Abstract
Although the protein content of swine diets is formulated based on the ileal digestibility of protein and amino acids (AA) under current nutrition requirements, the nitrogen utilization efficiency of swine varies based on protein source, which may be related to AA release kinetics. In this experiment, a 2 × 2 factorial arrangement with casein (CAS)-enriched or corn gluten meal (CGM)-enriched protein sources at different digestible crude protein levels (normal [N], 13%; and low [L], 11%) were applied to 24 crossbred (Duroc × Landrace × Yorkshire) growing pigs (average body weight = 43.3 ± 3.5 kg) in 4 treatments (N.CAS, L.CAS, N.CGM, L.CGM, respectively) to investigate the effects of AA release kinetics on nitrogen deposition in growing pigs. Standardized ileal digestible AA in all diets were balanced by adding individual AA to meet the nutrient requirements. The AA release kinetics were detected in vitro by measuring the hydrolysis of various protein diets under pepsin and trypsin conditions. The results demonstrated that the time of AA release peak in the CGM diet was 12 h later than that in the CAS diet. The synchronization indices of dietary AA release in N.CAS, N.CGM, L.CAS, and L.CGM were 23.73%, 29.37%, 23.40%, and 26.07%, respectively. The N.CGM had the poorest AA release synchronism while the N.CAS had the greatest among the 4 diets. However, within the pigs, L.CAS and N.CGM showed the highest (81.08%) and lowest (73.54%) nitrogen biological values, respectively, despite the standard ileal digestible AA levels being equal for all diets. These results indicate that the release kinetics of dietary AA had great effect on nitrogen deposition. To optimize nitrogen deposition, AA release kinetics and composition should be taken into consideration when formulating diets for growing pigs.
Keywords: Amino acid, Growing pig, Nitrogen deposition, Release kinetics, Synchronization
1. Introduction
The ideal protein composition of pig diets is determined based on the individual amino acid (AA) content of dietary protein at the ileal standard digestibility level. However, when diets composed of different protein sources have the same protein content and ileum-digestible AA, the free amino acid (FAA) levels vary during the process of digestion in the intestine or absorption into the bloodstream (Dangin et al., 2001; Zhong et al., 2017; Liu et al., 2019). When compared with AA released from proteins in feedstuffs, individual AA are preferentially absorbed by animals (Zhang et al., 2011; Rezaei et al., 2013); this problem is especially relevant in low-nitrogen diets due to the excessive use of individual AA to optimize protein content (Nunes et al., 2014). Thus, poor synchronization of AA absorption from different sources leads to different levels of AA absorbed into the blood (Yen et al., 2014).
Due to the complexity of metabolic processes and changing metabolic demands throughout an animal's lifespan, the current definition for ideal dietary protein composition being based simply on statistical calculations is not exactly “ideal” for the efficient utilization of AA, or their spatial–temporal equilibrium supply. Recent findings showed that the kinetics of AA released from dietary protein have substantial effects on protein synthesis in muscle (Reidy et al., 2013, 2014; Kanda et al., 2016; Berrazaga et al., 2020), suggesting that AA release kinetics are closely related to nitrogen deposition (ND) in animals. However, relatively few studies have been conducted to confirm whether AA release kinetics affect nitrogen utilization in pigs. Corn gluten meal (CGM) is a by-product of starch production or distilling-industry purification, with poor water solubility, low levels of essential AA (Zhuang et al., 2013), slow release of AA (Abdallah et al., 2019), and low utilization rates in animals (Jiang et al., 2000). By contrast, casein (CAS) has the highest standard ileal digestibility of AA than other protein sources and a better AA composition (Boirie et al., 1997; Cervantes-Pahm and Stein, 2010). In the present study, 2 diets with different AA release synchronization rates (which are based on a traditional corn-soybean meal diet with CAS and CGM partially replaced soybean meal) were formulated to explore the relationship between different rates of AA release kinetics for efficient swine nitrogen utilization.
2. Materials and methods
This study was conducted according to the guidelines of the Animal Care and Use Committee of Jilin Agricultural University in Jilin Province, China. All experimental procedures were in accordance with the Guidelines for the Care and Use of Experimental Animals of Jilin Agricultural University. This animal study was approved by the Ethics Committee of Jilin Agricultural University (approval number: KT2019012).
2.1. Diets
Factors and treatments were designed in a 2 × 2 factorial treatment arrangement, with the main factors being 2 different protein content levels and 2 different dietary protein sources. Dietary and nutritional compositions are presented in Table 1. The normal protein level groups were fed diets formulated according to National Research Council (2012) nutrient requirements, and the low protein level groups were fed diets formulated with protein requirements reduced by 15% as compared to the NRC. In total, 22% of the standardized ileal digestible (SID) crude protein in all experimental diets were derived from CAS or CGM. According to standard practice, the 4 dietary groups (N.CAS, diet with casein at normal protein levels; N.CGM, diet with corn gluten meal at normal protein levels; L.CAS, diet with casein at low protein levels; and L.CGM, diet with corn gluten meal at low protein levels) were supplemented with 12 crystalline AA to balance SID values.
Table 1.
