Abstract
Acid-sensing ion channels (ASICs) are abundantly expressed in the nucleus accumbens core (NAcore), a region of the mesolimbocortical system that has an established role in regulating drug-seeking behavior. Previous work shows that a single dose of cocaine reduced the AMPA-to-NMDA ratio in Asic1a−/− mice, an effect observed after withdrawal in wild-type mice, whereas ASIC1A overexpression in the NAcore of rats decreases cocaine self-administration. However, whether ASIC1A overexpression in the NAcore alters measures of drug-seeking behavior after the self-administration period is unknown. To examine this issue, the ASIC1A subunit was overexpressed in male Sprague-Dawley rats by injecting them with adeno-associated virus, targeted at the NAcore, after completion of two weeks of cocaine or food self-administration. After 21 days of homecage abstinence, rats underwent a cue-/context-driven drug/food-seeking test, followed by extinction training and then drug/food-primed, cued, and cued + drug/food-primed reinstatement tests. The results indicate that ASIC1A overexpression in the NAcore enhanced cue-/context-driven cocaine seeking, cocaine-primed reinstatement, and cued + cocaine-primed reinstatement but had no effect on food-seeking behavior, indicating a selective effect for ASIC1A in the processes underlying extinction and cocaine-seeking behavior.
Keywords: abstinence, incubation, reinstatement, self-administration, withdrawal
Introduction
Chronic exposure to drugs of abuse, including cocaine, causes a variety of synaptic and molecular alterations in the nucleus accumbens (NA), many of which contribute to the potential for relapse, as assessed in models of drug-seeking behavior. Studies, including our own, indicate that the NA abundantly expresses acid-sensing ion channels (ASICs) (Jiang et al., 2009; Wemmie et al., 2003), which provide a depolarizing current when activated by decreases in extracellular pH. ASICs include multiple channel subunits, and our prior work suggests that the ASIC1A subunit plays a critical role in drug-related behaviors, including for cocaine. Deletion of the Asic1a gene in mice enhances cocaine-conditioned place preference (CPP), an effect reversed by rescue of ASIC1A in the NA (Kreple et al., 2014). Local deletion of Asic1a in the NA also enhances cocaine CPP. Conversely, overexpression of ASIC1A in the NA of rats decreases the number of infusions taken during cocaine self-administration and produces a rightward shift in the cocaine dose-response curve during self-administration (Kreple et al., 2014). Together, these findings suggest that ASIC1A negatively regulates the reinforcing properties of cocaine.
On a physiological level, ASIC1A disruption appears to produce long-lasting synaptic changes similar to those observed following withdrawal from chronic cocaine administration. Previous findings indicate that Asic1a−/− mice exhibit an increased AMPA/NMDA receptor ratio, akin to cocaine-experienced rats and mice following a period of abstinence (Kourrich et al., 2007; Kreple et al., 2014; Moussawi et al., 2011). Asic1a−/− mice also have an increased rectification index in NA medium-spiny neurons, which is thought to reflect insertion of GluA2-lacking AMPA receptors and mirrors observations made in the NA of rodents following abstinence from cocaine self-administration (Conrad et al., 2008; Kreple et al., 2014). Evidence suggests that experimenter-administered cocaine produces diminished motor responses in Asic1a−/− mice compared to that observed in wild-type mice (Jiang et al., 2009). Together, the prior findings indicate that ASICs, and particularly the ASIC1A subunit, contribute to a variety of cocaine-related behaviors and synaptic changes in the NA related to cocaine exposure.
Of note, one study reported that 5 days of experimenter-administered cocaine followed by 14 days of abstinence caused the upregulation of ASIC1 protein in the striatum, including the NAcore (Zhang et al., 2009). However, this work does not reveal whether increased ASIC1A expression opposes or mediates the long-term effects of cocaine exposure. Moreover, prior work did not investigate how the ASIC1A subunit influences measures of relapse and craving following the drug self-administration period. Therefore, the present study investigated how ASIC1A overexpression, targeted at the NAcore of rats, alters cocaine-seeking behavior following the self-administration period. Rats underwent cocaine self-administration for at least 12 days and, following the last day of cocaine administration, received an intra-NA injection of an adeno-associated virus (AAV) overexpressing ASIC1A. After 21 days of homecage abstinence, the effects of ASIC1A overexpression on cue-/context-driven drug/food seeking, extinction and reinstatement were tested. Behavioral effects of ASIC1A overexpression following food self-administration were also examined for comparison purposes.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River, ~300 g at the time of surgery, n = 28) were individually housed and maintained on a 12-h reverse light-dark cycle. Rats were given ~20 g of food per day except during the 21-d homecage period following self-administration, when they remained in their homecages and were given ad libitum food. Methods were approved by the University of Iowa Institutional Animal Care and Use Committee and were in compliance with NIH guidelines for the care of laboratory animals.
Surgery
Rats underwent surgery for double-barreled cannulae targeted to the NAcore and, in some cases, implantation of intravenous jugular catheter. Surgical details are provided in the online supporting documents.
Self-administration
Self-administration sessions were carried out 6 d per week in standard operant boxes housed within sound-attenuating chambers (Med Associates, Fairfield, VT). Operant boxes were equipped with a central reward receptacle flanked by two levers. A cue light was positioned above each lever, and a 4500 Hz Sonalert module was used as the tone generator. A house light was positioned on the wall opposite the levers and was illuminated during the behavioral sessions. In Experiments 1, rats were food deprived for 24 h and then trained during a 15 h overnight session to lever press on a FR1 schedule of reinforcement for 45 mg food pellets (Bio-serve Dustless Precision Pellets, Flemington, NJ). One day after food training, rats began training 6 d per week on cocaine self-administration (Experiment 1). Rats in Experiment 2 did not undergo overnight food training but instead entered daily 2-h food self-administration sessions (described more below).
