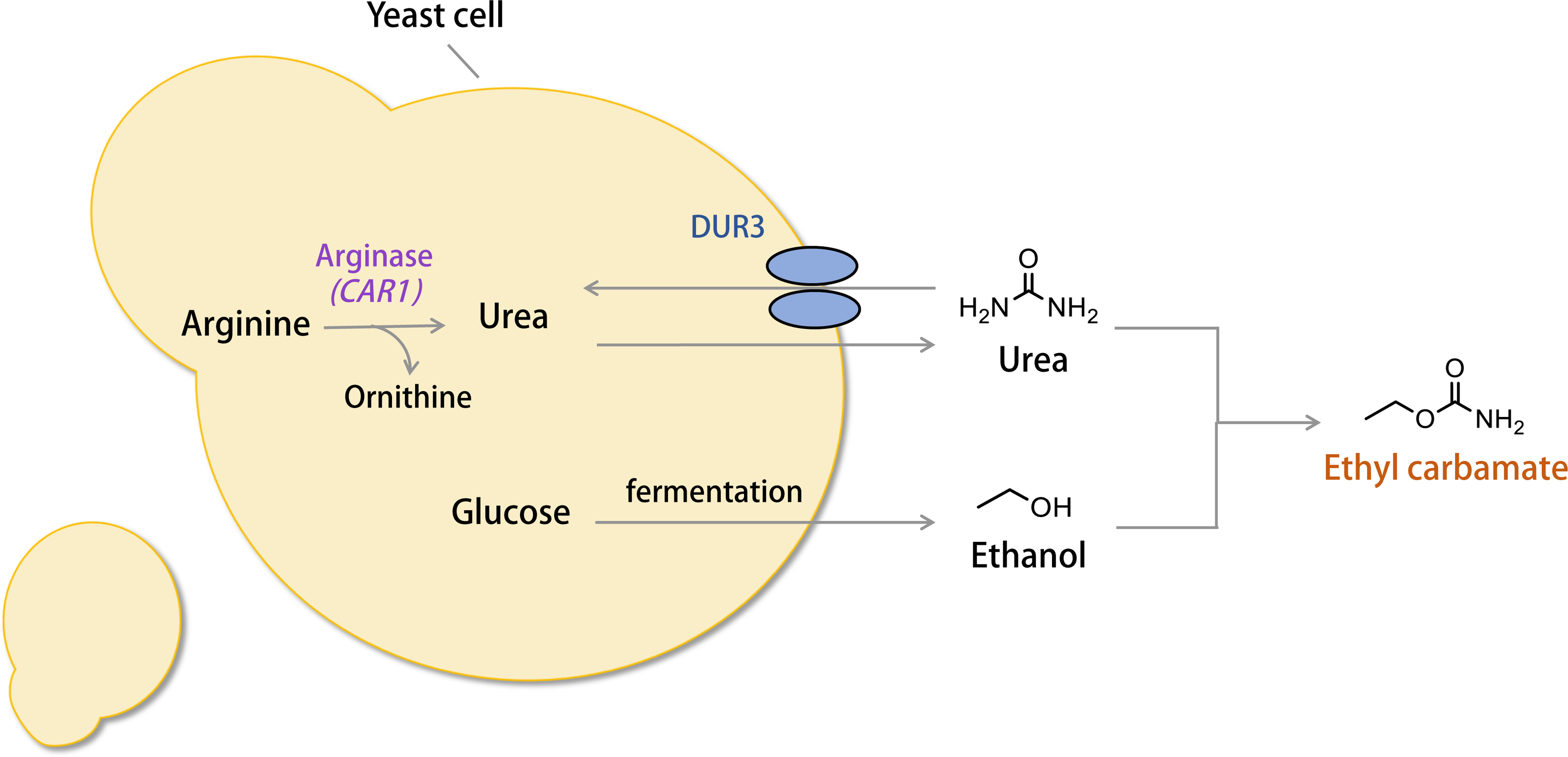
Overview of molecular targets under consideration for manipulation to reduce EC formation in yeast. Deletion of arginase (encoded by CAR1) reduces amounts of the EC precursor urea. Increased expression of DUR3 transporter enhances uptake of urea into the cell, which can subsequently reduce amounts of available urea to form EC.
Distillation.
The distillation process can be leveraged to reduce EC levels in distilled spirits through fractionation (15). During distillation of an alcoholic beverage, the condensed spirits collected at the beginning (the “heads”) and at the end of the process (the “tails”) typically contain undesirable or even toxic components, including methanol. The fraction between these two ends (the “hearts”) is the most desirable with regards to quality and purity. An analysis of all three major distillation fractions from sugar cane spirits found that the mean EC content was ~60,000 µg/L, 50 µg/L, and 1,600 µg/L, for the heads, hearts, and tails fractions, respectively (9). Similar results were replicated by two other authors, with the heads and tails fractions containing significantly higher EC concentrations compared to the hearts (12, 50). To increase efficiency of EC removal from distilled spirits by this fractionation process, increases in distillation time have been recommended (45).
Distilling spirits multiple times also has been shown to control EC levels. One study found that single distilled sugar cane spirits containing ~60 µg/L EC had levels reduced to non-detectable levels after being distilled an additional two times, due, in part, to increased ability to remove the undesirable heads and tails fractions (179). This reduction has been observed by other authors who found that double distillation removed ~95% of EC from sugar cane spirits (3, 4). These results demonstrate that although the distillation conditions can increase levels of EC in spirits, it also has the capability to be used to reduce EC levels via fractionation. However, increasing the number of times a product is distilled has the disadvantage of increasing the time to generate the product and reducing yield.
Mitigation in Stone Fruit Spirits.
EC formation in stone fruit spirits appears to be largely driven by the presence of cyanate precursors in the pit of stone fruits, but this relationship is complex and can be influenced by different production processes (40). In a study in Germany, small-scale distilleries generally had higher EC levels compared to large-scale distilleries, which may be related to larger-scale operations using good manufacturing practices such as removing the pit from stone fruits (113).
To test the extent to which destoning is effective as a method to prevent EC formation, Schehl et al. produced pilot-scale amounts of cherry and plum spirits with and without the pits included during fermentation (174). Both cherry and plum spirits made with and without pits contained EC levels below quantifiable levels (<10 µg/L), so the results were inconclusive. However, a separate test beverage made with only plum pits had EC levels of 3,100 µg/L and elevated levels of cyanate precursors. These results may indicate that other factors involved in production of distilled spirits, such as distillation practices and fruit quality, in addition to levels of cyanate from stone fruits, influence EC levels.
Controlled Storage Conditions.
Because high temperatures and light/UV exposure can accelerate EC formation, keeping finished product under controlled storage conditions can limit EC formation. Stone fruit spirits, in particular, should be protected from direct light exposure and elevated temperatures due to its potential to contain high levels of EC precursors (39). Specific target storage temperatures have not yet been determined, but EC has been found to increase in table wine and rice wine at temperatures ranging from 37–43 °C (182, 204).
Input Ingredients.
Quality control of ingredients has been shown to be important in keeping EC low in beverages. In wine making, use of grapes with high amounts of nitrogen or overly ripe fruit can lead to elevated levels of arginine and, consequently, increased EC concentrations in the finished product (115, 160). High nitrogen levels in grapes are generally due to excessive fertilization of a vineyard. Hence, it is important to measure available nitrogen in the juice before fermentation and potentially alter vineyard fertilization practices, if possible, to control grape nitrogen levels (22).
EXPERIMENTAL METHODS FOR MITIGATION OF EC IN FOODS AND BEVERAGES
In addition to established EC mitigation strategies, several experimental methods are under development to enhance current capabilities. The CRISPR-Cas9 technology is perhaps the most important due to its ability to modify target genes in yeast or bacteria with precision. Using this methodology, off-target effects that are more probable with legacy genetic modification techniques are largely reduced or avoided. These advantages can allow for relatively shorter times to develop a variety of starter culture strains modified using CRISPR-Cas9. Also showing promise as mitigation methods are enzymatic treatments (urethanase) and filtration using activated carbon. No less interesting is use of naturally occurring phenolic compounds or plant extracts that may prevent formation of EC or its precursors.
Experimental Genetically Engineered and Traditionally Bred Yeast.
CRISPR-Cas9 and other genetic engineering techniques have been used to delete the gene (CAR1) that codes for arginase in Saccharomyces cerevisiae to prevent yeast from metabolizing arginine to urea (33, 215). Pilot experiments with the low-arginase strain demonstrate that EC formation in distilled spirits and wine can be reduced up to 70% compared to controls (44, 175, 200). Genetic engineering has also been used to delete urea transporter proteins in Saccharomyces cerevisiae, leading urea to accumulate within the cell, where it can subsequently be degraded (193, 199).
Use of genetically engineered yeast to reduce EC levels in foods and beverages has limitations because microorganisms have multiple routes for generating EC precursors due to redundancies in metabolic pathways (224, 228). These redundancies often require suppression of multiple genes to reduce synthesis of target compounds (202). For example, no differences in EC levels were observed in rice wine fermented using genetically engineered yeast with reduced capacity to synthesize citrulline (201). Further, Guo et al. demonstrated that deletion of one copy of the CAR1 gene in Saccharomyces cerevisiae reduced EC formation in rice wine by only ~20%, but deletion of two CAR1 alleles reduced EC formation by 74% (75).
Efforts have also been made to use traditional breeding to select for desirable traits in yeast. Using this approach, yeast have been bred to produce sake and rice wine with reduced levels of EC precursors (100, 104, 225). One benefit of traditional breeding over modern genetic engineering techniques is the avoidance of potential product labeling requirements for genetic engineering, which may contribute to greater acceptance in certain consumer segments.
