Abstract
The number of available biological therapies have doubled over the last 10 years and the arrival of novel molecules (interleukin 23p19 inhibitors) is ongoing alongside the development of small molecules. As a result of this vast landscape of treatment, positioning advanced therapies (according to clinical situation, efficacy and safety) is of paramount importance to providing personalized, appropriate IBD treatment.
In this publication the recent available literature is summarized for practical integration into clinical practice including comparative efficacy data, patient and disease demographics. We refer to recent publications and expert opinion in order to facilitate the decision making process of positioning biologicals IBD treatment.
Keywords: Biological therapy, Monoclonal antibodies, Infliximab, Adalimumab, Vedolizumab, Ustekinumab
Graphical abstract
Abbreviations
- CD
Crohn's disease
- IBD
inflammatory bowel disease
- OR
odd ratio
- TNF
Tumor Necrosis Factor alpha
- UC
ulcerative colitis
1. Introduction
Biological therapies have revolutionized the management of inflammatory bowel diseases (IBD). Monoclonal antibodies against tumor necrosis factor alpha (anti-TNF) have been the cornerstone of IBD therapy since the start of the century. In 1998, infliximab was the first biological medication approved for the use of Crohn's disease (CD), followed by adalimumab. Numerous studies have demonstrated their efficacy and cost effectiveness for the induction and maintenance of remission in CD (Townsend et al., 2020) and ulcerative colitis (UC) (Colombel et al., 2007; Pantavou et al., 2019). Golimumab for UC (Sandborn et al., 2014) and Certolizumab pegol for CD (Schreiber et al., 2010; Yamazaki et al., 2019) are other anti-TNF inhibitors that are currently available. After over 20 years of experience of prescribing anti-TNF agents, their safety profile has been well described and dose optimization has been incorporated into daily clinical practice although proactive monitoring remains a question of debate (Argollo et al., 2020).
More recently, two additional biologicals, with different mechanisms of action, have complemented the IBD armamentarium. The first is vedolizumab, a humanized monoclonal antibody to the homing receptor α4β7 integrin complex, blocking the interaction of the surface homing molecules of activated immune cells with the endothelium to reduce diapedesis. The second is ustekinumab, a humanized monoclonal antibody to the interleukin (IL) p40 subunit common to IL12/23. These new agents have now proved their efficacy not only against placebo but also in head-to-head trials against anti-TNF agents in specific indications (Sands et al., 2019, 2021; Irving et al., 2021). Consequently, they possess a comfortable market position compared to the historical anti-TNF agents, when not surpassing them for certain targeted groups of patients. However, striking efficacy differences have not been shown to support their superiority and anti-TNF agents remain the most appropriate medications in presence of most concomitant extraintestinal manifestations or in fistulizing CD (Juillerat et al., 2020; Papamichael et al., 2021; Singh et al., 2021). Additionally, other biologicals such as etrolizumab (Danese et al., 2021; Peyrin-Biroulet et al., 2021; Rubin et al., 2021) (monoclonal antibody against the B7 subunit of integrins α4β7 and αΕβ7) as well as guselkumab, mirikizumab and risankizumab, other monoclonal antibodies which binds to p19 subunit of interleukin 23 (Sandborn et al., 2020; Schett et al., 2021) are emerging in the market.
As treatment choices expand with the introduction of newer biologicals with proven efficacy against placebo decision making pathways become necessary in order to differentiate between the biologicals. The aim of this publication is to provide an evidence based guidance which aids the decision making process in positioning biologicals according to the efficacy, patient characteristics, safety profile and specific indications. The positioning of the novel small molecules in the treatment of IBD is beyond the scope of this article.
2. Comparative efficacy of available biological agents
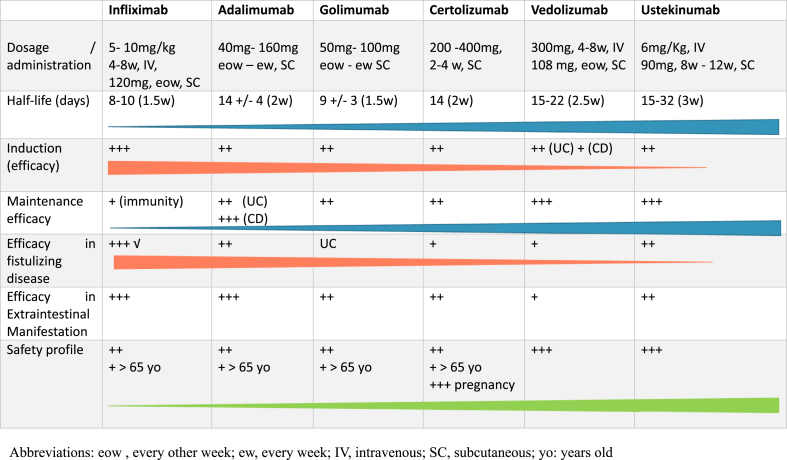
The biological agents available for IBD treatment and their individual characteristics are summarized in Table 1.
Table 1.
Characteristics of the available biologics.
2.1. Head to head trials
Direct comparison of drugs are uncommon in the IBD literature. Randomized, double blinded, double dummy, head to head comparative trials in IBD provide evidence comparing two drugs and overcome the problem of bias that is often present in observational studies. In the IBD field, the first head to head published was the VARSITY trial (Sands et al., 2019). This trial, conducted over 1 year, compared 383 UC patients treated with vedolizumab to 386 UC patients treated with adalimumab. The primary outcome was reached. At week 52 the clinical remission rate (31.3% vs. 22.5%; P = 0.006) and endoscopic improvement (39.7% vs. 27.7%; P < 0.001) was significantly higher with vedolizumab compared to adalimumab. There were no striking safety differences between the two drugs over the 1-year period.
The first head to head in Crohn's disease was recently presented at Digestive Disease Week (DDW) 2021(Sands et al., 2021) and European Crohn's and Colitis Organization (ECCO) congress 2021(Irving et al., 2021). The SEAVUE study compared ustekinumab with adalimumab in 386 bio-naïve patients with moderate to severe CD over 52 weeks. Participants were required to discontinue immunomodulators prior to initiation. This study showed similar efficacy between the 2 groups at week 52: 65% in the ustekinumab group (n124/N191) vs. 61% in the adalimumab (n119/N195), p = 0.47. Interestingly, the rapidity of response was also similar between the two groups. The subanalyses reported a trend toward more safety, less use of concomitant steroids and a slightly better endoscopic response (particularly in patients with higher endoscopic scores at baseline) in the ustekinumab group.
Additional head to head trials have been conducted in the etrolizumab study program (GARDENIA vs. infliximab (Danese et al., 2021) and HIBISCUS vs. adalimumab (Rubin et al., 2021)) and Guselkumab phase II study (GALAXY vs. Ustekinumab (Sandborn et al., 2022)) which were presented at the United European Gastroenterology (UEG) conference in 2020. These studies didn't show significant differences in the primary endpoints. More head to head trials are certainly under way and will help us better position biological agents and small molecules in the treatment algorithm (Pouillon et al., 2020). Of note, the result of the first positive head to head study (VARSITY) had been foreseen by prior indirect comparison information from network meta-analyses.
2.2. Network meta-analysis
When no head to head trials are available, network meta-analysis are used to compare efficacy of biologics agents against placebo.
One of the first meta-analyses, published in 2015, focused on the efficacy of first line biological therapy in UC (Danese et al., 2014). The authors suggested that in naïve UC patients infliximab and vedolizumab are more effective in inducing clinical response (Odd ratios (OR) 5.33 and 4.51, resp.) and maintaining remission (OR 2.78 and 5.19 resp.) compared to other regimens. These observations were confirmed in the most recent meta-analysis from the same group (Bonovas et al., 2018) and another analysis that included ustekinumab (Singh et al., 2018a). This latter meta-analysis reported some benefit of ustekinumab over adalimumab or vedolizumab after anti-TNF exposure in UC patients. Among anti-TNF agents, infliximab remains the most efficient agent in UC (Thorlund et al., 2015), but not in terms of cost effectiveness (Toor et al., 2015).
In CD, a meta-analysis of 9 randomized trials with mixed first-line and second line indications confirmed the superiority of infliximab alone for induction (OR 2.8) or in combination with azathioprine for maintenance of remission (OR 3.0) (Hazlewood et al., 2015). A recent pooled analysis of 4 clinical trial programs (EXTEND, UNITY, VERSIFY, CPT-13 BIOSIMILAR) was presented at ECCO congress 2022 and confirmed that infliximab achieved a higher proportion of 1-year endoscopic healing compared to adalimumab, ustekinumab and vedolizumab in CD patients, but not in bio-naïve patients (Narula et al., 2022).
As second line therapy, it is also important to consider that the efficacy of vedolizumab will be influenced by previous anti-TNF exposure, a numerical difference was seen in the response to therapy in UC (Feagan et al., 2017) and CD patients (Sands et al., 2017). An impact which was clearly significant at week 6 after induction (Sands et al., 2014).
In summary, the most recent meta-analysis in CD which included ustekinumab concluded that infliximab or adalimumab are the best first-line agents, and ustekinumab a preferred second-line agent in patients with prior anti-TNF alpha agents’ exposure (Singh et al., 2018b).
