Abstract
Protein kinase A (PKA) is a holoenzyme consisting of a regulatory (R)-subunit dimer and two catalytic (C)-subunits. There are two major families of C-subunits, Cα and Cβ, and four functionally nonredundant R-subunits (RIα, RIβ, RIIα, RIIβ). In addition to binding to and being regulated by the R-subunits, the C-subunits are regulated by two tail regions that each wrap around the N- and C-lobes of the kinase core. Although the C-terminal (Ct-) tail is classified as an intrinsically disordered region (IDR), the N-terminal (Nt-) tail is dominated by a strong helix that is flanked by short IDRs. In contrast to the Ct-tail, which is a conserved and highly regulated feature of all PKA, PKG, and protein kinase C protein kinase group (AGC) kinases, the Nt-tail has evolved more recently and is highly variable in vertebrates. Surprisingly and in contrast to the kinase core and the Ct-tail, the entire Nt-tail is not conserved in nonmammalian PKAs. In particular, in humans, Cβ actually represents a large family of C-subunits that are highly variable in their Nt-tail and also expressed in a highly tissue-specific manner. Although we know so much about the Cα1-subunit, we know almost nothing about these Cβ isoforms wherein Cβ2 is highly expressed in lymphocytes, and Cβ3 and Cβ4 isoforms account for ∼50% of PKA signaling in brain. Based on recent disease mutations, the Cβ proteins appear to be functionally important and nonredundant with the Cα isoforms. Imaging in retina also supports nonredundant roles for Cβ as well as isoform-specific localization to mitochondria. This represents a new frontier in PKA signaling.
SIGNIFICANCE STATEMENT
How tails and adjacent domains regulate each protein kinase is a fundamental challenge for the biological community. Here we highlight how the N- and C-terminal tails of PKA (Nt-tails/Ct-tails) affect the structure and regulate the function of the kinase core and show the combinatorial variations that are introduced into the Nt-tail of the Cα- and Cβ-subunits in contrast to the Ct-tail, which is conserved across the entire AGC subfamily of protein kinases.
Introduction
The eukaryotic protein kinases have evolved to be tightly regulated molecular switches where the highly conserved kinase domain is often controlled by flanking domains and other regulatory proteins that allow for recruitment to membranes after activation, as is the case for protein kinase C (PKC) and Src. In the case of other kinases, however, such as the extracellular signal-regulated kinases and cAMP-dependent protein kinase A (PKA), the kinase domain is surrounded by N- and C-terminal tails that are an integral part of the active kinase. These tails are typically classified mostly as intrinsically disordered regions (IDRs), and embedded within these tails are small linear motifs (SLiMs) that provide key functional contact points for allosteric regulation of the kinase core reviewed in van der Lee et al. (2014). In some ways, one can think of these tails as an “Allosteric Shell” that contributes to the overall activation and regulation of the kinase core. This is completely analogous to the ways in which contiguous domains and heterologous proteins regulate the kinase core of other kinases. We focus here on the tails of the PKA catalytic (C)-subunit in which the conserved kinase core is flanked by two tails—an N-terminal (Nt-) tail and a C-terminal (Ct-) tail. We compare and contrast the conserved and variable features of the Nt- and Ct-tails and show how the motifs embedded in the two tails contribute to the assembly of the active and allosterically sensitized C-subunit. We will then discuss the evolution of the Nt-tail and highlight the differences between the Cα and Cβ isoforms. We will emphasize, in particular, the N-terminal tail and how the variability of exon 1 creates an expanded family of Cβ-subunits that are introducing so much new and previously unappreciated biology into our understanding of PKA signaling. Finally, we will show how disease mutations in Cβ are validating the functional nonredundancy of this subfamily of PKA C-subunit isoforms.
C-Terminal Tail of PKA
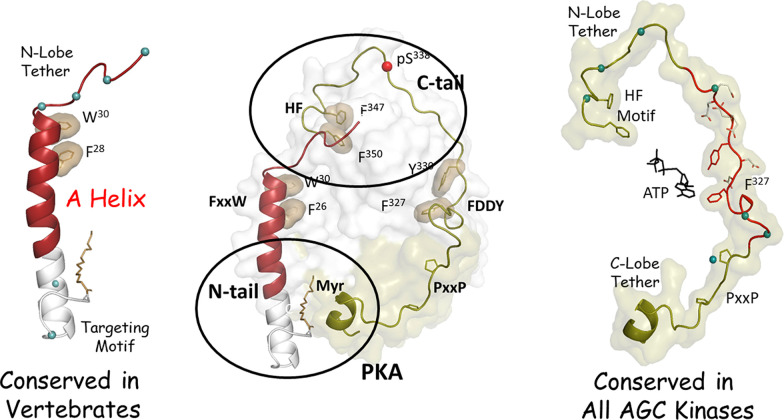
The entire Ct-tail of the PKA C-subunit is classified as an IDR, and it is conserved throughout the entire AGC family of kinases (Kannan et al., 2007). As indicated in Fig. 1, the Ct-tail contains many SLiMs that interface in distinct ways with the N- and C-lobes of the kinase core (Batkin et al., 2000; Gógl et al., 2019). The Ct-tail is divided into three functional units each defined by a set of SLiMs. The C-lobe tether includes the proline-rich motif that is a stable anchor to the C-lobe even when the kinase is inactive. The C-terminal hydrophobic (HF) motif that binds to the αC-Helix in the active kinase is dynamically assembled as part of the activation process and serves as a stable anchor to the N-lobe when the kinase is in an active conformation. It is referred to as the N-lobe tether. In contrast to the two stable motifs at each end, the highly dynamic Phe-Asp-Asp-Tyr motif is localized in the active site tether region. This segment in the free C-subunit is very dynamic in the absence of nucleotide but is very stable and contributes to nucleotide binding in the ternary complex. The assembly of the Ct-tail is complex and highly regulated by both autophosphorylation and by heterologous kinases, such as 3-phosphoinositide-dependent protein kinase-1 (Romano et al., 2009; Leroux and Biondi, 2020). Although each AGC kinase is regulated in unique ways by phosphorylation, the assembled Ct-tail and the ways that it contributes to stabilizing the N-lobe of the kinase core when it is in its active conformation are conserved. The ways in which mammalian target of rapamycin complex contributes to the assembly of the PKC Ct-tail and the newly discovered TIM motif in the Ct-tail are just the newest chapter to a long story (Baffi et al., 2021).
