Abstract
The offspring of super-multiparous sows face problems such as decreased growth performance, poor meat quality and even diseases in animal husbandry. Gama-aminobutyric acid (GABA) has long been known to promote growth and suppress inflammation, but little is known about the mechanisms. A total of 72 growing-finishing pigs from the 8th generation were randomly allotted to 2 groups with 6 replicates per treatment to receive a corn–soybean basal diet or the basal diet supplemented 20 mg/kg GABA for 60 d. After the animal-trial period, samples of serum and liver were collected for further analysis. Additionally, a lipopolysaccharide (LPS)-induced inflammatory model using HepG2 cells was established to explore the role of GABA on regulating hepatic inflammation. The results indicated that inflammatory cell infiltration occurs in the liver of progeny of super-multiparous sows, and dietary supplementation with GABA influenced liver morphology, increased activities of antioxidant enzymes and decreased the expression abundance of pro-inflammatory cytokines, including tumor necrosis factor-α (TNFα) and interleukin (IL)-1β, in the liver of growing-finishing pigs (P < 0.05). In addition, GABA supplementation increased mRNA expressions of peroxisome proliferator-activated receptor γ (PPARγ) and GABA receptors (GABARs), and reduced the expression of toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling (P < 0.05). Additionally, an in vitro experiment demonstrated that GABA decreased the expressions of hepatic TLR4/NF-κB signaling via activating GABARs under LPS-stress (P < 0.05). In summary, liver injury may affect the growth performance of growing-finishing pigs by changing hepatic mitochondrial metabolism, the expression of pro-inflammatory cytokines and TLR4/NF-κB pathway and that GABA supplementation has a restorative effect by acting on GABARs.
Keywords: Gama-aminobutyric acid, Growing-finishing pig, Liver inflammation, Lipopolysaccharide, NF-κB
1. Introduction
The health of growing-finishing pigs is important for improving productivity and hence attaining economic benefits; the best performance of the sows usually came from 2 to 4 parities. However, in order to improve the utilization rate of sows and reduce production costs, the service life of sows will be extended to 8 to 9 parities during production. The offspring of super-multiparous sows are prone to problems such as decreased growth performance, are susceptible to disease and face elimination from breeding production. The gestating sow parity has been reported to affect growth performance of offspring throughout its productive life, resulting in important differences by the time pigs reach market weight (Pineiro et al., 2019). There are many factors contributing to this result, that may be related to chronic exposure to antigens, the genetic effects of mutations in mitochondrial DNA (Zhang et al., 2019a), or changes in environmental conditions. In this study, chronic inflammation was observed in the liver of progeny bred by super-multiparous sows. However, data on production traits of growing-finishing pigs of super-multiparous sows is extremely scarce and the mechanism of poor body condition of growing-finishing pigs generated by sows with higher parity is still unclear.
Nutrition is a basic need for growth and plays an important role in the metabolic regulation of the body. More recently, the effects of nutrients on immune regulation or disease prevention have been recorded and are gradually becoming more valued. As an amino acid with neuro-inhibitory effects, γ-aminobutyric acid (GABA) has great effect on the mammalian central nervous system (Sarkar et al., 2016; Bi et al., 2020). GABA is released by GABAergic interneurons to suppress neuronal activities through acting on GABA receptors (GABARs) at presynaptic terminals, or acting on postsynaptic GABARs in neurons (Yan et al., 2015). Therefore, there is a theoretical basis that GABA regulates food intake. In animal husbandry, GABA is usually used to promote the growth performance of animals. Several studies have reported that dietary GABA has beneficial effects on promoting daily gain or food intake of weaned piglets (Chen et al., 2019), lambs (Wang et al., 2015) and cows (Wang et al., 2013). Supplementation with GABA can improve antioxidant status of growing-finishing pigs under transportation stress conditions (Bi et al., 2020). Similarly, dietary supplementation with GABA could increase the antioxidant activity of heat-stressed hens (Zhang et al., 2012) and transition dairy cows (Wang et al., 2013). Other studies have demonstrated that GABA has benefits in reversing the inflammatory response (Dias et al., 2014; Hwang et al., 2019). A synthetic analogue of GABA was also reported to play roles in anti-inflammation and anti-oxidant in mice (Dias et al., 2014). Mice pretreated with GABA could prevent abnormal immune reactivity (Feng et al., 2017). Those studies suggest that GABA signal has a potential therapeutic role in protecting from inflammatory injury and improving growth performance of animals under stress. However, the molecular mechanism of GABA induced growth performance improvement in animals is unclear.
In the previous study, we observed the growth performance, antioxidant function, immune function and many other indices of growing-finishing pigs generated by super-multiparous sows (unpublished), and found the progeny of super-multiparous sows have lipopolysaccharide (LPS)-induced chronic inflammation especially in liver and the activation of toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) pathway. Thus, we hypothesize that GABA can improve the growth performance of growing-finishing pigs undergoing multiple-generation stress via alleviating the inflammatory response. Therefore, the objective of this study was to estimate that whether dietary GABA supplementation has beneficial effects on growth performance, antioxidant indices, tissue morphology and the abundance of inflammatory signaling in the liver of growing-finishing pigs, and to explore whether the regulation of GABA on TLR4/NF-κB is mediated by GABA receptors in vitro.
2. Materials and methods
2.1. Animal ethics
All animal procedures were approved by the Animal Care and Use Committee of China Agricultural University and performed in strict accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
2.2. Animal experiment and cell culture
A total of 72 crossbred Duroc × Landrace × Large White growing-finishing pigs (72.9 ± 6.75 kg) from the 8th generation were chosen and randomly allotted to 1 of 2 treatments. Pigs were fed a corn–soybean meal basal diet (as control; Control) or the basal diet supplemented with 20 mg GABA/kg of diet (as treatment; GABA), with 6 replicates of 6 pigs per pen based on body weight. The experiment was carried out in Feng Ning Swine Research Unit of China Agricultural University (Academician Workstation in Chengdejiuyun Agricultural and livestock Co., Ltd) and lasted 60 d. The feed-grade GABA product was supplied by a commercial company (Sanway Biotech Co., LTD, Shanghai, China). The corn–soybean meal basal diet was formulated without any additives according to the National Research Council (2012) for growing-finishing pigs (Table 1). All pigs had free access to feed and water during the experiment. At the end of the feed trial, the average daily gain (ADG) was calculated by weighing, and diet disappearance was determined to calculate the average daily feed intake (ADFI) and feed-to-gain ratio (F:G).
Table 1.