During self-administration sessions, a lever press on the active (right) lever resulted in drug or food delivery and the presentation of light and tone cues. A 20 s time out period followed each cocaine or food pellet reward, during which active lever presses had no consequence. In all experiments, lever presses on the inactive (left) lever had no consequence. All rats underwent at least 12 d of self-administration, followed by a virus microinjection, and 21 d spent in the homecage.
Microinjections and homecage period
On the day immediately following the final day of self-administration, AAV2/1-CMV-ASIC1A(mouse)-eGFP or AAV2/1-CMV-eGFP (0.3 μl infused at a rate of 0.1 μl/min) was infused via injectors that extended 2 mm beyond the cannulae. Injectors were left in place for 2 min to allow the virus to diffuse. Rats were counterbalanced so that GFP and ASIC1A groups earned equivalent levels of cocaine or food pellets on the last 3 d of self-administration. Rats were kept in their home cage for a 21-d homecage period to allow for robust ASIC1A overexpression prior to the tests measuring relapse-like behavior. This time period has previously been shown to be sufficient to induce incubation of craving following 2-h daily self-administration sessions (Neisewander et al., 2000). Food restriction (~20 g/day) was resumed one day prior to the first drug/food-seeking test after the homecage period (described for each experiment below).
Experiment 1: Cocaine self-administration
This experiment examined whether ASIC1A overexpression in the NAcore affects the first cue-/context-driven drug-seeking test after the homecage period, the extinction of cocaine seeking, and the reinstatement of cocaine-seeking behavior following extinction training. Rats underwent daily 2 h self-administration sessions in which active lever presses produced a 50 μl intravenous cocaine infusion (200 μg cocaine per infusion, dissolved in 0.9% sterile saline; cocaine kindly provided by National Institute on Drug Abuse). Cocaine infusions were capped at 35 during the first 3 d of self-administration. Rats self-administered cocaine for at least 12 d, and the criteria for entering the homecage phase was at least 10 d of greater than 10 infusions, with greater than 15 infusions on each of the final two days (Gutman et al., 2017).
Following the homecage period, rats underwent a 2-h cue-/context-driven drug-seeking test, during which active lever presses resulted in the delivery of light and tone cues but no cocaine infusion. Rats then underwent at least 7 d of 2-h extinction sessions, during which both active and inactive lever presses had no consequences. Rats were required to make fewer than 25 active lever presses on the final 2 d of extinction training in order to begin the reinstatement tests. Rats underwent the three reinstatement tests in the following order: cued, cocaine-primed, and cued + cocaine-primed. During cocaine-primed reinstatement, rats received an injection of cocaine immediately before the session (10 mg/kg, i.p.), and active lever presses had no consequences. During cued reinstatement, active lever presses resulted in the presentation of light and tone cues. During cued + cocaine-primed reinstatement, rats received the cocaine prime prior to the session and underwent the cued reinstatement session described above. During both the cue-/context-driven drug-seeking test and the reinstatement tests, active lever pressing served as a measure of cocaine-seeking behavior. At least 3 d of extinction training were included between reinstatement tests, and rats were required to make fewer than 25 active lever presses during the two extinction sessions immediately preceding a reinstatement test.
Experiment 2: Food self-administration
This experiment examined whether ASIC1A overexpression in the NAcore affects the cue-/context-driven food-seeking test after the homecage period, extinction learning, and the reinstatement of food-seeking behavior following extinction. During daily 2-h sessions, rats were trained to lever press for a 45 mg food pellet (described above) on an FR1 schedule of reinforcement. Rats were required to earn ≥ 100 pellets per session for at least 3 d in order to advance to an FR3 schedule of reinforcement. Rats self-administered food for at least 12 d, and the criteria for entering the homecage phase was at least 3 d of self-administration on the FR3 schedule, with ≥ 100 pellets earned on each of these days (McFarland and Kalivas, 2001).
Following the 21-d homecage period, rats underwent a 2-h cue-/context-driven food-seeking test, as described for Experiment 1. Rats were then trained to extinguish active lever pressing during at least 7 d of 2-h extinction sessions. The criterion for reinstatement testing was 2 consecutive days of extinction training during which rats executed fewer than 10% of the average number of lever presses made during the last 2 d of food self-administration. Reinstatement tests occurred in the following order: food primed, cued, and cued + food primed. During the food-primed reinstatement test, one 45 mg pellet was passively delivered every 2 min for the first 30 min of the session, but active lever presses had no consequence. During the cued reinstatement test, active lever presses resulted in the delivery of the tone and light cue previously associated with the food pellet. During cued + food-primed reinstatement, rats received the food primes for the first 30 min of the session and tone and light cue presentations following active lever presses throughout the entire session.
Histological analysis
Brains were removed and analyzed using immunohistochemistry to confirm expression of the EGFP virus in the NAcore. Details of histological analysis are provided in the online supporting documents
Statistical analysis
Unpaired t-tests were used to examine the rewards earned on the last 3 d of self-administration and the lever presses during the initial post-homecage drug/food-seeking tests. Within-session lever pressing was examined during the test by dividing the session into 15-min bins and performing a two-way repeated measures ANOVA with bin as the within-subjects variable and group (GFP or ASIC1A) as the between-subjects variable. For reinstatement tests, lever pressing during the extinction baseline (an average of the two days immediately preceding the reinstatement) and reinstatement test were analyzed using two-way ANOVAs with day as a repeated measure. Holm-Sidak’s multiple comparisons test was used for all post-hoc analyses.
Results
Figure 1A shows the experimental timeline used for all experiments. Figure 1B shows a representative immunohistochemistry image with virus expression in the NAcore. Figure 1C shows the observed minimum and maximum virus spread. As pictured, spread of the virus occurred in some rats, and as such, it cannot be ruled out that ASIC1A overexpression in nearby striatal regions contributed to the observed results. In particular, two rats showed evidence of expression in the lateral NAshell and many rats showed spread into the portions of the dorsal striatum closest to the NAcore.