2.3. Real life propensity score weighted data
Another way to compare efficacy between drugs is a retrospective analysis of prospective data from epidemiological cohort studies using the best adjustment of confounders. A good example of this a the Mayo Clinic study using propensity score matching to evaluate the efficacy of anti-TNF agents in >3000 biologic-naïve CD patients, extracted from a national administrative claims database (Singh et al., 2016). The authors demonstrated that infliximab was associated with less hospitalization, abdominal surgery and corticosteroid use compared to adalimumab and certolizumab.
Another recent propensity score matched post hoc analysis of clinical trial programs compared infliximab with the newcomer ustekinumab (Narula et al., 2021a). This suggested a similar efficacy of the two compounds as first line biological agents in CD patients. The same analysis was conducted in naïve UC patients, which showed that infliximab and had similar efficacy based on clinical response, but a significant higher rate of steroid free clinical remission (30% vs. 15%) and endoscopic remission (36% vs. 26%) was achieved in infliximab treated patients (Narula et al., 2021b). The Sicilian Network for IBD reported similar efficacy for adalimumab and vedolizumab in CD patients in a propensity score matched analysis of a cohort conducted between 2016 and 2019 (Macaluso et al., 2021), but superiority of vedolizumab in UC patients (Macaluso et al., 2020). Finally, a propensity score matched analysis of a cohort conducted in Germany (the VEDO IBD study from Kompetenznetz Darmerkrankungen) has been presented at the recent ECCO congress 2022 comparing vedolizumab and anti-TNF agents in UC patients. This work confirmed a more persistent clinical response (61.7% vs. 40.3%; OR 2.39 (95% CI 1.39–4.10)) and steroid-free remission (36.5% vs. 24.0%; OR 1.82 (95% CI 1.00–3.34)) at one year under vedolizumab (Plachta-Danielzik et al., 2022).
2.4. Available data on treatment sequences in algorithms and guidelines
Data from the literature on strategies to position biological therapy is scarce. Guidance and consensus statements exist to help aid the decision making process. However there is a tendency to position all biologics on the same level and to consider them as interchangeable in the ECCO Guidelines (Torres et al., 2020; Raine et al., 2021). These guidelines, used the novel Grade methodology which prioritizes meta-analyses (or perform their own) to extract the necessary data to answer specific (so call PICO) questions on IBD management (e.g. for anti-TNF agents in UC) (Raine et al., 2021)).
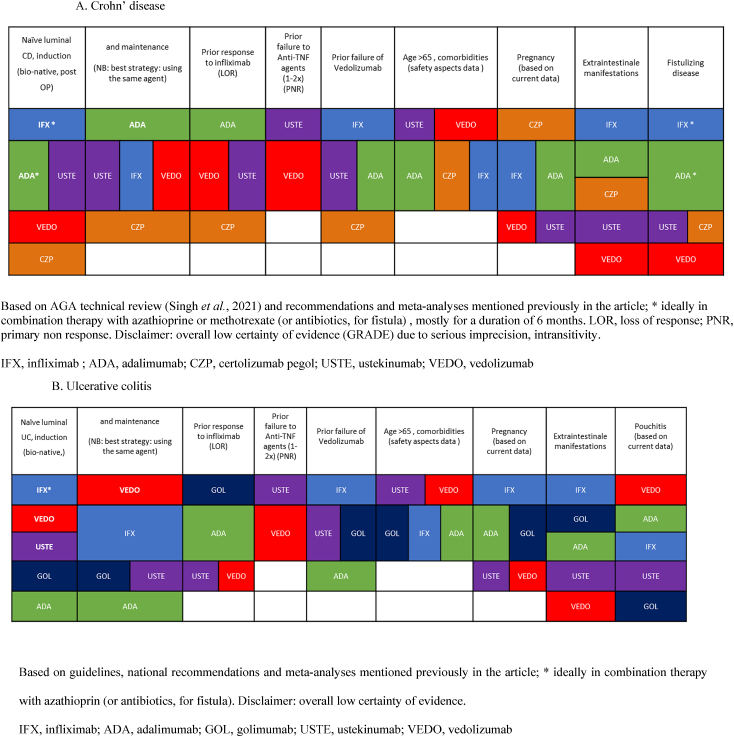
In the most recent publication from the American Gastroenterology Association: “Technical Review, the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease”, the question of comparing the efficacy between all available biologics is addressed (Singh et al., 2021). The conclusion was that in biologic-naïve patients with moderate to severely active CD, infliximab, adalimumab, and ustekinumab are the most effective compared to vedolizumab and certolizumab pegol. Infliximab was considered the strongest for induction. However, in patients exposed to anti-TNF agents (mostly to infliximab), the benefit of one biological agent over the other remains uncertain. A summary of the efficacies of the various biological agents, according to the line of treatment and previous treatment failures and patients' phenotype, is provided in Table 2. The final word about the efficacy of biologics in IBD and the theoretical “therapeutic ceiling” which cannot be broken (Alsoud et al., 2021) could potentially come from combining these agents such as demonstrated by the recent VEGA study. This 3-arm trial presented as one of the highlights of the recent ECCO congress 2022, compared golimumab (GOL), guselkumab (GUS) and their combined use during induction period of 12 weeks. This showed a significant higher rate of clinical response (83% for combination therapy vs. 75% for GUS (p = 0.215) and 61% for GOL (p = 0.003)) and clinical remission (47% for combination therapy vs. 24% for GUS (p = 0.005) and 25% for GOL (p = 0.007) based on modified Mayo score) without added risks for safety in moderate to severely active UC patients naïve to biologics (Sands et al., 2022).
Table 2.
Efficacy of biological treatments according to the line of treatment, earlier exposure, disease phenotype and patient characteristics.
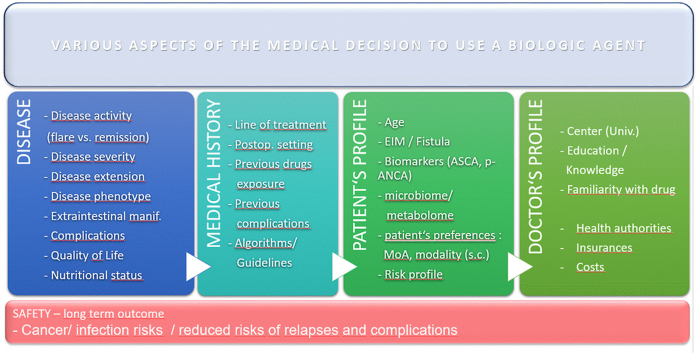
3. Personalizing choice of biologic
3.1. Prior biologic exposure
In current clinical practice we are confronted with numerous options of treatment sequencing. Personalizing IBD treatment depends on previous drug exposure IBD (1st, 2nd, 3rd line of treatment), prior treatment response, comorbidities, safety profile, patient preferences and patient risk for future IBD complications (disease severity). For example, primary non response to an anti-TNF was found to be a predictor for diminished treatment efficacy with an anti-integrin biologic (Singh et al., 2018c). First and second line agent treatment sequences and treatment response have been presented in the previous chapter. However, data are even scarcer concerning third line of treatment after two anti-TNFs exposure. A retrospective small series of 67 patients with Crohn's disease treated with 3rd line anti-TNF agent (Allez et al., 2010) reported a lower response rate and the lack of late responders at 6 months. A recent multicenter retrospective study reported the outcome of patients with late CD who had failed treatment with one anti-TNF agent and either vedolizumab or ustekinumab. After 48 weeks on a third line of biologic therapy the remission rate was 30.7% whereas the surgery rate was 23.5% (Kassouri et al., 2020).
There is a mechanistic role for anti-IL 23 agents in TNF refractory IBD patients. During anti TNF treatment, in non-responders compared to responders, there is upregulation of apoptosis resistant IL23p19, IL23R, and IL17 A as demonstrated by studies using immunophenotyping of T cells. (Bek et al., 2016; Schmitt et al., 2019). This phenomenon suggests that ustekinumab (as IL-12/IL-23 inhibitor) and other IL-23 inhibitor agents may have a mechanistic potential in late CD (Eftychi et al., 2019) when an expansion of Th1 and Th17 cells (Veny et al., 2010) may have occurred.
3.2. Disease characteristics and patient profile
3.2.1. Comorbidities, age, gender and body mass index and muscle mass
The decision on drug selection in clinical practice is often guided by disease severity that requires efficacious treatment with rapid onset of action, an acceptable safety profile balanced against potential adverse effects, in particular when the decision results in the use of high dose of corticosteroids (Ford et al., 2011) (Graphical Abstract). When the situation is less acute, depending patient comorbidities, the safety profile of the biologic is one of the major factor influencing the decision. For example the risk of specific biologic adverse events, such as infections or lymphomas is higher in the elderly population (Sturm et al., 2017; Hruz et al., 2020). This is also a key point in the management of frail or malnourished patients (Kochar et al., 2020). A pooled analysis of data from four randomized trials including 2257 UC patients reported that older patients have an increased baseline risk of adverse effects not related to anti-TNF therapy. In older IBD patients, a protective effect against the development of severe adverse events have been demonstrated in UC (OR 0.54 (0.35–0.83, p < 0.01) but not CD (OR 1.3 (0.8–1.20)) when administering vedolizumab compared to anti-TNF agents (Cheng et al., 2021). Earlier publications, also suggested a very good safety of anti-TNF agents in older populations (Bonovas et al., 2016). At this stage, we can thus consider all biological agents, when indicated, as safe in all populations.