Analyzed chemical composition and ingredients of the basal diet (as-fed basis, %).
| Item | Initial period (50 to 80 kg) | Later period (80 to100 kg) |
|---|---|---|
| Analyzed chemical composition | ||
| Gross energy, MJ/kg | 89.5 | 90.2 |
| Crude protein | 15.9 | 12.1 |
| Ash | 4.1 | 4.0 |
| Organic matter | 84.2 | 83.9 |
| Calcium | 0.64 | 0.60 |
| Total phosphorus | 0.56 | 0.51 |
| Digestible phosphorus | 0.27 | 0.24 |
| Ingredients | ||
| Corn | 77.2 | 82.3 |
| Soybean meal | 20 | 15 |
| CaHPO4 | 0.65 | 0.57 |
| Stone powder | 0.85 | 0.80 |
| NaCl | 0.35 | 0.35 |
| Premix1 | 0.50 | 0.50 |
| L-Lysine hydrochloride | 0.30 | 0.30 |
| DL-Methionine | 0.03 | 0.03 |
| L-Threonine | 0.06 | 0.06 |
| L-Tryptophan | 0.04 | 0.03 |
| L-Valine | 0.02 | 0.02 |
| Total | 100 | 100 |
Premix (antibiotic-free) provided the following vitamins and minerals per kilogram of the complete diet for growing-finishing pigs: vitamin A 15,000 IU, vitamin D3 3,000 mg, vitamin E 40 mg, vitamin K 2 mg, niacin 32 mg, pantothenic acid 16 mg, folic acid 1.6 mg, vitamin B12 0.2 mg, choline chloride 1,000 mg, Fe 100 mg, Cu 8 mg, Mn 30 mg, Zn 100 mg, Se 0.3 mg, I 0.5 mg.
Human hepatocellular carcinoma cell line (HepG2, HB-8065, ATCC, USA) was selected as the model for studying liver inflammation. Cells were prepared according to the instructions of the manufacturer and incubated in DMEM supplemented with 10% fetal bovine serum, at 37 °C in a humidified 5% CO2 atmosphere until confluence and passaged at regular intervals. 10 h before experimentation, the medium was replaced with serum-free medium to eliminate non-specific cell cycle effects by serum proteins. Next, HepG2 cells were subjected to inflammatory treatments during the logarithmic phase. We firstly used different LPS concentrations (0, 0.5, 1.0, 2.0 μg/mL) to pick out an optimum concentration as the condition to establish hepatic inflammation model of HepG2 cell. On the basis of hepatic inflammation model, we further set the concentration gradient of GABA (0, 0.5, 1.0, 2.0 mmol/L) to screen the lowest GABA concentration that alleviates LPS-induced hepatitis. Besides, HepG2 cells were treated by CGP52432 (a GABAR inhibitor) and acamprosate calcium (Campral EC; a GABAR agonist) to explore the effect of GABA on TLR4/NF-κB pathway.
2.3. Histological examination of liver morphology
After the trial period, pigs were slaughtered in accordance with routine procedure (shock stunning followed by exsanguination). Samples of liver were dehydrated with xylene and ethanol, and then paraffin embedded. Embedded blocks were sectioned into 3 μm thick slices for hematoxylin and eosin (H&E) staining. The nucleus was stained with hematoxylin and cytoplasm with eosin. An optical microscope (Olympus CX53) was used to observe tissue morphology.
2.4. Determination of inflammatory biomarkers and antioxidant indices
The concentration of malondialdehyde (MDA) (#A003-1) and activities of alanine aminotransferase (ALT) (#C009-2-1), aspartate aminotransferase (AST) (#C010-2-1), superoxide dismutase (SOD) (#A001-3), glutathione peroxidase (GSH-Px) (#A005-1) and catalase (CAT) (#A007-1) in serum and liver were analyzed using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and the results were expressed as units per milliliter or per milligram of protein. Total protein concentrations in the supernatants of liver homogenates were measured using a BCA Protein Assay kit (Thermo Scientific, Rockford, IL). All procedures were carried out according to the manufacturers' instructions.
2.5. Quantitative real-time PCR assay
Total RNA from both liver and HepG2 cell were extracted using the Trizol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Both the quality and integrity of total RNA were assessed by electrophoresis on 1% agarose gel, and the concentration of RNA products was determined using a NanoDrop Spectrophotometer (NanoDrop 1000; Thermo Fisher). A total of 0.5 μg RNA was used to synthesis cDNA using the reagent kit (Prime Script RT reagent Kit with gDNA Eraser). The reaction system was 42 °C for 20 min, followed by inactivating at 85 °C for 5 s. The quantitative real-time PCR reactions were conducted on a Light Cycler 96 real-time PCR system (Roche, Basel, Switzerland) using Real-time PCR Super Mix (SYBRGreen, with anti-Taq) (Mei5 Biotechnology, Co., Ltd, Beijing, CHN). The primer sequences of TLR4, NF-κB, tumor necrosis factor-α (TNFα), interleukin (IL)-1β, IL-6, IL-10, peroxisome proliferator-activated receptor γ (PPARγ), peroxisome proliferator-activated receptor gamma coactivator-1 α (PGC-1α), type A1 GABA receptor (GABrA1), GABrB1, GABrB2, GABrD, GABrQ, GABBR1 and β-actin used in the study were listed in Table 2. The procedure of PCR is described as follows: denaturation at 95 °C for 2 min followed by 40 cycles of 95 °C for 60 s, 60 °C for 30 s, and 72 °C for 30 s. Each gene was performed in duplicate and β-actin was used as the reference gene to normalize the expression level of the targeted gene using the 2−△△CT method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences for real-time PCR amplification.
| Genes | GenBank ID | Primer sequences (5´ to 3´) | |
|---|---|---|---|
| pig-β-actin | XM_005671704.2 | Forward | TCTGGCACCACACCTTCT |
| Reverse | TGATCTGGGTCATCTTCTCAC | ||
| pig-PGC-1α | NM_213963.2 | Forward | GTCCTTCCTCCATGCCTGAC |
| Reverse | TGGTTTGCATGGTTCTGGGT | ||
| pig-PPARγ | NM_214379.1 | Forward | ATTCCCGAGAGCTGATCCAA |
| Reverse | TGGAACCCCGAGGCTTTAT | ||
| pig-TLR4 | NM_001113039.2 | Forward | CTCCAGCTTTCCAGAACTGC |
| Reverse | AGGTTTGTCTCAACGGCAAC | ||
| pig-NF-κB | NM_001048232.1 | Forward | CTCGCACAAGGAGACATGAA |
| Reverse | ACTCAGCCGGAAGGCATTAT | ||
| pig-TNFα | NM_214022.1 | Forward | CCCCTCTGAAAAAGACACCA |
| Reverse | TCGAAGTGCAGTAGGCAGAA | ||
| pig-IL-1β | NM_214055.1 | Forward | CAGCCATGGCCATAGTACCT |
| Reverse | CCACGATGACAGACACCATC | ||
| pig-IL-6 | NM_001252429.1 | Forward | ATGGCAGAAAAAGACGGATG |
| Reverse | GTGGTGGCTTTGTCTGGATT | ||
| pig-IL-10 | NM_214041.1 | Forward | AGCCAGCATTAAGTCTGAGAA |
| Reverse | CCTCTCTTGGAGCTTGCTAA | ||
| pig-GABRB1 | NM_001123114.1 | Forward | GCCCGTGGACTATGAGATTG |
| Reverse | CTTGGAGCAGATTCGGACAC | ||
| pig-GABRB2 | XM_021066509.1 | Forward | CATCACCCTTTGCCTGGTGT |
| Reverse | AGGTGGTCTTTTCTGGTGTGT | ||
| pig-GABrD | XM_021095199.1 | Forward | ACGTGTACTTCTGGATCTGCTA |
| Reverse | TTGGCCTTTTGCTTCTTCCGGTA | ||
| pig-GABrQ | XM_0.13986506.2 | Forward | CTTGCCCACCTCCTGCGTA |
| Reverse | AAGTTCCCTAGAGCCGTCCTG | ||
| homo-β-actin | NM_001101 | Forward | ATCATTGCTCCTCCTGAGCG |
| Reverse | CGGACTCGTCATACTCCTGC | ||
| homo-GABrA1 | NM_001127647 | Forward | AGCCGTCATTACAAGATGAACTT |
| Reverse | TGGTCTCAGGCGATTGTCATAA | ||
| homo-GABrB1 | NM_000812 | Forward | AAGGATATGACATTCGCTTGCG |
| Reverse | AGAGTTGGTCAGCTACCCTATT | ||
| homo-GABrB2 | NM_000813 | Forward | TGACCCTAGTAATATGTCGCTGG |
| Reverse | GGTCTCAGACGAATGTCATAGC | ||
| homo-GABBR1 | NM_021903.3 | Forward | GCCACACTCCACAACCCTAC |
| Reverse | ACTCTGGCGGAAAGTAATCTCAA | ||
ID = identity; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator-1 α; PPARγ = peroxisome proliferator-activated receptor γ; TLR4 = toll-like receptor 4; NF-κB = nuclear factor kappa-B; TNFα = tumor necrosis factor-α; IL = interleukin; GABRB1, GABR2, GABrD, GABrA1, GABrB1, GABrB2 = different types of γ-aminobutyric acid receptors.