Figure 1.
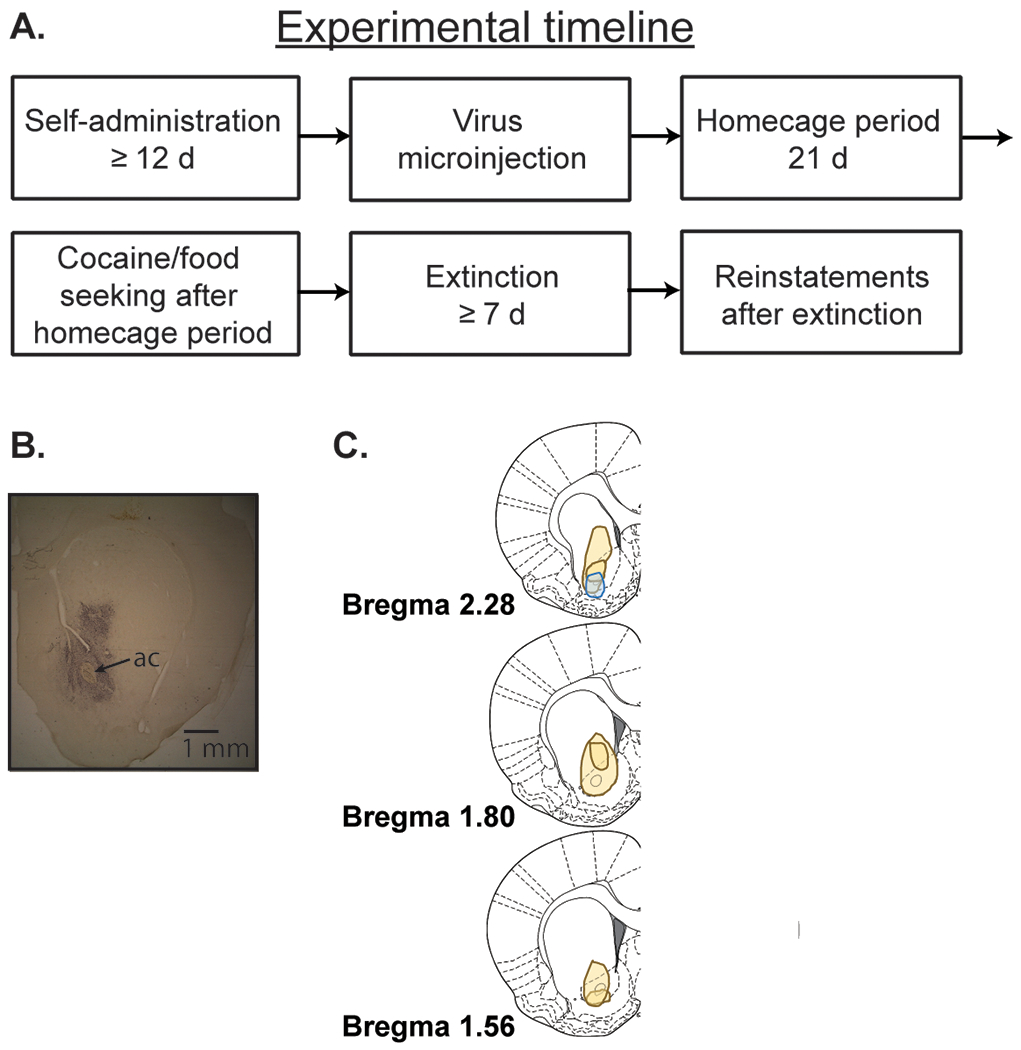
A. Timeline for all experiments. B. Representative immunohistochemistry image showing virus expression in the NAcore. ac, anterior commissure. C. The left panel is a schematic showing the maximum and minimum observed spread of virus, as visualized by immunohistochemistry. The blue shape shows rats (n = 2) with viral spread into the NAshell.
Experiment 1: Cocaine-self administration
Figure 2 shows the results from Experiment 1, which examined the effect of ASIC1A overexpression in the NAcore on cue-/context-driven cocaine-seeking behavior following 21 d in the homecage, the extinction of cocaine seeking, and the reinstatement of cocaine-seeking behavior following extinction training. A t-test examining cocaine infusions averaged across the last 3 d of self-administration revealed no difference between GFP and ASIC1A groups (Figure 2A, t(12) = 0.33, p > 0.05). A t-test comparing inactive lever presses averaged across the last 3 d of self-administration similarly revealed no difference between the groups (t(12) = 0.33, p > 0.05). A t-test for the initial cue-/context-driven cocaine-seeking test found that following 21 d of abstinence, active lever pressing of rats overexpressing ASIC1A was significantly higher than that of GFP-control rats (Figure 2B, t(12) = 6.02, p < 0.0001). A t-test of inactive lever presses during the initial cue-/context-driven cocaine-seeking test likewise revealed significantly higher levels of lever pressing for ASIC1A-overexpressing rats compared to those of the GFP-control rats (Figure 2C, t(12) = 2.97, p < 0.05).
Figure 2.