In some large clinical trials, initiation of biologic therapy at a younger age was associated with better response, for example infliximab in both CD and UC patients (Vermeire et al., 2002; Billiet et al., 2015). However, other studies, (particularly in long term follow-up of patients cohorts), did not find such an association as well as no differences in response according to gender (Ferrante et al., 2008; Sprakes et al., 2012; Arias et al., 2015; Iborra et al., 2017). Although much fewer studies are available, remission rates observed with vedolizumab in elderly and pediatric population appear to match those reported in the general adult population (Asscher et al., 2020; Ibraheim et al., 2020). The same observations were also made for ustekinumab (Asscher et al., 2020; Conrad and Kelsen, 2020; Gisbert and Chaparro, 2020).
Obesity may contribute to the development and to the course of IBD through altered pharmacokinetics and obesity-mediated chronic inflammation (Singh et al., 2020). However, it remains unclear whether obesity influences response to biological therapies. On the one hand, a recent meta-analysis that investigated the outcome of anti-TNF therapy in IBD patients stratified by BMI, Dai et al. observed a higher rate of treatment failure in UC but not in CD patients with higher BMI (Dai et al., 2020). On the other hand, other reports failed to find an association between BMI and treatment response in IBD (Singh et al., 2018d). At this stage, there is little data on the impact of BMI on the response to vedolizumab or ustekinumab in IBD, but response to the second was lower in a study of obese patients with psoriasis (Pirro et al., 2021).
Sarcopenia, often associated with malnutrition, has an important impact in IBD patients on quality of life, prognosis, outcome of surgical interventions and treatment with biologics and immunomodulators (Ryan et al., 2019). Only two studies investigated the direct effect of biological agents on sarcopenia: the first reported an improvement of quadriceps muscle volume and strength in CD patients after 25 weeks of infliximab treatment (Subramaniam et al., 2015), whereas Csontos et al. observed that UC and CD patients on anti-TNF agents had an improvement in fat free mass index after 12 week already (Csontos et al., 2016).
3.2.2. Disease duration, phenotype, genetics, biomarkers, and extraintestinal manifestations
Short duration of disease has long been associated with better response to infliximab as well as other anti-TNF agents. This was reported in the first pediatric randomized trial by Hyams et al., which yielded better response and remission rates than observed in adults with longer duration of disease. This observation later confirmed in others pediatric studies (Hyams et al., 2007; Walters et al., 2014). Similarly, registration trials with adalimumab and certolizumab pegol, which stratified according to disease duration showed better responses in patients with shorter disease duration (Colombel et al., 2007; Schreiber et al., 2010). A large meta-analysis of 6′168 CD patients, confirmed and extended this observation for other biological therapies, including golimumab and vedolizumab (Ben-Horin et al., 2021). However, the same analyses, failed to show an association between disease duration and response in 3′227 UC patients. Regarding vedolizumab, a retrospective analysis of the Swiss IBD cohort showed an impact of disease duration on response in UC but not in CD patients (Mader et al., 2021). Very limited data are available regarding ustekinumab in early CD. In a retrospective pediatric cohort, treated with ustekinumab, the rate of remission was 61.1% in patients despite prior anti-TNF failure, suggesting a high potential with this agent in early disease (Kim et al., 2021).
Differentiation can also be made on the extension and location of disease which may have a direct impact on the response to biological treatment (Atreya and Siegmund, 2021). In a recent genetic association study, Cleynen et al. determined that the separation of IBD between ileal CD, colonic CD and UC could be considered as a continuum of phenotypes, to which specific risk score could be applied (Cleynen et al., 2016). Concerning disease behavior, better results were obtained in CD with anti-TNF agents in patients with non-stricturing, non-penetrating disease (B1 according to the Montréal classification), as compared to stricturing disease (B2) or fistulizing disease (B3) (Moran et al., 2014; Bouhnik et al., 2018; Nunez-Gomez et al., 2018). In UC, only limited data are available to guide therapy of isolated proctitis with biological agents. In a national retrospective study in France, half of 104 patients with ulcerative proctitis achieved remission with various anti-TNF therapies and 60% achieved mucosal healing (Pineton de Chambrun et al., 2020). In a large retrospective single center cohort, clinical response to biological therapy (mostly infliximab) was obtained in 70% of 118 5-ASA refractory ulcerative proctitis patients, as compared to 11% with azathioprine (Dubois et al., 2020).
Concerning pouch patients, a recent large randomized, double-blind placebo-controlled study presented at ECCO congress, the EARNEST trial, was the first significant benefits across multiple treatment outcomes in patients with chronic pouchitis after IPAA for UC (Travis et al., 2022). Till then our best options for pouchitis refractory to antibiotics were mostly anti-TNF agents but without very high level of evidence (Kayal and Dubinsky, 2022): only one randomized controlled trial with adalimumab (Kjær et al., 2019).
Recent studies have linked the risk of antibody development against biologic therapies, in particular anti-TNF agents, to the HLA profile of patients (Sazonovs et al., 2020, 2021). The authors associated the carriage of the HLA-DQA1∗05 allele, common to 40% of Europeans, with a higher rate of immunogenicity to infliximab and adalimumab. Although details of the evidence has been discussed, the same association was found in the ABIRISK consortium in autoimmune diseases (Hassler et al., 2020). Whether information on HLA allele carriage can be used prospectively to identify patients at risk of developing antibodies for a specific prospective intervention remains to be seen.
Although the serological biomarkers ASCA and pANCA have long been used to help classify indeterminate colitis, their value in guiding medical therapy is still limited. A recent study associated presence of ASCA with extensive and severe disease phenotype and the need for an early use of biologics for a better prognosis in a cohort of 273 CD pediatric patients (Chandrakumar et al., 2019), whereas a meta-analysis found that pANCA positive IBD patients had a lower response rate to infliximab therapy (Nguyen et al., 2015).
Extraintestinal manifestations occur in half of IBD patients and represent an important cause of morbidity and disability (Vavricka et al., 2011; Juillerat et al., 2020). Among EIMs, articular manifestations affect 30% of patients. They include non-inflammatory joint pain as well as inflammatory joint manifestations including axial arthritis considered as spondyloarthropathies. Since their approval in IBD, infliximab and later the other anti-TNF agents represent the best treatment choice in IBD patient with rheumatological manifestations, supported by multiple trials in both CD and UC, in addition to their well-established efficacies in most rheumatological indications (Herfarth et al., 2002; Generini et al., 2004; Lofberg et al., 2012; Louis et al., 2018).
Vedolizumab may not represent the most appropriate biologic in presence of articular manifestations of IBD. Indeed, by virtue of its binding to α4β7 integrin, this antibody may not impede the entry of proinflammatory cells in joints. In fact, it may even contribute to higher influx of these cells to distant sites, as suggested by Diaz et al., who observed de novo extraintestinal manifestations in vedolizumab-treated IBD patients (Diaz et al., 2020). An increased incidence of extraintestinal manifestation in vedolizumab treated IBD patients have also been observed, compared to anti-TNF agents in a large American claims database (Dubinsky et al., 2018). However, by improving disease activity these drugs could still have an indirect impact on associated EIM, mostly peripheral arthritis, as suggested by the recent EMOTIVE retrospective analysis (Kopylov et al., 2021). Ustekinumab, however, has also limited role in this indication, as this drug is also indicated in rheumatoid arthritis (Kerschbaumer et al., 2020). Dermatological extraintestinal manifestations occur in up to 15% of IBD patients (Vavricka et al., 2011). The most common, erythema nodosum, depends on the underlying disease activity, whereas pyoderma gangrenosum may be associated with active or inactive intestinal disease and requires rapid management, most commonly with systemic medication. Treatment includes oral corticosteroids, cyclosporine, tacrolimus and anti-TNF therapy (infliximab or adalimumab), drugs that have good efficacy in several cases reports and small case series (Juillerat et al., 2007). Cases reports and a recent semi-systematic review suggest that IBD cutaneous lesions may respond to the newer IBD drugs. This is the case for ustekinumab and less frequently for vedolizumab (Phillips et al., 2020; Ben Abdallah et al., 2021). Uveitis also appears to respond to anti-TNF agents (Leal et al., 2019), whereas no biological agents, despite high expectations for vedolizumab, has shown efficacy in primary sclerosing cholangitis (Christensen et al., 2018; Lynch et al., 2020).