2.6. Western blot analysis
Frozen liver (100 mg) was powdered in liquid nitrogen and lysed in 1.0 mL of ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology, CHN) and a Halt protease inhibitor cocktail (Thermo Fisher Scientific, US). The homogenates were centrifuged at 14,000 × g for 10 min at 4 °C, and the supernatant was collected. Total protein concentration was determined using a BCA Protein Assay kit (Thermo Fisher Scientific, US). Protein extracts (30 μg) from each sample were then loaded onto 10% SDS-PAGE gels, and the separated proteins were transferred onto nitrocellulose membranes (Merck-Millipore, US). After the transfer, membranes were blocked for 1 h at room temperature in blocking buffer with 5% skimmed milk, and then incubated overnight at 4 °C with the following primary antibodies from Cell Signaling Technology: TLR4 (1:1,000, #14358), NF-κB p65 (1:1,000, #8242), phospho-NF-κB p65 (1:1,000, #3033) and β-actin (1:1,000, #8457). After 3 washes for 10 min each with Tris-buffered saline containing Tween, membranes were incubated with anti-rabbit IgG (H + L) DyLight 800 4× PEG conjugated secondary antibodies (1:5,000, #5151) for 1 h at room temperature. After three washes for 10 min each, the signals were recorded by an infrared fluorescence scanner (Odyssey) and were analyzed with Image J (National Institutes of Health, MD). Beta-actin protein was used to normalize the expression levels of the targeted proteins, and the average expression of proteins in the Control was used as a calibrator.
2.7. Statistical analyses
The data in Tables and Fig. 1, Fig. 2, Fig. 3 were analyzed by the two-tailed unpaired Student's t-test, and the data in Fig. 4, Fig. 5, Fig. 6 were analyzed by one-way analysis of variance (ANOVA) using SAS 9.2 (SAS Institute, Cary, NC), and expressed as mean values and standard errors. Each replicate served as the experimental unit. Differences in means among treatments were tested by the least significant difference method. P < 0.05 was considered statistically significant. GraphPad Prism 7.04 software was used to create the artwork.
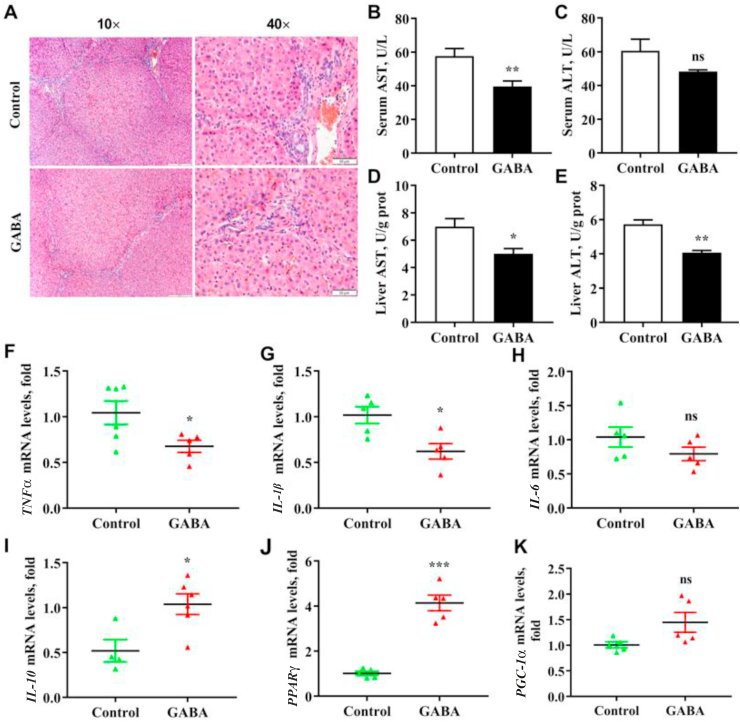
Fig. 1.
Effects of γ-aminobutyric acid (GABA) supplementation on inflammatory response in the liver of growing-finishing pigs. (A) The H&E staining of liver in growing-finishing pig. The nucleus was stained with hematoxylin, and cytoplasm with eosin. (B and C) The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the serum in growing-finishing pigs. The results in serum were expressed as units per liter. (D and E) The levels of AST and ALT in the liver of growing-finishing pigs. The results in the liver were expressed as units per gram of protein. (F to K) The mRNA expressions of nuclear factor-κB (NF-κB) signaling, inflammatory cytokines pathway and mitochondrial metabolism pathway in the liver of growing-finishing pigs. All data were obtained by real-time PCR and normalized by β-actin. Control, pigs were fed the corn–soybean basal diet; GABA, pigs were fed GABA-containing diet. Data are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. TLR4 = toll-like receptor 4; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator-1 α; PPARγ = peroxisome proliferator-activated receptor γ; TNFα = tumor necrosis factor-α; IL-1β = interleukin-1β.
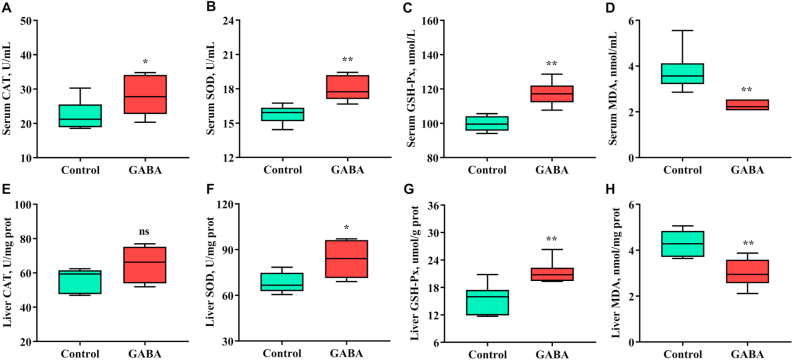
Fig. 2.