Fig. 1.
The tails of PKA. The N- and C-lobes of the PKA Cα1-subunit are surrounded by Nt- and Ct-tails that wrap around both lobes of the kinase core (center). The Ct-tail (right) is conserved in all AGC kinases. It is an IDR that contains many SLiMs that interact with both lobes of the kinase core. The Ct-segment is a stable anchor to the C-lobe, whereas the Nt segment contains an HF motif that serves as a stable anchor to the N-lobe in the mature and fully active C-subunit. The intervening region (red) is a tether to the active site and is disordered in the absence of ATP. A dominant feature of the Nt-tail is a stable helix (red) that is flanked by two IDRs (left). W30 and F26 (tan shell) provide an important hydrophobic anchor to the kinase core. Crosstalk between the Ct-tail and the N-tail on the N-lobe (upper oval) is an important allosteric regulatory site, whereas crosstalk in the C-lobe (lower oval) contributes to the acyl pocket where the Nt myristyl moiety binds in the free C-subunit. Blue dots correspond to residues that differ in Cα1 and Cβ1.
N-Terminal Tail of PKA
In contrast to the highly conserved Ct-tail, the N-terminal regions that flank the kinase core of the AGC kinases are highly variable. In most AGC kinases, such as PKC, the N-terminal flanking region is not a small tail but instead consists of flanking domains that are responsive to second messenger signals, such as inositol trisphosphate, diacyl glycerol, calcium, and cGMP. This is in fact true for most of the >500 kinases in the human kinome (Manning et al., 2002). Biophysical studies show that the Nt-tail is important for stability, although deletion of the Nt-tail does not influence activity (Herberg et al., 1997; Vetter et al., 2011).
In terms of its physical properties, the dominant motif in the mammalian Nt-tail is a helix (A-helix), which is also in striking contrast to the intrinsically disordered Ct-tail with its multiple SLiMs (Fig. 2). The A-helix is flanked by two sequences that are classified as IDRs, and these flanking regions have distinctly different roles. The C-terminal IDR is an integral part of the N-lobe of the kinase core and is critical for the regulation and assembly of the N-lobe, in particular the C-helix, whereas the N-terminal IDR in the Cα1-subunit is myristylated and associated with targeting to membranes. We will focus first on the αA-helix, including the pockets in the kinase core that are filled by the A-helix, and then on the two flanking regions.
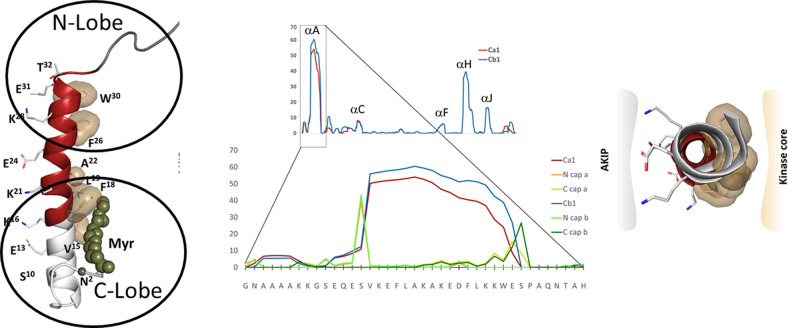
Fig. 2.
The amphipathic A-helix is a very stable protein interaction motif that is flanked by IDRs. The strong helix propensity is for the helix A is seen in the center. The A-helix (red on the left) is anchored by hydrophobic residues to the N- and C-lobes of the kinase core. The flanking region that follows the A-helix wraps around the N-lobe of the kinase core, whereas the first 14 residues encoded for by exon 1 are disordered in most crystal structures. The N-terminal myristyl group, when present, is anchored to the hydrophobic surface of the A-helix. The hydrophobic surface of the A-helix (right) binds to the kinase core, whereas the amphiphilic surface binds to AKIP1 that targets the C-subunit to the nucleus.
The A-Helix
The dominant feature of the Nt-tail is a strong amphipathic helix that mediates protein:protein interactions (Knighton et al., 1991). As seen in Fig. 2, the Nt-tail has a very high propensity to form a helix (residues 14–30). This is striking and in contrast to the rest of the protein where only the αH-Helix is comparable in spite of the high helix content of the C-lobe. It is thus likely that this helix forms as the protein is being synthesized on the ribosome and that some of the interacting proteins bind to the nascent polypeptide chain and protect it while the rest of the protein is being synthesized. Once the fully synthesized and folded C-subunit is released from the ribosome, however, the amphipathic surface appears to be tightly anchored to the kinase core.
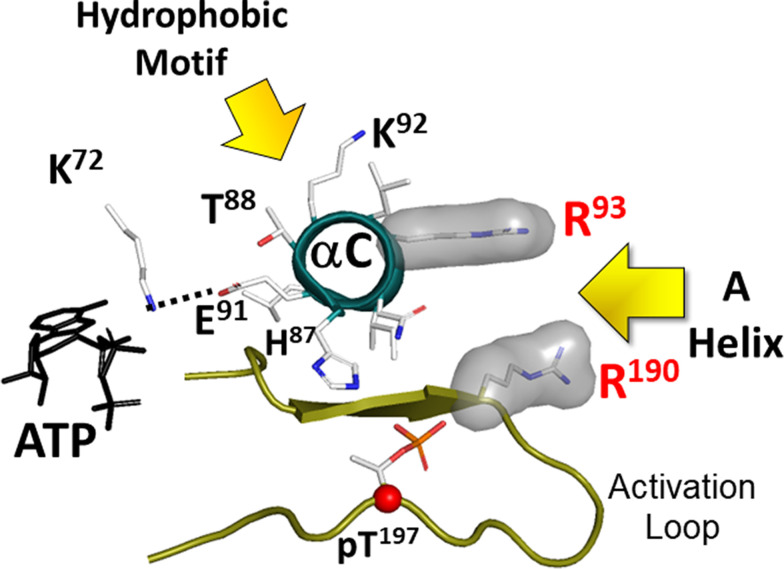
The A-helix is amphipathic; the hydrophobic surface is anchored to the kinase core while the amphiphilic surface is exposed to solvent and serves as a docking site for other proteins (Fig. 2). Two hydrophobic motifs anchor the A-helix to the kinase core; one bridges the N-and C-lobes, whereas the other faces the acyl pocket in the C-lobe. Trp30 and Phe26 [FxxxW motif (PhexxxTrp motif where x represents any amino acid)] create the hydrophobic node that fills a crucial pocket that lies between the N-lobe and the C-lobe. The importance of the A-helix in filling this pocket was described in 1993 (Veron et al., 1993), although neither the dynamic features of the kinase domain nor the importance of phosphorylation of the activation loop (AL) (Nolan et al., 2004; Taylor and Kornev, 2011; Steichen et al., 2012) were fully appreciated at that time. Although the early paper focused on the A-helix, it was of course not the A-helix that was conserved in every kinase but rather the pocket that is conserved and filled in a very precise way when the kinase is in its active conformation (Fig. 3) (Thompson et al., 2009). When the PKA C-subunit is in its active conformation and phosphorylated on Thr197 in the activation loop, Arg193 in the activation loop forms the other surface of this pocket where the FxxxW motif binds (see Fig. 1). Although the A-helix is not a conserved feature of all kinases, the helix is conserved in mammalian PKA Cα and Cβ, and most importantly the pocket that it fills is a highly conserved feature of all active protein kinases (Thompson et al., 2009).