Ingredients and chemical composition of experimental diets1 (%, DM basis).
| Item | N.CAS | L.CAS | N.CGM | L.CGM |
|---|---|---|---|---|
| Ingredients | ||||
| Corn | 68.01 | 71.80 | 62.65 | 67.82 |
| Wheat bran | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean powder | 14.23 | 9.55 | 15.09 | 10.29 |
| Casein | 3.13 | 2.66 | – | – |
| Corn gluten meal | – | – | 6.52 | 5.47 |
| Sucrose | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean oil | 1.54 | 1.59 | 2.53 | 2.25 |
| L-Arginine | 0.03 | 0.18 | 0.00 | 0.07 |
| L-Histidine | 0.00 | 0.06 | 0.02 | 0.07 |
| L-Isoleucine | 0.04 | 0.14 | 0.00 | 0.11 |
| L-Leucine | 0.35 | 0.50 | 0.00 | 0.21 |
| L-Lysine | 0.24 | 0.39 | 0.38 | 0.51 |
| L-Methionine | 0.00 | 0.04 | 0.00 | 0.04 |
| L-Cysteine | 0.00 | 0.12 | 0.04 | 0.14 |
| L-Phenylalanine | 0.09 | 0.19 | 0.00 | 0.11 |
| L-Tyrosine | 0.00 | 0.36 | 0.40 | 0.46 |
| L-Threonine | 0.09 | 0.17 | 0.09 | 0.17 |
| L-Tryptophan | 0.01 | 0.04 | 0.02 | 0.05 |
| L-Valine | 0.00 | 0.10 | 0.01 | 0.12 |
| Limestone | 1.00 | 0.87 | 1.02 | 1.09 |
| Dicalcium phosphate | 1.39 | 1.38 | 1.38 | 1.18 |
| Salt | 0.85 | 0.85 | 0.84 | 0.84 |
| Vitamin and mineral premix2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calculated nutrition content3 | ||||
| Net energy, MJ/kg | 10.35 | 10.35 | 10.35 | 10.35 |
| Crude protein (SID)4 | 15.69 (13.18) | 15.69 (11.21) | 15.69 (13.18) | 15.69 (11.21) |
| Crude fiber | 1.88 | 1.79 | 1.89 | 1.85 |
| Ether extract | 2.81 | 2.87 | 2.97 | 3.02 |
| Carbohydrate | 60.77 | 62.00 | 58.36 | 60.36 |
| Arginine (SID) | 0.93 (0.85) | 0.92 (0.85) | 0.94 (0.85) | 0.85 (0.93) |
| Histidine (SID) | 0.42 (0.36) | 0.41 (0.36) | 0.43 (0.36) | 0.36 (0.42) |
| Isoleucine (SID) | 0.67 (0.59) | 0.66 (0.59) | 0.68 (0.59) | 0.59 (0.67) |
| Leucine (SID) | 1.80 (1.62) | 1.79 (1.62) | 1.82 (1.62) | 1.62 (1.80) |
| Lysine (SID) | 1.09 (0.98) | 1.06 (0.98) | 1.09 (0.98) | 0.98 (1.07) |
| Methionine (SID) | 0.31 (0.28) | 0.31 (0.28) | 0.33 (0.28) | 0.28 (0.32) |
| Methionine + Cysteine (SID) | 0.58 (0.98) | 0.63 (0.98) | 0.68 (0.98) | 0.98 (0.71) |
| Phenylalanine + Tyrosine (SID) | 1.40 (2.42) | 0.87 (2.42) | 0.89 (2.42) | 2.42 (0.87) |
| Threonine (SID) | 0.70 (0.59) | 0.68 (0.59) | 0.71 (0.59) | 0.59 (1.81) |
| Tryptophan (SID) | 0.20 (0.17) | 0.19 (0.17) | 0.26 (0.17) | 0.17 (0.18) |
| Valine (SID) | 0.80 (0.68) | 0.78 (0.68) | 0.81 (0.68) | 0.68 (0.80) |
| Calcium | 0.80 | 0.80 | 0.80 | 0.80 |
| Available phosphorus | 0.40 | 0.40 | 0.40 | 0.40 |
N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet with corn gluten meal at low protein levels.
Supplied the following per kilogram complete diet, vitamin A, 28,500 IU; vitamin D, 36,000 IU; vitamin E, 67.5 IU; vitamin K, 37.5 mg; vitamin B1, 17.5 mg; vitamin B2, 215 mg; vitamin B6, 69 mg; vitamin B12, 0.075 mg, nicotinic acid, 70 mg, folic acid, 3 mg, calcium pantothenate, 0.375 mg, antioxidant, 0.15 mg, choline chloride, 105 mg; Co as CoSO₄, 1 mg; Cu as CuSO4•5H2O, 155 mg; Fe as FeSO4•H2O, 145 mg; Mn as MnO, 75 mg; Zn as ZnSO4, 125 mg; I as KI, 0.3 mg; Se as Na2SeO3, 0.3 mg.
The data are calculated according to NRC (2012) standards.
SID = standardized ileal digestible values.
2.2. Determination of dietary AA release
2.2.1. In vitro digestion experiments
According to the methods of Boisen and Fernandez (1995), 1 g feed samples were added to 100-mL conical flasks, with 3 replicates for each sample. Porcine pepsin solution (10 mL; pH = 2, 1 mg/mL) and chloramphenicol solution (0.5 mL; 1 mg/mL) were mixed with each sample and sealed with a sealing membrane before incubating in a thermostatic water bath oscillator (HZS-H, Harbin, China) at 37 °C for 0, 2, and 4 h.
The solution was adjusted to pH = 6.8 with 0.2 mol/L NaOH before adding 10 mL phosphate buffer (0.2 mol/mL, pH = 6.8) and 1 mL porcine trypsin solution (50 mg/mL) to the pepsin hydrolysate, and the flasks were sealed again. Samples were incubated in a thermostatic water bath oscillator at 39 °C for 0, 1, 2, 4, 8, 12, 16, 20, 24, and 28 h. After centrifugation (Eppendorf, 5810R, Germany) at 4 °C for 1,157 × g, 800 μL of the supernatant was removed and 200 μL of 10% sulfosalicylic acid was added before the sample was filtered through a 0.22 μm membrane. After incubation at 4 °C for 1 h, the supernatant was centrifuged (Eppendorf, 5430, Germany) at 2,350 × g for 10 min. The supernatant was again filtered through a 0.22 μm membrane and stored at −80 °C for free AA detection.