Fistula development affects up to 50% of CD patients over 20 years of disease course. Half of these patients experience perianal fistula (Rubbino et al., 2021). After surgical drainage of pelvic sepsis, medical therapy may be undertaken. Best results so far are obtained with anti-TNF agents. Among them, only infliximab has been evaluated in a large, dedicated placebo-randomized trial. Out of 366 patients who received induction with 5 mg/kg infliximab at Week 0, 2 and 6, 195 responders were randomized to maintenance therapy or placebo. At Week 54, 36% of infliximab treated patients had a complete absence of fistula against 19% in the placebo group (Sands et al., 2004). Regarding the other biologicals, subanalyses of randomized trials showed effectiveness of adalimumab, vedolizumab and ustekinumab in fistula closure, however the patient numbers are limited. All the data regarding these studies are put in perspective in the previously cited AGA Technical Review (Singh et al., 2021). Recently, a small, randomized trial prospectively tested two vedolizumab regimens in fistulizing CD patients with better results achieved in the high dose group. However, the trial was prematurely interrupted due to recruitment challenges (Schwartz et al., 2021). These results are in keeping with others, suggesting that therapeutic drug monitoring to achieve high trough levels is of prime importance to achieve the therapeutic goal of fistula closure (Nones et al., 2021).
4. Conclusions
Providing personalized IBD treatment by appropriately positioning biologics relies on numerous factors. Treatment decision is based not only on drug efficacy but also depends on IBD disease severity, activity and phenotype, the patient's current situation and wishes. The most appropriate drug should combine a rapid resolution of symptoms, good potential on pre-set targets, as well as an optimal long term safety profile. In this regard, perhaps the current available biological agents will be challenged by the development of the novel small molecules and a new generation of antibodies which are more focused on the key inflammatory pathways and involved in the pathogenesis of IBD.
Sources of support
None.
Disclosure of funding received for this work
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Pascal Juillerat: Conceptualization, Data curation, Methodology, Writing – original draft, Funding acquisition. Maude Martinho Grueber: Conceptualization, Methodology, Investigation, Writing – review & editing. Roseline Ruetsch: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition. Giulia Santi: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition. Marianne Vuillèmoz: Methodology, Investigation, Writing – review & editing, Funding acquisition. Pierre Michetti: Conceptualization, Data curation, Methodology, Writing – original draft.
Declaration of competing interest
Pascal Juillerat received consulting fees from AbbVie, Arena Pharma, Amgen, BMS, Ferring, Gilead, Janssen, Lilly, MSD, Pfizer, Pierre Fabre, Roche, Takeda, and Vifor Pharma. Lecture fees from AbbVie, Amgen, Janssen, Pfizer, Takeda, UCB pharma and Vifor Pharma and research grants from Vifor Pharma. Maude Martinho Grueber, Roseline Ruetsch, Giulia Santi and Marianne Vullièmoz have no conflict of interest to declare. Pierre Michetti received consulting fees from AstraZeneca, AbbVie, Ferring Pharmaceuticals, Janssen, MSD, Nestlé Health Sciences, Pfizer, Pierre Fabre, Takeda, UCB Pharma, and Vifor Pharma, lecture fees from Ferring Pharmaceuticals, Janssen, Hospira, MSD, Pfizer, Takeda, UCB Pharma, and Vifor Pharma and research grants from iQone.
References
- Allez M., Vermeire S., Mozziconacci N., Michetti P., Laharie D., Louis E., Bigard M.A., Hebuterne X., Treton X., Kohn A., Marteau P., Cortot A., Nichita C., van Assche G., Rutgeerts P., Lemann M., Colombel J.F. The efficacy and safety of a third anti-tnf monoclonal antibody in crohn's disease after failure of two other anti-tnf antibodies. Aliment. Pharmacol. Ther. 2010;31(1):92–101. doi: 10.1111/j.1365-2036.2009.04130.x. [DOI] [PubMed] [Google Scholar]
- Alsoud D., Verstockt B., Fiocchi C., Vermeire S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. 2021;6(7):589–595. doi: 10.1016/S2468-1253(21)00065-0. [DOI] [PubMed] [Google Scholar]
- Argollo M., Kotze P.G., Kakkadasam P., D'Haens G. Optimizing biologic therapy in ibd: how essential is therapeutic drug monitoring? Nat. Rev. Gastroenterol. Hepatol. 2020;17(11):702–710. doi: 10.1038/s41575-020-0352-2. [DOI] [PubMed] [Google Scholar]
- Arias M.T., Vande Casteele N., Vermeire S., de Buck van Overstraeten A., Billiet T., Baert F., Wolthuis A., Van Assche G., Noman M., Hoffman I., D'Hoore A., Gils A., Rutgeerts P., Ferrante M. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015;13(3):531–538. doi: 10.1016/j.cgh.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Asscher V.E.R., Biemans V.B.C., Pierik M.J., Dijkstra G., Lowenberg M., van der Marel S., de Boer N.K.H., Bodelier A.G.L., Jansen J.M., West R.L., Haans J.J.L., van Dop W.A., Weersma R.K., Hoentjen F., Maljaars P.W.J., Dutch Initiative on C., Colitis Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease-a prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2020;52(8):1366–1376. doi: 10.1111/apt.16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R., Siegmund B. Location is important: differentiation between ileal and colonic crohn's disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18(8):544–558. doi: 10.1038/s41575-021-00424-6. [DOI] [PubMed] [Google Scholar]
- Bek S., Nielsen J.V., Bojesen A.B., Franke A., Bank S., Vogel U., Andersen V. Systematic review: genetic biomarkers associated with anti-tnf treatment response in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2016;44(6):554–567. doi: 10.1111/apt.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Horin S., Novack L., Mao R., Guo J., Zhao Y., Sergienko R., Zhang J., Kobayashi T., Hibi T., Chowers Y., Peyrin-Biroulet L., Colombel J.F., Kaplan G.G., Chen M.H. Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology. 2021;162(2):482–494. doi: 10.1053/j.gastro.2021.10.037. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah H., Fogh K., Vestergaard C., Bech R. Pyoderma gangrenosum and interleukin inhibitors: a semi-systematic review. Dermatology. 2021:1–8. doi: 10.1159/000519320. [DOI] [PubMed] [Google Scholar]
- Billiet T., Papamichael K., de Bruyn M., Verstockt B., Cleynen I., Princen F., Singh S., Ferrante M., Van Assche G., Vermeire S. A matrix-based model predicts primary response to infliximab in crohn's disease. J Crohns Colitis. 2015;9(12):1120–1126. doi: 10.1093/ecco-jcc/jjv156. [DOI] [PubMed] [Google Scholar]
- Bonovas S., Fiorino G., Allocca M., Lytras T., Nikolopoulos G.K., Peyrin-Biroulet L., Danese S. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin. Gastroenterol. Hepatol. 2016;14(10):1385–1397. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- Bonovas S., Lytras T., Nikolopoulos G., Peyrin-Biroulet L., Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47(4):454–465. doi: 10.1111/apt.14449. [DOI] [PubMed] [Google Scholar]
- Bouhnik Y., Carbonnel F., Laharie D., Stefanescu C., Hebuterne X., Abitbol V., Nachury M., Brixi H., Bourreille A., Picon L., Bourrier A., Allez M., Peyrin-Biroulet L., Moreau J., Savoye G., Fumery M., Nancey S., Roblin X., Altwegg R., Bouguen G., Bommelaer G., Danese S., Louis E., Zappa M., Mary J.Y., Group G.C.S. Efficacy of adalimumab in patients with crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (creole) study. Gut. 2018;67(1):53–60. doi: 10.1136/gutjnl-2016-312581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakumar A., Georgy M., Agarwal P., Jong G.W. t, El-Matary W. Anti-saccharomyces cerevisiae antibodies as a prognostic biomarker in children with crohn disease. J. Pediatr. Gastroenterol. Nutr. 2019;69(1):82–87. doi: 10.1097/MPG.0000000000002311. [DOI] [PubMed] [Google Scholar]
- Cheng D., Cushing K.C., Cai T., Ananthakrishnan A.N. Safety and efficacy of tumor necrosis factor antagonists in older patients with ulcerative colitis: patient-level pooled analysis of data from randomized trials. Clin. Gastroenterol. Hepatol. 2021;19(5):939–946. doi: 10.1016/j.cgh.2020.04.070. [DOI] [PubMed] [Google Scholar]