Fig. 3.
Two conserved hydrophobic pockets anchor the C-helix in its active conformation. The two hydrophobic anchors to the N-lobe come from the A-helix (residues F26 and W30/FxxxW motif) and from the Ct-tail (HF motif). By filling the space between the N-lobe and the C-lobe, the FxxxW motif stabilizes the closed conformation in PKA. In the active phosphorylated C-subunit the hydrophobic pocket is lined by Arg93 in the C-helix of the N-lobe and Arg190 in the activation loop of the C-lobe. The HF motif is anchored to Lys92 in the C-helix. These two motifs keep the C-Helix in an αC-IN position, which is a characteristic feature of active kinases (Taylor and Kornev, TIBS 2011). Glu91 is a conserved residue in the C-helix of all kinases. It reaches across to another conserved residue, Lys72 in β3 strand. Together these residues stabilize the α- and β-phosphates of ATP, positioning the γ-phosphate for transfer.
The hydrophilic surface of the A-helix binds to a scaffold protein referred to as A kinase–interacting protein (AKIP) 1 (Sastri et al., 2005). Although PKI, which is a pseudosubstrate that binds to the active site of the C-subunit (Knighton et al., 1991; Zheng et al., 1993), can be considered as a scaffold protein that drives the C-subunit out of the nucleus (Wen et al., 1995), AKIP1 was the first scaffold protein that was shown to interact directly with the C-subunit in a way that does not involve the active site. AKIP1 binding is also in contrast to the more commonly recognized A kinase anchoring proteins that bind directly to the dimerization/docking domain of the R-subunits (Gold et al., 2006; Kinderman et al., 2006). AKIP1 binds to many other proteins, including nuclear factor κ-light-chain-enhancer of activated B cells, apoptosis interacting factor, heat shock protein 70, and many others (King et al., 2011; Sastri et al., 2013). In addition, a peptide corresponding to this helix is capable of preventing the C-subunit from entering the nucleus and inducing gene transcription (Choi et al., 1991; Gao et al., 2008; King et al., 2011). Binding to the R-subunits does not involve the exposed surface of the A-helix, so AKIP can bind to the holoenzyme state as well as to the free C-subunit.
C-Terminal Intrinsically Disordered Region of the Nt-Tail
This segment reaches across the N-lobe of the kinase core and is integrated with the end of the Ct-tail (Fig. 1). The crosstalk between this region and the kinase core is complex and still not well understood, but this region is extremely important for the assembly and regulation of every protein kinase. Embedded within the Ct-tail in this region is the HF motif, and this motif docks onto another important pocket that is also spatially conserved across the kinome (Fig. 3) (Thompson et al., 2009). In contrast to the W30/F26 motif in the A-helix, which is anchored between the C-helix of the N-lobe and the activation loop of the C-lobe, the HF motif docks on top of the C-helix. This is a critical allosteric node that is conserved across the AGC family (Leroux and Biondi, 2020). The common function of these two motifs that dock to pockets on the N-lobe of the kinase, the W30/F26 motif in the A-helix of the Nt-tail and the HF motif in the Ct-tail, is to lock the C-helix into an active conformation that will be productive for phospho-transfer.
N-Terminal Intrinsically Disordered Region
The αA-helix in Cα1 is preceded by residues 1–14 that are encoded for by exon 1 and include a number of key regulatory motifs. Most prominent is the myristylation motif at the N terminus (Fig. 4). (Zheng et al., 1993; Bastidas et al., 2012, 2013). The free C-subunit is not strongly anchored to membranes nor is the RIα holoenzyme, and in these two cases the acyl group is bound into the acyl pocket. In the RIIβ holoenzyme, however, the myristylated N-terminal 1–14 residues are disordered in the crystal structure and mediate binding to nanodisks independent of A kinase anchoring proteins (Zhang et al., 2015). The close proximity of the acylated N-tails to membranes is also seen in the recent cryo electron microscopy (cryoEM) structure of the RIIβ holoenzyme (Lu et al., 2020). An important next structural challenge will be to see how the acyl groups and patches of nearby basic residues are embedded in the membrane and how this influences the structure, function, and dynamics of the holoenzyme. Much remains still to be elucidated about the mechanisms that regulate trafficking of the C-subunit, and much of that information will most likely be embedded in the flexible and highly dynamic N terminus encoded for by exon 1.
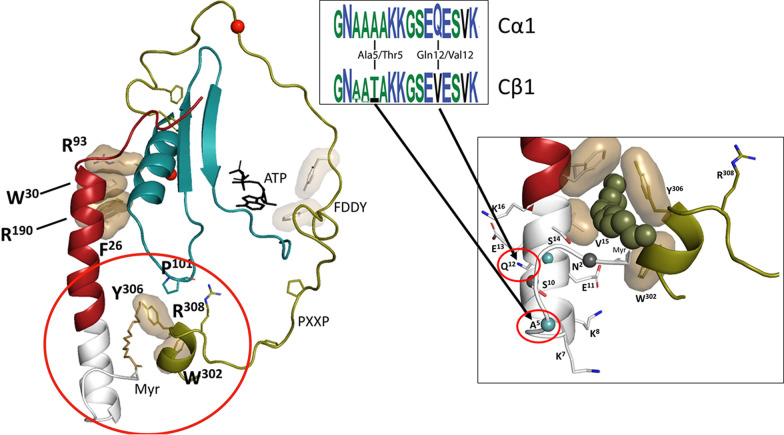
Fig. 4.