2.2.2. Free AA determination
Step 1: Preparation of derivatives
A micropipette was used to accurately transfer 1 mL AccQ·Tag Ultra Reagent Diluent from vial 2B to the AccQ·Tag Ultra Reagent powder at the bottom of vial 2A. After vortexing until the powder had dissolved, the solution was sealed and put into an electro-thermostatic blast oven (Boxun, BGZ-70, China) at 55 °C for up to 15 min.
Step 2: Sample derivation
With a clean micropipette, 10 μL of 10-fold diluted sample was dispensed into the bottom of a clean derivative tube (P/NWT007571), and 70 μL AccQ·Tag Ultra-1 Borate buffer was added before swirling to mix. Then, 20 μL of reconstituted AccQ·Tag Ultra Reagent derivative (2A) was added to the derivative tube during vortexing; after which, the sample was vortexed for a further 15 s. After standing for 1 min at room temperature, the derivation tube was sealed and heated in a heating block for 10 min at 55 °C before derivatives were transferred to ultra-performance liquid chromatography (UPLC) sample bottle for analysis.
Step 3: Sample determination
A Waters ACQUITY UPLC TUV system (Waters, Milford, MA, USA) and an AccQ·Tag Ultra column (2.1 mm × 100 mm, P/N:186003837) were used with the following parameters: mobile phase A, 10% AccQ·Tag Ultra eluent A; mobile phase B, AccQ·Tag Ultra eluent B; flow rate, 0.7 mL/min; injection volume, 1 μL; column temperature, 55 °C; sample temperature, 15 °C; detection wavelength, 260 nm; collection rate, 20 points/s; time constant, 0.1 s; and run time, 10 min.
2.2.3. Determination of AA release kinetics
Total amino acid (TAA) release of each diet was calculated as a percentage of final TAA release (28 h). The TAA release percentage at each time point was obtained by calculating the difference between the TAA release at 2 adjacent time points. The individual AA release percentage was calculated every 4 h for each diet to measure the synchronicity of AA release. Release kinetics of individual AA were plotted by Origin 9.1 (USA). Synchronization of AA release was quantified according to the method of Wang et al. (2021). The standard deviation of TAA and individual AA release (%) at each time point was calculated to indicate the synchronization sub index of AA release. The synchronization index (SI) of each diet was the sum of the synchronization sub index at each time point. The lower the SI, the better the synchronization.
2.3. Animals
All pigs used were purchased from the breeding branch of Jilin Huazheng Agriculture and Animal Husbandry Development Co., Ltd. The statistical model design used was completely randomized in a 2 × 2 factorial arrangement. Twenty-four cross-bred (Duroc × Landrace × Yorkshire) growing pigs with similar body weights (43.27 ± 3.51 kg) were randomly divided into 4 dietary treatment groups. The animals were kept in the livestock house of the College of Animal Science and Technology, Jilin Agricultural University, Changchun, China.
2.4. Experimental design and sample collection
During the nitrogen balance experiment, all pigs were placed in stainless steel metabolic pens. During the experimental period, they were fed twice a day at 08:00 and 20:00. After 7 d of acclimation to the environment and diet. Feed intakes were maintained at 2.8 times metabolic body weight (460 kJ digestible energy × metabolic weight) from the beginning of the experiment (Adeola, 2000). All feces and urine from the pigs were collected in plastic bags or bottles at 08:00 from 8 to 12 d to record the weight and volume, respectively.
Every 100 g feces or 100 mL urine was added to 10 mL of 10% hydrochloric acid for nitrogen fixation and stored at −20 °C. The feces from all 5 d were combined for each pig, of which 400 g feces was air dried, weighed, and stored in a dry bottle at room temperature. The urine from all 5 d was combined for each pig, of which 50 mL was taken and stored at −80 °C. At 2 h after the first meal on the 12 d, the blood of pigs was collected from the precaval vein using a 10-mL gel vacuum collection tube. After centrifugation at 4 °C and 1,157 × g for 15 min, the supernatant was removed and stored at −80 °C.
2.5. Nitrogen balance experiment
Nitrogen content was determined with the automatic Kjeldahl nitrogen determination apparatus (FOSS Kjeltec 8420 Kjeldahl nitrogen determination apparatus, FOSS [Technology & Trade Co., Ltd., Beijing, China]). The formulas used were as follows: fecal nitrogen excretion (FN, g/d) = average daily fecal weight × fecal nitrogen content, urinary nitrogen (UN, g/d) = average daily urine volume × urinary nitrogen content, nitrogen intake (NI, g/d) = average daily feed intake × feed nitrogen content, nitrogen emission (NE, g/d) = UN + FN, nitrogen deposition (ND, g/d) = NI − NE, nitrogen deposition rate (NDR, %) = (NI − FN)/NI × 100, and protein apparent biological value (ABV, %) = RN/(NI − FN) × 100.
2.6. Serum analysis
Concentrations of total protein (TP), albumin (ALB), blood urea nitrogen (BUN), glutamic oxaloacetic transaminase (GOT), and glutamic-pyruvic transaminase (GPT) were determined by an automatic biochemical analyzer (Mindray BS-400 Chemical Analyzer, Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) and assay kits (Shanghai Enzyme Union Biotechnology Co., Ltd., Shanghai, China).