Effects of γ-aminobutyric acid (GABA) supplementation on antioxidant functions of serum and liver in growing-finishing pigs. Control, pigs were fed the corn–soybean basal diet; GABA, pigs were fed GABA-containing diet. Values are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05). ∗, P < 0.05; ∗∗, P < 0.01. CAT = catalase; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; MDA = malondialdehyde.
Fig. 3.
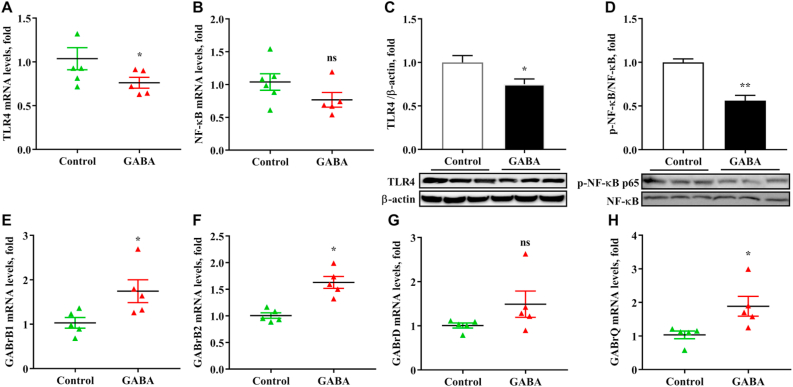
Effects of γ-aminobutyric acid (GABA) supplementation on mRNA expressions of GABA receptors and protein expressions of toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling in the liver of growing-finishing pigs. (A and B) The mRNA expressions of TLR4 and NF-κB in the liver of growing-finishing pigs. The data were obtained by real-time PCR and normalized by β-actin. (C and D) Western blot of immune-precipitated TLR4 and the phosphorylation of NF-κB in the liver and normalized β-actin densities of these proteins. (E to H) The mRNA expressions of GABA receptors in the liver of growing-finishing pigs. Control, pigs were fed the corn–soybean basal diet; GABA, pigs were fed GABA-containing diet. Values are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05). ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 4.
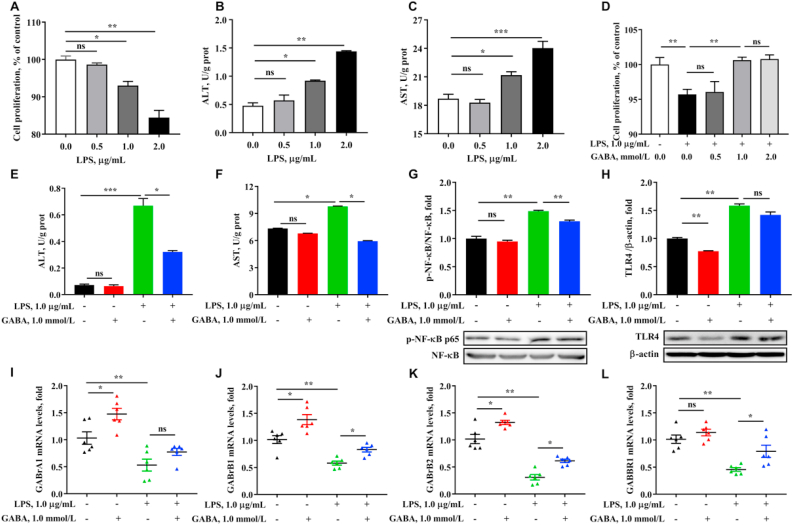
Lipopolysaccharides (LPS)-induced inflammation of HepG2 cell could be alleviated by γ-aminobutyric acid (GABA). (A) Cell proliferation were tested by using CCK8 kit after treatment by lipopolysaccharides (LPS) (0.0, 0.5, 1.0 and 2.0 μg/mL) for 12 h. (B and C) The levels of ALT and AST of the cell, which were expressed as units per gram of protein. (D) Cell proliferation were tested after treatment by GABA (0.0, 0.5, 1.0 and 2.0 mmol/L) for 12 h and LPS (1.0 μg/mL) for 12 h. (E and F) The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) of the cell. (G) The phosphorylation of nuclear factor-κB (NF-κB) in cell. (H) Western blot of immune-precipitated toll-like receptor 4 (TLR4) and normalized β-actin densities of these proteins. (I to L) The mRNA expressions of GABA receptors. Values are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Fig. 5.
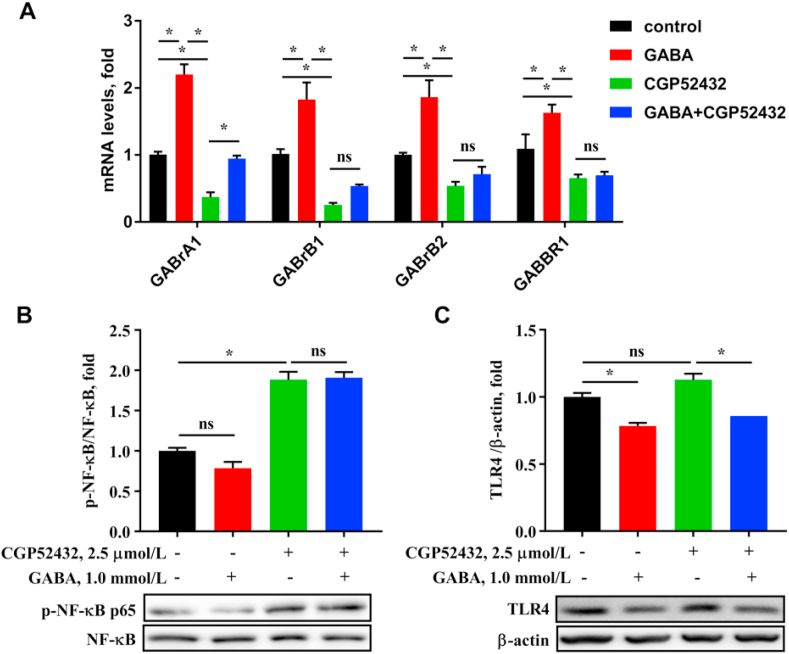
Effects of γ-aminobutyric acid (GABA) and GABA inhibitor on GABARs/TLR4/NF-κB signaling of hepatocytes. (A) The mRNA expressions of GABA receptors (including GABrA1, GABrB1, GABrB2, and GABBR1) in HepG2 cell which were treated by 2.5 μmol/L CGP52432 for 12 h and 1.0 mmol/L GABA for 12 h. (B and C) Protein expressions of immune-precipitated toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling tested by western blotting. Values are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05)∗, P < 0.05.
Fig. 6.