- Christensen B., Micic D., Gibson P.R., Yarur A., Bellaguarda E., Corsello P., Gaetano J.N., Kinnucan J., Rao V.L., Reddy S., Singh S., Pekow J., Rubin D.T. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment. Pharmacol. Ther. 2018;47(6):753–762. doi: 10.1111/apt.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleynen I., Boucher G., Jostins L., Schumm L.P., Zeissig S., Ahmad T., Andersen V., Andrews J.M., Annese V., Brand S., Brant S.R., Cho J.H., Daly M.J., Dubinsky M., Duerr R.H., Ferguson L.R., Franke A., Gearry R.B., Goyette P., Hakonarson H., Halfvarson J., Hov J.R., Huang H., Kennedy N.A., Kupcinskas L., Lawrance I.C., Lee J.C., Satsangi J., Schreiber S., Theatre E., van der Meulen-de Jong A.E., Weersma R.K., Wilson D.C., International Inflammatory Bowel Disease Genetics C., Parkes M., Vermeire S., Rioux J.D., Mansfield J., Silverberg M.S., Radford-Smith G., McGovern D.P., Barrett J.C., Lees C.W. Inherited determinants of crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387(10014):156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J.F., Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Panaccione R., Schreiber S., Byczkowski D., Li J., Kent J.D., Pollack P.F. Adalimumab for maintenance of clinical response and remission in patients with crohn's disease: the charm trial. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Conrad M.A., Kelsen J.R. The treatment of pediatric inflammatory bowel disease with biologic therapies. Curr. Gastroenterol. Rep. 2020;22(8):36–51. doi: 10.1007/s11894-020-00773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csontos A.A., Molnar A., Piri Z., Katona B., Dako S., Palfi E., Miheller P. The effect of anti-tnfalpha induction therapy on the nutritional status and dietary intake in inflammatory bowel disease. J Gastrointestin Liver Dis. 2016;25(1):49–56. doi: 10.15403/jgld.2014.1121.251.tnf. [DOI] [PubMed] [Google Scholar]
- Dai Z.H., Xu X.T., Ran Z.H. Associations between obesity and the effectiveness of anti-tumor necrosis factor-alpha agents in inflammatory bowel disease patients: a literature review and meta-analysis. Ann. Pharmacother. 2020;54(8):729–741. doi: 10.1177/1060028019900660. [DOI] [PubMed] [Google Scholar]
- Danese S., Colombel J.F., Lukas M., Gisbert J.P., D’Haens G., Hayee B., Panaccione R., Kim H.S., Reinisch W., Tyrrell H., Oh Y.S., Tole S., Chai A., Chamberlain-James K., Tang M.T., Schreiber S., Group G.S. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (gardenia): a randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol Hepatol. 2021;7(2):118–127. doi: 10.1016/S2468-1253(21)00294-6. [DOI] [PubMed] [Google Scholar]
- Danese S., Fiorino G., Peyrin-Biroulet L., Lucenteforte E., Virgili G., Moja L., Bonovas S. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann. Intern. Med. 2014;160(10):704–711. doi: 10.7326/M13-2403. [DOI] [PubMed] [Google Scholar]
- Diaz L.I., Keihanian T., Schwartz I., Bin Kim S., Calmet F., Alejandra Quintero M., Abreu M.T. Vedolizumab-induced de novo extraintestinal manifestations. Gastroenterol. Hepatol. 2020;16(2):75–81. [PMC free article] [PubMed] [Google Scholar]
- Dubinsky M.C., Cross R.K., Sandborn W.J., Long M., Song X., Shi N., Ding Y., Eichner S., Pappalardo B., Ganguli A., Wang A. Extraintestinal manifestations in vedolizumab and anti-tnf-treated patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24(9):1876–1882. doi: 10.1093/ibd/izy065. [DOI] [PubMed] [Google Scholar]
- Dubois E.A.-O., Moens A., Geelen R., Sabino J., Ferrante M., Vermeire S.A.-O. Long-term outcomes of patients with ulcerative proctitis: analysis from a large referral centre cohort. United Eur. Gastroenterol. J. 2020;8(8):933–941. doi: 10.1177/2050640620941345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftychi C., Schwarzer R., Vlantis K., Wachsmuth L., Basic M., Wagle P., Neurath M.F., Becker C., Bleich A., Pasparakis M. Temporally distinct functions of the cytokines il-12 and il-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity. 2019;51(2):367–380. doi: 10.1016/j.immuni.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Feagan B.G., Rubin D.T., Danese S., Vermeire S., Abhyankar B., Sankoh S., James A., Smyth M. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin. Gastroenterol. Hepatol. 2017;15(2):229–239. doi: 10.1016/j.cgh.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Ferrante M., Vermeire S., Fidder H., Schnitzler F., Noman M., Van Assche G., De Hertogh G., Hoffman I., D'Hoore A., Van Steen K., Geboes K., Penninckx F., Rutgeerts P. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2(3):219–225. doi: 10.1016/j.crohns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ford A.C., Bernstein C.N., Khan K.J., Abreu M.T., Marshall J.K., Talley N.J., Moayyedi P. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106(4):590–599. doi: 10.1038/ajg.2011.70. [DOI] [PubMed] [Google Scholar]
- Generini S., Giacomelli R., Fedi R., Fulminis A., Pignone A., Frieri G., Del Rosso A., Viscido A., Galletti B., Fazzi M., Tonelli F., Matucci-Cerinic M. Infliximab in spondyloarthropathy associated with crohn's disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann. Rheum. Dis. 2004;63(12):1664–1669. doi: 10.1136/ard.2003.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert J.P., Chaparro M. Predictors of primary response to biologic treatment [anti-tnf, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020;14(5):694–709. doi: 10.1093/ecco-jcc/jjz195. [DOI] [PubMed] [Google Scholar]
- Hassler S., Bachelet D., Duhaze J., Szely N., Gleizes A., Hacein-Bey Abina S., Aktas O., Auer M., Avouac J., Birchler M., Bouhnik Y., Brocq O., Buck-Martin D., Cadiot G., Carbonnel F., Chowers Y., Comabella M., Derfuss T., De Vries N., Donnellan N., Doukani A., Guger M., Hartung H.P., Kubala Havrdova E., Hemmer B., Huizinga T., Ingenhoven K., Hyldgaard-Jensen P.E., Jury E.C., Khalil M., Kieseier B., Lauren A., Lindberg R., Loercher A., Maggi E., Manson J., Mauri C., Mohand Oumoussa B., Montalban X., Nachury M., Nytrova P., Richez C., Ryner M., Sellebjerg F., Sievers C., Sikkema D., Soubrier M., Tourdot S., Trang C., Vultaggio A., Warnke C., Spindeldreher S., Donnes P., Hickling T.P., Hincelin Mery A., Allez M., Deisenhammer F., Fogdell-Hahn A., Mariette X., Pallardy M., Broet P., consortium A. Clinicogenomic factors of biotherapy immunogenicity in autoimmune disease: a prospective multicohort study of the abirisk consortium. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood G.S., Rezaie A., Borman M., Panaccione R., Ghosh S., Seow C.H., Kuenzig E., Tomlinson G., Siegel C.A., Melmed G.Y., Kaplan G.G. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in crohn's disease: a network meta-analysis. Gastroenterology. 2015;148(2):344–354. doi: 10.1053/j.gastro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Herfarth H., Obermeier F., Andus T., Rogler G., Nikolaus S., Kuehbacher T., Schreiber S. Improvement of arthritis and arthralgia after treatment with infliximab (remicade) in a German prospective, open-label, multicenter trial in refractory crohn's disease. Am. J. Gastroenterol. 2002;97(10):2688–2690. doi: 10.1111/j.1572-0241.2002.06064.x. [DOI] [PubMed] [Google Scholar]
- Hruz P., Juillerat P., Kullak-Ublick G.A., Schoepfer A.M., Mantzaris G.J., Rogler G., a.o.w.g.o.t.S.S.o.G. on behalf of Swiss Ibdnet Management of the elderly inflammatory bowel disease patient. Digestion. 2020;101(Suppl. 1):105–119. doi: 10.1159/000503099. [DOI] [PubMed] [Google Scholar]
- Hyams J., Crandall W., Kugathasan S., Griffiths A., Olson A., Johanns J., Liu G., Travers S., Heuschkel R., Markowitz J., Cohen S., Winter H., Veereman-Wauters G., Ferry G., Baldassano R., Group R.S. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe crohn's disease in children. Gastroenterology. 2007;132(3):863–873. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Iborra M., Perez-Gisbert J., Bosca-Watts M.M., Lopez-Garcia A., Garcia-Sanchez V., Lopez-Sanroman A., Hinojosa E., Marquez L., Garcia-Lopez S., Chaparro M., Aceituno M., Calafat M., Guardiola J., Belloc B., Ber Y., Bujanda L., Beltran B., Rodriguez-Gutierrez C., Barrio J., Cabriada J.L., Rivero M., Camargo R., van Domselaar M., Villoria A., Schuterman H.S., Hervas D., Nos P., Spanish Working Group on Crohn's D., Ulcerative C. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naive and non-naive patients. J. Gastroenterol. 2017;52(7):788–799. doi: 10.1007/s00535-016-1274-1. [DOI] [PubMed] [Google Scholar]