2.7. Statistical analysis
Data were analyzed using the model, where was the population mean, was the effect of dietary protein level ( = 1, 2), was the source of dietary protein ( = 1, 2), was the interaction between dietary protein level and source of protein, and was the residual effect. The data were analyzed using SPSS version 19.0 (IBM, Inc., Armonk, NY, USA). Protein content, protein source, and interaction effects were analyzed with a 2-way ANOVA. Differences between the 4 treatments were analyzed by multiple comparisons using the Tukey method. P < 0.05 was considered statistically significant. The results were expressed as the mean and standard error of the mean.
3. Results
3.1. AA release rates in vitro
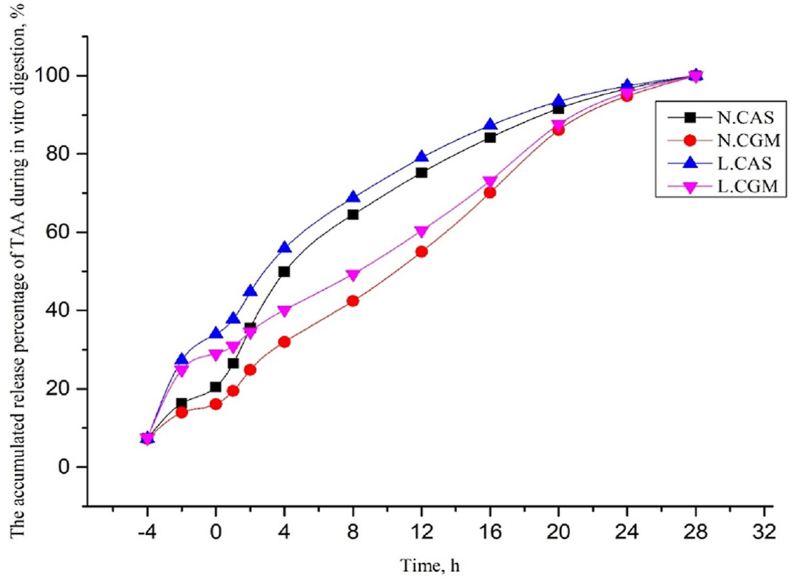
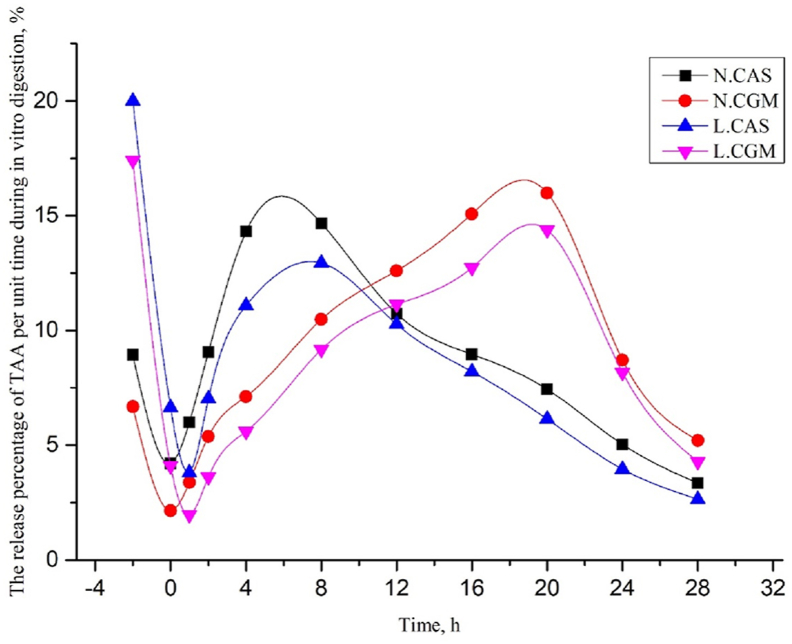
When expressed as a percentage of final TAA release, the release of TAA in the L.CAS and L.CGM diets increased rapidly between −4 and 0 h, and was higher than the N.CAS and N.CGM diets during this period. The release of TAA in all 4 diets increased continuously from 0 to 28 h, with variation in release rate higher in CAS diets than CGM diets (Fig. 1). During trypsin digestion, when calculated over individual time points, the release of TAA in the CAS diets and CGM diets was highest at 4 and 20 h, respectively (Fig. 2).
Fig. 1.
Total amino acid (TAA) accumulate release expressed as a percentage of final TAA release over 32 h via in vitro digestion. Digestion was recorded between −4 and 0 h by pepsin digestion and between 0 and 28 h by trypsin digestion. N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet with corn gluten meal at low protein levels.
Fig. 2.
Total amino acid (TAA) accumulate release at individual time points expressed as a percentage of final TAA release over 32 h via in vitro digestion. Digestion was recorded between −4 and 0 h by pepsin digestion and between 0 and 28 h by trypsin digestion. N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet with corn gluten meal at low protein levels.