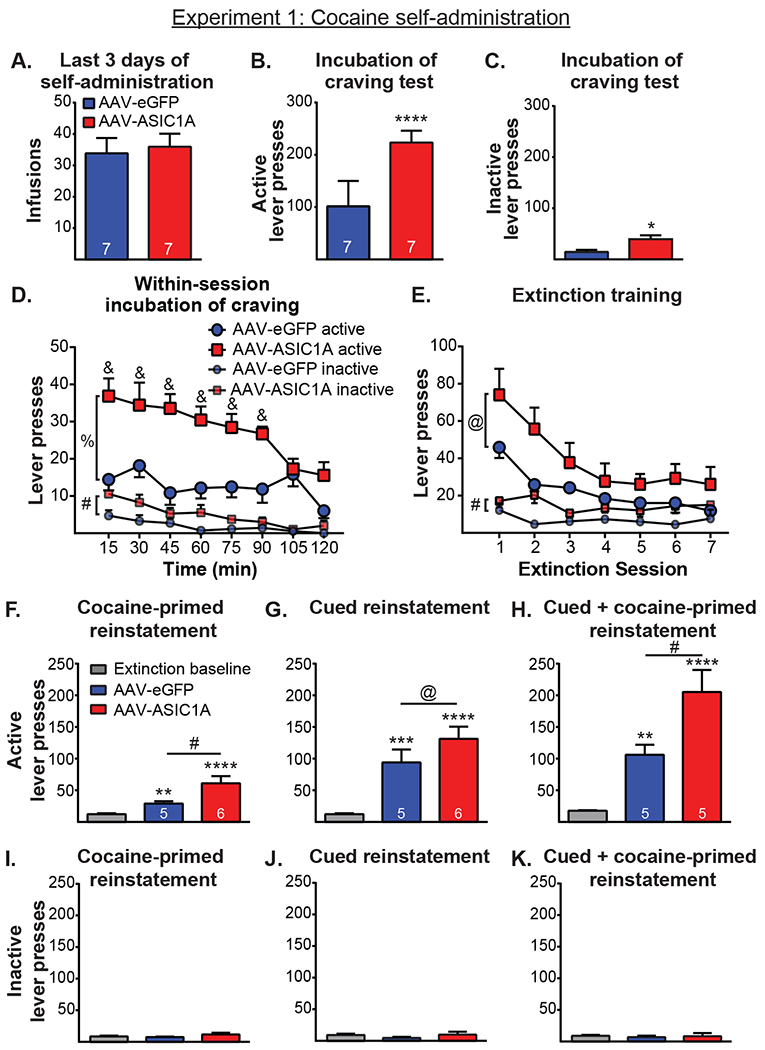
A. Groups did not differ in the total number of cocaine infusions during the last 3 days of self-administration. B. ASIC1A overexpression in the NAcore increased active lever pressing during the cue-/context-driven cocaine-seeking test after the homecage period. ****, p < 0.0001. C. ASIC1A overexpression increased inactive lever pressing during the cue-/context-driven cocaine seeking. *, p < 0.05. D. ASIC1A-overexpressing rats showed greater lever pressing during the first 90 min of the session. %, p < 0.0001 and #, p < 0.05 for overall between-group difference; &, p < 0.05 for group difference on that day. E. During the extinction of cocaine seeking, ASIC1A overexpression marginally increased active lever pressing and increased inactive lever pressing. @, p < 0.1; #, p < 0.05. F. ASIC1A overexpression increased active lever pressing during cocaine-primed reinstatement. @, p < 0.1 and ***, p < 0.001 relative to extinction baseline; #, p < 0.01 relative to GFP. G. ASIC1A overexpression had no significant effect on active lever pressing during cued reinstatement. **, p < 0.01; and ***, p < 0.001 relative to extinction baseline. H. ASIC1A overexpression increased active lever pressing during cued + cocaine-primed reinstatement. *, p < 0.05 and ***, p < 0.001 relative to extinction baseline; #, p < 0.01 for GFP vs. ASIC1A. I-K. ASIC1A overexpression had no effect on inactive lever presses during reinstatement tests.
Lever pressing during the initial cue-/context-driven cocaine-seeking test was broken down into 15-min bins and examined to determine whether ASIC1A overexpression altered lever pressing in a time-dependent manner. A two-way repeated measures ANOVA examining within-session active lever presses during the test revealed a significant main effect of group (Figure 2D, F(1, 12) = 36.21, p < 0.0001), a significant effect of day (F(7, 84) = 4.78, p < 0.001), and a significant interaction (F(7, 84) = 2.34, p < 0.05). Post-hoc tests indicated a significant difference in active lever presses between GFP and ASIC1A-overexpressing groups for the first 90 min of the session (p < 0.001 for 0-15 min and 31-45 min bins; p < 0.01 for 16-30 min, 45-60 min, and 61-75 min bins; p < 0.05 for 76-90 min bin), with ASIC1A-overexpressing rats showing higher lever pressing during that time compared to their control counterparts. Within-session analysis of inactive lever pressing during the cocaine-seeking test revealed a main effect of group (Figure 2D, F(1,12) = 8.94, p < 0.05) and a main effect of day (F(7, 84) = 7.37, p < 0.0001) but no interaction (F(7, 84) = 1.20, p > 0.05). Post-hoc tests indicated that ASIC1A-overexpressing rats had significantly higher inactive lever pressing during the 0-15 min bin (p < 0.05) and marginally higher during the 16-30 and 45-60 min bins (p < 0.08) compared to their control counterparts.
A two-way repeated measures ANOVA of active lever presses during extinction revealed a marginally significant main effect of group (Figure 2E, F(1, 11) = 3.30, p < 0.1), a significant main effect of day (F(6, 66) = 16.79, p < 0.0001), and no significant interaction (F(6, 66) = 1.38, p > 0.05). A two-way repeated measures ANOVA of inactive lever presses during the same extinction training revealed a main effect of group (Figure 2E, F(1, 11) = 5.53, p < 0.05), no main effect of day (F(6, 66) = 1.44, p > 0.05), and no interaction (F(6, 66) = 1.14, p > 0.05). Post-hoc tests indicated that GFP and ASIC1A groups significantly differed in inactive lever presses on the second day of extinction (p < 0.05). Note that only the first 7 d of extinction training are shown on the graph. Although the ASIC1A group required more days than the GFP group to reach reinstatement criteria (7.17 ± 0.17 for GFP vs. 8.14 ± 0.59 for ASIC1A), a t-test comparing the number of days found no between-group differences (t(11) = 1.47, p > 0.05).