EC Degrading Enzymes.
Use of urethanase to directly degrade EC has been proposed, with ongoing experiments testing how well this treatment can scale up. Several authors have found that urethanase can reduce EC in rice wine and soy sauce by 15–50% (95, 129, 210). An alternate approach to direct addition of a purified enzyme is inoculation of beverages with microorganisms possessing similar degradation activity to the purified enzymes. In one case, Cui et al. used Lysinibacillus sphaericus MT33 to degrade EC in distilled spirits by 30–60% (42). Lactobacillus coryniformis BBE-H3 displays EC hydrolase activity, and has potential as a starter culture for pickled vegetables (58).
Preservatives.
Although limited work has been done using the preservative potassium metabisulfite, levels of EC in plum wine were approximately 30% lower compared to controls with the addition of this preservative (80). The mechanism for this effect has not been completely elucidated, but it is possible that this preservative inhibits growth of microorganisms that metabolize arginine into urea. Potassium metabisulfite is a widely used preservative that has undergone several safety assessments and has GRAS status (52, 187).
Filtration.
Activated charcoal has been explored as a method to reduce EC in alcoholic beverages and soy sauce. Preliminary research conducted has shown that filtering distilled spirits and soy sauce through activated charcoal reduced EC levels by ~45% (156). A limitation of this process is that the activated charcoal can also remove important flavor compounds, affecting sensory attributes of the treated product.
Phenolic Compounds and Plant Extracts.
It was previously mentioned that certain phenolic compounds (e.g., gallic acid) can inhibit the activity of enzyme treatments that reduce urea in wine. However, some research suggests that certain classes of these compounds can prevent EC formation in fermented beverages, possibly due to their ability to inhibit growth of certain strains of microorganisms involved in increased EC formation. Researchers found that addition of 200 mg/L of gallic acid reduced EC formation in yellow rice wine by ~90%, while another reported a ~40% reduction in EC formation in soy sauce with the addition of 10 mg/L quercetin (231, 234). Plant extracts rich in phenolic compounds have also been able to achieve reductions in EC formation (235).
CONCLUSION AND FUTURE DIRECTIONS
Considerable progress has been made in developing robust analytical methods to better characterize dietary exposure to EC. In addition, mechanistic work on EC formation has enabled innovation in approaches to mitigate EC in the food supply. Survey data have demonstrated overall reductions in levels of EC in certain beverage types (113), but it continues to be important to monitor EC because of the compound’s probable carcinogenicity and the elevated levels that have been occasionally identified in certain samples.
On the whole, there are several aspects of this field that would benefit from further development. With no EC standard reference material available, it is difficult to ensure analytical consistency across laboratories. However, there have been recent efforts by certain institutions to produce a reference material (192). Availability of a reference material becomes increasingly important as laboratories shift from using legacy GC-MS to other methodologies, such as LC-MS or FI-MS.
Additional data are needed on niche foods and beverages that increasingly constitute a larger segment of commercially available products. For example, there are limited data on EC concentrations in a number of fermented/cultured products that have become more widely available in many markets. Because levels of EC in foods and beverages are largely dependent on input ingredients and processing, it is difficult to generalize EC levels found in one type of product to an entire category on the market.
Nanosensors for EC detection show promise for use in the development of easily transported testing kits that could be used in the field for food inspection or quality control purposes by sampling and analyzing food rapidly on site. Nanosensors could also be embedded in food/beverage packaging, which could alert a consumer if a product contains elevated levels of EC. Use of packaging embedded with sensors may be especially relevant to products that have historically contained elevated levels of EC, such as stone fruit spirits.
Finally, robust mitigation methods should continue to be explored and developed. Some enzymatic methods to degrade urea are commercially available, but further refinement of this technology is needed to minimize the influence of constituents commonly found in wine (such as organic acids) and to produce an enzyme that is economical. Ongoing work on testing and mitigation technologies will allow for continued monitoring of EC from dietary exposure and the capability to prevent elevated levels of EC in the food supply.
Acknowledgements
The authors would like to thank Jesse Lunzer (FDA/OFS) for providing helpful feedback on the manuscript.
REFERENCES
- 1.Ajtony Z, Szoboszlai N, Bencs L, Viszket E, and Mihucz VG. 2013. Determination of ethyl carbamate in wine by high performance liquid chromatography. Food Chem. 141:1301–1305. [DOI] [PubMed] [Google Scholar]
- 2.Alberts P, Stander MA, and De Villiers A. 2011. Development of a novel solid-phase extraction, LC-MS/MS method for the analysis of ethyl carbamate in alcoholic beverages: application to South African wine and spirits. Food Addit. Contam. Part A. 28:826–839. [DOI] [PubMed] [Google Scholar]
- 3.Alcarde A, Souza L, and Bortoletto A. 2012. Ethyl carbamate kinetics in double distillation of sugar cane spirit. Part 2: influence of type of pot still. J. Inst. Brew. 118:352–355. [Google Scholar]
- 4.Alcarde AR, Souza L, and Bortoletto A. 2012. Ethyl carbamate kinetics in double distillation of sugar cane spirit. J. Inst. Brew. 118:27–31. [Google Scholar]
- 5.Araque I, Reguant C, Rozès N, and Bordons A. 2011. Influence of wine-like conditions on arginine utilization by lactic acid bacteria. Int. Microbiol. 14:225–33. [DOI] [PubMed] [Google Scholar]
- 6.Aresta M, Boscolo M, and Franco DW. 2001. Copper(II) catalysis in cyanide conversion into ethyl carbamate in spirits and relevant reactions. J. Agric. Food Chem. 49:2819–2824. [DOI] [PubMed] [Google Scholar]
- 7.Aylott R, Cochrane G, Leonard MJ, MacDonald L, MacKenzie W, McNeish A, and Walker D. 1990. Ethyl carbamate formation in grain based spirits: Part I: post‐distillation ethyl carbamate formation in maturing grain whisky. J. Inst. Brew. 96:213–221. [Google Scholar]
- 8.Aylott R, McNeish A, and Walker D. 1987. Determination of ethyl carbamate in distilled spirits using nitrogen specific and mass spectrometric detection. J. Inst. Brew. 93:382–386. [Google Scholar]
- 9.Baffa Júnior JC, Mendonça RCS, Kluge J. M. d. A. T., Pereira JAM, and Soares N. d. F. F. 2011. Ethyl-carbamate determination by gas chromatography-mass spectrometry at different stages of production of a traditional Brazilian spirit. Food Chem. 129:1383–1387. [Google Scholar]
- 10.Bai W, Sun S, Zhao W, Qian M, Liu X, and Chen W. 2017. Determination of ethyl carbamate (EC) by GC-MS and characterization of aroma compounds by HS-SPME-GC-MS during wine frying status in Hakka yellow rice wine. Food Anal. Methods. 10:2068–2077. [Google Scholar]
- 11.Bailey R, North D, Myatt D, and Lawrence J. 1986. Determination of ethyl carbamate in alcoholic beverages by methylation and gas chromatography with nitrogen-phosphorus thermionic detection. J. Chromatogr. A. 369:199–202. [Google Scholar]
- 12.Balcerek M, Pielech‐Przybylska K, Patelski P, Dziekońska‐Kubczak U, and Strąk E. 2017. The effect of distillation conditions and alcohol content in ‘heart’ fractions on the concentration of aroma volatiles and undesirable compounds in plum brandies. J. Inst. Brew. 123:452–463. [Google Scholar]
- 13.Beland FA, Benson RW, Mellick PW, Kovatch RM, Roberts DW, Fang J-L, and Doerge DR. 2005. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 43:1–19. [DOI] [PubMed] [Google Scholar]
- 14.Benito Á, Jeffares D, Palomero F, Calderón F, Bai F-Y, Bähler J, and Benito S. 2016. Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLOS ONE. 11:e0151102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges GBV, Gomes F. d. C. O., Badotti F, Silva ALD, and de Resende Machado AM 2014. Selected Saccharomyces cerevisiae yeast strains and accurate separation of distillate fractions reduce the ethyl carbamate levels in alembic cachaças. Food Control. 37:380–384. [Google Scholar]
- 16.Bortoletto AM, and Alcarde AR. 2015. Assessment of chemical quality of Brazilian sugar cane spirits and cachaças. Food Control. 54:1–6. [Google Scholar]
- 17.Brazil Ministry of Agriculture. 2014. Normative Instruction No. 28 of August 8, 2014. Available at: http://www.lex.com.br/legis_25819224_INSTRUCAO_NORMATIVA_N_28_DE_8_DE_AGOSTO_DE_2014.aspx. Accessed 26 October 2020.