Exon 1 encodes for a dynamic targeting motif. Exon 1 corresponds to residues 1–14 in Cα1 and Cβ1. In Cα1 and Cβ1 the N-terminal Gly is myristylated, and this serves as a targeting motif (left panel). The myristyl moiety is buried in an acyl pocket in the free Cα1-subunit (right panel) but is flexible and targeted to membranes in the RIIβ holoenzyme. The allosteric acyl pocket is lined by residues from the Ct-tail. The conservation of key residues in this region suggests that Cβ1 will also be myristylated and that Ser10 will be an autophosphorylation site. The N-terminal sequence of Cα1 and Cβ1 is encoded with amino acid 5 being Ala in Cα1 and Thr in Cβ1 and amino acid 12 being Gln in Cα1 and Val in Cβ1, respectively. The sequence logos highlight the differences between exon 1–encoded residues in Eutherian Cα1 and Cβ1 (Søberg et al., 2017). Exon 1 is highly variable in the other Cα and Cβ isoforms, and several oncogenic fusion proteins have also been described for Cα and Cβ, wherein a heterologous domain is fused to exon 2.
Evolution of the PKA C-Subunit
By delving into the evolution of the PKA C-subunit, we find that the kinase core and the Ct-tail have been highly conserved. In contrast, the emergence of the Nt-tail that characterizes the mammalian Cα1-subunit has been relatively recent. Along the way is PrKX, which belongs to an ancient family of cAMP-dependent serine/threonine kinases and has evolved independently from PKA Cα and Cβ (Li et al., 2002) (Fig. 5). However, PrKX lacks the A-helix and contains instead a unique proline-rich region, and kinase activity is only regulated by pseudosubstrate inhibitors of PKA, including the type I PKA R-subunits and PKI (Zimmermann et al., 1999). Although PrKX is involved in disease phenotypes, its physiologic role remains unclear. Here we focus, however, on how the mammalian Cα and Cβ isoforms evolved.
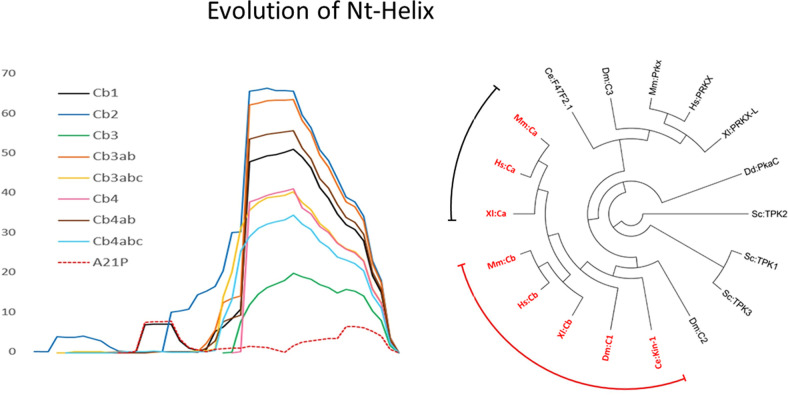
Fig. 5.
Evolution of the A-helix. Although the Ct-tail is a conserved feature of all AGC kinases, the A-helix is only a conserved feature of the PKA Cα- and Cβ-subunit isoforms. Although exon 1 varies for all the Cα/Cβ isoforms, exon 2 encodes for a sequence that has a high propensity to form the strong A-helix, as is seen for Cβ (left). The PRKACA and PRKACB genes arose due to a duplication of an ancestor C-subunit gene around the evolution of jawed vertebrates (Søberg et al., 2013). The tree illustrates that the PKA C-subunit genes make up a separate protein family distinct from the more distantly related PrKX. Sequences in red (i.e., PKA Cα and Cβ) contain a conserved A-helix, whereas this helix is not conserved in PrKX and more distantly related sequences. (Hs: Homo sapiens, Mm: Mus musculus, Xl: Xenopus laevis, Dm: Drosophila melanogaster, Ce: Caenorhabditis elegans, Sc: Saccharomyces cerevisiae, Dd: Dictyostelium discoideum; Identifiers of sequences used for phylogenetic analysis: Mm:Cα P05132; Hs:Cα P17612; Xl:Cα Q90WN3; Mm:Cβ P68181; Hs:Cβ P22694; Xl:Cβ Q7ZWV0; Dm:C1 P12370; Ce:Kin-1 P21137; Dm:C2 P16911; Sc:TPK3 P05986; Sc:TPK1 P06244; Sc:TPK2 P06245; Dd:PkaC P34099; Xl:PRKX-L XP_018101650.1; Hs:PRKX P51817; Mm:Prkx Q922R0; Dm:C3 P16912; Ce:F47F2.1 Q7JP68).
The principal C-subunit genesPRKACA and PRKACB arose relatively recently due to a duplication of an ancient C-subunit gene in a common ancestor of vertebrates that had a different N terminus (Fig. 5) (Søberg et al., 2013, 2017). Since then, each gene has evolved into subsets of Cα and Cβ proteins that differ by only 25 amino acids and likely serve a range of nonredundant functions that, to a large extent, remain unexplored. Within the subset of Cα and Cβ isoforms the Nt-tail is the most variable region (Fig. 5). Variable Nt-tails in PKA C arose through two mechanisms. Exons 2 through 11 in both PRKACA and PRKACB were conserved except for the brain specifically expressed Cβ variants missing the exon 4–encoded sequence (Larsen et al., 2008). The Cα and Cβ isoforms vary exclusively in the first exon (Fig. 6). Exon 2 starts with Lys15 so that the A-helix is a conserved feature of all Cα- and Cβ-subunit isoforms. In summary, the human PRKACA gene contains two variants at the N terminus, Cα1 and Cα2, whereas the PRKACB gene contains a number of variants, including Cβ1, Cβ2, Cβ3, Cβ4, and variants of Cβ3 and Cβ4 with inclusions of an a, b, and c exon cassette (Fig. 6) (Søberg et al., 2013).