Figures 2F–H show active lever presses for the tests of reinstatement of cocaine seeking. Two rats from each group were excluded from one or more reinstatement tests due to death before the test was completed. A two-way repeated measures ANOVA of active lever presses during the cocaine-primed reinstatement revealed a significant effect of day (Figure 2F, F(1,9) = 29.18, p < 0.001), a strong trend for an effect of group (F(1,9) = 5.01, p = 0.05), and a significant interaction (F(1,9) = 7.02, p < 0.05). Post-hoc analysis indicated the GFP group marginally reinstated cocaine seeking relative to extinction baseline (p < 0.1) and that ASIC1A-overexpressing rats significantly reinstated cocaine seeking (p < 0.001). Moreover, the ASIC1A-overexpressing group had significantly greater active lever presses relative to GFP controls on the cocaine-primed reinstatement day (p < 0.01). A two-way repeated measures ANOVA of active lever presses during the cued reinstatement indicated a significant main effect of day (Figure FG, F(1,9) = 55.30, p < 0.0001), no main effect of group (F(1,9) = 1.23, p > 0.05), and no interaction (F(1,9) = 2.07, p > 0.05). Post-hoc tests revealed that both groups significantly reinstated cocaine seeking relative to extinction baseline (p < 0.01 for GFP and p < 0.001 for ASIC1A). Although ASIC1A overexpression increased active lever pressing during the cued reinstatement test compared to that of the GFP control group, the effect was not statistically significant (p > 0.05). A power analysis, assuming a desired power of 0.8, indicated that an n of ~25 per group would be required to observe statistical significance in the difference between the groups during the cued reinstatement test. A two-way repeated measures ANOVA of active lever presses during cued + cocaine-primed reinstatement revealed a significant main effect of day (Figure 2H, F(1,8) = 50.79, p < 0.0001), a significant effect of group (F(1,8) = 7.32, p < 0.05), and a significant interaction (F(1,8) = 6.32, p < 0.05). Post-hoc tests indicated that both groups showed significantly more active lever presses on the reinstatement day relative to the extinction day (p < 0.05 for GFP and p < 0.001 for ASIC1A), and the ASIC1A group had significantly more active lever presses relative to the GFP group on the cued + cocaine-primed reinstatement day (p < 0.01).
Figures 2I–K shows inactive lever presses for the same sessions. A two-way repeated measures ANOVA of inactive lever pressing during the cocaine-primed reinstatement revealed no effect of group (Figure 2I, F(1,9) = 0.48, p > 0.05), no effect of day (F(1,9) = 0.64, p > 0.05), and no interaction (F(1,9) = 0.86, p > 0.05). A two-way repeated measures ANOVA of inactive lever pressing during the cued reinstatement showed no effect of group (Figure 2J, F(1,9) = 1.81, p > 0.05), no effect of day (F(1,9) = 0.86, p > 0.05), and no interaction (F(1,9) = 0.49, p > 0.05). Similarly, a two-way repeated measures ANOVA for inactive lever pressing during cued + cocaine-primed reinstatement indicated no main effect of group (Figure 2K, F(1,8) = 0.21, p > 0.05), no main effect of day (F(1,8) = 0.50, p > 0.05), and no interaction (F(1,8) = 0.03, p > 0.05). Taken together, Experiment 1 showed that ASIC1A overexpression in the NAcore increased cocaine seeking during tests for cue-driven cocaine seeking after the homecage period, the extinction of cocaine seeking, and the cocaine-primed and cued + cocaine-primed reinstatement of cocaine-seeking behavior after extinction.
Experiment 2: Food self-administration
Figure 3 shows the results from Experiment 2, which examined the effect of ASIC1A overexpression in the NAcore on the cue-/context-driven food-seeking behavior following 21 d in the homecage, the extinction of food seeking, and the reinstatement of food seeking after extinction training. This experiment tested whether the results from Experiment 1 generalize to non-drug rewards. A t-test examining food pellets earned, averaged across the last 3 d of self-administration, revealed no difference between GFP and ASIC1A-overexpressing rats (Figure 3A, t(12) = 0.95, p > 0.05). A t-test comparing active lever presses after the 21-d homecage period showed no between-group differences (Figure 3B, t(12) = 0.94, p > 0.05). A two-way repeated measures ANOVA of active lever presses during the extinction of food-seeking behavior revealed no main effect of group (Figure 3C, F(1, 12) = 1.01, p > 0.05), a significant main effect of day (F(6, 72) = 35.48, p < 0.05), and no interaction (F(6. 72) = 0.83, p > 0.05). A two-way repeated measures ANOVA of inactive lever presses during extinction also revealed no main effect of group (Figure 3C, F(1, 12) = 0.03, p > 0.05), a trend for an effect of day, (F(6. 72) = 1.97, p < 0.09), and no interaction (F(6, 72) = 0.76, p > 0.05).
Figure 3.