- 18.Brumley WC, Canas BJ, Perfetti GA, Mossoba MM, Sphon JA, and Corneliussen PE. 1988. Quantitation of ethyl carbamate in whiskey, sherry, port, and wine by gas chromatography/tandem mass spectrometry using a triple quadrupole mass spectrometer. Anal. Chem. 60:975–978. [DOI] [PubMed] [Google Scholar]
- 19.Bruno SNF, Vaitsman DS, Kunigami CN, and Brasil MG. 2007. Influence of the distillation processes from Rio de Janeiro in the ethyl carbamate formation in Brazilian sugar cane spirits. Food Chem. 104:1345–1352. [Google Scholar]
- 20.Bueno RC, Tonin AP, Poliseli CB, Sinosaki N, Oliveira CC, Visentainer JV, Ribeiro MA, Silva VM, and Meurer EC. 2020. Two years monitoring of ethyl carbamate in sugar cane spirit from Brazilian distilleries. J. Braz. Chem. Soc. 31:1461–1466. [Google Scholar]
- 21.Bujake JE 1992. Beverage spirits, distilled. In Kirk‐Othmer Encyclopedia of Chemical Technology, vol. 4. Wiley, Hoboken, NJ. [Google Scholar]
- 22.Butzke C, and Bisson L. 1997. Ethyl carbamate preventative action manual. University of California, Davis. [Google Scholar]
- 23.Cairns T, Siegmund EG, Luke MA, and Doose GM. 1987. Residue levels of ethyl carbamate in wines and spirits by gas chromatography and mass spectrometry/mass spectrometry. Anal. Chem. 59:2055–2059. [DOI] [PubMed] [Google Scholar]
- 24.Canas BJ, Diachenko GW, and Nyman PJ. 1997. Ethyl carbamate levels resulting from azodicarbonamide use in bread. Food Addit. Contam. 14:89–94. [DOI] [PubMed] [Google Scholar]
- 25.Canas BJ, Havery DC, and Joe FL Jr. 1988. Rapid gas chromatographic method for determining ethyl carbamate in alcoholic beverages with thermal energy analyzer detection. J. AOAC Int. 71:509–511. [PubMed] [Google Scholar]
- 26.Canas BJ, Havery DC, Robinson LR, Sullivan MP, Joe FL Jr, and Diachenko GW. 1989. Ethyl carbamate levels in selected fermented foods and beverages. J. AOAC Int. 72:873–876. [PubMed] [Google Scholar]
- 27.Canas BJ, Joe FL Jr., Diachenko GW, and Burns G. 1994. Determination of ethyl carbamate in alcoholic beverages and soy sauce by gas chromatography with mass selective detection: collaborative study. J. AOAC Int. 77:1530–6. [PubMed] [Google Scholar]
- 28.Cañas PI, Romero EG, Alonso SG, González MF, and Herreros MP. 2008. Amino acids and biogenic amines during spontaneous malolactic fermentation in Tempranillo red wines. J. Food Compos. Anal. 21:731–735. [Google Scholar]
- 29.Cao G, Li K, Guo J, Lu M, Hong Y, and Cai Z. 2020. Mass spectrometry for analysis of changes during food storage and processing. J. Agric. Food Chem. [DOI] [PubMed] [Google Scholar]
- 30.Cepeda-Vázquez M, Camel V, Blumenthal D, and Rega B. 2019. Quality-driven design of sponge cake: insights into reactivity, furan mitigation and consumer liking. Food Chem. 285:94–103. [DOI] [PubMed] [Google Scholar]
- 31.Cerreti M, Fidaleo M, Benucci I, Liburdi K, Tamborra P, and Moresi M. 2016. Assessing the potential content of ethyl carbamate in white, red, and rosé wines as a key factor for pursuing urea degradation by purified acid urease. J. Food Sci. 81:C1603-C1612. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Ren Y, Zhong Q, Shao Y, Zhao Y, and Wu Y. 2017. Ethyl carbamate in alcoholic beverages from China: levels, dietary intake, and risk assessment. Food Control. 72:283–288. [Google Scholar]
- 33.Chin Y-W, Kang W-K, Jang HW, Turner TL, and Kim HJ. 2016. CAR1 deletion by CRISPR/Cas9 reduces formation of ethyl carbamate from ethanol fermentation by Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 43:1517–1525. [DOI] [PubMed] [Google Scholar]
- 34.Choi B, Jang Y, and Koh E. 2018. Determination of ethyl carbamate in soy sauce from Korean market. Food Control. 93:56–60. [Google Scholar]
- 35.Choi B, and Koh E. 2016. Changes of ethyl carbamate and its precursors in maesil (Prunus mume) extract during one-year fermentation. Food Chem. 209:318–322. [DOI] [PubMed] [Google Scholar]
- 36.Choi B, Ryu D, Kim C, Lee J, Choi A, and Koh E. 2017. Probabilistic dietary exposure to ethyl carbamate from fermented foods and alcoholic beverages in the Korean population. Food Addit. Contam. Part A. 34:1885–1892. [DOI] [PubMed] [Google Scholar]
- 37.Chung SW-C, Kwong KP, and Chen BL-S. 2010. Determination of ethyl carbamate in fermented foods by GC-HRMS. Chromatographia. 72:571–575. [Google Scholar]
- 38.Clegg BS, and Frank R. 1988. Detection and quantitation of trace levels of ethyl carbamate in alcoholic beverages by selected ion monitoring. J. Agric. Food Chem. 36:502–505. [Google Scholar]
- 39.Codex Alimentarius Commission. 2011. Code of practice for the prevention and reduction of ethyl carbamate contamination in stone fruit distillates - CAC/RCP70–2011. In Prevention and reduction of food and feed contamination World Health Organization. [Google Scholar]
- 40.Codex Committee on Contaminants in Foods. 2009. Discussion paper on ethyl carbamate in alcoholic beverages. [Google Scholar]
- 41.Cook R, McCaig N, McMillan J, and Lumsden W. 1990. Ethyl carbamate formation in grain‐based spirits: part III. The primary source. J. Inst. Brew. 96:233–244. [Google Scholar]
- 42.Cui K, Wu Q, and Xu Y. 2018. Biodegradation of ethyl carbamate and urea with Lysinibacillus sphaericus MT33 in Chinese liquor fermentation. J. Agric. Food Chem. 66:1583–1590. [DOI] [PubMed] [Google Scholar]
- 43.d’Avila GB, Cardoso M. d. G., Santiago WD, Rodrigues LMA, da Silva BL, Cardoso RR, Caetano ARS, de Fatima e Silva Ribeiro C, and Nelson DL 2016. Quantification of ethyl carbamate in cachaça produced in different agro‐industrial production systems. J. Inst. Brew. 122:299–303. [Google Scholar]
- 44.Dahabieh M, Husnik J, and Van Vuuren H. 2010. Functional enhancement of sake yeast strains to minimize the production of ethyl carbamate in sake wine. J. Appl. Microbiol. 109:963–973. [DOI] [PubMed] [Google Scholar]
- 45.de Almeida Lima U, Teixeira CG, Bertozzi JC, Serafim FAT, and Alcarde AR. 2012. Influence of fast and slow distillation on ethyl carbamate content and on coefficient of non‐alcohol components in Brazilian sugarcane spirits. J. Inst. Brew. 118:305–308. [Google Scholar]
- 46.Deák E, Gyepes A, Stefanovits-Bányai É, and Dernovics M. 2010. Determination of ethyl carbamate in pálinka spirits by liquid chromatography–electrospray tandem mass spectrometry after derivatization. Food Res. Int. 43:2452–2455. [Google Scholar]
- 47.Dennis M, Howarth N, Massey R, Parker I, Scotter M, and Startin J. 1986. Method for the analysis of ethyl carbamate in alcoholic beverages by capillary gas chromatography. J. Chromatogr. A. 369:193–198. [Google Scholar]
- 48.Dennis M, Massey R, Ginn R, Parker I, Crews C, Zimmerli B, Zoller O, Rhyn P, and Osborne B. 1997. The effect of azodicarbonamide concentrations on ethyl carbamate concentrations in bread and toast. Food Addit. Contam. 14:95–100. [DOI] [PubMed] [Google Scholar]
- 49.Diachenko GW, Canas BJ, Joe FL, and DiNovi M. 1992. Ethyl carbamate in alcoholic beverages and fermented foods. p. 419–428. In Food safety assessment, vol. 484. American Chemical Society. [Google Scholar]
- 50.Ding X, Huang J, Wu C, and Zhou R. 2017. Effects of different distillation patterns on main compounds of Chinese Luzhou‐flavour raw liquors. J. Inst. Brew. 123:442–451. [Google Scholar]
- 51.Du H, Song Z, and Xu Y. 2018. Ethyl carbamate formation regulated by lactic acid bacteria and nonconventional yeasts in solid-state fermentation of Chinese moutai-flavor liquor. J. Agric. Food Chem. 66:387–392. [DOI] [PubMed] [Google Scholar]
- 52.EFSA Panel on Food Contact Materials, Enzymes, Flavourings, and Processing Aids (CEF). 2016. Safety assessment of the active substance potassium metabisulfite, for use in active food contact materials. EFSA J. 14:e04465. [Google Scholar]
- 53.Environment and Climate Change Canada and Health Canada. 2016. Screening assessment: ethyl carbamate. [Google Scholar]
- 54.Esti M, Fidaleo M, Moresi M, and Tamborra P. 2007. Modeling of urea degradation in white and rosé wines by acid urease. J. Agric. Food Chem. 55:2590–2596. [DOI] [PubMed] [Google Scholar]
- 55.European Commission. 2004. Commission Directive 2004/1/EC of 6 January 2004 amending Directive 2002/72/EC as regards the suspension of the use of azodicarbonamide as blowing agent. [Google Scholar]
- 56.European Commission. 2016. Commission recommendation (EU) 2016/22 of 7 January 2016 on the prevention and reduction of ethyl carbamate contamination in stone fruit spirits and stone fruit marc spirits, repealing recommendation 2010/133/EU. [Google Scholar]
- 57.European Food Safety Authority. 2007. Ethyl carbamate and hydrocyanic acid in food and beverages: scientific opinion of the panel on contaminants. EFSA J. 5:551. [Google Scholar]
- 58.Fang F, Feng T, Du G, and Chen J. 2016. Evaluation of the impact on food safety of a Lactobacillus coryniformis strain from pickled vegetables with degradation activity against nitrite and other undesirable compounds. Food Addit. Contam. Part A. 33:623–630. [DOI] [PubMed] [Google Scholar]
- 59.Fang F, Qiu Y, Du G, and Chen J. 2018. Evaluation of ethyl carbamate formation in luzhou-flavor spirit during distillation and storage processes. Food Biosci. 23:137–141. [Google Scholar]
- 60.Fang F, Zhang J, Zhou J, Zhou Z, Li T, Lu L, Zeng W, Du G, and Chen J. 2018. Accumulation of citrulline by microbial arginine metabolism during alcoholic fermentation of soy sauce. J. Agric. Food Chem. 66:2108–2113. [DOI] [PubMed] [Google Scholar]
- 61.Fang RS, Dong YC, Li HJ, and Chen QH. 2015. Ethyl carbamate formation regulated by Saccharomyces cerevisiae ZJU in the processing of Chinese yellow rice wine. Int. J. Food Sci. Technol. 50:626–632. [Google Scholar]
- 62.Fauhl C, Catsburg R, and Wittkowski R. 1993. Determination of ethyl carbamate in soy sauces. Food Chem. 48:313–316. [Google Scholar]
- 63.Register Federal. 1990. Urethane in alcoholic beverages; research and survey reports; availability. p. 10816–10817, vol. 55. United States Government Printing Office. [Google Scholar]
- 64.Ferreira DC, Hernandes KC, Nicolli KP, Souza-Silva ÉA, Manfroi V, Zini CA, and Welke JE. 2019. Development of a method for determination of target toxic carbonyl compounds in must and wine using HS-SPME-GC/MS-SIM after preliminary GC×GC/TOFMS analyses. Food Anal. Methods.12:108–120. [Google Scholar]
- 65.Field K, and Lang C. 1988. Hazards of urethane (ethyl carbamate): a review of the literature. Lab. Anim. 22:255–262. [DOI] [PubMed] [Google Scholar]
- 66.Flamini R, and Panighel A. 2006. Mass spectrometry in grape and wine chemistry. Part II: the consumer protection. Mass Spectrom. Rev. 25:741–774. [DOI] [PubMed] [Google Scholar]
- 67.Food Standards Australia New Zealand. 2007. Ethyl carbamate in Australian foods. Available at: https://www.foodstandards.gov.au/science/surveillance/documents/Final%20Ethyl%20Carbamate%20report%20for%20web.pdf. Accessed 26 October 2020.