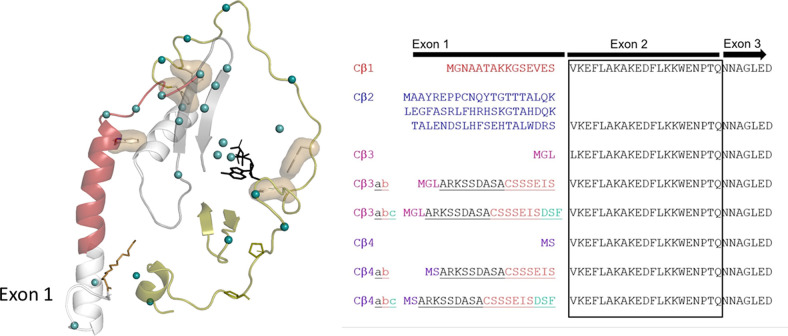
Fig. 6.
Diversity of the Cβ isoforms. The variability of the Cβ isoforms is determined by exon 1 (right). None of the Cβ isoforms, with the exception of Cβ1, are myristylated, and none have so far been characterized biochemically or at the cellular level. There are only 25 sequence differences between Cα1 and Cβ1 (green dots), and half of these are localized to the tails. The rest are mostly in the N-lobe and in regions that interact with the Nt- or Ct-tail. None are in catalytic residues or near the active site, suggesting the differences between Cα and Cβ will correlate with differences in regulation.
Functional Features of the Exon1-Encoded Sequences
Exons 1-1 of PRKACA and PRKACB are paralogous exons (Søberg et al., 2017). Surprisingly, however, almost all studies of PKA so far have been performed only on the Cα1 isoform. Residues 1–14 are located in proximity to the hydrophobic pocket that in Cα1 serves as a site for the Nt-myristic acid attached to Gly1 (Fig. 4). Gly1 is a conserved feature of all Cα1/Cβ1 paralogs, suggesting that N-terminal myristylation occurs also on Cβ1 and is an ancient post-translational modification of both Cα1 and Cβ1. Further support for this hypothesis is the fact that several residues in both Cα1/Cβ1 are highly conserved and constitute substrate recognition sites for N-myristoyl transferase, the enzyme responsible for myristylation, (M)GNXXXXRR (Thompson and Okuyama, 2000; Dumonceaux et al., 2004). This includes the conservation of basic residues at position 7 and 8, which constitutes a classic Myr-in/Myr-out motif that serves to regulate subcellular localization and function of proteins (Resh, 1999). Ser10, a putative autophosphorylation site (Yonemoto et al., 1993), is another conserved feature of mammalian Cα1/Cβ1 and can in principle contribute to the regulation of the Myr-in/Myr-out conformation. Phylogenetic analyses suggest that this residue evolved into Ser10 independently in Cα1 and Cβ1 in mammals as a case of convergent evolution (Søberg et al., 2013). A signature logo (Fig. 4) highlights the conserved features of the exon 1–encoded sequences in Cα1 and Cβ1 (Søberg et al., 2017), which indicates that both proteins will have common functions. Residues Gly1, Asn2, and Ser10 as well as the “Cα1/Cβ1-determining” residues at position 5 and 12 will likely contribute in multiple ways to functions, such as stability, myristylation, and membrane anchoring. Post-translational modifications, such as phosphorylation and deamidation, will also be important (Tholey et al., 2001).
Short Cα and Cβ Variants
“Short” forms of Cα and Cβ are Cα2, Cβ3, and Cβ4 and abc variants of Cβ3 and Cβ4. Given the weak phylogenetic signal of short sequences, determining whether exon 1-2 of PRKACA has a paralog in PRKACB, has proven highly difficult. However, it is possible that one or more of exon 1-3, 1-4, or any of the a, b, and c exons of PRKACB are paralogs of PRKACA exon 1-2. None of the resulting Cβ proteins have an N-tail that is compatible with myristylation (Guthrie et al., 1997), and all variants are specifically expressed in neural cells (Guthrie et al., 1997; Ørstavik et al., 2001; Higuchi et al., 2003; Kvissel et al., 2004; Larsen et al., 2008). Apart from this, very little is known of potential functions of specific residues in the N-tail of these C-subunit isoforms.
PKA Cβ-Subunits and the Combinatorial Expansion of Exon 1
Although we find that the Nt-tail of the mammalian PKA C-subunit has varied considerably throughout evolution, we also are now realizing that even within the mammalian PKA C-subunits, there is far more diversity than we had appreciated. This is quite distinct from the PKA R-subunits (RIα, RIβ, RIIα, RIIβ) in which there is not this range of splice variants, although there are differences in tissue expression. In general, RIα and RIIα are expressed constitutively, whereas RIβ and RIIβ are highly enriched in neuronal tissues (Ilouz et al., 2017). It is widely appreciated also that the R-subunits are functionally and structurally nonredundant (Taylor et al., 2012). In the case of the Cα isoforms, Cα1 is ubiquitously expressed and is the most abundant PKA C-subunit in most tissues, and it was thus the first to be discovered after purification from muscle and heart (Walsh et al., 1968). Subsequent cloning revealed that there were three families of C-subunit isoforms (Uhler et al., 1986; Beebe et al., 1990). Cγ is highly specialized, and its mRNA is found in mature sperm cells (Beebe et al., 1990), and Cα2 is also specific for sperm cells. Although Cβ represents a large subfamily of proteins (Fig. 6), these Cβ isoforms have been largely ignored at the protein level. Cβ1 and Cα1 are most similar and are expressed ubiquitously. They are myristylated and have the same number of residues (350 amino acids). Moreover, if one maps the 25 amino acid differences between Cα1 and Cβ1 (Fig. 6), one finds that they localize primarily to the N- and C-terminal tails and to the regions in the core that flank these tails (Taylor et al., 2021). Their catalytic residues are conserved, so it is likely that these isoforms are regulated differently. Selective depletion of Cα versus Cβ in kidney supports the conclusions that Cα and Cβ are functionally nonredundant, but nothing is known yet about the array of splice variants (Raghuram et al., 2020). Their expression is also highly tissue-specific and will likely be much more relevant for disease. Cβ2, for example, is highly expressed in immune cells and may potentially represent an important marker for inflammation (Funderud et al., 2009; Moen et al., 2017). In contrast, Cβ3 and Cβ4 are expressed almost exclusively in brain tissues and neurons, which together with Cβ1 account for ∼50% of PKA signaling (Ørstavik et al., 2001). We are now using the retina as a window into the brain where we have a set of highly differentiated neurons as well as the myelinated retinal glioma cells that return to the brain. Imaging of the Cα and Cβ isoforms confirms that the two families are spatially different and also shows that the Cβ isoforms are exclusively localized to mitochondria (submitted Roa et al., 2021).