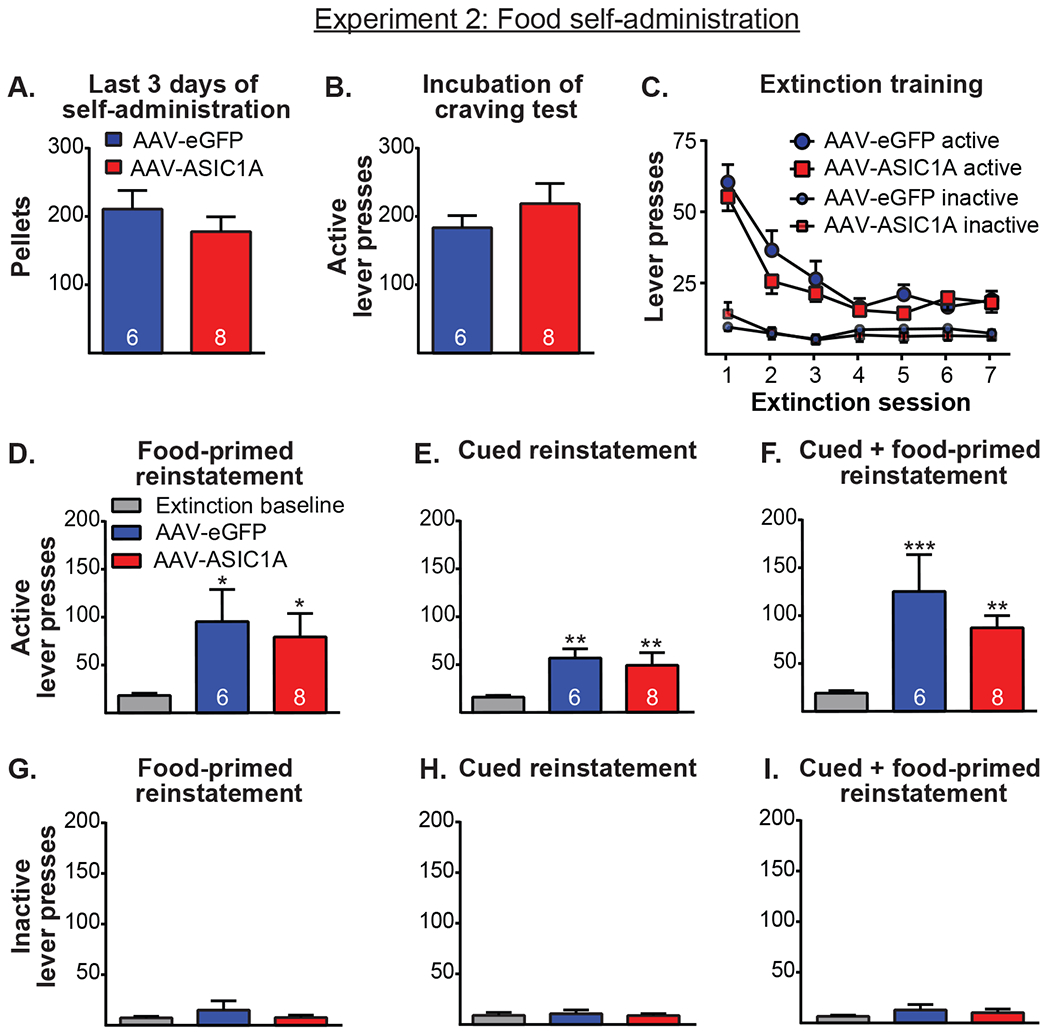
A. Groups did not differ in the number of food pellets earned during the last 3 sessions of self-administration. B. ASIC1A overexpression in the NAcore had no effect on food seeking during the cue-/context-driven food-seeking test after the homecage period. C. ASIC1A overexpression had no effect on active or inactive lever presses during extinction training. D-F. Rats showed significantly increased active lever pressing during food-primed, cued, and cued + food-primed reinstatement tests, respectively, but GFP and ASIC1A overexpressing rats did not differ from one another. *, p < 0.05 and **, p < 0.01 relative to extinction baseline. G-I. No differences existed in inactive lever presses during food-primed, cued, and cued + food-primed reinstatement, respectively.
Figures 3D–F show active lever presses for the reinstatement of food seeking tests. A two-way repeated measures ANOVA of active lever presses during the food-primed reinstatement revealed a significant main effect of day (Figure 3D, F(1,12) = 13.24, p < 0.01), no effect of group (F(1,12) = 0.11, p > 0.05), and no interaction (F(1,12) = 0.22, p > 0.05). Post-hoc tests indicated that both groups significantly reinstated cocaine seeking relative to baseline (p < 0.05 for both) but did not differ from one another (p > 0.05). A two-way repeated measures ANOVA of active lever pressing during cued reinstatement indicated a significant main effect of day (Figure 3E, F(1,12) = 23.29, p < 0.001) but no significant main effect of group (F(1,12) = 0.19, p > 0.05) and no interaction (F(1,12) = 0.17, p > 0.05). Post-hoc tests showed both ASIC1A-overexpressing rats and GFP rats significantly reinstated cocaine seeking (p < 0.01) but did not differ from each other (p > 0.05). A two-way repeated measures ANOVA examining active lever pressing during cued + food-primed reinstatement showed a significant main effect of day (Figure 3F, F(1,12) = 29.48, p < 0.001) but no effect of group (F(1,12) = 0.95, p > 0.05) and no interaction (F(1,12) = 1.28, p > 0.05). Post-hoc tests indicated rats in both groups reinstated food-seeking behavior (p < 0.01) but did not differ from one another (p > 0.05). Figures 3G–I show inactive lever presses for the same sessions. A two-way repeated measures ANOVA examining inactive lever presses during food-primed reinstatement revealed no significant effect of day (Figure 3G, F(1,12) = 1.97, p > 0.05), no effect of group (F(1,12) = 0.62, p > 0.05), and no interaction (F(1,12) = 0.87, p > 0.05). A two-way repeated measures ANOVA of inactive lever pressing during cued reinstatement indicated no significant effect of day (Figure 3H, F(1,12) = 0.12, p > 0.05), no effect of group (F(1,12) = 0.04, p > 0.05), and no interaction (F(1,12) = 0.25, p > 0.05). Similarly, a two-way repeated measures ANOVA of inactive lever pressing during cued + food-primed reinstatement revealed no significant effect of day (Figure 3I, F(1,12) = 3.23, p > 0.05), no effect of group (F(1,12) = 0.52, p > 0.05), and no interaction (F(1,12) = 0.0061, p > 0.05).