- 68.Fu H-J, Chen Z-J, Wang H, Luo L, Wang Y, Huang R-M, Xu Z-L, and Hammock B. 2021. Development of a sensitive non-competitive immunoassay via immunocomplex binding peptide for the determination of ethyl carbamate in wine samples. J. Haz. Mater. 406:124288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu ML, Liu J, Chen QH, Liu XJ, He GQ, and Chen JC. 2010. Determination of ethyl carbamate in Chinese yellow rice wine using high‐performance liquid chromatography with fluorescence detection. Int. J. Food Sci. Technol. 45:1297–1302. [Google Scholar]
- 70.Fu Z, Yang L, Ma L, Liu X, and Li J. 2016. Occurrence of ethyl carbamate in three types of Chinese wines and its possible reasons. Food Sci. Biotechnol. 25:949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galinaro CA, Ohe TH, da Silva AC, da Silva SC, and Franco DW. 2015. Cyanate as an active precursor of ethyl carbamate formation in sugar cane spirit. J. Agric. Food Chem. 63:7415–7420. [DOI] [PubMed] [Google Scholar]
- 72.Giachetti C, Assandri A, and Zanolo G. 1991. Gas chromatographic-mass spectrometric determination of ethyl carbamate as the xanthylamide derivative in Italian aqua vitae (grappa) samples. J. Chromatogr. A. 585:111–115. [DOI] [PubMed] [Google Scholar]
- 73.Gowd V, Su H, Karlovsky P, and Chen W. 2018. Ethyl carbamate: an emerging food and environmental toxicant. Food Chem. 248:312–321. [DOI] [PubMed] [Google Scholar]
- 74.Guo M, Hu Y, Wang L, Brodelius PE, and Sun L. 2018. A facile synthesis of molecularly imprinted polymers and their properties as electrochemical sensors for ethyl carbamate analysis. RSC Adv. 8:39721–39730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo X-W, Li Y-Z, Guo J, Wang Q, Huang S-Y, Chen Y-F, Du L-P, and Xiao D-G. 2016. Reduced production of ethyl carbamate for wine fermentation by deleting CAR1 in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 43:671–679. [DOI] [PubMed] [Google Scholar]
- 76.Ha M-S, Hu S-J, Park H-R, Lee H-M, Kwon K-S, Han E-M, Kim K-M, Ko E-J, Ha S-D, and Bae D-H. 2006. Estimation of Korean adult’s daily intake of ethyl carbamate through Korean commercial alcoholic beverages based on the monitoring. Food Sci. Biotechnol. 15:112–116. [Google Scholar]
- 77.Haddon WF, Mancini ML, McLaren M, Effio A, and Harden L. 1994. Occurrence of ethyl carbamate (urethane) in US and Canadian breads: measurements by gas chromatography-mass spectrometry. Cereal Chem. 71:207–215. [Google Scholar]
- 78.Hamlet CG, Jayaratne SM, and Morrison C. 2005. Application of positive ion chemical ionisation and tandem mass spectrometry combined with gas chromatography to the trace level analysis of ethyl carbamate in bread. Rapid Commun. Mass Spectrom. 19:2235–2243. [DOI] [PubMed] [Google Scholar]
- 79.Hasegawa Y, Nakamura Y, Tonogai Y, Terasawa S, Ito Y, and Uchiyama M. 1990. Determination of ethyl carbamate in various fermented foods by selected ion monitoring. J. Food Prot. 53:1058–1061. [DOI] [PubMed] [Google Scholar]
- 80.Hashiguchi T, Horii S, Izu H, and Sudo S. 2010. The concentration of ethyl carbamate in commercial ume (Prunus mume) liqueur products and a method of reducing it. Biosci. Biotechnol. Biochem. 74:2060–2066. [DOI] [PubMed] [Google Scholar]
- 81.Hashiguchi T, Izu H, and Sudo S. 2012. Lignin is linked to ethyl-carbamate formation in ume (Prunus mume) liqueur. Biosci. Biotechnol. Biochem. 76:148–152. [DOI] [PubMed] [Google Scholar]
- 82.Hasnip S, Caputi A, Crews C, and Brereton P. 2004. Effects of storage time and temperature on the concentration of ethyl carbamate and its precursors in wine. Food Addit. Contam. 21:1155–1161. [DOI] [PubMed] [Google Scholar]
- 83.Hasnip S, Crews C, Potter N, Christy J, Chan D, Bondu T, Matthews W, Walters B, and Patel K. 2007. Survey of ethyl carbamate in fermented foods sold in the United Kingdom in 2004. J. Agric. Food Chem. 55:2755–2759. [DOI] [PubMed] [Google Scholar]
- 84.Health Canada. 2007. Novel Food Information: Saccharomyces cerevisiae Yeast Strain ECMo01. Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/saccharomyces-cerevisiae-yeast-strain-ecmo01.html. Accessed 04 MAY 2021.
- 85.Health Canada. 2020. Health Canada’s maximum levels for chemical contaminants in foods. [Google Scholar]
- 86.Heller L 2006. Canada approves GM yeast that combats cancer compound. Available at: https://www.foodnavigator.com/Article/2006/10/06/Canada-approves-GM-yeast-that-combats-cancer-compound. Accessed 26 MAR 2021.
- 87.Herbert P, Santos L, Bastos M, Barros P, and Alves A. 2002. New HPLC method to determine ethyl carbamate in alcoholic beverages using fluorescence detection. J. Food Sci. 67:1616–1620. [Google Scholar]
- 88.Hernandes KC, Souza-Silva ÉA, Assumpção CF, Zini CA, and Welke JE. 2020. Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam. Part A. 37:61–68. [DOI] [PubMed] [Google Scholar]
- 89.Horii S, and Goto K. 2010. Determination of ethyl carbamate in sake using headspace solid phase microextraction. J. Inst. Brew. 116:177–181. [Google Scholar]
- 90.International Agency for Research on Cancer. 2010. IARC monographs on the evaluation of carcinogenic risks to humans: alcohol consumption and ethyl carbamate. vol. 96. International Agency for Research on Cancer, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- 91.International Organization of Vine and Wine. 2017. Determination of ethyl carbamate in spirituous beverages by gas chromatography/mass spectrometry (GC/MS) coupling. [Google Scholar]
- 92.International Organization of Vine and Wine. 2017. List of OIV Admitted Compounds and Their Status as Additives and Processing Aids and the Use Levels or Residual Limits. Available at: https://www.oiv.int/public/medias/5523/list-of-oiv-admitted-compounds.pdf. Accessed 12 FEB 2021.