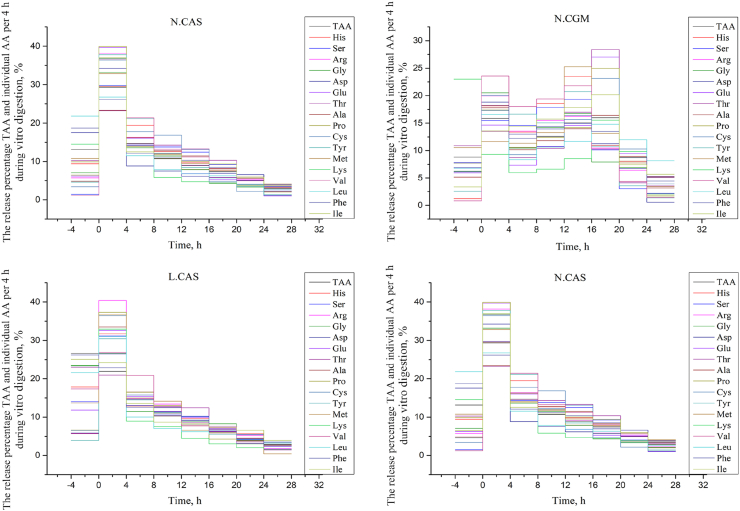
For TAA and 17 individual AA, a vertical step curve of AA release in every 4 h, expressed as a percentage of the final concentration, was plotted to indicate AA release synchronization. Variation in AA release in the low protein diets during the early period of pepsin digestion was higher than in the normal protein diets, indicating less synchronization. The AA release in CAS diets had lower variation, and therefore better synchronization, than the CGM diets in the middle and later digestion periods. In general, AA release synchronization was best in the L.CAS diet and worst in the N.CGM diet (Fig. 3).
Fig. 3.
Total amino acid (TAA) and individual AA release expressed as a percentage of final TAA and individual AA release every 4 h over 32 h via in vitro digestion. Digestion was recorded between −4 and 0 h by pepsin digestion and between 0 and 28 h by trypsin digestion. N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet with corn gluten meal at low protein levels. The AA release rate of each diet was calculated as a percentage of the final AA release by dividing the AA release at individual time points by the AA release at the final time point (28 h). The AA release percentage at each time point was obtained by calculating the difference between the TAA release at 2 adjacent time points.
Synchronization subindices of AA release every 4 h in the 4 diets are shown in Table 2. Synchronization indexes of the N.CAS, N.CGM, L.CAS, and L.CGM diets were 23.73%, 29.37%, 23.40%, and 26.07%, respectively. Thus, the SI of the diets gave the same results as the vertical step curve: the L.CAS diet exhibited a lower AA release SI, and the N.CGM diet exhibited the highest AA release SI.
Table 2.
Synchronization index (SI) of the 4 experimental diets1 (%).
| Time points, h | N.CAS | L.CAS | L.CGM | N.CGM |
|---|---|---|---|---|
| −4−0 | 5.93 | 8.22 | 6.43 | 4.62 |
| 0−4 | 5.46 | 5.66 | 4.79 | 3.53 |
| 4−8 | 3.36 | 2.66 | 2.31 | 3.16 |
| 8−12 | 2.55 | 1.91 | 2.25 | 3.23 |
| 12−16 | 2.41 | 1.84 | 2.81 | 4.11 |
| 16−20 | 1.81 | 1.19 | 4.38 | 6.32 |
| 20−24 | 1.16 | 1.04 | 1.48 | 2.45 |
| 24−28 | 1.04 | 0.88 | 1.64 | 1.96 |
| Summation2 | 23.73 | 23.40 | 26.07 | 29.37 |
N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet with corn gluten meal at low protein levels.
The summation was calculated from the sum of the SI at each time point. The lower the summation value, the better the synchronization.
3.2. Nitrogen balance
Growing pigs in the low protein groups had significantly lower NI, UN, FN, and NE values than those in the high protein groups (Table 3; P < 0.05). The NI, NDR, and ABV values in the CAS group were significantly higher than those in the CGM group (P < 0.05), but the UN and NE were significantly lower in the CAS group than in the CGM group (P < 0.05). When all 4 dietary conditions were compared, there were significant differences between one or more diets in NI, UN, FN, NE, NDR, and ABV (P < 0.05), although no significant interaction effects were observed.
Table 3.
Effects of different dietary protein levels and protein sources on nitrogen deposition in growing pigs.
| Item | Factors and treatments |
SEM |
P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein content |
Protein source |
Dietary treatment |
||||||||||
| Normal (N) | Low (L) | CAS | CGM | N.CAS | L.CAS | L.CGM | N.CGM | PC | PS | PC × PS | ||
| NI, g/d | 41.49m | 36.23n | 38.24x | 39.77y | 40.61b | 35.88d | 37.41c | 42.36a | 0.16 | <0.001 | <0.001 | 0.150 |
| UN, g/d | 7.74m | 5.92n | 6.18y | 7.65x | 6.83ab | 5.52b | 6.44b | 8.65a | 0.28 | 0.005 | 0.023 | 0.429 |
| FN, g/d | 8.84m | 6.88n | 7.33 | 8.53 | 8.03ab | 6.62b | 7.19b | 9.64a | 0.29 | 0.003 | 0.074 | 0.374 |
| NE, g/d | 16.58m | 12.82n | 13.50y | 16.18x | 14.86b | 12.14b | 13.63b | 18.30a | 0.37 | <0.001 | 0.003 | 0.201 |
| ND, g/d | 24.67 | 23.77 | 24.59 | 23.85 | 25.42 | 23.73 | 23.77 | 24.07 | 0.42 | 0.194 | 0.383 | 0.355 |
| NDR, % | 59.96n | 64.97m | 64.63x | 59.69y | 63.10a | 66.15a | 63.55a | 56.81b | 0.93 | 0.016 | 0.027 | 0.332 |
| ABV, % | 76.17m | 79.99n | 79.94x | 75.86y | 78.79ab | 81.08a | 78.70ab | 73.54b | 0.87 | 0.045 | 0.040 | 0.418 |
N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet supplemented with corn gluten meal at low protein levels; SEM = standard error of the mean; PC = effect of protein content; PS = effect of protein source; PC × PS = interaction effect between protein content and source; NI = nitrogen intake; UN = urine nitrogen; FN = fecal nitrogen; NE = nitrogen excretion; ND = nitrogen deposition; NDR = nitrogen deposition rate; ABV = apparent biological value.
a,b,c,d Different letters denote significant differences between experimental diets within each nitrogen deposition variable (P < 0.05).
m,n Different letters denote significant differences between the normal and low protein groups within each nitrogen deposition variable (P < 0.05).
x,y Different letters denote significant differences between the CAS and CGM groups within each nitrogen deposition variable (P < 0.05).