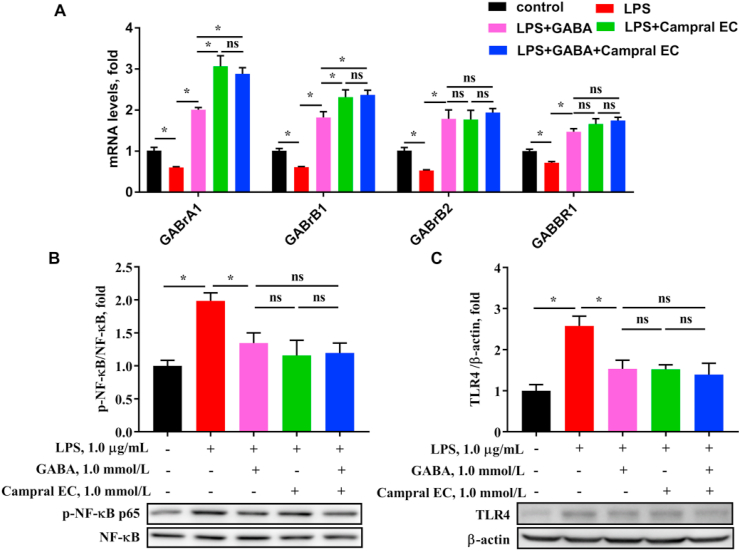
Effects of γ-aminobutyric acid (GABA) and GABA activator on GABARs/TLR4/NF-κB signaling of hepatocytes. (A) The mRNA expressions of GABA receptors (including GABrA1, GABrB1, GABrB2, and GABBR1) in HepG2 cell which were treated by 1.0 μg/mL lipopolysaccharides (LPS) for 12 h and 1.0 mmol/L GABA or 1.0 mmol/L acamprosate calcium (Campral EC) for 12 h. (B and C) Protein expressions of immune-precipitated toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling tested by Western blotting. Values are presented as mean ± SEM, n = 6. nsThere was no difference between the groups (P > 0.05). ∗, P < 0.05.
3. Results
3.1. GABA improved growth performance of growing-finishing pigs
The effects of GABA on the growth performance of multi-generation growing-finishing pigs were shown in Table 3. At beginning of the trial, the initial BW in the Control group was the same as that in GABA group (P > 0.05). After the trial period, no statistical difference in finial BW was observed between the pigs fed the control diet and the GABA-supplemented diet (P > 0.05). Meanwhile, there was no difference in ADG between Control and GABA groups (P = 0.89). However, the pigs fed the diet supplemented with GABA had lower ADFI and F:G than those fed the basal diet (P < 0.05). Overall, the above data suggested that dietary supplementation with 20 mg/kg GABA influenced the feed intake of growing-finishing pigs from super-multiparous sows.
Table 3.
Effects of dietary γ-aminobutyric acid (GABA) on growth performance in growing-finishing pigs.1
| Item | Groups |
SEM | P-value | |
|---|---|---|---|---|
| Control | GABA | |||
| Initial BW, kg | 72.9 | 72.9 | 1.65 | 1.00 |
| Final BW, kg | 104.0 | 104.2 | 2.11 | 0.95 |
| ADFI, kg | 2.22 | 2.05 | 0.05 | 0.03 |
| ADG, kg | 0.74 | 0.74 | 0.02 | 0.89 |
| F:G | 3.04 | 2.77 | 0.08 | 0.05 |
BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; F:G = feed-to-gain ratio.
The values represented the means of 6 replicates (n = 6).
3.2. GABA increased hepatic anti-inflammation function
To explore whether the liver was affected by dietary nutrition and systemic conditions, liver morphology was observed using H&E staining to evaluate the health (Fig. 1). The structure of the hepatic lobule was obvious and natural in both Control and GABA groups. Dietary supplementation with GABA affected the morphology of the liver. The pigs fed the diet supplemented with the GABA had less inflammatory cell infiltration between hepatocytes than those fed the basal diet.
To confirm the effect of GABA on anti-inflammation function of the liver, we tested the activities of AST and ALT in serum and liver and evaluated the expressions of inflammatory cytokines in the liver of growing-finishing pigs (Fig. 1). The results showed that GABA dramatically decreased ALT activities of both serum and liver and AST activity of serum (P < 0.05). There was a trend to decrease, but no significant difference in serum AST activity (P > 0.05). In addition, the expression of inflammatory cytokines also fully illustrated this view. Both decreased mRNA expressions of pro-inflammatory cytokines including TNFα and IL-1β and increased anti-inflammatory cytokines IL-10 were observed by dietary GABA in the liver (P < 0.05). There was no difference in the mRNA expression of IL-6 by dietary supplementation with GABA (P > 0.05). These results demonstrate that GABA alleviated liver inflammation by decreasing the expression of pro-inflammatory cytokines.
To further explore the repair effect of GABA on cell damage, the related gene expression of mitochondrial metabolism were detected. Dietary GABA dramatically increased the relative abundance of PPARγ in the liver (P < 0.01, Fig. 1J and K), and tended to affect the expression of PGC-1α (P < 0.05). Dietary GABA could significantly improve mitochondrial metabolism in the liver under the condition of liver inflammation.
3.3. GABA improved antioxidant indices of serum and liver
GABA is a well-known antioxidant and may have beneficial effects on alleviating inflammation. Therefore, we detected the activities of antioxidant enzymes and concentrations of MDA in serum and liver of growing-finishing pigs (Fig. 2) to verify the effects of GABA on antioxidant function. Pigs fed the diet supplemented with GABA significantly increased activities of SOD and GSH-Px enzymes in serum and liver (P < 0.05). CAT is an enzyme that catalyzes the breakdown of hydrogen peroxide, the activity of which was also increased in serum by dietary GABA (P < 0.05). No difference of the activity of CAT was observed in the liver (P > 0.05). The activities of the most of important enzymes that play important roles in exerting major antioxidant activities in the liver could be increased by dietary GABA. In addition, MDA is a product of intracellular lipid peroxidation, which easily causes further oxidative stress in the case of tissue damage. In this study, dietary GABA dramatically decreased MDA concentrations in serum and liver of growing-finishing pigs from super-multiparous sows (P < 0.05). Increased activities of antioxidant enzymes and decreased lipid peroxidation have suggested that supplementation with GABA could alleviate oxidative stress occurring in the body of growing-finishing pigs from super-multiparous sows, which provided sound evidence that GABA reduces inflammation.
3.4. GABA changed GABARs/TLR4/NF-κB pathway of the liver
The upregulation of NF-κB signal is closely related to liver inflammation. From the results (Fig. 3), the phosphorylation of NF-κB in the liver was significantly decreased by dietary supplementation with GABA (P < 0.001), despite that the mRNA expression of NF-κB was not affected by GABA (P > 0.05). In addition, GABA significantly decreased the mRNA and protein relative abundance of TLR4 in the liver of growing-finishing pigs (P < 0.05). The results demonstrated that GABA down-regulated TLR4/NF-κB signal to alleviate liver inflammation. In order to determine how GABA plays an anti-inflammatory and anti-oxidant function, the levels of GABA receptors (GABARs; including GABrB1, GABrB2, GABrD and GABrQ) in the liver of pigs were observed. The mRNA expressions of GABrB1, GABrB2 and GABrQ were significantly increased by dietary supplementation with GABA (P < 0.05, Fig. 3).
3.5. GABA alleviates LPS-induced inflammation of hepatocyte through acting on GABARs
To further explore the mechanism of GABA on alleviating liver inflammation, a series of experiments in vitro using HepG2 cells were conducted. From Fig. 4, we observed significantly reduced cell proliferation and increased ALT and AST activities when LPS concentration was in 1.0 μg/mL (P < 0.05). Based on the previous results, when GABA concentration was 1.0 mmol/L, GABA significantly increased cell proliferation under LPS-stimulated conditions (P < 0.05). Meanwhile, 1.0 mmol/L GABA significantly reduced LPS-induced the increase of AST and ALT activities (P < 0.04), and both the protein expression of TLR4 and the phosphorylation of NF-κB were also decreased by GABA (P < 0.05), which were consistent with the results of the in vivo study. Meanwhile, we examined the expression levels of GABARs of HepG2 cell under the treatment of LPS and GABA. From the results, LPS decreased the mRNA expression levels of GABARs (P < 0.05), which could be improved by GABA under LPS-stress, in accordance with the previous animal trial.