- 93.Jagerdeo E, Dugar S, Foster GD, and Schenck H. 2002. Analysis of ethyl carbamate in wines using solid-phase extraction and multidimensional gas chromatography/mass spectrometry. J. Agric. Food Chem. 50:5797–5802. [DOI] [PubMed] [Google Scholar]
- 94.Jayakody LN, Lane S, Kim H, and Jin Y-S. 2016. Mitigating health risks associated with alcoholic beverages through metabolic engineering. Curr. Opin. Biotechnol. 37:173–181. [DOI] [PubMed] [Google Scholar]
- 95.Jia Y, Zhou J, Du G, Chen J, and Fang F. 2020. Identification of an urethanase from Lysinibacillus fusiformis for degrading ethyl carbamate in fermented foods. Food Bioscience:100666. [Google Scholar]
- 96.Jiang X, Xie Y, Wan D, Zheng F, and Wang J. 2020. Simultaneously detecting ethyl carbamate and its precursors in rice wine based on a pH-responsive electrochemical impedance sensor. Anal. Chim. Acta. 1126:124–132. [DOI] [PubMed] [Google Scholar]
- 97.Jiao Z, Dong Y, and Chen Q. 2014. Ethyl carbamate in fermented beverages: presence, analytical chemistry, formation mechanism, and mitigation proposals. Compr. Rev. Food Sci. Food Saf. 13:611–626. [DOI] [PubMed] [Google Scholar]
- 98.Jung S, Kim S, Kim I, Chung M-S, Moon B, Shin S, and Lee J. 2021. Risk assessment of ethyl carbamate in alcoholic beverages in Korea using the margin of exposure approach and cancer risk assessment. Food Control. 124:107867. [Google Scholar]
- 99.Kim YG, Lyu J, Kim MK, and Lee K-G. 2015. Effect of citrulline, urea, ethanol, and urease on the formation of ethyl carbamate in soybean paste model system. Food Chem. 189:74–79. [DOI] [PubMed] [Google Scholar]
- 100.Kitagaki H, and Kitamoto K. 2013. Breeding research on sake yeasts in Japan: history, recent technological advances, and future perspectives. Annu. Rev. Food Sci. Technol. 4:215–235. [DOI] [PubMed] [Google Scholar]
- 101.Koh E, and Kwon H. 2007. Quantification of ethyl carbamate in soy sauce consumed in Korea and estimated daily intakes by age. J. Sci. Food Agric. 87:98–102. [Google Scholar]
- 102.Koontz JL, Liggans GL, and Redan BW. 2020. Temperature and pH affect copper release kinetics from copper metal foil and commercial copperware to food simulants. Food Addit. Contam. Part A. 37:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar A, Loughran T, Alsina M, Durie BG, and Djulbegovic B. 2003. Management of multiple myeloma: A systematic review and critical appraisal of published studies. Lancet Oncology. 4:293–304. [DOI] [PubMed] [Google Scholar]
- 104.Kuribayashi T, Tamura H, Sato K, Nabekura Y, Aoki T, Anzawa Y, Katsumata K, Ohdaira S, Yamashita S, and Kume K. 2013. Isolation of a non-urea-producing sake yeast strain carrying a discriminable molecular marker. Biosci. Biotechnol. Biochem. 77:2505–2509. [DOI] [PubMed] [Google Scholar]
- 105.Kwon DY, Nyakudya E, and Jeong YS. 2014. Fermentation: food products. p. 113–123. Van Alfen NK (ed.), In Encyclopedia of agriculture and food systems Academic Press, Oxford. [Google Scholar]
- 106.Lachenmeier DW 2005. Rapid screening for ethyl carbamate in stone-fruit spirits using FTIR spectroscopy and chemometrics. Anal. Bioanal. Chem. 382:1407–1412. [DOI] [PubMed] [Google Scholar]
- 107.Lachenmeier DW, Frank W, and Kuballa T. 2005. Application of tandem mass spectrometry combined with gas chromatography to the routine analysis of ethyl carbamate in stone‐fruit spirits. Rapid Commun. Mass Spectrom. 19:108–112. [DOI] [PubMed] [Google Scholar]
- 108.Lachenmeier DW, Ganss S, Rychlak B, Rehm J, Sulkowska U, Skiba M, and Zatonski W. 2009. Association between quality of cheap and unrecorded alcohol products and public health consequences in Poland. Alcohol. Clin. Exp. Res. 33:1757–1769. [DOI] [PubMed] [Google Scholar]
- 109.Lachenmeier DW, Kanteres F, Kuballa T, López MG, and Rehm J. 2009. Ethyl carbamate in alcoholic beverages from Mexico (tequila, mezcal, bacanora, sotol) and Guatemala (cuxa): market survey and risk assessment. Int. J. Environ. Res. Public Health. 6:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lachenmeier DW, Lima MC, Nóbrega IC, Pereira JA, Kerr-Corrêa F, Kanteres F, and Rehm J. 2010. Cancer risk assessment of ethyl carbamate in alcoholic beverages from Brazil with special consideration to the spirits cachaça and tiquira. BMC Cancer. 10:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lachenmeier DW, Samokhvalov AV, Leitz J, Schoeberl K, Kuballa T, Linskiy IV, Minko OI, and Rehm J. 2010. The composition of unrecorded alcohol from eastern Ukraine: is there a toxicological concern beyond ethanol alone? Food Chem. Toxicol. 48:2842–2847. [DOI] [PubMed] [Google Scholar]
- 112.Lachenmeier DW, Sarsh B, and Rehm J. 2009. The composition of alcohol products from markets in Lithuania and Hungary, and potential health consequences: a pilot study. Alcohol Alcohol. 44:93–102. [DOI] [PubMed] [Google Scholar]
- 113.Lachenmeier DW, Schehl B, Kuballa T, Frank W, and Senn T. 2005. Retrospective trends and current status of ethyl carbamate in German stone-fruit spirits. Food Addit. Contam. 22:397–405. [DOI] [PubMed] [Google Scholar]
- 114.Lachenmeier DW, Schoeberl K, Kanteres F, Kuballa T, Sohnius EM, and Rehm J. 2011. Is contaminated unrecorded alcohol a health problem in the European Union? A review of existing and methodological outline for future studies. Addiction. 106:20–30. [DOI] [PubMed] [Google Scholar]
- 115.Lago LO, Nicolli KP, Marques AB, Zini CA, and Welke JE. 2017. Influence of ripeness and maceration of the grapes on levels of furan and carbonyl compounds in wine–simultaneous quantitative determination and assessment of the exposure risk to these compounds. Food Chem. 230:594–603. [DOI] [PubMed] [Google Scholar]
- 116.Lau BP-Y, Weber D, and Page BD. 1987. Gas chromatographic-mass spectrometric determination of ethyl carbamate in alcoholic beverages. J. Chromatogr. A. 402:233–241. [DOI] [PubMed] [Google Scholar]
- 117.Leça JM, Pereira V, Miranda A, Vilchez JL, and Marques JC. 2021. New insights into ethyl carbamate occurrence in fortified wines. LWT - Food Science and Technology:111566. [Google Scholar]
- 118.Leça JM, Pereira V, Pereira AC, and Marques JC. 2014. Rapid and sensitive methodology for determination of ethyl carbamate in fortified wines using microextraction by packed sorbent and gas chromatography with mass spectrometric detection. Anal. Chim. Acta. 811:29–35. [DOI] [PubMed] [Google Scholar]
- 119.Leça JM, Pereira V, Pereira AC, and Marques JC. 2018. A sensitive method for the rapid determination of underivatized ethyl carbamate in fortified wine by liquid chromatography-electrospray tandem mass spectrometry. Food Anal. Methods. 11:327–333. [Google Scholar]
- 120.Lee G. h., Bang D.-y., Lim J.-h., Yoon S.-m., Yea M-J, and Chi Y.-m. 2017. Simultaneous determination of ethyl carbamate and urea in Korean rice wine by ultra-performance liquid chromatography coupled with mass spectrometric detection. J. Chromatogr. B. 1065:44–49. [DOI] [PubMed] [Google Scholar]
- 121.Lee J-B, Kim MK, Kim B-K, Chung Y-H, and Lee K-G. 2018. Analysis of ethyl carbamate in plum wines produced in Korea. Food Sci. Biotechnol. 27:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee Kim Y-K, Koh E, Chung H-J, and Kwon H. 2000. Determination of ethyl carbamate in some fermented Korean foods and beverages. Food Addit. Contam. 17:469–475. [DOI] [PubMed] [Google Scholar]
- 123.Li G, Zhong Q, Wang D, and Gao H. 2017. A survey of ethyl carbamate in beer from Chinese market. Food Control. 79:254–257. [Google Scholar]
- 124.Li M, Zhao Y, Cui M, Wang C, and Song Q. 2016. SERS-active Ag nanostars substrates for sensitive detection of ethyl carbamate in wine. Anal. Sci. 32:725–728. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Wang P, Wu D, and Lu J. 2014. Effects of sterilization temperature on the concentration of ethyl carbamate and other quality traits in Chinese rice wine. J. Inst. Brew. 120:512–515. [Google Scholar]
- 126.Liao QG, Li WH, and Luo LG. 2013. Ultrasound-assisted emulsification-microextraction for the sensitive determination of ethyl carbamate in alcoholic beverages. Anal. Bioanal. Chem. 405:6791–6797. [DOI] [PubMed] [Google Scholar]
- 127.Liao QG, and Luo LG. 2014. Fast and selective pressurized liquid extraction with simultaneous in-cell cleanup for the analysis of ethyl carbamate in fermented solid foods. Chromatographia. 77:963–967. [Google Scholar]
- 128.Lim H-S, and Lee K-G. 2011. Development and validation of analytical methods for ethyl carbamate in various fermented foods. Food Chem. 126:1373–1379. [Google Scholar]