3.3. Blood biochemical index
Serum biochemical parameters are presented in Table 4. The concentrations of TP, ALB, GPT, and BUN were significantly lower in the low protein groups than in the normal protein groups (P < 0.05). The concentration of GOT and BUN in the group supplemented with CGM was significantly higher than in the group supplemented with CAS (P < 0.05). When all 4 dietary conditions were compared, there were significant differences between one or more diets in TP, ALB, GPT, GOT, and BUN (P < 0.05), although no significant interaction effects were evident.
Table 4.
Effects of different dietary protein levels and protein sources on the blood biochemistry of growing pigs.
| Item | Factors and treatments |
SEM |
P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein content |
Protein source |
Dietary treatment |
||||||||||
| Normal (N) | Low (L) | CAS | CGM | N.CAS | L.CAS | N.CGM | L.CGM | PC | PS | PC × PS | ||
| TP, g/L | 65.53m | 62.57n | 64.13 | 64.10 | 65.57a | 62.70b | 65.50a | 62.42b | 0.28 | <0.001 | 0.762 | 0.852 |
| ALB, g/L | 42.43m | 39.08n | 40.85 | 40.80 | 42.77a | 38.93b | 42.10a | 39.26b | 0.34 | <0.001 | 0.805 | 0.474 |
| GPT, U/L | 55.00m | 50.82n | 53.33 | 52.64 | 55.67a | 51.00b | 54.33a | 50.60b | 0.76 | <0.001 | 0.293 | 0.568 |
| GOT, U/L | 62.00 | 61.10 | 57.17y | 66.36x | 57.33b | 57.00b | 66.67a | 66.00a | 1.23 | 0.841 | 0.001 | 0.947 |
| BUN, mg/dL | 5.88m | 3.24n | 4.14y | 5.13x | 5.51a | 2.78d | 6.25b | 3.79d | 0.11 | <0.001 | 0.001 | 0.560 |
N.CAS = diet with casein at normal protein levels; L.CAS = diet with casein at low protein levels; N.CGM = diet with corn gluten meal at normal protein levels; L.CGM = diet supplemented with corn gluten meal at low protein levels; SEM = standard error of the mean; PC = effect of protein content; PS = effect of protein source; PC × PS = interaction effect between protein content and source; TP = total protein; ALB = albumin; GPT = glutamic-pyruvic transaminase; GOT = glutamic-oxalacetic transaminase; BUN = blood urea nitrogen.
a,b,c,d Different letters denote significant differences between experimental diets within each nitrogen deposition variable (P < 0.05).
m,n Different letters denote significant differences between the normal and low protein groups within each nitrogen deposition variable (P < 0.05).
x,y Different letters denote significant differences between the CAS and CGM groups within each nitrogen deposition variable (P < 0.05).
4. Discussion
Due to the complex digestion properties of animals, the conditions of digestion cannot be completely simulated in vitro. But over the years, numerous in vitro digestion methods have been reported. In a successful in vitro technique, there will be a strong correlation between the in vitro prediction (Clunies and Leeson, 1984; Graham, 1991; Boisen and Fernandez, 1995) and the in vivo true values. The in vitro digestion is a rapid and low-cost method, and it can greatly reduce the inter-individual differences (Zaefarian et al., 2021). Therefore, this study used a model that simulated the digestion and fermentation process of the stomach stage, small intestine, and large intestine (Boisen and Fernandez, 1995) to explain the loss of nutrients in the entire digestive tract.
Overall, we have demonstrated that diets with different compositions vary in their AA release pattern. This is consistent with a previous study, which found that piglets displayed different digestive patterns depending on variations of TAA and polypeptides along the digestive tract (Zhong et al., 2017). It is worth noting that the TAA release peak of the CAS diets in this study was 12 h earlier than that of CGM diets, which is consistent with the results of a previous study (Abdallah et al., 2019). This is likely due to the higher digestibility of CAS (Reidy et al., 2013; Park et al., 2021). Another difference in AA release between the 4 diets in the present study was the peaks of individual AA release during gastric digestion. The release peak of individual AA in the low protein diet was higher than that in the normal protein diet, due to the higher individual AA levels in the low protein diet. Generally, protein digestion begins with the breakdown of pepsin in the stomach before transportation to the small intestine to be further broken down into free AA and peptides (Laerke and Hedemann, 2012). Individual AA do not need to be digested and can be absorbed in the small intestine without enzymatic digestion. The release peak of AA in the CGM group was later than that in the CAS group, possibly due to the low water solubility of CGM and the imbalance of AA (Zhuang et al., 2013). Higher levels of individual AA should therefore be added to the CGM diet to ensure the diet meets the nutritional requirements of animals.
An important finding in our study was the difference in synchrony of AA release between the 4 experimental diets, despite the proportion of digestible protein and AA in the terminal ileum being the same. A better synchronization was apparent in the CAS diet, probably because casein is a high-quality protein and the diet contains higher concentrations of digestible crude protein and AAs than other diets (Eklund et al., 2008). Thus, this would account for the high release rate at earlier time points during trypsin digestion, rather than during the protein-specific pepsin digestion (Miralles et al., 2021). The variation in AA release from the CAS diet gradually decreased at later time points, indicating that the protein was almost completely digested. By contrast, the CGM diet exhibited poor synchronization, as seen by the variation in AA release. This was likely due to the slower AA release rate from the CGM diet (Liu et al., 2015), meaning the variation in the release by trypsin via hydrolysis at later time points was higher.