Most PKA disease mutations so far are associated with RIα, wherein Carney Complex disease is linked to hyperactive PKA signaling, whereas Acrodysostosis mutations are associated with reduced PKA activity (Bruystens et al., 2016). In contrast, several mutations in Cα and one in Cβ are associated with Cushing disease (Fig. 7) (Beuschlein et al., 2014; Espiard et al., 2018; Weigand et al., 2021). Most of these mutations lie in the activation segment and disrupt binding to R-subunits. One recently identified that Cushing disease mutation (E31V) lies in the Nt-tail of Cα and leads to severe and correlated changes in the activation segment (Walker et al., 2021) The recent discovery of seven different patients having mutations in five different sites, one mutation in PRKACA and four in PRKACB, gave new credence to the importance of the Cβ isoforms (Palencia-Campos et al., 2020). Three different patients each had the same mutation in PRKACA, whereas four different patients had different mutations in PRKACB all leading to the same phenotype. These patients presented with a severe and complex phenotype that included cardiomyopathies, endocrine disorders, skeletal defects, polydactyly, and intellectual disability. In addition, these proteins when expressed in heterologous cells inhibited Sonic Hedgehog signaling, implicating the importance of Cβ for neurodevelopment (Palencia-Campos et al., 2020). Another recent report of a Gly137Arg mutation in PRKACA links the disorder to ciliopathies, so clearly there is an important role for both Cα and Cβ in cilia biology (Hammarsjö et al., 2021). These disease mutations coupled with the distinct localization of Cα and Cβ in the retina strongly suggest not only that Cα and Cβ are likely to be functionally nonredundant but that the Cβ variants may also mediate distinct and physiologically important functions within cells and, in particular, in neuronal cells.
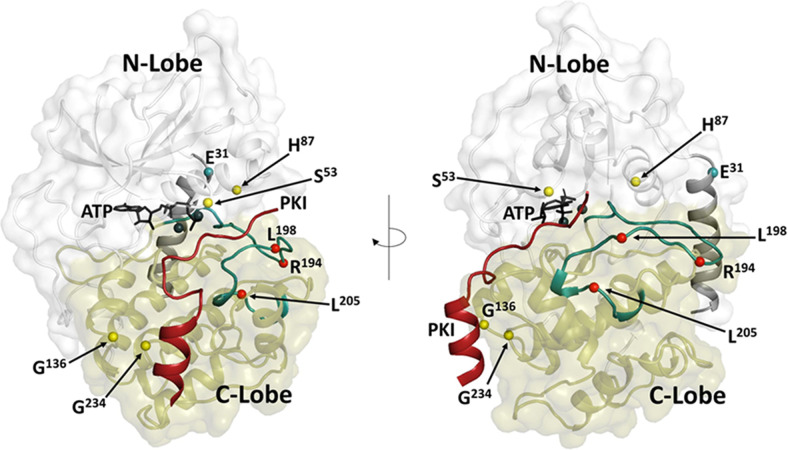
Fig. 7.
Disease mutations associated with the PKA C-subunits. Cushing disease mutations (red dots) are associated mostly with the Cα-subunit. They cluster around the activation segment and interfere with regulation by the R-subunits. A newly discovered Cushing disease mutation (teal dot) is located in the Nt-helix and shows correlated changes in the activation segment (Walker et al., 2021). Mutations that correlate with Sonic Hedgehog signaling (yellow dots) have been identified in Cβ and cluster around the active site cleft and the docking surface for the PKI helix (Palencia-Campos et al., 2020). The N-lobe of the kinase core is in white; the C-lobe is in tan. The A-helix and the activation segment are highlighted in teal, and the PKI peptide is red. ATP and the two Mg++ ions are black.
Abbreviations
- AGC
PKA, PKG, and PKC protein kinase group
- AKIP
A kinase–interacting protein
- C
catalytic subunit of PKA
- Ct-
C-terminal
- HF
hydrophobic
- IDR
intrinsically disordered region
- Nt-
N-terminal
- PKA
protein kinase A
- PKC
protein kinase C
- PKI
protein kinase inhibitor
- PrKX
protein kinase X–linked
- R
regulatory
- SLiM
small linear motif
Authorship Contributions
Conducted experiments: Søberg, Pautz.
Wrote or contributed to the writing of the manuscript: Taylor, Søberg, Kobori, Wu, Pautz, Herberg, Skålhegg.
Footnotes
S.S.T. was supported by funding from National Institutes of Health (NIH) National Institute of General Medical Sciences [Grant R35-GM130389] and [Grant R03-TR002947]. F.W.H. was supported by the Deutsche Forschungsgemeinschaft [He1818/12]; S.P. is supported by the Kassel Programme “PhosMOrg.” B.S.S. and K.S. were supported by the Throne Holst Foundation [2019-2021] and Universitetet i Oslo [Grant 412805].