- 129.Liu Q, Yao X, Liang Q, Li J, Fang F, Du G, and Kang Z. 2018. Molecular engineering of Bacillus paralicheniformis acid urease to degrade urea and ethyl carbamate in model Chinese rice wine. J. Agric. Food Chem. 66:13011–13019. [DOI] [PubMed] [Google Scholar]
- 130.Liu X, Qian M, Dong H, Bai W, Zhao W, Li X, and Liu G. 2020. Effect of ageing process on carcinogen ethyl carbamate (EC), its main precursors and aroma compound variation in hakka huangjiu produced in southern China. Int. J. Food Sci. Technol. 55:1773–1780. [Google Scholar]
- 131.Liu Y, Dong B, Qin Z, Yang N, Lu Y, Yang L, Chang F, and Wu Y. 2011. Ethyl carbamate levels in wine and spirits from markets in Hebei province, China. Food Addit. Contam. 4:1–5. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y, Wang S, and Hu P. 2013. A survey of levels of ethyl carbamate in alcoholic beverages in 2009–2012, Hebei province, China. Food Addit. Contam. Part B. 6:214–217. [DOI] [PubMed] [Google Scholar]
- 133.Lu X, Zhou N, and Tian Y. 2015. Spectrophotometric determination of ethyl carbamate through bi-enzymatic cascade reactions. Anal. Methods. 7:1261–1264. [Google Scholar]
- 134.Luo L, Lei H-T, Yang J-Y, Liu G-L, Sun Y-M, Bai W-D, Wang H, Shen Y-D, Chen S, and Xu Z-L. 2017. Development of an indirect ELISA for the determination of ethyl carbamate in Chinese rice wine. Anal. Chim. Acta. 950:162–169. [DOI] [PubMed] [Google Scholar]
- 135.Luo L, Song Y, Zhu C, Fu S, Shi Q, Sun Y-M, Jia B, Du D, Xu Z-L, and Lin Y. 2018. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sens. Actuators B Chem. 255:2742–2749. [Google Scholar]
- 136.Ma L, Tong W, Du L, Huang S, Wei J, and Xiao D. 2019. Optimization of an aqueous two-phase system for the determination of trace ethyl carbamate in red wine. J. Food Prot. 82:1377–1383. [DOI] [PubMed] [Google Scholar]
- 137.Ma Y-P, Deng F-Q, Chen D-Z, and Sun S-W. 1995. Determination of ethyl carbamate in alcoholic beverages by capillary multi-dimensional gas chromatography with thermionic specific detection. J. Chromatogr. A. 695:259–265. [Google Scholar]
- 138.Machado AM Cardoso d. R., M. d. G., Emídio ES, Prata V. d. M., Dórea HS, Anjos J. P. d., Magriotis ZM, and Nelson DL 2012. Experimental design methodology to optimize the solid phase microextraction procedure prior to GC/MS determination of ethyl carbamate in samples of homemade cachaça. Anal. Lett. 45:1143–1155. [Google Scholar]
- 139.Mackenzie W, Clyne A, and MacDonald L. 1990. Ethyl carbamate formation in grain based spirits: part II. The identification and determination of cyanide related species involved in ethyl carbamate formation in Scotch grain whisky J. Inst. Brew. 96:223–232. [Google Scholar]
- 140.Madrera RR, and Valles BS. 2009. Determination of ethyl carbamate in cider spirits by HPLC-FLD. Food Control. 20:139–143. [Google Scholar]
- 141.Masson J, das Graças Cardoso M, Zacaroni LM, dos Anjos JP, Santiago WD, Machado A. M. d. R., Saczk AA, and Nelson DL. 2014. GC‐MS analysis of ethyl carbamate in distilled sugar cane spirits from the northern and southern regions of Minas Gerais. J. Inst. Brew. 120:516–520. [Google Scholar]
- 142.McGill D, and Morley A. 1990. Ethyl carbamate formation in grain spirits: part IV—Radiochemical studies. J. Inst. Brew. 96:245–246. [Google Scholar]
- 143.Mendonça JGP, Cardoso MDG, Santiago WD, Rodrigues LMA, Nelson DL, Brandão RM, and da Silva BL. 2016. Determination of ethyl carbamate in cachaças produced by selected yeast and spontaneous fermentation. J. Inst. Brew. 122:63–68. [Google Scholar]
- 144.Mira de Orduña R, Liu S-Q, Patchett M, and Pilone G. 2000. Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol. Lett. 183:31–35. [DOI] [PubMed] [Google Scholar]
- 145.Mirzoian A, and Mabud M. 2006. Comparison of methods for extraction of ethyl carbamate from alcoholic beverages in gas chromatography/mass spectrometry analysis. J. AOAC Int. 89:1048–1051. [PubMed] [Google Scholar]
- 146.Mo W. m., He H.-l., Xu X.-m., Huang B.-f., and Ren Y.-p.. 2014. Simultaneous determination of ethyl carbamate, chloropropanols and acrylamide in fermented products, flavoring and related foods by gas chromatography-triple quadrupole mass spectrometry. Food Control. 43:251–257. [Google Scholar]
- 147.Mossoba M, Chen J, Brumley W, and Page S. 1988. Application of gas chromatography/matrix isolation/Fourier transform infrared spectrometry to the determination of ethyl carbamate in alcoholic beverages and foods. Anal. Chem. 60:945–948. [DOI] [PubMed] [Google Scholar]
- 148.National Toxicology Program. 2016. 14th report on carcinogens. US Department of Health and Human Services. [Google Scholar]
- 149.Nóbrega IC, Pereira GE, Silva M, Pereira EV, Medeiros MM, Telles DL, Albuquerque EC Jr, Oliveira JB, and Lachenmeier DW. 2015. Improved sample preparation for GC-MS-SIM analysis of ethyl carbamate in wine. Food Chem. 177:23–28. [DOI] [PubMed] [Google Scholar]
- 150.Nóbrega IC, Pereira JA, Paiva JE, and Lachenmeier DW. 2011. Ethyl carbamate in cachaça (Brazilian sugarcane spirit): extended survey confirms simple mitigation approaches in pot still distillation. Food Chem. 127:1243–1247. [DOI] [PubMed] [Google Scholar]
- 151.Nout M, Ruikes M, Bouwmeester H, and Beljaars P. 1993. Effect of processing conditions on the formation of biogenic amines and ethyl carbamate in soybean tempe. J. Food Saf. 13:293–303. [Google Scholar]
- 152.Ough C, Crowell E, and Gutlove B. 1988. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 39:239–242. [Google Scholar]
- 153.Ough CS 1976. Ethyl carbamate in fermented beverages and foods. I. Naturally occurring ethylcarbamate. J. Agric. Food Chem. 24:323–328. [DOI] [PubMed] [Google Scholar]
- 154.Pang X-N, Li Z-J, Chen J-Y, Gao L-J, and Han B-Z. 2017. A comprehensive review of spirit drink safety standards and regulations from an international perspective. J. Food Prot. 80:431–442. [DOI] [PubMed] [Google Scholar]
- 155.Park S-K, Kim CT, Lee J-W, Jhee OH, Om AS, Kang JS, and Moon TW. 2007. Analysis of ethyl carbamate in Korean soy sauce using high-performance liquid chromatography with fluorescence detection or tandem mass spectrometry and gas chromatography with mass spectrometry. Food Control. 18:975–982. [Google Scholar]
- 156.Park S-R, Ha S-D, Yoon J-H, Lee S-Y, Hong K-P, Lee E-H, Yeom H-J, Yoon N-G, and Bae D-H. 2009. Exposure to ethyl carbamate in alcohol-drinking and nondrinking adults and its reduction by simple charcoal filtration. Food Control. 20:946–952. [Google Scholar]
- 157.Patrignani F, Ndagijimana M, Belletti N, Gardini F, Vernocchi P, and Lanciotti R. 2012. Biogenic amines and ethyl carbamate in primitivo wine: survey of their concentrations in commercial products and relationship with the use of malolactic starter. J. Food Prot. 75:591–596. [DOI] [PubMed] [Google Scholar]
- 158.Perestrelo R, Petronilho S, Câmara JS, and Rocha SM. 2010. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A. 1217:3441–3445. [DOI] [PubMed] [Google Scholar]
- 159.Pflaum T, Hausler T, Baumung C, Ackermann S, Kuballa T, Rehm J, and Lachenmeier DW. 2016. Carcinogenic compounds in alcoholic beverages: an update. Arch. Toxicol. 90:2349–2367. [DOI] [PubMed] [Google Scholar]
- 160.Pozo-Bayón MÁ, Monagas M, Bartolomé B, and Moreno-Arribas MV. 2012. Wine features related to safety and consumer health: an integrated perspective. Critic. Rev. Food Sci. Nutr. 52:31–54. [DOI] [PubMed] [Google Scholar]
- 161.Qi H, Chen H, Wang Y, and Jiang L. 2018. Detection of ethyl carbamate in liquors using surface-enhanced Raman spectroscopy. R. Soc. Open Sci. 5:181539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin Y, Duan B, Shin J-A, So H-J, Hong E-S, Jeong H-G, Lee J-H, and Lee K-T. 2021. Effect of fermentation on cyanide and ethyl carbamate contents in cassava flour and evaluation of their mass balance during lab-scale continuous distillation. Foods. 10:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reche RV, Leite Neto AF, Da Silva AA, Galinaro CA, De Osti RZ, and Franco DW. 2007. Influence of type of distillation apparatus on chemical profiles of Brazilian cachaças. J. Agric. Food Chem. 55:6603–6608. [DOI] [PubMed] [Google Scholar]
- 164.Redan BW 2020. Processing aids in food and beverage manufacturing: potential source of elemental and trace metal contaminants. J. Agric. Food Chem. 68:13001–13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Riachi L, Santos A, Moreira R, and De Maria C. 2014. A review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaça and other Brazilian sugarcane spirits. Food Chem. 149:159–169. [DOI] [PubMed] [Google Scholar]