In the in vivo trial, 4 diets were formulated that resulted in the same digestible nitrogen in the terminal ileum, indicating that the amount of nitrogen absorbed by the pigs in each group was basically the same. Moreover, although the protein digestibility of casein differs from that of corn gluten meal, the total nitrogen content differs between the two diets for the same amount of protein, which could also explain the different NDR. The casein diets showed a higher ABV than corn gluten meal diets, indicating that nitrogen deposition, based on a similar level of absorbed nitrogen, was better in pigs fed the casein diets. Thus, on the premise that the amount of digestible nitrogen between diets was similar but total nitrogen was not, ABV was a more reliable reference for making comparisons between the nitrogen utilization rate of each of the 4 diets during the process of metabolism.
In the present study, the UN and BUN contents in pigs fed the CAS diet were significantly lower than in those fed the CGM diet, indicating that nitrogen utilization from CGM diets was inferior to other diets at the same level of digestible nitrogen. Moreover, the above findings, combined with the NDR and BUN results, suggest that the efficiency of ND in the CAS group was better not due to higher NI levels but higher nitrogen absorption. A previous study found that with better dietary AA balance, UN was lower because the nitrogen utilization rate of the body was higher, and fewer blood AA were catabolized (Malmlof, 1988), supporting an effect of AA balance on nitrogen utilization. In addition, the higher NE in pigs fed CGM diets were likely due to asynchronization of AA release in CGM diets, where the derived nitrogen is excreted in the form of urea (Wang et al., 2018), resulting in AA waste. The results of this study indicated that dietary protein level and amino acid balance can affect the content of GPT and GOT in serum, which is consistent with previous studies (Zhang et al., 2012). This may have an effect on the pathways of amino acid synthesis that some amino acids were diverted to non-protein synthesis.
Our hypothesis that the protein release kinetics pattern had a significant effect on ABV was verified from in vitro and in vivo trials. The other important discovery in our study was that good synchronization of AA release in diets generated perfect nitrogen utilization. Impaired synchronization of AA release could have serious adverse effects on the efficiency of body protein synthesis according to the “the bucket effect”. Because the body cannot store AA for a long time, excessive AA is rapidly excreted through urine, resulting in AA waste (Trommelen et al., 2021). At the same time, the SI of AA was 23.4% and 29.37% in the L.CAS and N.CGM diets, respectively, consistent with the results of ND. Therefore, the SI of AA can be used as a reference value to evaluate AA release patterns.
To ensure consistent levels of terminal ileal AA digestibility, the NI of pigs in the CGM group was higher than that in pigs fed the CAS diet, due to the low digestibility of CGM. The similar FN observed between diets with different protein sources obtained in this study confirms that the diets were well matched for terminal ileal AA digestibility because the SI of AA was taken into consideration when formulating the diets. Because we ensured AA balance across various diets, the primary cause of the differences in nitrogen deposition in our study was likely due to the differences in the synchronization of AA release.
5. Conclusions
The release kinetics of dietary AA have important effects on the ABV of nitrogen in pigs. Diets with a lower SI of dietary AA release can improve nitrogen utilization and reduce NE in pigs. Therefore, the release synchronization of dietary AA is an important reference for feeding standards in pig production and provides a scientific basis for the preparation of pig diets.
Author contributions
Qiyu Zhang: Formal analysis, Writing – original draft. Bin Wang: Formal analysis. Nianzhi Hu: Formal analysis. Li Pan: Conceptualization. Nan Bao: Conceptualization. Yuan Zhao: Conceptualization, Supervision, Writing – review & editing. Guixin Qin: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31572439 and No .31572415) , the Natural Science Foundation of Jilin Province of China (No. 20160101348JC), and the Key technology research and development program of Jilin Province of China (No. 20180201018NY).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Yuan Zhao, Email: zhaoyuan4CL52@126.com.
Guixin Qin, Email: qgx@jlau.edu.cn.