- Ibraheim H., Samaan M.A., Srinivasan A., Brain O., Digby-Bell J., Irving P.M., Norman I., Jawad I., Biedermann J., Ibarra A., Kok K.B., Parkes G., Rimmer J., Compot E., Parkes M., Segal J., Oppong P., Hart A., Hayee B., Powell N. Effectiveness and safety of vedolizumab in inflammatory bowel disease patients aged 60 and over: an observational multicenter UK experience. Ann. Gastroenterol. 2020;33(2):170–177. doi: 10.20524/aog.2020.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P.M., Sands B.E., Hoops T., Izanec J.L., Gao L.L., Gasink C., Greenspan A., Allez M., Danese S., Hanauer S.B., Jairath V., Kuehbacher T., Lewis J.D., Loftus E.V., Jr., Mihaly E., Panaccione R., Scherl E., Shchukina O., Sandborn W.J. Op02 ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe crohn's disease: the seavue study. J. Crohn's Colitis. 2021;15(Suppl. ment_1) S001-002. [Google Scholar]
- Juillerat P., Christen-Zach S., Troillet F.X., Gallot-Lavallee S., Pannizzon R.G., Michetti P. Infliximab for the treatment of disseminated pyoderma gangrenosum associated with ulcerative colitis. Case report and literature review. Dermatology. 2007;215(3):245–251. doi: 10.1159/000106584. [DOI] [PubMed] [Google Scholar]
- Juillerat P., Manz M., Sauter B., Zeitz J., Vavricka S.R., S.I.a.o.w.g.o.t.S.S.o. Gastroenterology Therapies in inflammatory bowel disease patients with extraintestinal manifestations. Digestion. 2020;101(Suppl. 1):83–97. doi: 10.1159/000502816. [DOI] [PubMed] [Google Scholar]
- Kassouri L., Amiot A., Kirchgesner J., Treton X., Allez M., Bouhnik Y., Beaugerie L., Carbonnel F., Meyer A. The outcome of crohn's disease patients refractory to anti-tnf and either vedolizumab or ustekinumab. Dig. Liver Dis. 2020;52(10):1148–1155. doi: 10.1016/j.dld.2020.07.031. [DOI] [PubMed] [Google Scholar]
- Kayal M., Dubinsky M.C. Medical management of chronic pouch inflammation. Curr. Res. Pharmacol. Drug Discov. 2022;3:100095. doi: 10.1016/j.crphar.2022.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaumer A., Sepriano A., Smolen J.S., van der Heijde D., Dougados M., van Vollenhoven R., McInnes I.B., Bijlsma J.W.J., Burmester G.R., de Wit M., Falzon L., Landewe R. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the eular recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2020;79(6):744–759. doi: 10.1136/annrheumdis-2019-216656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F.S., Patel P.V., Stekol E., Ali S., Hamandi H., Heyman M.B., Verstraete S.G. Experience using ustekinumab in pediatric patients with medically refractory crohn disease. J. Pediatr. Gastroenterol. Nutr. 2021;73(5):610–614. doi: 10.1097/MPG.0000000000003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjær M.D., Qvist N., Nordgaard-Lassen I., Christensen L.A., Kjeldsen J. Adalimumab in the treatment of chronic pouchitis. A randomized double-blind, placebo-controlled trial. Scand. J. Gastroenterol. 2019;54(2):188–193. doi: 10.1080/00365521.2019.1569718. [DOI] [PubMed] [Google Scholar]
- Kochar B., Cai W., Cagan A., Ananthakrishnan A.N. Pretreatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology. 2020;158(8):2104–2111. doi: 10.1053/j.gastro.2020.02.032. [DOI] [PubMed] [Google Scholar]
- Kopylov U., Burisch J., Ben-Horin S., Braegger F., Fernandez-Nistal A., Lara N., Vavricka S. A retrospective analysis of the efficacy of vedolizumab on extra-intestinal manifestations in patients with inflammatory bowel disease across five european countries. J. Crohns Colitis. 2021;15:S412–S413. [Google Scholar]
- Leal I., Rodrigues F.B., Sousa D.C., Duarte G.S., Romão V.C., Marques-Neves C., Costa J., Fonseca J.E. Anti-tnf drugs for chronic uveitis in adults-a systematic review and meta-analysis of randomized controlled trials. Front. Med. 2019;6:104. doi: 10.3389/fmed.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofberg R., Louis E.V., Reinisch W., Robinson A.M., Kron M., Camez A., Pollack P.F. Adalimumab produces clinical remission and reduces extraintestinal manifestations in crohn's disease: results from care. Inflamm. Bowel Dis. 2012;18(1):1–9. doi: 10.1002/ibd.21663. [DOI] [PubMed] [Google Scholar]
- Louis E.J., Reinisch W., Schwartz D.A., Lofberg R., Robinson A.M., Berg S., Wang A.W., Maa J.F., Huang B., Pappalardo B. Adalimumab reduces extraintestinal manifestations in patients with crohn's disease: a pooled analysis of 11 clinical studies. Adv. Ther. 2018;35(4):563–576. doi: 10.1007/s12325-018-0678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.D., Chapman R.W., Keshav S., Montano-Loza A.J., Mason A.L., Kremer A.E., Vetter M., de Krijger M., Ponsioen C.Y., Trivedi P., Hirschfield G., Schramm C., Liu C.H., Bowlus C.L., Estes D.J., Pratt D., Hedin C., Bergquist A., de Vries A.C., van der Woude C.J., Yu L., Assis D.N., Boyer J., Ytting H., Hallibasic E., Trauner M., Marschall H.U., Daretti L.M., Marzioni M., Yimam K.K., Perin N., Floreani A., Beretta-Piccoli B.T., Rogers J.K., International Primary Sclerosing Cholangitis Study G., Levy C. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020;18(1):179–187. doi: 10.1016/j.cgh.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso F.S., Ventimiglia M., Fries W., Viola A., Cappello M., Scrivo B., Magnano A., Pluchino D., Camilleri S., Garufi S., Mitri R.D., Mocciaro F., Magri G., Ferracane C., Citrano M., Graziano F., Bertolami C., Renna S., Orlando R., Rizzuto G., Cottone M., Orlando A., Sicilian Network for Inflammatory Bowel D. A propensity score weighted comparison of vedolizumab, adalimumab, and golimumab in patients with ulcerative colitis. Dig. Liver Dis. 2020;52(12):1461–1466. doi: 10.1016/j.dld.2020.06.014. [DOI] [PubMed] [Google Scholar]
- Macaluso F.S., Ventimiglia M., Fries W., Viola A., Sitibondo A., Cappello M., Scrivo B., Busacca A., Privitera A.C., Camilleri S., Garufi S., Di Mitri R., Mocciaro F., Belluardo N., Giangreco E., Bertolami C., Renna S., Orlando R., Rizzuto G., Cottone M., Orlando A., Sicilian Network for Inflammatory Bowel D. A propensity score weighted comparison of vedolizumab and adalimumab in crohn's disease. J. Gastroenterol. Hepatol. 2021;36(1):105–111. doi: 10.1111/jgh.15107. [DOI] [PubMed] [Google Scholar]
- Mader O., Juillerat P., Biedermann L., Michetti P., Hruz P., Pittet V., Rogler G., Zahnd-Straumann N., Seibold F. Factors influencing the outcome of vedolizumab treatment: real-life data with objective outcome measurements. United Eur. Gastroenterol. J. 2021;9(3):398–406. doi: 10.1177/2050640620965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran G.W., Dubeau M.F., Kaplan G.G., Yang H., Seow C.H., Fedorak R.N., Dieleman L.A., Barkema H.W., Ghosh S., Panaccione R., Alberta Inflammatory Bowel Disease C. Phenotypic features of crohn's disease associated with failure of medical treatment. Clin. Gastroenterol. Hepatol. 2014;12(3):434–442. doi: 10.1016/j.cgh.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Narula N., Wong E., Dulai P., Marshall J., Jairath V., Reinisch W. Op10 comparative efficacy of biologics for endoscopic healing of the ileum and colon in crohn's disease. J. Crohn's Colitis. 2022;16(Suppl. ment_1):i010–i011. doi: 10.14309/ajg.0000000000001795. [DOI] [PubMed] [Google Scholar]
- Narula N., Wong E.C.L., Dulai P.S., Sengupta N.K., Marshall J.K., Colombel J.F., Reinisch W. Comparative efficacy and rapidity of action for infliximab vs ustekinumab in biologic naive crohn's disease. Clin. Gastroenterol. Hepatol. 2021 doi: 10.1016/j.cgh.2021.04.006. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Narula N., Wong E.C.L., Marshall J.K., Colombel J.F., Dulai P.S., Reinisch W. Comparative efficacy for infliximab vs vedolizumab in biologic naive ulcerative colitis. Clin. Gastroenterol. Hepatol. 2021 doi: 10.1016/j.cgh.2021.07.038. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Nguyen D.L., Nguyen E.T., Bechtold M.L. Panca positivity predicts lower clinical response to infliximab therapy among patients with ibd. South. Med. J. 2015;108(3):139–143. doi: 10.14423/SMJ.0000000000000253. [DOI] [PubMed] [Google Scholar]