Discussion
The present findings indicate that ASIC1A overexpression targeted at the NAcore following the completion of cocaine self-administration increased cocaine seeking during the cue-/context-driven test following the homecage abstinence period, marginally impaired the extinction of cocaine seeking, and increased cocaine-primed and cued + cocaine-primed reinstatement. In contrast, the current work found that ASIC1A overexpression did not alter food-seeking behavior. Together, the present findings indicate a selective role for ASIC1A in the NA in regulating relapse-like behavior following a period of abstinence from cocaine self-administration.
Our prior work reported that ASIC1A overexpression decreases the number of cocaine infusions and shifts the dose-response curve to the right during self-administration (Kreple et al., 2014). As a result, it is unclear whether the present results would differ if the ASIC1A overexpression was present throughout the self-administration and abstinence phases, as such an experiment would be inherently confounded by the differences in cocaine self-administration levels. Although the current results would appear to be in contrast to those with overexpression during self-administration (Kreple et al., 2014), several important differences exist between the studies that likely account for the apparent discrepancies. First, different neural systems appear to mediate self-administration vs. relapse-like behaviors. During cocaine self-administration, blocking dopamine receptors in the NA alters the number of cocaine infusions (Caine et al., 1995; Robledo et al., 1992; Veeneman et al., 2012; Woolverton and Virus, 1989). In contrast, during cued and cocaine-primed reinstatement tests, evidence suggests that blocking AMPA glutamate receptors, but not dopamine receptors, in the NAcore reduces cocaine seeking (Cornish and Kalivas, 2000). Together, these results suggest that the neurobiological substrates mediating the reinforcing properties of cocaine differ from those required to drive reinstatement of cocaine seeking following self-administration. Moreover, Kreple et al. overexpressed ASIC1A prior to cocaine self-administration, whereas the present study overexpressed ASIC1A on the first day of abstinence. A 3-week period was required in the current experiment in order to achieve the desired expression of ASIC1A. Because the transduction and upregulation of ASIC1A occurred during the same time period as abstinence from cocaine self-administration, it is possible that changes in ASIC1A signaling interacted with the molecular mechanisms underlying abstinence in an unforeseen manner.
Prior findings indicate that relapse-like behavior for cocaine seeking depends on glutamatergic transmission in the NAcore (Backstrom and Hyytia, 2007; Cornish and Kalivas, 2000; Wang et al., 2013). As ASICs contribute to excitatory synaptic transmission in the NAcore and elsewhere (Du et al., 2014; Gonzalez-Inchauspe et al., 2017; Kreple et al., 2014), the present findings may result from alterations in excitatory signaling as a consequence of changes in ASIC1A expression or functioning. Such changes in excitatory transmission may cause structural remodeling in ASIC1A-overexpressing rats (Mattison et al., 2014) that contributes to enhanced cocaine-seeking behavior. Consistent with this possibility, ASIC1A-null mice show increased spine density in the NAcore (Kreple et al., 2014), and therefore ASIC1A-overexpressing rats may have decreased spine density. A prior study using 3D imaging and analysis showed that cocaine self-administration followed by abstinence reduces spine density in the mPFC (Radley et al., 2015). Taken together, these studies suggest that ASIC1A-mediated changes in spine density in the mPFC-to-NA pathway may contribute to enhanced cocaine-seeking behavior in the present experiment. (Of note, due to viral spread, overexpression was observed outside the NAcore as well and, therefore, overexpression in nearby striatal regions may have contributed to the present findings.)
In support of the involvement of NAcore glutamate transmission, ASIC1A-null mice show increased expression of GluA2-lacking AMPA receptors in the NA, suggesting that, in this model, ASIC1A negatively regulates the expression of these receptors (Kreple et al., 2014). GluA2-lacking AMPA receptors are inserted into the NA during withdrawal and mediate the incubation of cocaine craving (Conrad et al., 2008; Ma et al., 2014; Pascoli et al., 2014). Moreover, disruption of ASIC1A increases AMPA/NMDA receptor ratio in mice (Kreple et al., 2014). Evidence suggests this neural adaptation contributes to the incubation of craving, as rats exposed to 2-h per day cocaine self-administration sessions followed by a period of abstinence also showed an enhanced AMPA/NMDA receptor ratio (Moussawi et al., 2011; Ortinski et al., 2012). These previous findings would lead to a prediction of ASIC1A overexpression decreasing, rather than enhancing, the cocaine seeking following the homecage period in the present experiment. That we did not see such an effect may be accounted for by differences in the temporal and spatial expression provided by the techniques. Global knockouts, as were used in the Kreple et al study, lack ASIC1A during development, and as such, ASIC1A-dependent neurodevelopment would also be disrupted. In both the ASIC1A knockout mice and the ASIC1A-overexpressing rats, compensatory mechanisms may be present, although these changes are likely distinct in the two models.
A caveat of the present study is that overexpressed ASIC1A protein may function differently than the endogenous ASIC1A protein or cause compensatory changes in neural circuits. Although AAV-CMV-ASIC1A was targeted to the NAcore (with spread of expression to nearby striatal regions), this method of overexpression lacks spatial specificity within the synapse. It is unknown whether ASIC1A overexpression is localized to the synapse, extrasynaptic space, or both. Protons released from vesicles during neurotransmission are thought to activate synaptic ASIC1A and create a depolarizing postsynaptic current (Kreple et al., 2014). As the NAcore mediates the reinstatement of cocaine-seeking (Ito et al., 2004), increased ASIC1A expression in the NAcore could drive cocaine seeking by promoting firing of these neurons. Another possibility is that increased extrasynaptic ASIC1A expression and the consequent increase in ASIC1A currents may place cells in a more depolarized state, making them more sensitive to glutamatergic inputs promoting cocaine seeking. Whereas the spatial expression of AAV-ASIC1A within the synapse is unknown, endogenous ASIC1A is preferentially localized to dendrites and dendritic spines (Zha et al., 2006). It therefore remains to be determined whether disrupting endogenous ASIC1A signaling, rather than overexpressing ASIC1A, would reduce cocaine-seeking behavior after abstinence. Moreover, it is unknown whether ASIC1A overexpression causes an increase in ASIC1A heteromeric channels (contain ASIC2A and/or ASIC2B) or ASIC1A homomeric channels. Future studies examining sensitivity to the ASIC1A-specific blocker Psalmotoxin-1 (PcTx1) could be used to discriminate between these possibilities.