3.6. GABA regulates GABARs/TLR4/NF-κB signaling through receptors
To further explore if the effect of GABA on TLR4/NF-κB pathway was regulated by GABARs, GABARs inhibitor and agonist were used. When CGP52432 was used to inhibit GABARs activities, we tested the decreased mRNA expressions of GABAR (P < 0.05, Fig. 5), and increased expressions of GABARs with GABA treatment (P < 0.05). Interestingly, GABrA1 expression could be rescued by GABA in the presence of the inhibitor (P < 0.05), but there were no difference in expressions of GABrB1, GABrB2, and GABBR1 (P > 0.05). Additionally, phosphorylation of NF-κB were increased by the inhibitor (P < 0.05), but protein expression of TLR4 was not affected by the inhibitor (P > 0.05). GABA could significantly decrease the protein expression of TLR4 under CGP52432-surpressed conditions (P < 0.05), but had no effect on phosphorylation of NF-κB (P > 0.05). The expression of TLR4 was decreased by combination with GABA and the inhibitor compared with inhibitor alone (P < 0.05); but the phosphorylation of NF-κB was not affected (P > 0.05). In summary, the activation of TLR4/NF-κB signaling by the inhibitor was similar with that by LPS.
Inversely, when Campral EC, a GABAR activator treated HepG2 cell, GABAR expressions were dramatically increased as expected under LPS-stimulated condition (P < 0.05, Fig. 6). Similar results were obtained with GABA treatment (P < 0.05). Treatment with both GABA and activator at the same time did not result in a stronger effect (P > 0.05). Immunoblotting showed significantly increased expression of TLR4 and phosphorylation of NF-κB in HepG2 cell with LPS treatment (P < 0.05), which is similar with Fig. 4. After treatment with GABA, Campral EC, or GABA and Campral EC combination, both the protein expression of TLR4 and phosphorylation of NF-κB were significantly decreased under LPS-stimulated condition (P < 0.05). The decrease of TLR4/NF-κB signaling by Campral EC was not different from that of GABA, or the GABA and Campral EC combination (P > 0.05), suggesting that the GABAR activator had similar effect on inflammatory signals with GABA. Taken together, GABA decreases TLR4/NF-κB signaling by activating GABARs to alleviate hepatic inflammation.
4. Discussion
In production, when sows produce more than eight parities, the growth performance of offspring from the super-multiparous sows is dramatically decreased, and elimination and mortality begin to increase. From the results of this study, both finial BW and ADG were not affected by dietary GABA. However, GABA supplementation decreased ADFI and F:G of growing-finishing pigs produced by super-multiparous sows. Because of the neuro-regulatory properties, GABA could theoretically be involved in the digestive process of food involving the intestinal nerves. Our results showed the inconsistent impact of GABA on feed intake within previous studies. For example, dietary GABA has promoted feed intake of growing lambs (Wang et al., 2015). Additionally, dietary supplementation with 30 mg/kg GABA improved ADG but did not affect ADFI of growing-finishing pigs (Bi et al., 2020). From the previous study, the difference in animal models may be one of the reasons for the differing results; that GABA does not always regulate feed intake behavior under different environmental conditions. As Chen et al. reports, GABA supplementation had no effect on both ADFI and F:G of weaning piglets (Chen et al., 2019).
Studies on the effect of parity of the gestating sow on the growth performance of their offspring were reported, but more attention has been paid to the comparison between the offspring growth of primiparous sows than that of multiparous sows (Wu et al., 2019). Pigs are born with limited energy reserves and underdeveloped immune systems, and most of the nutrients needed for growth are derived from breast milk. The parities of gestating sows were reported to affect growth performance and the humoral immune response of their progeny (Pineiro et al., 2019). Claudio et al. (Franceschi, 2007) reported that age-related inflammation has the close relationship with the genetic effects of mitochondrial DNA variants, which was confirmed by recent study using lactating Holstein cows (Zhang et al., 2019a). In this study, we observed chronic inflammation in the liver of progeny bred by super-multiparous sows and a disorder of mitochondrial metabolism, suggesting that the parities of gestating sow could affect intracellular metabolism through the genetic effect of altering mitochondrial DNA variation, in addition to influencing phenotypic indicators such as the growth of offspring.
Liver, an important central organ of energy metabolism and detoxification, is susceptible to nutrients and systemic conditions and undergoes many remodeling processes except for energy synthesis and decomposition; such as endoplasmic reticulum stress, inflammatory responses and mitochondrial dysregulation. In the present study, no change of liver morphology was observed, but an increase of inflammatory cell infiltration in the liver of the Control group suggested that the chronic inflammatory symptoms in growing-finishing pigs produced by super-multiparous sows is likely to be an important cause of poor growth performance or even elimination. Inflammation and decreased antioxidant enzyme activity-induced oxidative stress are closely related (Ren et al., 2018). The close relationship between oxidative stress and inflammation is demonstrated (Kim et al., 2019; Zheng et al., 2019). Dietary GABA increased activities of antioxidant enzymes (SOD, CAT and GSH-Px) and decreased MDA concentrations of serum and liver. The results demonstrated that both chronic liver inflammation and increased oxidative stress could be alleviated by dietary GABA. Similarly, dietary supplementation with GABA have benefits to increase the activity of GSH-Px and decrease MDA content in serum of growing-finishing pigs undergoing transport stress (Bi et al., 2020). As the product of lipid peroxidation, MDA is considered an indicator of oxidative stress. Excess oxidative stress would result in decreased activities of antioxidant enzymes and mitochondrial injury. A decrease in MDA concentration was observed in the case of increased GABA synthesis (Dias et al., 2014). Combined with the results of ALT, AST and liver morphology, these results fully indicated that liver injury was serious in the Control group and GABA supplementation has a restorative effect that could inhibit hepatitis, play an anti-inflammatory role, and restore antioxidant capacity of growing-finishing pigs from super-multiparous sows. The change of mitochondrial metabolism and morphological results of liver visually confirmed this theory. Additionally, activation of PPARγ is related with inhibition of LPS-induced inflammation (Ma et al., 2018; He et al., 2018).