- Nones R.B., Fleshner P.R., Queiroz N.S.F., Cheifetz A.S., Spinelli A., Danese S., Peyrin-Biroulet L., Papamichael K., Kotze P.G. Therapeutic drug monitoring of biologics in ibd: essentials for the surgical patient. J. Clin. Med. 2021;10(23):5642. doi: 10.3390/jcm10235642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Gomez L., Mesonero-Gismero F., Albillos-Martinez A., Lopez-Sanroman A. Anti-tumor necrosis factor agents in crohn's disease and ulcerative colitis: beyond luminal disease. Gastroenterol. Hepatol. 2018;41(9):576–582. doi: 10.1016/j.gastrohep.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Pantavou K., Yiallourou A.I., Piovani D., Evripidou D., Danese S., Peyrin-Biroulet L., Bonovas S., Nikolopoulos G.K. Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe ulcerative colitis: a systematic overview of meta-analyses. United Eur. Gastroenterol. J. 2019;7(10):1285–1303. doi: 10.1177/2050640619883566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichael K., Vande Casteele N., Jeyarajah J., Jairath V., Osterman M.T., Cheifetz A.S. Higher postinduction infliximab concentrations are associated with improved clinical outcomes in fistulizing crohn's disease: an accent-ii post hoc analysis. Am. J. Gastroenterol. 2021;116(5):1007–1014. doi: 10.14309/ajg.0000000000001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Hart A., Bossuyt P., Long M., Allez M., Juillerat P., Armuzzi A., Loftus E.V., Jr., Ostad-Saffari E., Scalori A., Oh Y.S., Tole S., Chai A., Pulley J., Lacey S., Sandborn W.J., Group H.S. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (hickory): a phase 3, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2021;7(2):128–140. doi: 10.1016/S2468-1253(21)00298-3. [DOI] [PubMed] [Google Scholar]
- Phillips F.M., Verstockt B., Sebastian S., Ribaldone D., Vavricka S., Katsanos K., Slattery E., de Suray N., Flores C., Fries W., Vincenzi F., Capoferro E., Bachmann O., Kopylov U. Inflammatory cutaneous lesions in inflammatory bowel disease treated with vedolizumab or ustekinumab: an ecco confer multicentre case series. J Crohns Colitis. 2020;14(10):1488–1493. doi: 10.1093/ecco-jcc/jjaa078. [DOI] [PubMed] [Google Scholar]
- Pineton de Chambrun G., Amiot A., Bouguen G., Viennot S., Altwegg R., Louis E., Collins M., Fumery M., Poullenot F., Armengol L., Buisson A., Abitbol V., Laharie D., Seksik P., Nancey S., Blanc P., Bouhnik Y., Pariente B., Peyrin-Biroulet L., Nachury M., Boschetti G., Flourié B., Danion P., Savoye G., brazier F., Loreau J., Beaugerie L., Sokol H., Nion-Larmurier I., Bourrier A., Landman C., Lefèvre J., Chafai N., Bouta N., Funakoshi N. Efficacy of tumor necrosis factor antagonist treatment in patients with refractory ulcerative proctitis. Clin. Gastroenterol. Hepatol. 2020;18(3):620–627. doi: 10.1016/j.cgh.2019.05.060. [DOI] [PubMed] [Google Scholar]
- Pirro F., Caldarola G., Chiricozzi A., Burlando M., Mariani M., Parodi A., Peris K., De Simone C. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin. Drug Invest. 2021;41(10):917–925. doi: 10.1007/s40261-021-01080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachta-Danielzik S., di Giuseppe R., Bokemeyer B., Efken P., Mohl W., Krause T., Hoffstadt M., Ehehalt R., Trentmann L., Schweitzer A., Jessen P., Franzenburg S., Hartmann P., Schreiber S. Op17 maintenance phase propensity score adjusted effectiveness and persistence at week-52 in biologic-naïve ulcerative colitis patients treated with vedolizumab or anti-tnf (vedo ibd-study) J. Crohn's Colitis. 2022;16(Suppl. ment_1):i018–i019. [Google Scholar]
- Pouillon L., Travis S., Bossuyt P., Danese S., Peyrin-Biroulet L. Head-to-head trials in inflammatory bowel disease: past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2020;17(6):365–376. doi: 10.1038/s41575-020-0293-9. [DOI] [PubMed] [Google Scholar]
- Raine T., Bonovas S., Burisch J., Kucharzik T., Adamina M., Annese V., Bachmann O., Bettenworth D., Chaparro-Sanchez M., Czuber-Dochan W., Eder P., Ellul P., Fidalgo C., Fiorino G., Gionchetti P., Gisbert J.P., Gordon H., Hedin C., Holubar S., Iacucci M., Karmiris K., Katsanos K., Kopylov U., Lakatos P., Lytras T., Lyutakov I., Noor N., Pellino G., Piovani D., Savarino E., Selvaggi F., Verstockt B., Spinelli A., Panis Y., Doherty G. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J. Crohns Colitis. 2021;16(1):2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- Rubbino F., Greco L., di Cristofaro A., Gaiani F., Vetrano S., Laghi L., Bonovas S., Piovani D. Journey through crohn's disease complication: from fistula formation to future therapies. J. Clin. Med. 2021;10(23):5548. doi: 10.3390/jcm10235548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.T., Dotan I., DuVall A., Bouhnik Y., Radford-Smith G., Higgins P.D.R., Mishkin D.S., Arrisi P., Scalori A., Oh Y.S., Tole S., Chai A., Chamberlain-James K., Lacey S., McBride J., Panes J., Group H.S. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (hibiscus): two phase 3 randomised, controlled trials. Lancet Gastroenterol Hepatol. 2021;7(1):17–27. doi: 10.1016/S2468-1253(21)00338-1. [DOI] [PubMed] [Google Scholar]
- Ryan E., McNicholas D., Creavin B., Kelly M.E., Walsh T., Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 2019;25(1):67–73. doi: 10.1093/ibd/izy212. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Chan D., Johanns J., G L., Adedokun O.J., Afzali A., Andrews J.M., D'Haens G., Danese S., Hisamatsu T., Panaccione R., Panés J., Reinisch W., Rubin D.T., Sands B.E., Feagan B.G., Investigators o.b.o.t.G. The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active crohn's disease: week 12 interim analyses from the phase 2 galaxi 1 study. UEG Journal. 2020;8(8):64. [Google Scholar]
- Sandborn W.J., D’Haens G.R., Reinisch W., Panés J., Chan D., Gonzalez S., Weisel K., Germinaro M., Frustaci M.E., Yang Z., Adedokun O.J., Han C., Panaccione R., Hisamatsu T., Danese S., Rubin D.T., Sands B.E., Afzali A., Andrews J.M., Feagan B.G. Guselkumab for the treatment of crohn’s disease: induction results from the phase 2 galaxi-1 study. Gastroenterology. 2022;162(6):1650–1664. doi: 10.1053/j.gastro.2022.01.047. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Feagan B.G., Marano C., Zhang H., Strauss R., Johanns J., Adedokun O.J., Guzzo C., Colombel J.F., Reinisch W., Gibson P.R., Collins J., Jarnerot G., Hibi T., Rutgeerts P., P.-S.S. Group Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- Sands B.E., Anderson F.H., Bernstein C.N., Chey W.Y., Feagan B.G., Fedorak R.N., Kamm M.A., Korzenik J.R., Lashner B.A., Onken J.E., Rachmilewitz D., Rutgeerts P., Wild G., Wolf D.C., Marsters P.A., Travers S.B., Blank M.A., van Deventer S.J. Infliximab maintenance therapy for fistulizing crohn's disease. N. Engl. J. Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- Sands B.E., Feagan B.G., Rutgeerts P., Colombel J.F., Sandborn W.J., Sy R., D'Haens G., Ben-Horin S., Xu J., Rosario M., Fox I., Parikh A., Milch C., Hanauer S. Effects of vedolizumab induction therapy for patients with crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618–627. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Sands B.E., Feagan B.G., Sandborn W.J., Shipitofsky N., Marko M., Sheng S., Johanns J., Germinaro M., Vetter M., Panés J., Vega Op36 efficacy and safety of combination induction therapy with guselkumab and golimumab in participants with moderately-to-severely active ulcerative colitis: results through week 12 of a phase 2a randomized, double-blind, active-controlled, parallel-group, multicenter, proof-of-concept study. J. Crohn's Colitis. 2022;16(Suppl. ment_1):i042–i043. [PMC free article] [PubMed] [Google Scholar]
- Sands B.E., Irving P.M., Hoops T., Izanec J.L., Gao L.-L., Gasink C., Greenspan A., Allez M., Danese S., Hanauer S.B., Jairath V., Kuehbacher T., Lewis J.D., Loftus E.V., Mihaly E., Panaccione R., Scherl E.J., Shchukina O., Sandborn W.J. 775d ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe crohn's disease: the seavue study. Gastroenterology. 2021;161(2):e30–e31. [Google Scholar]
- Sands B.E., Peyrin-Biroulet L., Loftus E.V., Danese S., Colombel J.-F., Törüner M., Jonaitis L., Abhyankar B., Chen J., Rogers R., Lirio R.A., Bornstein J.D., Schreiber S. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 2019;381(13):1215–1226. doi: 10.1056/NEJMoa1905725. [DOI] [PubMed] [Google Scholar]
- Sands B.E., Sandborn W.J., Van Assche G., Lukas M., Xu J., James A., Abhyankar B., Lasch K. Vedolizumab as induction and maintenance therapy for crohn's disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm. Bowel Dis. 2017;23(1):97–106. doi: 10.1097/MIB.0000000000000979. [DOI] [PubMed] [Google Scholar]
- Sazonovs A., Ahmad T., Anderson C.A. Underpowered pants: a response to the conclusions of "extended analysis identifies drug-specific association of two distinct hla class ii haplotypes for development of immunogenicity to adalimumab and infliximab. Gastroenterology. 2021;160(1):470–471. doi: 10.1053/j.gastro.2020.05.102. [DOI] [PubMed] [Google Scholar]