CMV is a general promoter, and evidence suggests that this promoter may target non-neuronal cells with glial morphology (Kugler et al., 2003). Previous work shows that depolarization of perineuronal oligodendrocytes can decrease the firing latency of neighboring neurons (Yamazaki et al., 2007), whereas activation of perineuronal astrocytes suppresses the activity of neighboring interneurons (Yamazaki et al., 2005). Moreover, astrocytes influence synaptic functioning and efficacy, for example, by regulating extrasynaptic glutamate levels and activation of presynaptic glutamate receptors, which, in turn, influence drug-seeking behavior (Moussawi et al., 2009; Reissner and Kalivas, 2010). Therefore, the present findings may result from changes not only in ASIC1A expression in neurons but also alterations in glial cell functioning.
Cocaine self-administration followed by abstinence may increase ASIC1A expression or acid-evoked currents, and if ASIC1A contributes to drug seeking as reported here, then these data may suggest an ASIC1A-mediated feedforward loop that perpetuates addiction. Indeed, previous work reported that 5 d of experimenter-administered cocaine followed by 14 d of abstinence enhances ASIC1 protein expression in the NA of mice (Zhang et al., 2009). However, this previous study did not examine a causative relationship between ASIC1A expression and cocaine-seeking behavior, and ASIC1A could act as a compensatory mechanism to oppose cocaine seeking.
ASIC1A overexpression increased responding on the inactive lever during the first cocaine seeking test after the homecage period and the extinction training. This can likely be accounted for by rats engaging in alternative drug-seeking behavior. It is possible that overexpression of ASIC1A caused a loss of discrimination capacity between the active and inactive levers, although this seems unlikely because the number of active lever presses is still higher than inactive lever presses in all of the tests. Another possibility is that ASIC1A overexpression caused a general increase in motor behavior. This seems unlikely, however, because GFP and ASIC1A groups showed equivalent levels of both active and inactive lever pressing during the cue-/context-driven food-seeking test in Experiment 2 (food seeking).
Together with previous findings, the present study suggests that ASIC1A has a dynamic role in cocaine-taking and -seeking behavior, such that it negatively regulates cocaine taking but promotes cocaine seeking. However, it appears these findings are not generalizable to all types of reward-seeking behavior, as ASIC1A overexpression in the NAcore had no effect on food-seeking behavior.
Supplementary Material
Funding and Disclosure
The authors declare no competing financial interests. The research was supported by NIH grants DA034684 (RTL), DA037216 (RTL and JAW), and DA043364-01 (CVC).
References
- Backstrom P, Hyytia P (2007) Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 192:571–580. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF (1995) Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res 692:47–56. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ (2014) Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America 111:8961–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Inchauspe C, Urbano FJ, Di Guilmi MN, Uchitel OD (2017) Acid-Sensing Ion Channels Activated by Evoked Released Protons Modulate Synaptic Transmission at the Mouse Calyx of Held Synapse. J Neurosci 37:2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman AL, Nett KE, Cosme CV, Worth WR, Gupta SC, Wemmie JA, LaLumiere RT (2017) Extinction of Cocaine Seeking Requires a Window of Infralimbic Pyramidal Neuron Activity after Unreinforced Lever Presses. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:6075–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ (2004) Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7:389–397. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP (2009) Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience 162:55–66. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ (2007) Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 27:7921–7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreple CJ, Lu Y, Taugher RJ, Schwager-Gutman AL, Du J, Stump M, Wang Y, Ghobbeh A, Fan R, Cosme CV, Sowers LP, Welsh MJ, Radley JJ, LaLumiere RT, Wemmie JA (2014) Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci 17:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler S, Kilic E, Bahr M (2003) Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther 10:337–347. [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison HA, Popovkina D, Kao JP, Thompson SM (2014) The role of glutamate in the morphological and physiological development of dendritic spines. Eur J Neurosci 39:1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature neuroscience 12:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW (2011) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America 108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Vassoler FM, Carlson GC, Pierce RC (2012) Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology 37:1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C (2014) Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509:459–464. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT (2015) The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:11897–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW (2010) Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 21:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Maldonado-Lopez R, Koob GF (1992) Role of dopamine receptors in the nucleus accumbens in the rewarding properties of cocaine. Ann N Y Acad Sci 654:509–512. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ (2012) Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology 37:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW (2013) Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol 18:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr., Welsh MJ (2003) Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 23:5496–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM (1989) The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav 32:691–697. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Li J, Fujii S, Miyakawa H, Kudo Y, Kato H (2005) Direct evidence for mutual interactions between perineuronal astrocytes and interneurons in the CA1 region of the rat hippocampus. Neuroscience 134:791–802. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Sugihara T, Fujii S, Goto K, Kato H (2007) Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol 3:325–334. [DOI] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ (2006) Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proceedings of the National Academy of Sciences of the United States of America 103:16556–16561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Wang JQ, Chu XP (2009) Upregulation of acid-sensing ion channel 1 protein expression by chronic administration of cocaine in the mouse striatum in vivo. Neurosci Lett 459:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