- 166.Ribeiro M, Tonin A, Poliseli C, Oliveira C, Visentainer J, Silva V, and Meurer E. 2019. Determination of ethyl carbamate in sugar cane spirit by direct injection electrospray ionization tandem mass spectrometry using 18-crown-6/trifluoroacetic acid spiking additives. Food Anal. Methods. 12:69–75. [Google Scholar]
- 167.Riffkin HL, Wilson R, and Bringhurst TA. 1989. The possible involvement of Cu2+ peptide/protein complexes in the formation of ethyl carbamate. J. Inst. Brew. 95:121–122. [Google Scholar]
- 168.Riffkin HL, Wilson R, Howie D, and Muller SB. 1989. Ethyl carbamate formation in the production of pot still whisky. J. Inst. Brew. 95:115–119. [Google Scholar]
- 169.Ruiz‐Bejarano M, Castro‐Mejías R, Rodríguez‐Dodero M, and García‐Barroso C. 2015. Effect of ageing of sweet Sherry wines obtained from cvs Muscat and Pedro Ximénez on ethyl carbamate concentration. Aust. J. Grape Wine Res. 21:396–403. [Google Scholar]
- 170.Ryu D, Choi B, Kim E, Park S, Paeng H, Kim C.-i., Lee J.-y, Yoon HJ, and Koh E. 2015. Determination of ethyl carbamate in alcoholic beverages and fermented foods sold in Korea. Toxicol. Res. 31:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ryu D, Choi B, Kim N, and Koh E. 2016. Validation of analytical methods for ethyl carbamate in nine food matrices. Food Chem. 211:770–775. [DOI] [PubMed] [Google Scholar]
- 172.Santiago WD, Cardoso M. d. G., Lunguinho A. d. S., Barbosa RB, Cravo FDC, Goncalves G. d. S., and Nelson DL. 2017. Determination of ethyl carbamate in cachaça stored in newly made oak, amburana, jatobá, balsa and peroba vats and in glass containers. J. Inst. Brew. 123:572–578. [Google Scholar]
- 173.Santiago WD, Das Graças Cardoso M, Duarte FC, Saczk AA, and Nelson DL. 2014. Ethyl carbamate in the production and aging of cachaça in oak (Quercus sp.) and amburana (Amburana cearensis) barrels. J. Inst. Brew. 120:507–511. [Google Scholar]
- 174.Schehl B, Lachenmeier D, Senn T, and Heinisch JJ. 2005. Effect of the stone content on the quality of plum and cherry spirits produced from mash fermentations with commercial and laboratory yeast strains. J. Agric. Food Chem. 53:8230–8238. [DOI] [PubMed] [Google Scholar]
- 175.Schehl B, Senn T, Lachenmeier DW, Rodicio R, and Heinisch JJ. 2007. Contribution of the fermenting yeast strain to ethyl carbamate generation in stone fruit spirits. Appl. Microbiol. Biotechnol. 74:843–850. [DOI] [PubMed] [Google Scholar]
- 176.Schlatter J, DiNovi M, and Setzer RW. 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: example: ethyl carbamate (CAS 51–79-6). Food Chem. Toxicol. 48:S63–S68. [DOI] [PubMed] [Google Scholar]
- 177.Sen NP, Seaman SW, Boyle M, and Weber D. 1993. Methyl carbamate and ethyl carbamate in alcoholic beverages and other fermented foods. Food Chem. 48:359–366. [Google Scholar]
- 178.Serafim FA, and Lanças FM. 2019. Sugarcane spirits (cachaça) quality assurance and traceability: an analytical perspective. p. 335–359. In Production and Management of Beverages. Elsevier. [Google Scholar]
- 179.Silva FA, Vendruscolo F, Carvalho WR, Soares Junior MS, Pinheiro MV, and Caliari M. 2013. Influence of the number of distillations on the composition of organic sugarcane spirit. J. Inst. Brew. 119:133–138. [Google Scholar]
- 180.Solodun Y, Monakhova Y, Kuballa T, Samokhvalov A, Rehm J, and Lachenmeier D. 2011. Unrecorded alcohol consumption in Russia: toxic denaturants and disinfectants pose additional risks. Interdiscip. Toxicol. 4:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tang A, Chung S, Kwong K, Xiao Y, Chen M, Ho Y, and Ma S. 2011. Ethyl carbamate in fermented foods and beverages: dietary exposure of the Hong Kong population in 2007–2008. Food Addit. Contam. Part B. 4:195–204. [DOI] [PubMed] [Google Scholar]
- 182.Tegmo‐Larsson I-M, and Spittler TD. 1990. Temperature and light effects on ethyl carbamate formation in wine during storage. J. Food Sci. 55:1166–1167. [Google Scholar]
- 183.Tredoux A, and Ferreira AS. 2012. Fortified wines: styles, production and flavour chemistry. p. 159–179. In Alcoholic beverages. Elsevier. [Google Scholar]
- 184.U.S. Food and Drug Administration. 1993. Direct food substances affirmed as generally recognized as safe; urease enzyme derived from Lactobacillus fermentum. p. 57 FR 60470. Federal Register. [Google Scholar]
- 185.U.S. Food and Drug Administration. 2006. GRN No. 175: Saccharomyces cerevisiae strain ECMo01 with enhanced expression of urea amidolyase. Available at: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=175. Accessed 04 MAY 2021.
- 186.U.S. Food and Drug Administration. 2018, Azodicarbonamide (ADA) frequently asked questions. Available at: https://www.fda.gov/food/food-additives-petitions/azodicarbonamide -ada-frequently-asked-questions. Accessed 29 MAR 2021.
- 187.U.S. Food and Drug Administration. 2019. Food additive status list. Available at: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list. Accessed 31 MAR 2021.
- 188.Ubeda C, Balsera C, Troncoso A, Callejón R, and Morales M. 2012. Validation of an analytical method for the determination of ethyl carbamate in vinegars. Talanta. 89:178–182. [DOI] [PubMed] [Google Scholar]
- 189.Uthurry C, Varela F, Colomo B, Lepe JS, Lombardero J. d., and Del Hierro JG. 2004. Ethyl carbamate concentrations of typical Spanish red wines. Food Chem. 88:329–336. [Google Scholar]
- 190.Vahl M 1993. A survey of ethyl carbamate in beverages, bread and acidified milks sold in Denmark. Food Addit. Contam. 10:585–592. [DOI] [PubMed] [Google Scholar]
- 191.Valente IM, Ramos RM, Gonçalves LM, and Rodrigues JA. 2014. Determination of ethyl carbamate in spirits using salting-out assisted liquid-liquid extraction and high performance liquid chromatography with fluorimetric detection. Anal. Methods. 6:9136–9141. [Google Scholar]
- 192.Vicentim MP, Monteiro TM, de Almeida RRR, Soares ADA, Rodrigues JM, and do Rego ECP. 2019. Isotope dilution gas chromatography-mass spectrometry for the development of certified reference material of ethyl carbamate in hydroalcoholic matrix. Microchem. J. 147:497–506. [Google Scholar]
- 193.Vigentini I, Gebbia M, Belotti A, Foschino R, and Roth FP. 2017. CRISPR/Cas9 system as a valuable genome editing tool for wine yeasts with application to decrease urea production. Front. Microbiol. 8:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Walker G, Winterlin W, Fouda H, and Seiber J. 1974. Gas chromatographic analysis of urethane (ethyl carbamate) in wine. J. Agric. Food Chem. 22:944–947. [DOI] [PubMed] [Google Scholar]
- 195.Wang P, Sun J, Li X, Wu D, Li T, Lu J, Chen J, and Xie G. 2014. Contribution of citrulline to the formation of ethyl carbamate during Chinese rice wine production. Food Addit. Contam. Part A. 31:587–592. [DOI] [PubMed] [Google Scholar]
- 196.Wang R, Wu H, Zhou X, and Chen L. 2014. Simultaneous detection of ethyl carbamate and urea in Chinese yellow rice wine by HPLC-FLD. J. Liq. Chromatogr. Relat. Technol. 37:39–47. [Google Scholar]
- 197.Wei X, Huang Z, Zhang W, and Du Y. 2007. Improving the sensitivity of NIR spectroscopy with an enrichment technique: determining a trace analyte of ethyl carbamate. Anal. Sci. 23:853–856. [DOI] [PubMed] [Google Scholar]
- 198.World Health Organization. 2006. Safety evaluation of certain contaminants in food: sixty-fourth report of the Joint FAO/WHO expert committee on food additives. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 199.Wu D, Li X, Lu J, Chen J, Zhang L, and Xie G. 2016. Constitutive expression of the DUR1,2 gene in an industrial yeast strain to minimize ethyl carbamate production during Chinese rice wine fermentation. FEMS Microbiol. Lett. 363:fnv214. [DOI] [PubMed] [Google Scholar]
- 200.Wu D, Li X, Shen C, Lu J, Chen J, and Xie G. 2014. Decreased ethyl carbamate generation during Chinese rice wine fermentation by disruption of CAR1 in an industrial yeast strain. Int. J. Food Microbiol. 180:19–23. [DOI] [PubMed] [Google Scholar]
- 201.Wu D, Li X, Sun J, Cai G, Xie G, and Lu J. 2018. Effect of citrulline metabolism in Saccharomyces cerevisiae on the formation of ethyl carbamate during Chinese rice wine fermentation. J. Inst. Brew. 124:77–84. [Google Scholar]
- 202.Wu D, Xie W, Li X, Cai G, Lu J, and Xie G. 2020. Metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas9 system to minimize ethyl carbamate accumulation during Chinese rice wine fermentation. Appl. Microbiol. Biotechnol. 104:4435–4444. [DOI] [PubMed] [Google Scholar]