GABA has been considered to suppress inflammatory responses and immune cells, particularly through inhibiting the release of pro-inflammatory cytokines. For example, fat-derived stem cells were stimulated by GABA to inhibit secretion of inflammatory cytokines, such as TNFα (Cherng et al., 2014), thereby suppressing the inflammatory response of subcutaneous adipose in obesity (Hwang et al., 2019). Additionally, GABA suppress production of IL-1β in inflammatory macrophages through increasing the abundance of GABA transporters (Xia et al., 2021). Increased expression of pro-inflammatory IL-17 in hepatocytes and macrophages promotes hepatocellular carcinoma (Ma et al., 2020; Zhang et al., 2021). In a previous study, we observed obvious liver inflammation and increased serum LPS concentration (unpublished). Therefore, we further explored LPS-induced liver inflammation models in vitro, which generated results consistent with those results in pigs. TLR4 protein, as a single target of LPS, plays a role in promoting inflammatory process by regulating downstream protein NF-κB. Also, pro-inflammatory cytokines stimulate an NF-κB-dependent signaling cascade that contributes to establishing an inflammatory milieu. Our study found that GABA suppressed hepatic inflammatory cytokines expression and inhibited the expression of TLR4/NF-κB pathway at the same time. Generally, the activation of TLR4/NF-κB signaling occurs in the injury tissues and causes the release of inflammatory cytokines. Rats with TLR4 knockout were reported to reduce the abundance of inflammatory cytokines in the spinal cord (Tanga et al., 2005). The recent study reported that the involvement of NF-κB pathway participates in attenuating acute intestinal injury which was accompanied by the high levels of inflammatory cytokines in weaned piglets (Huang et al., 2019) and cells (Lai et al., 2017; Mangali et al., 2019). Furthermore, in lactating ruminants, GABA inhibited the abundance of inflammatory cytokines and TLR4/NF-κB signaling induced by LPS (Wang et al., 2018). Herein, the activation of GABARs by GABA could suppress the expression of TLR4/NF-κB pathway, suggesting a blocking effect of GABA on the combination of LPS and TLR4, or an inhibitory effect on the activation of TLR4. The liver is a huge immune organ; macrophages of which make up 80 to 90% of the macrophages in the whole body (Li et al., 2017). In the liver, an association between activation and NF-κB signaling and injury or death of hepatocyte stimulated by TNFα has been described (Zhang et al., 2019b, 2021; de Gregorio et al., 2020). In addition, the involvement of NF-κB signaling is well participated in the activation and survival of hepatic stellate cells to promote the induction and secretion of inflammatory mediators (He et al., 2019; de Gregorio et al., 2020). The mechanism of GABAergic system on TLR4/NF-κB pathway still needs to be further studied.
Consistently, reduced GABA synthesis is likely to be related with the condition of TLR4 activation. Yan et al. reported that TLR4 activation induced the release of IL-1β from microglia by decreasing GABAergic synaptic activities, thereby inhibiting GABA synthesis (Yan et al., 2015). Moreover, IL-1β has been shown to reduce glutamate supply in the glutamate–glutamate cycle by inhibiting glial glutamate transporter expression (Yan and Weng, 2013). Generally, GABA can be synthesized in the body through the glutamate–glutamine cycle to maintain relative GABA homeostasis. Gabapentin, another one synthetic analogue of GABA, has been reported to reverse systemic acute inflammatory response and oxidative stress in mice (Dias et al., 2014). These reports suggest the close relationship between the inflammatory response and decreased GABA concentration in vivo. However, the mechanisms of inhibition of GABAergic synaptic activities induced by inflammatory cytokines is still unclear.
Although studies on GABA in immune function are in the early stages, its immune-regulatory effects seem to be triggered via GABARs. As an inhibitory neurotransmitter, GABA has been reported to modulate the differentiation and proliferation of neuronal cells by activating the GABARs (Li et al., 2012). Therefore, it is speculated that GABA is likely to block the release of pro-inflammatory cytokines through GABARs seizing. Our results revealed a negative relationship between GABARs and inflammatory response. When HepG2 cell exposed to LPS, cells had altered the expression of TLR4/NF-κB pathway, and GABA alleviated LPS-induced hepatic inflammation probably by acting on GABARs in return. To explore if the effect of GABA on TLR4/NF-κB pathway was regulated by GABARs, we carried out experiments where cells were treated by an inhibitor or activator combined with GABA. Combined GABA and GABARs inhibitor application is likely to reduce the expression of TLR4 to the levels seen for GABA treatment alone. In turn, the agonist alone or combined with GABA could simultaneously reduce TLR4/NF-κB signaling to the levels seen for GABA treatment alone under LPS-pressure. Therefore, GABA may block LPS-TLR4 binding by activating GABARs, thus affecting downstream inflammatory processes, which is consistent with previous reports (Liu et al., 2018, 2019; Seifi et al., 2018). Overall, our results revealed that GABA decreases TLR4/NF-κB signaling by activating GABARs to alleviate LPS-induced hepatic inflammation; thus, affecting the phosphorylation of downstream NF-κB and reducing liver inflammation.
5. Conclusions
Our study revealed that chronic inflammation exists in growing-finishing pigs generated by super-multiparous sows, and dietary supplemental GABA promoted antioxidant function and alleviated liver inflammation. Moreover, GABA decreases TLR4/NF-κB signaling by acting on GABARs. Importantly, dietary supplementation with 20 mg/kg GABA could alleviate the inflammatory state and promote feed conversion in the case of growing-finishing pigs from super-multiparous sows. These findings help us to better understand the mechanism of GABA alleviating liver inflammation and contribute to the use of GABA in commercial pig diets.
Author contributions
Shumin Zhang: Conceptualization, Methodology, Software, Data curation, Writing-Original draft preparation. Jinbiao Zhao: Conceptualization, Resources. Jinhua Hu: Methodology, Investigation. Hengxun He: Data Curation, Investigation. Yihan Wei: Investigation. Linbao Ji: Methodology. Xi Ma: Conceptualization, Writing-Review& Editing, Project administration, Funding acquisition.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was founded by the National Natural Science Foundation of China (31930106, 31829004 and 31722054), the 2115 Talent Development Program of China Agricultural University (1041-00109019), the China National Postdoctoral Program for Innovation Talents (BX20200365), China Postdoctoral Science Foundation (2020M680771), the National Ten-thousand Talents Program of China (23070201), and the project of Academician Workstation in Chengdejiuyun Agricultural and livestock Co., Ltd (199A7310H).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Bi C., Yin J., Yang W., Shi B., Shan A. Effects of dietary gamma-aminobutyric acid supplementation on antioxidant status, blood hormones and meat quality in growing-finishing pigs undergoing transport stress. J Anim Physiol Anim Nutr (Berl) 2020;104(2):590–596. doi: 10.1111/jpn.13280. [DOI] [PubMed] [Google Scholar]
- Chen S., Tan B., Xia Y., Liao S., Wang M., Yin J., et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct. 2019;10(1):366–378. doi: 10.1039/c8fo02161a. [DOI] [PubMed] [Google Scholar]
- Cherng S.H., Huang C.Y., Kuo W.W., Lai S.E., Tseng C.Y., Lin Y.M., et al. GABA tea prevents cardiac fibrosis by attenuating TNF-alpha and Fas/FasL-mediated apoptosis in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2014;65:90–96. doi: 10.1016/j.fct.2013.12.022. [DOI] [PubMed] [Google Scholar]