References
- Abdallah A., Wang J., Elemba E., Abubakari A.H., Zhong Q.Z., Sun Z.W. Amino acid release patterns of growing pig diets formulated with different dietary protein sources. J Appl Anim Res. 2019;1:417–422. [Google Scholar]
- Adeola O. vol. 40. 2000. (Digestion and balance techniques in pigs). [Google Scholar]
- Berrazaga I., Salles J., Laleg K., Guillet C., Patrac V., Giraudet C., et al. Anabolic properties of mixed wheat-legume pasta products in old rats: impact on whole-body protein retention and skeletal muscle protein synthesis. Nutrients. 2020;6:1596. doi: 10.3390/nu12061596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y., Dangin M., Gachon P., Vasson M.P., Maubois J.L., Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci Unit States Am. 1997;26:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen S., Fernandez J.A. Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mixtures for pigs by in vitro analyses. Anim Feed Sci Technol. 1995;51:29–43. [Google Scholar]
- Cervantes-Pahm S.K., Stein H.H. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J Anim Sci. 2010;8:2674–2683. doi: 10.2527/jas.2009-2677. [DOI] [PubMed] [Google Scholar]
- Clunies M., Leeson S. In vitro estimation of dry matter and crude protein digestibility. Poultry Sci. 1984;63:89–96. [Google Scholar]
- Dangin M., Boirie Y., Garcia-Rodenas C., Gachon P., Fauquant J., Callier P., et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;2:E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Eklund M., Mosenthin R., Piepho H.P., Rademacher M. Estimates of basal ileal endogenous losses of amino acids by regression analysis and determination of standardized ileal amino acid digestibilities from casein in newly weaned pigs. J Sci Food Agric. 2008;88:641–651. [Google Scholar]
- Graham H. In: In vitro digestion for pigs and poultry. Fuller M.F., editor. CAB International; UK: 1991. The physical and chemical constitution of foods: effects on carbohydrate digestion; p. 35e44. [Google Scholar]
- Jiang R.H., Chang X.Y., Stoll B., Ellis K.J., Shypailo R.J., Weaver E., et al. Dietary plasma protein is used more efficiently than extruded soy protein for lean tissue growth in early-weaned pigs. J Nutr. 2000;8:2016–2019. doi: 10.1093/jn/130.8.2016. [DOI] [PubMed] [Google Scholar]
- Kanda A., Nakayama K., Sanbongi C., Nagata M., Ikegami S., Itoh H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients. 2016;6:339. doi: 10.3390/nu8060339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laerke H.N., Hedemann M.S. In: Bach Knudsen K.E., Kjeldsen N.J., Poulsen H.D., Jensen B.B., editors. Chapter 5. University of Copenhagan; 2012. The digestive system of the pig; pp. 1–37. (Nutritional Physiology of Pigs (online publication)). [Google Scholar]
- Liu J., Klebach M., Visser M., Hofman Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: a double-blind, cross-over trial. Nutrients. 2019;11:2613. doi: 10.3390/nu11112613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.L., Zheng X.Q., Song Z.L., Liu X.F., Kopparapu N.K., Wang X.J., et al. Preparation of enzymatic pretreated corn gluten meal hydrolysate and in vivo evaluation of its antioxidant activity. J Funct Foods. 2015;18:1147–1157. [Google Scholar]
- Malmlof K. Amino acid in farm animal nutrition metabolism, partition and consequences of imbalance. Swed J Agric Res. 1988;4:191–193. [Google Scholar]
- Miralles B., Sanchón J., Sánchez-Rivera L., Martínez-Maqueda D., Gouar Y.L., Dupont D., et al. Digestion of micellar casein in duodenum cannulated pigs. Correlation between in vitro simulated gastric digestion and in vivo data. Food Chem. 2021;343:128424. doi: 10.1016/j.foodchem.2020.128424. [DOI] [PubMed] [Google Scholar]
- NRC . 11th ed. National Academies Press; Washington DC, USA: 2012. Nutrient requirements of swine. [Google Scholar]
- Nunes A.J.P., Sá M.V.C., Browdy C.L., Vazquez-Anon M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture. 2014;20:20–27. [Google Scholar]
- Park C.S., Ragland D., Adeola O. Amino acid digestibility in corn distillers' dried grains with solubles in pigs at different dietary levels of casein and test ingredient. Animal. 2021;15:100147. doi: 10.1016/j.animal.2020.100147. [DOI] [PubMed] [Google Scholar]
- Rezaei R., Wang W.W., Wu Z.L., Dai Z.L., Wang J.J., Wu G.T. Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol. 2013;4:7. doi: 10.1186/2049-1891-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy P.T., Walker D.K., Dickinson J.M., Gundermann D.M., Drummond M.J., Timmerman K.L. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr. 2013;4:410–416. doi: 10.3945/jn.112.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy P.T., Walker D.K., Dickinson J.M., Gundermann D.M., Drummond M.J., Timmerman K.L. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol. 2014;11:1353–1364. doi: 10.1152/japplphysiol.01093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommelen J., Tomé D., Loon L.J.C.V. Gut amino acid absorption in humans: concepts and relevance for postprandial metabolism. Clin Nutr. 2021;36:43–55. [Google Scholar]
- Wang B., Mi M.M., Zhang Q.Y., Bao N., Pan L., Zhao Y. Relationship between the amino acid release kinetics of feed proteins and nitrogen balance in finishing pigs. Animal. 2021;10:100359. doi: 10.1016/j.animal.2021.100359. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou J., Wang G., Cai S., Zeng X., Qiao S. Advances in low-protein diets for swine. J Anim Sci Biotechnol. 2018;9:60. doi: 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J.T., Kerr B.J., Easter R.A., Parkhurst A.M. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J Anim Sci. 2014;4:1079–1090. doi: 10.2527/2004.8241079x. [DOI] [PubMed] [Google Scholar]
- Zaefarian F., Cowieson A.J., Pontoppidan K., Abdollahi M.R., Ravindran V. Trends in feed evaluation for poultry with emphasis on in vitro techniques. Anim Nutr. 2021;7:268–281. doi: 10.1016/j.aninu.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.J., Yi X.W., Chu L.C., Lu N., Htoo J., Qiao S.Y. Effects of dietary net energy density and standardized ileal digestible lysine: net energy ratio on the performance and carcass characteristic of growing-finishing pigs fed low crude protein supplemented with crystalline amino acids diets. Agric Sci China. 2011;4:602–610. [Google Scholar]
- Zhang Y., Han C.X., Zhu Y.J., Ji W.Y., Shao C.M. Coated methionine: effects on growth performance, blood biochemical indices and nutrient apparent ileal digestibility in broilers. Chin J Anim Nutr. 2012;24:739–746. [Google Scholar]
- Zhong R.Z., Xia J.Q., Sun H., Qin G.X. Effects of different sources of protein on the growth performance, blood chemistry and polypeptide profiles in the gastrointestinal tract digesta of newly weaned piglets. J Anim Physiol Anim Nutr. 2017;101:e312–e322. doi: 10.1111/jpn.12607. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Tang N., Yuan Y. Purification and identification of antioxidant peptides from corn gluten meal. J Funct Foods. 2013;5:1810–1821. [Google Scholar]