- 203.Wu L, Wang Y, Zhou S, Zhu Y, and Chen X. 2021. Enzyme-induced Cu2+/Cu+ conversion as the electrochemical signal for sensitive detection of ethyl carbamate. Anal. Chim. Acta. 1151:338256. [DOI] [PubMed] [Google Scholar]
- 204.Wu P, Cai C, Shen X, Wang L, Zhang J, Tan Y, Jiang W, and Pan X. 2014. Formation of ethyl carbamate and changes during fermentation and storage of yellow rice wine. Food Chem. 152:108–112. [DOI] [PubMed] [Google Scholar]
- 205.Wu P, Pan X, Wang L, Shen X, and Yang D. 2012. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control. 23:286–288. [Google Scholar]
- 206.Wu P, Zhang L, Shen X, Wang L, Zou Y, Zhang J, Tan Y, Tang J, Ma B, and Pan X. 2015. Determination of ethyl carbamate in Chinese yellow rice wine by diatomaceous earth extraction and GC/MS method. J. AOAC Int. 98:834–838. [DOI] [PubMed] [Google Scholar]
- 207.Wu P, Zhang L, Wang L, Zhang J, Tan Y, Tang J, Ma B, Pan X, and Jiang W. 2014. Simultaneous determination of ethyl carbamate and 4‐(5‐)methylimidazole in yellow rice wine and soy sauce by gas chromatography with mass spectrometry. J. Sep. Sci. 37:2172–2176. [DOI] [PubMed] [Google Scholar]
- 208.Wu Q, Cui K, Lin J, Zhu Y, and Xu Y. 2017. Urea production by yeasts other than Saccharomyces in food fermentation. FEMS Yeast Res. 17:fox072. [DOI] [PubMed] [Google Scholar]
- 209.Wu Q, Lin J, Cui K, Du R, Zhu Y, and Xu Y. 2017. Effect of microbial interaction on urea metabolism in Chinese liquor fermentation. J. Agric. Food Chem. 65:11133–11139. [DOI] [PubMed] [Google Scholar]
- 210.Wu Q, Zhao Y, Wang D, and Xu Y. 2013. Immobilized Rhodotorula mucilaginosa: a novel urethanase-producing strain for degrading ethyl carbamate. Appl. Biochem. Biotechnol. 171:2220–2232. [DOI] [PubMed] [Google Scholar]
- 211.Wu Z, Xu E, Li J, Long J, Jiao A, and Jin Z. 2016. Highly sensitive determination of ethyl carbamate in alcoholic beverages by surface-enhanced Raman spectroscopy combined with a molecular imprinting polymer. RSC Adv. 6:109442–109452. [Google Scholar]
- 212.Xia Q, Yang C, Wu C, Zhou R, and Li Y. 2018. Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: an overview. Food Control. 84:499–512. [Google Scholar]
- 213.Xian Y, Wu Y, Dong H, Liang M, Wang B, Wang L, Bai W, Zeng X, Qian M, and Zhao X. 2019. Ice-bath assisted sodium hydroxide purification coupled with GC–MS/MS analysis for simultaneous quantification of ethyl carbamate and 12 N-nitrosoamines in yellow rice wine and beer. Food Chem. 300:125200. [DOI] [PubMed] [Google Scholar]
- 214.Yang D, Zhou H, Ying Y, Niessner R, and Haisch C. 2013. Surface-enhanced Raman scattering for quantitative detection of ethyl carbamate in alcoholic beverages. Anal. Bioanal. Chem. 405:9419–9425. [DOI] [PubMed] [Google Scholar]
- 215.Yang HF, Zeng XA, Wang LH, Yu SJ, and Brennan MA. 2017. Ethyl carbamate control by genomic regulation of arginase in Saccharomyces cerevisiae EC1118 in sugarcane juice fermentation. J. Food Process. Preserv. 41:e13261. [Google Scholar]
- 216.Yang T, and Duncan TV. 2021. Challenges and potential solutions for nanosensors intended for use with foods. Nat. Nanotechnol. 16:251–265. [DOI] [PubMed] [Google Scholar]
- 217.Yang T, Paulose T, Redan BW, Mabon JC, and Duncan TV. 2021. Food and beverage ingredients induce the formation of silver nanoparticles in products stored within nanotechnology-enabled packaging. ACS Appl. Mater. Interfaces. 13:1398–1412. [DOI] [PubMed] [Google Scholar]
- 218.Ye C-W, Zhang X-N, Gao Y-L, Wang Y.-l., Pan S-Y, and Li X-J 2012. Multiple headspace solid-phase microextraction after matrix modification for avoiding matrix effect in the determination of ethyl carbamate in bread. Anal. Chim. Acta. 710:75–80. [DOI] [PubMed] [Google Scholar]
- 219.Yu W, Xie G, Wu D, Li X, and Lu J. 2020. A Lactobacillus brevis strain with citrulline re-uptake activity for citrulline and ethyl carbamate control during Chinese rice wine fermentation. Food Biosci.:100612. [Google Scholar]
- 220.Zacaroni LM, Cardoso M. d. G., Santiago WD, de Souza Gomes M, Duarte FC, and Nelson DL. 2015. Effect of light on the concentration of ethyl carbamate in cachaça stored in glass bottles. J. Inst. Brew. 121:238–243. [Google Scholar]
- 221.Zhang J, Fang F, Chen J, and Du G. 2014. The arginine deiminase pathway of koji bacteria is involved in ethyl carbamate precursor production in soy sauce. FEMS Microbiol. Lett. 358:91–97. [DOI] [PubMed] [Google Scholar]
- 222.Zhang J, Liu G, Zhang Y, Gao Q, Wang D, and Liu H. 2014. Simultaneous determination of ethyl carbamate and urea in alcoholic beverages by high-performance liquid chromatography coupled with fluorescence detection. J. Agric. Food Chem. 62:2797–2802. [DOI] [PubMed] [Google Scholar]
- 223.Zhang P, Du G, Zou H, Xie G, Chen J, Shi Z, and Zhou J. 2017. Mutant potential ubiquitination sites in Dur3p enhance the urea and ethyl carbamate reduction in a model rice wine system. J. Agric. Food Chem. 65:1641–1648. [DOI] [PubMed] [Google Scholar]
- 224.Zhang P, Li B, Wen P, Wang P, Yang Y, Chen Q, Chang Y, and Hu X. 2018. Metabolic engineering of four GATA factors to reduce urea and ethyl carbamate formation in a model rice wine system. J. Agric. Food Chem. 66:10881–10889. [DOI] [PubMed] [Google Scholar]
- 225.Zhang W, Cheng Y, Li Y, Du G, Xie G, Zou H, Zhou J, and Chen J. 2018. Adaptive evolution relieves nitrogen catabolite repression and decreases urea accumulation in cultures of the Chinese rice wine yeast strain Saccharomyces cerevisiae XZ-11. J. Agric. Food Chem. 66:9061–9069. [DOI] [PubMed] [Google Scholar]
- 226.Zhang W, Si G, Ye M, Feng S, Cheng F, Li J, Mei J, Zong S, Wang J, and Zhou P. 2017. An efficient assay for simultaneous quantification of ethyl carbamate and phthalate esters in Chinese liquor by gas chromatography-mass spectrometry. Food Anal. Methods. 10:3487–3495. [Google Scholar]
- 227.Zhao X, and Jiang C. 2015. Determination of ethyl carbamate in fermented liquids by ultra high performance liquid chromatography coupled with a Q Exactive hybrid quadrupole-orbitrap mass spectrometer. Food Chem. 177:66–71. [DOI] [PubMed] [Google Scholar]
- 228.Zhao X, Zou H, Fu J, Zhou J, Du G, and Chen J. 2014. Metabolic engineering of the regulators in nitrogen catabolite repression to reduce the production of ethyl carbamate in a model rice wine system. Appl. Environ. Microbiol. 80:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhao X, Zuo J, Qiu S, Hu W, Wang Y, and Zhang J. 2017. Reduced graphene oxide-modified screen-printed carbon (rGO-SPCE)-based disposable electrochemical sensor for sensitive and selective determination of ethyl carbamate. Food Anal. Methods. 10:3329–3337. [Google Scholar]
- 230.Zhou K, Liu Y, Li W-Q, Liu G-L, Wei N, Sun Y-M, Bai W-D, and Xu Z-L. 2017. An improved HPLC-FLD for fast and simple detection of ethyl carbamate in soy sauce and prediction of precursors. Food Anal. Methods. 10:3856–3865. [Google Scholar]
- 231.Zhou K, Patrignani F, Sun Y-M, Lanciotti R, and Xu Z-L. 2021. Inhibition of ethyl carbamate accumulation in soy sauce by adding quercetin and ornithine during thermal process. Food Chem. 343:128528. [DOI] [PubMed] [Google Scholar]
- 232.Zhou K, Siroli L, Patrignani F, Sun Y, Lanciotti R, and Xu Z. 2019. Formation of ethyl carbamate during the production process of Cantonese soy sauce. Molecules. 24:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou K, Wang Z-L, Luo L, Dong Y-Z, Yang J-Y, Lei H-T, Wang H, Shen Y-D, and Xu Z-L. 2020. Development of Cu(II)/Cu(I)-induced quantum dot-mediated fluorescence immunoassay for the sensitive determination of ethyl carbamate. Microchim. Acta. 187:1–10. [DOI] [PubMed] [Google Scholar]
- 234.Zhou W, Fang R, and Chen Q. 2017. Effect of gallic and protocatechuic acids on the metabolism of ethyl carbamate in Chinese yellow rice wine brewing. Food Chem. 233:174–181. [DOI] [PubMed] [Google Scholar]
- 235.Zhou W, Hu J, Zhang X, and Chen Q. 2020. Application of bamboo leaves extract to Chinese yellow rice wine brewing for ethyl carbamate regulation and its mitigation mechanism. Food Chem. 319:126554. [DOI] [PubMed] [Google Scholar]
- 236.Žilić S, Aktağ IG, Dodig D, Filipović M, and Gökmen V. 2020. Acrylamide formation in biscuits made of different wholegrain flours depending on their free asparagine content and baking conditions. Food Res. Int. 132:109109. [DOI] [PubMed] [Google Scholar]
- 237.Zimmerli B, and Schlatter J. 1991. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat. Res. 259:325–350. [DOI] [PubMed] [Google Scholar]