- Sazonovs A., Kennedy N.A., Moutsianas L., Heap G.A., Rice D.L., Reppell M., Bewshea C.M., Chanchlani N., Walker G.J., Perry M.H., McDonald T.J., Lees C.W., Cummings J.R.F., Parkes M., Mansfield J.C., Irving P.M., Barrett J.C., McGovern D., Goodhand J.R., Anderson C.A., Ahmad T., Consortium P. Hla-dqa1∗05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with crohn's disease. Gastroenterology. 2020;158(1):189–199. doi: 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- Schett G., McInnes I.B., Neurath M.F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 2021;385(7):628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Billmeier U., Dieterich W., Rath T., Sonnewald S., Reid S., Hirschmann S., Hildner K., Waldner M.J., Mudter J., Hartmann A., Grutzmann R., Neufert C., Munster T., Neurath M.F., Atreya R. Expansion of il-23 receptor bearing tnfr2+ t cells is associated with molecular resistance to anti-tnf therapy in crohn's disease. Gut. 2019;68(5):814–828. doi: 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Colombel J.F., Bloomfield R., Nikolaus S., Scholmerich J., Panes J., Sandborn W.J., Investigators P.R.S. Increased response and remission rates in short-duration crohn's disease with subcutaneous certolizumab pegol: an analysis of precise 2 randomized maintenance trial data. Am. J. Gastroenterol. 2010;105(7):1574–1582. doi: 10.1038/ajg.2010.78. [DOI] [PubMed] [Google Scholar]
- Schwartz D.A., Peyrin-Biroulet L., Lasch K., Adsul S., Danese S. Efficacy and safety of 2 vedolizumab intravenous regimens for perianal fistulizing crohn’s disease: enterprise study. Clin. Gastroenterol. Hepatol. 2021;20(5):1059–1067. doi: 10.1016/j.cgh.2021.09.028. [DOI] [PubMed] [Google Scholar]
- Singh S., Fumery M., Sandborn W.J., Murad M.H. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47(2):162–175. doi: 10.1111/apt.14422. [DOI] [PubMed] [Google Scholar]
- Singh S., Fumery M., Sandborn W.J., Murad M.H. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe crohn's disease. Aliment. Pharmacol. Ther. 2018;48(4):394–409. doi: 10.1111/apt.14852. [DOI] [PubMed] [Google Scholar]
- Singh S., George J., Boland B.S., Vande Casteele N., Sandborn W.J. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis. 2018;12(6):635–643. doi: 10.1093/ecco-jcc/jjy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Heien H.C., Sangaralingham L.R., Schilz S.R., Kappelman M.D., Shah N.D., Loftus E.V. Comparative effectiveness and safety of anti–tumor necrosis factor agents in biologic-naive patients with crohn's disease. Clin. Gastroenterol. Hepatol. 2016;14(8):1120–1129. doi: 10.1016/j.cgh.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Picardo S., Seow C.H. Management of inflammatory bowel diseases in special populations: obese, old, or obstetric. Clin. Gastroenterol. Hepatol. 2020;18(6):1367–1380. doi: 10.1016/j.cgh.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Proctor D., Scott F.I., Falck-Ytter Y., Feuerstein J.D. Aga technical review on the medical management of moderate to severe luminal and perianal fistulizing crohn's disease. Gastroenterology. 2021;160(7):2512–2556. doi: 10.1053/j.gastro.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Proudfoot J., Xu R., Sandborn W.J. Obesity and response to infliximab in patients with inflammatory bowel diseases: pooled analysis of individual participant data from clinical trials. Am. J. Gastroenterol. 2018;113(6):883–889. doi: 10.1038/s41395-018-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprakes M.B., Ford A.C., Warren L., Greer D., Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for crohn's disease: a large single centre experience. J Crohns Colitis. 2012;6(2):143–153. doi: 10.1016/j.crohns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Sturm A., Maaser C., Mendall M., Karagiannis D., Karatzas P., Ipenburg N., Sebastian S., Rizzello F., Limdi J., Katsanos K., Schmidt C., Jeuring S., Colombo F., Gionchetti P. European crohn's and colitis organisation topical review on ibd in the elderly. J Crohns Colitis. 2017;11(3):263–273. doi: 10.1093/ecco-jcc/jjw188. [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Fallon K., Ruut T., Lane D., McKay R., Shadbolt B., Ang S., Cook M., Platten J., Pavli P., Taupin D. Infliximab reverses inflammatory muscle wasting (sarcopenia) in crohn's disease. Aliment. Pharmacol. Ther. 2015;41(5):419–428. doi: 10.1111/apt.13058. [DOI] [PubMed] [Google Scholar]
- Thorlund K., Druyts E., Toor K., Mills E.J. Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expet Rev. Gastroenterol. Hepatol. 2015;9(5):693–700. doi: 10.1586/17474124.2015.1024657. [DOI] [PubMed] [Google Scholar]
- Toor K., Druyts E., Jansen J.P., Thorlund K. Cost per remission and cost per response with infliximab, adalimumab, and golimumab for the treatment of moderately-to-severely active ulcerative colitis. J. Med. Econ. 2015;18(6):437–446. doi: 10.3111/13696998.2015.1012513. [DOI] [PubMed] [Google Scholar]
- Torres J., Bonovas S., Doherty G., Kucharzik T., Gisbert J.P., Raine T., Adamina M., Armuzzi A., Bachmann O., Bager P., Biancone L., Bokemeyer B., Bossuyt P., Burisch J., Collins P., El-Hussuna A., Ellul P., Frei-Lanter C., Furfaro F., Gingert C., Gionchetti P., Gomollon F., Gonzalez-Lorenzo M., Gordon H., Hlavaty T., Juillerat P., Katsanos K., Kopylov U., Krustins E., Lytras T., Maaser C., Magro F., Marshall J.K., Myrelid P., Pellino G., Rosa I., Sabino J., Savarino E., Spinelli A., Stassen L., Uzzan M., Vavricka S., Verstockt B., Warusavitarne J., Zmora O., Fiorino G. Ecco guidelines on therapeutics in crohn's disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- Townsend C.M., Nguyen T.M., Cepek J., Abbass M., Parker C.E., MacDonald J.K., Khanna R., Jairath V., Feagan B.G. Adalimumab for maintenance of remission in crohn's disease. Cochrane Database Syst. Rev. 2020;5:CD012877. doi: 10.1002/14651858.CD012877.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S., Silverberg M.S., Danese S., Gionchetti P., Löwenberg M., Jairath V., Feagan B.G., Bressler B., Lindner D., Escher A., Jones S., Shen B. Op04 vedolizumab intravenous is effective across multiple treatment targets in chronic pouchitis: results of the randomised, double-blind, placebo-controlled earnest trial. J. Crohn's Colitis. 2022;16(Suppl. ment_1):i004–i005. [Google Scholar]
- Vavricka S.R., Brun L., Ballabeni P., Pittet V., Prinz Vavricka B.M., Zeitz J., Rogler G., Schoepfer A.M. Frequency and risk factors for extraintestinal manifestations in the swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 2011;106(1):110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- Veny M., Esteller M., Ricart E., Pique J.M., Panes J., Salas A. Late crohn's disease patients present an increase in peripheral th17 cells and cytokine production compared with early patients. Aliment. Pharmacol. Ther. 2010;31(5):561–572. doi: 10.1111/j.1365-2036.2009.04209.x. [DOI] [PubMed] [Google Scholar]
- Vermeire S., Louis E., Carbonez A., Van Assche G., Noman M., Belaiche J., De Vos M., Van Gossum A., Pescatore P., Fiasse R., Pelckmans P., Reynaert H., D’Haens G., Rutgeerts P., Belgian Group of Infliximab Expanded Access Program in Crohn’s D. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn’s disease. Am. J. Gastroenterol. 2002;97(9):2357–2363. doi: 10.1111/j.1572-0241.2002.05991.x. [DOI] [PubMed] [Google Scholar]
- Walters T.D., Kim M.O., Denson L.A., Griffiths A.M., Dubinsky M., Markowitz J., Baldassano R., Crandall W., Rosh J., Pfefferkorn M., Otley A., Heyman M.B., LeLeiko N., Baker S., Guthery S.L., Evans J., Ziring D., Kellermayer R., Stephens M., Mack D., Oliva-Hemker M., Patel A.S., Kirschner B., Moulton D., Cohen S., Kim S., Liu C., Essers J., Kugathasan S., Hyams J.S., Group P.-K.R. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with crohn's disease. Gastroenterology. 2014;146(2):383–391. doi: 10.1053/j.gastro.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., So R., Matsuoka K., Kobayashi T., Shinzaki S., Matsuura M., Okabayashi S., Kataoka Y., Tsujimoto Y., Furukawa T.A., Watanabe N. Certolizumab pegol for induction of remission in crohn's disease. Cochrane Database Syst. Rev. 2019;8:CD012893. doi: 10.1002/14651858.CD012893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]