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Baffi TRLordén GWozniak JMFeichtner AYeung WKornev APKing CCDel Rio JCLimaye AJBogomolovas J, et al. (2021) mTORC2 controls the activity of PKC and Akt by phosphorylating a conserved TOR interaction motif. Sci Signal 14:eabe4509 10.1126/scisignal.abe4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas AC, Deal MS, Steichen JM, Keshwani MM, Guo Y, Taylor SS (2012) Role of N-terminal myristylation in the structure and regulation of cAMP-dependent protein kinase. J Mol Biol 422:215–229 10.1016/j.jmb.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas AC, Pierce LC, Walker RC, Johnson DA, Taylor SS (2013) Influence of N-myristylation and ligand binding on the flexibility of the catalytic subunit of protein kinase A. Biochemistry 52:6368–6379 10.1021/bi400575k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkin M, Schvartz I, Shaltiel S (2000) Snapping of the carboxyl terminal tail of the catalytic subunit of PKA onto its core: characterization of the sites by mutagenesis. Biochemistry 39:5366–5373 10.1021/bi000153z. [DOI] [PubMed] [Google Scholar]
- Beebe SJ, Oyen O, Sandberg M, Frøysa A, Hansson V, Jahnsen T (1990) Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol 4:465–475 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- Beuschlein FFassnacht MAssié GCalebiro DStratakis CAOsswald ARonchi CLWieland TSbiera SFaucz FR, et al. (2014) Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med 370:1019–1028 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruystens JG, Wu J, Fortezzo A, Del Rio J, Nielsen C, Blumenthal DK, Rock R, Stefan E, Taylor SS (2016) Structure of a PKA RIα recurrent acrodysostosis mutant explains defective cAMP-dependent activation. J Mol Biol 428 (24 Pt B):4890–4904 10.1016/j.jmb.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Li B, Lin Z, Huang E, Liu AY (1991) cAMP and cAMP-dependent protein kinase regulate the human heat shock protein 70 gene promoter activity. J Biol Chem 266:11858–11865 10.1016/S0021-9258(18)99036-8. [DOI] [PubMed] [Google Scholar]
- Dumonceaux T, Rajala RVS, Sharma R, Selvaraj G, Datla R (2004) Molecular characterization of a gene encoding N-myristoyl transferase (NMT) from Triticum aestivum (bread wheat). Genome 47:1036–1042 10.1139/g04-074. [DOI] [PubMed] [Google Scholar]
- Espiard SKnape MJBathon KAssié GRizk-Rabin MFaillot SLuscap-Rondof WAbid DGuignat LCalebiro D, et al. (2018) Activating PRKACB somatic mutation in cortisol-producing adenomas. JCI Insight 3:1–11 10.1172/jci.insight.98296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderud A, Aas-Hanssen K, Aksaas AK, Hafte TT, Corthay A, Munthe LA, Orstavik S, Skålhegg BS (2009) Isoform-specific regulation of immune cell reactivity by the catalytic subunit of protein kinase A (PKA). Cell Signal 21:274–281 10.1016/j.cellsig.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gao N, Asamitsu K, Hibi Y, Ueno T, Okamoto T (2008) AKIP1 enhances NF-kappaB-dependent gene expression by promoting the nuclear retention and phosphorylation of p65. J Biol Chem 283:7834–7843 10.1074/jbc.M710285200. [DOI] [PubMed] [Google Scholar]
- Gógl G, Kornev AP, Reményi A, Taylor SS (2019) Disordered protein kinase regions in regulation of kinase domain cores. Trends Biochem Sci 44:300–311 10.1016/j.tibs.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Taskén K, Carlson CR, Scott JD, Barford D (2006) Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell 24:383–395 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Guthrie CR, Skâlhegg BS, McKnight GS (1997) Two novel brain-specific splice variants of the murine Cbeta gene of cAMP-dependent protein kinase. J Biol Chem 272:29560–29565 10.1074/jbc.272.47.29560. [DOI] [PubMed] [Google Scholar]
- Hammarsjö APettersson MChitayat DHanda AAnderlid B-MBartocci MBasel DBatkovskyte DBeleza-Meireles AConner P, et al. (2021) High diagnostic yield in skeletal ciliopathies using massively parallel genome sequencing, structural variant screening and RNA analyses. J Hum Genet 10.1038/s10038-021-00925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg FW, Zimmermann B, McGlone M, Taylor SS (1997) Importance of the A-helix of the catalytic subunit of cAMP-dependent protein kinase for stability and for orienting subdomains at the cleft interface. Protein Sci 6:569–579 10.1002/pro.5560060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Yamashita T, Yoshikawa H, Tohyama M (2003) PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J 22:1790–1800 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilouz R, Lev-Ram V, Bushong EA, Stiles TL, Friedmann-Morvinski D, Douglas C, Goldberg JL, Ellisman MH, Taylor SS (2017) Isoform-specific subcellular localization and function of protein kinase A identified by mosaic imaging of mouse brain. eLife 6:1–23 10.7554/eLife.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Haste N, Taylor SS, Neuwald AF (2007) The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc Natl Acad Sci USA 104:1272–1277 10.1073/pnas.0610251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, Spraggon G, Xuong NH, Jennings PA, Taylor SS (2006) A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell 24:397–408 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CC, Sastri M, Chang P, Pennypacker J, Taylor SS (2011) The rate of NF-κB nuclear translocation is regulated by PKA and A kinase interacting protein 1. PLoS One 6:e18713 10.1371/journal.pone.0018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton DZheng JTen Eyck LAshford VXuong NTaylor S, et al. (1991). Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253:407–414 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Kvissel AK, Ørstavik S, Øistad P, Rootwelt T, Jahnsen T, Skålhegg BS (2004) Induction of Cbeta splice variants and formation of novel forms of protein kinase A type II holoenzymes during retinoic acid-induced differentiation of human NT2 cells. Cell Signal 16:577–587 10.1016/j.cellsig.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Larsen ACV, Kvissel AK, Hafte TT, Avellan CIA, Eikvar S, Rootwelt T, Ørstavik S, Skålhegg BS (2008) Inactive forms of the catalytic subunit of protein kinase A are expressed in the brain of higher primates. FEBS J 275:250–262 10.1111/j.1742-4658.2007.06195.x. [DOI] [PubMed] [Google Scholar]
- Leroux AE, Biondi RM (2020) Renaissance of allostery to disrupt protein kinase interactions. Trends Biochem Sci 45:27–41 10.1016/j.tibs.2019.09.007. [DOI] [PubMed] [Google Scholar]