- de Gregorio E., Colell A., Morales A., Mari M. Relevance of SIRT1-NF-kappaB axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci. 2020;21(11) doi: 10.3390/ijms21113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J.M., de Brito T.V., de Aguiar Magalhaes D., da Silva Santos P.W., Batista J.A., do Nascimento Dias E.G., et al. Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation. 2014;37(5):1826–1836. doi: 10.1007/s10753-014-9913-2. [DOI] [PubMed] [Google Scholar]
- Feng A.L., Xiang Y.Y., Gui L., Kaltsidis G., Feng Q., Lu W.Y. Paracrine GABA and insulin regulate pancreatic alpha cell proliferation in a mouse model of type 1 diabetes. Diabetologia. 2017;60(6):1033–1042. doi: 10.1007/s00125-017-4239-x. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65(12 Pt 2):S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- He J., Gerstenlauer M., Chan L.K., Leithauser F., Yeh M.M., Wirth T., et al. Block of NF-kB signaling accelerates MYC-driven hepatocellular carcinogenesis and modifies the tumor phenotype towards combined hepatocellular cholangiocarcinoma. Cancer Lett. 2019;458:113–122. doi: 10.1016/j.canlet.2019.05.023. [DOI] [PubMed] [Google Scholar]
- He T., He L., Gao E., Hu J., Zang J., Wang C., et al. Fat deposition deficiency is critical for the high mortality of pre-weanling newborn piglets. J Anim Sci Biotechnol. 2018;9:66. doi: 10.1186/s40104-018-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., He X., Jiao N., Zhang X., Qiu K., et al. The involvement of NF-kappaB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets. Br J Nutr. 2019;122(2):152–161. doi: 10.1017/S0007114519000928. [DOI] [PubMed] [Google Scholar]
- Hwang I., Jo K., Shin K.C., Kim J.I., Ji Y., Park Y.J., et al. GABA-stimulated adipose-derived stem cells suppress subcutaneous adipose inflammation in obesity. Proc Natl Acad Sci U S A. 2019;116(24):11936–11945. doi: 10.1073/pnas.1822067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Feng D., Guillot A., Dai S., Liu F., Hwang S., et al. Adipocyte death preferentially induces liver injury and inflammation through the activation of chemokine (C-C Motif) receptor 2-positive macrophages and lipolysis. Hepatology. 2019;69(5):1965–1982. doi: 10.1002/hep.30525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 2017;40(1):1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- Li P., He K., Li J., Liu Z., Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222–229. doi: 10.1016/j.molimm.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Liu Y., Li Y.D., Liu Y.H., Li F., Ju Q., et al. GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World J Gastroenterol. 2012;18(21):2704–2711. doi: 10.3748/wjg.v18.i21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Zhang Y.Y., Song N., Lin J., Liu M.K., Huang C.L., et al. GABAB receptor activation attenuates inflammatory orofacial pain by modulating interleukin-1beta in satellite glial cells: role of NF-kappaB and MAPK signaling pathways. Brain Res Bull. 2019;149:240–250. doi: 10.1016/j.brainresbull.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Liu P., Yuan H.B., Zhao S., Liu F.F., Jiang Y.Q., Guo Y.X., et al. Activation of GABAB receptor suppresses diabetic neuropathic pain through toll-like receptor 4 signaling pathway in the spinal dorsal horn. Mediators Inflamm. 2018;2018:6016272. doi: 10.1155/2018/6016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma H.Y., Yamamoto G., Xu J., Liu X., Karin D., Kim J.Y., et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J Hepatol. 2020;72(5):946–959. doi: 10.1016/j.jhep.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Guo P., Zhang J., He T., Kim S.W., Zhang G., Ma X. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front Immunol. 2018;9:5. doi: 10.3389/fimmu.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangali S., Bhat A., Udumula M.P., Dhar I., Sriram D., Dhar A. Inhibition of protein kinase R protects against palmitic acid-induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J Cell Biochem. 2019;120(3):3651–3663. doi: 10.1002/jcb.27643. [DOI] [PubMed] [Google Scholar]
- Pineiro C., Manso A., Manzanilla E.G., Morales J. Influence of sows' parity on performance and humoral immune response of the offspring. Porcine Health Manag. 2019;5:1. doi: 10.1186/s40813-018-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Liu W., Song X., Qi Y., Zhang C., Gao Z., et al. Antioxidant and anti-inflammation of enzymatic-hydrolysis residue polysaccharides by Lentinula edodes. Int J Biol Macromol. 2018;120(Pt A):811–822. doi: 10.1016/j.ijbiomac.2018.08.114. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi M., Rodaway S., Rudolph U., Swinny J.D. GABAA receptor subtypes regulate stress-induced colon inflammation in mice. Gastroenterology. 2018;155(3):852–864 e853. doi: 10.1053/j.gastro.2018.05.033. [DOI] [PubMed] [Google Scholar]
- Tanga F.Y., Nutile-McMenemy N., DeLeo J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.M., Chacher B., Liu H.Y., Wang J.K., Lin J., Liu J.X. Effects of gamma-aminobutyric acid on feed intake, growth performance and expression of related genes in growing lambs. Animal. 2015;9(3):445–448. doi: 10.1017/S1751731114002651. [DOI] [PubMed] [Google Scholar]
- Wang D.M., Wang C., Liu H.Y., Liu J.X., Ferguson J.D. Effects of rumen-protected gamma-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. J Dairy Sci. 2013;96(5):3222–3227. doi: 10.3168/jds.2012-6285. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Sun S.P., Zhu H.S., Jiao X.Q., Zhong K., Guo Y.J., et al. GABA regulates the proliferation and apoptosis of MAC-T cells through the LPS-induced TLR4 signaling pathway. Res Vet Sci. 2018;118:395–402. doi: 10.1016/j.rvsc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Wu Y., Ma N., Song P., He T., Levesque C., Bai Y., et al. Grape seed proanthocyanidin affects lipid metabolism via changing gut microflora and enhancing propionate production in weaned pigs. J Nutr. 2019;149(9):1523–1532. doi: 10.1093/jn/nxz102. [DOI] [PubMed] [Google Scholar]
- Xia Y., He F., Wu X., Tan B., Chen S., Liao Y., et al. GABA transporter sustains IL-1beta production in macrophages. Sci Adv. 2021;7(15) doi: 10.1126/sciadv.abe9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Jiang E., Weng H.R. Activation of toll like receptor 4 attenuates GABA synthesis and postsynaptic GABA receptor activities in the spinal dorsal horn via releasing interleukin-1 beta. J Neuroinflammation. 2015;12:222. doi: 10.1186/s12974-014-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Weng H.R. Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J Biol Chem. 2013;288(42):30544–30557. doi: 10.1074/jbc.M113.495465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Wang Y., Luo H., Qiu W., Zhang H., Hu L., et al. The association between inflammaging and age-related changes in the ruminal and fecal microbiota among lactating holstein cows. Front Microbiol. 2019;10:1803. doi: 10.3389/fmicb.2019.01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zou X.T., Li H., Dong X.Y., Zhao W. Effect of dietary gamma-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim Sci J. 2012;83(2):141–147. doi: 10.1111/j.1740-0929.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhao J., Xie F., He H., Johnston L.J., Dai X., et al. Dietary fiber-derived short-chain fatty acids: a potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes Rev. 2021 doi: 10.1111/obr.13316. [DOI] [PubMed] [Google Scholar]
- Zhang T., Hu J., Wang X., Zhao X., Li Z., Niu J., et al. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-kappaB-TNFalpha pathway. J Hepatol. 2019;70(1):87–96. doi: 10.1016/j.jhep.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Qu H., Xiong X., Wang Y., Liu X., Zhang L., et al. Deficiency of mitochondrial glycerol 3-phosphate dehydrogenase contributes to hepatic steatosis. Hepatology. 2019;70(1):84–97. doi: 10.1002/hep.30507. [DOI] [PMC free article] [PubMed] [Google Scholar]