- Li X, Li H-P, Amsler K, Hyink D, Wilson PD, Burrow CR (2002) PRKX, a phylogenetically and functionally distinct cAMP-dependent protein kinase, activates renal epithelial cell migration and morphogenesis. Proc Natl Acad Sci USA 99:9260–9265 10.1073/pnas.132051799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TW, Aoto PC, Weng J-H, Nielsen C, Cash JN, Hall J, Zhang P, Simon SM, Cianfrocco MA, Taylor SS (2020) Structural analyses of the PKA RIIβ holoenzyme containing the oncogenic DnaJB1-PKAc fusion protein reveal protomer asymmetry and fusion-induced allosteric perturbations in fibrolamellar hepatocellular carcinoma. PLoS Biol 18:e3001018 10.1371/journal.pbio.3001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Moen LV, Sener Z, Volchenkov R, Svarstad AC, Eriksen AM, Holen HL, Skålhegg BS (2017) Ablation of the Cβ2 subunit of PKA in immune cells leads to increased susceptibility to systemic inflammation in mice. Eur J Immunol 47:1880–1889 10.1002/eji.201646809. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS (2004) Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 101:13483–13488 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik S, Reinton N, Frengen E, Langeland BT, Jahnsen T, Skålhegg BS (2001) Identification of novel splice variants of the human catalytic subunit Cbeta of cAMP-dependent protein kinase. Eur J Biochem 268:5066–5073 10.1046/j.0014-2956.2001.02429.x. [DOI] [PubMed] [Google Scholar]
- Palencia-Campos AAoto PCMachal EMFRivera-Barahona ASoto-Bielicka PBertinetti DBaker BVu LPiceci-Sparascio FTorrente I, et al. (2020) Germline and mosaic variants in PRKACA and PRKACB cause a multiple congenital malformation syndrome. Am J Hum Genet 107:977–988 10.1016/j.ajhg.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram V, Salhadar K, Limbutara K, Park E, Yang C-R, Knepper MA (2020) Protein kinase A catalytic-α and catalytic-β proteins have nonredundant regulatory functions. Am J Physiol Renal Physiol 319:F848–F862 10.1152/ajprenal.00383.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451:1–16 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Roa J, Ma Y, Mikulski Z, Xu Q, Ilouz R, Taylor S, Skowronska-Krawczyk D (2021) Subcellular localization of PKA catalytic subunits provides a basis for their distinct functions in the retina. bioRxiv 10.1101/2021.05.17.444584. [DOI] [Google Scholar]
- Romano RA, Kannan N, Kornev AP, Allison CJ, Taylor SS (2009) A chimeric mechanism for polyvalent trans-phosphorylation of PKA by PDK1. Protein Sci 18:1486–1497 10.1002/pro.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastri M, Barraclough DM, Carmichael PT, Taylor SS (2005) A-kinase-interacting protein localizes protein kinase A in the nucleus. Proc Natl Acad Sci USA 102:349–354 10.1073/pnas.0408608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastri MHaushalter KJPanneerselvam MChang PFridolfsson HFinley JCNg DSchilling JMMiyanohara ADay ME, et al. (2013) A kinase interacting protein (AKIP1) is a key regulator of cardiac stress. Proc Natl Acad Sci USA 110:E387–E396 10.1073/pnas.1221670110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søberg K, Jahnsen T, Rognes T, Skålhegg BS, Laerdahl JK (2013) Evolutionary paths of the cAMP-dependent protein kinase (PKA) catalytic subunits. PLoS One 8:e60935 10.1371/journal.pone.0060935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søberg K, Moen LV, Skålhegg BS, Laerdahl JK (2017) Evolution of the cAMP-dependent protein kinase (PKA) catalytic subunit isoforms. PLoS One 12:e0181091 10.1371/journal.pone.0181091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen JM, Kuchinskas M, Keshwani MM, Yang J, Adams JA, Taylor SS (2012) Structural basis for the regulation of protein kinase A by activation loop phosphorylation. J Biol Chem 287:14672–14680 10.1074/jbc.M111.335091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 36:65–77 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Ilouz R, Zhang P, Kornev AP (2012) Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13:646–658 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 36:65–77 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SSWallbott MMachal EMFSøberg KAhmed FBruystens JVu LBaker BWu JRaimondi F, et al. (2021) PKA Cβ: a forgotten catalytic subunit of cAMP-dependent protein kinase opens new windows for PKA signaling and disease pathologies. Biochem J 478:2101–2119 10.1042/BCJ20200867. [DOI] [PubMed] [Google Scholar]
- Tholey A, Pipkorn R, Bossemeyer D, Kinzel V, Reed J (2001) Influence of myristoylation, phosphorylation, and deamidation on the structural behavior of the N-terminus of the catalytic subunit of cAMP-dependent protein kinase. Biochemistry 40:225–231 10.1021/bi0021277. [DOI] [PubMed] [Google Scholar]
- Thompson EE, Kornev AP, Kannan N, Kim C, Ten Eyck LF, Taylor SS (2009) Comparative surface geometry of the protein kinase family. Protein Sci 18:2016–2026 10.1002/pro.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GA Jr, Okuyama H (2000) Lipid-linked proteins of plants. Prog Lipid Res 39:19–39 10.1016/s0163-7827(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Uhler MD, Chrivia JC, McKnight GS (1986) Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 261:15360–15363 10.1016/s0021-9258(18)66717-1. [DOI] [PubMed] [Google Scholar]
- van der Lee RBuljan MLang BWeatheritt RJDaughdrill GWDunker AKFuxreiter MGough JGsponer JJones DT, et al. (2014) Classification of intrinsically disordered regions and proteins. Chem Rev 114:6589–6631 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron M, Radzio-Andzelm E, Tsigelny I, Ten Eyck LF, Taylor SS (1993) A conserved helix motif complements the protein kinase core. Proc Natl Acad Sci USA 90:10618–10622 10.1073/pnas.90.22.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter MM, Zenn H-M, Méndez E, van den Boom H, Herberg FW, Skålhegg BS (2011) The testis-specific Cα2 subunit of PKA is kinetically indistinguishable from the common Cα1 subunit of PKA. BMC Biochem 12:40 10.1186/1471-2091-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Wang Y, Gao J, Bernlohr DA, Calebiro D, Taylor SS, Veglia G (2021) The allosteric E31V mutation disrupts the nucleotide-substrate cooper-2 ativity in protein kinase A: is there a common mechanism for Cushing’ssyndrome driving mutations? J Mol Biol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Perkins JP, Krebs EG (1968) An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem 243:3763–3765 10.1016/S0021-9258(19)34204-8. [DOI] [PubMed] [Google Scholar]
- Weigand IRonchi CLVanselow JTBathon KLenz KHerterich SSchlosser AKroiss MFassnacht MCalebiro D, et al. (2021) PKA Cα subunit mutation triggers caspase-dependent RIIβ subunit degradation via Ser114 phosphorylation. Sci Adv 7:eabd4176 10.1126/sciadv.abd4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS (1995) Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463–473 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Yonemoto W, Garrod SM, Bell SM, Taylor SS (1993) Identification of phosphorylation sites in the recombinant catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 268:18626–18632 10.1016/s0021-9258(17)46675-0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Ye F, Bastidas AC, Kornev AP, Wu J, Ginsberg MH, Taylor SS (2015) An isoform-specific myristylation switch targets type II PKA holoenzymes to membranes. Structure 23:1563–1572 10.1016/j.str.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Knighton DR, Xuong NH, Taylor SS, Sowadski JM, Ten Eyck LF (1993) Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci 2:1559–1573 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B, Chiorini JA, Ma Y, Kotin RM, Herberg FW (1999) PrKX is a novel catalytic subunit of the cAMP-dependent protein kinase regulated by the regulatory subunit type I. J Biol Chem 274:5370–5378 10.1074/jbc.274.9.5370. [DOI] [PubMed] [Google Scholar]