Abstract
Background
Serum uric acid (SUA) is a key determinant of cardiovascular diseases (CVDs). Studies have also shown that SUA independently impacts age-related health outcomes, although their findings differ between males and females. Furthermore, predictive data on all-cause mortality remain limited, particularly for the Japanese population. Thus, this study examined the association between SUA and survival prognosis among males and females based on a follow-up period of 7 or 19 years.
Methods
The study was based on 1,573 male (63 ± 14 years) and 1,980 female (65 ± 12 years) participants who participated in a Nomura Cohort Study in 2002 (Cohort 1) and 2014 (Cohort 2), and continued throughout the follow-up period. A basic resident register was referenced to derive the adjusted relative risk estimates for all-cause mortality. Finally, a Cox proportional hazards model analysis was conducted and was adjusted for possible confounders to estimate hazard ratios (HRs). 95% confidence intervals (CIs) were computed separately for male and female participants.
Results
Of the total 3,553 participants, 905 (25.5%) were deceased. Of these, 473 were male (30.1% of all males) and 432 were female (21.8% of all females). Hyperuricemia was defined in males with SUA levels of 8.5 mg/dL or higher, and in females with SUA levels of 7.5 mg/dL or higher, and was associated with a significantly increased HR for all-cause mortality (males: 1.67; 95% CI: 1.06–2.63; females: 2.17; 95% CI: 1.20–3.94). The data were further stratified based on age (< 65 years or ≥ 65 years), body mass index (BMI) (< 25.0 kg/m2 or ≥ 25.0 kg/m2), History of cardiovascular disease, estimated glomerular filtration rate (< 60 mL/min/1.73 m2 or ≥ 60 mL/min/1.73 m2), and presence of SUA-lowering medication. All stratified groups demonstrated a similar trend. The hyperuricemia group in particular reported a significant increase in HR. On the other hand, a U-shaped increase in HR was observed in those with BMI greater than 25 kg/m2 and SUA-lowering medication, but interaction effect was not significant.
Conclusions
Hyperuricemia is a key risk indicator for all-cause mortality in male and female community-dwelling individuals in Japan.
Keywords: Hyperuricemia, All-cause mortality, Community-dwelling individuals, Cohort study
1. Introduction
Uric acid (UA) is the end metabolite of endogenous purine catabolism in humans and plays a critical role in free radical damage [1]. The enzymes involved in UA production significantly contribute to oxidative stress [1]. Hyperuricemia is mainly caused by increased production or decreased excretion of UA, or both. Numerous experimental and epidemiological studies have shown that increased serum uric acid (SUA) in humans is associated with systemic inflammation [2], endothelial dysfunction [3], hypertension [4], and diabetes [5]. Moreover, several researchers highlighted hyperuricemia as a key factor associated with cardiovascular disease (CVD) [[6], [7], [8], [9], [10], [11], [12], [13]], renal dysfunction (e.g., acute kidney injury [14]), and chronic kidney disease [15].
SUA levels are strongly correlated with age, sex, renal function, obesity, and metabolic abnormalities. The causal role of SUA in all-cause mortality remains controversial. Studies reported a relationship between hyperuricemia and all-cause mortality. However, these results have been inconsistent, especially when considering the results across both sexes. For example, studies have reported that the relationship between SUA and all-cause mortality is observed only in males [7,8,16], females [6,11,17], and/or both [12,18,19]. Some studies indicated that the association between SUA levels and mortality is J-shaped for males [12] and U-shaped for females [[20], [21], [22]], rather than being a linear relationship. A long-term cohort study with an adequate number of events and various sex-specific correction factors is needed to address such inconsistencies.
Thus, this study aimed to investigate whether hyperuricemia is related to all-cause mortality based on a 7- or 19-year follow-up on male and female community-dwelling individuals in Japan.
2. Materials and methods
2.1. Study design and participants
This prospective cohort analysis is part of the Nomura Study conducted in 2002 (first cohort) and 2014 (second cohort). Participants were primarily rural residents of Ehime Prefecture who underwent a community-based annual health checkup. A total of 3,164 participants were in the first cohort and 1,832 participants were in the second cohort. Participants’ age ranged between 22 and 95 years. In the first cohort, 2,775 patients were followed, excluding 389 with missing data, of which 268 were lost to follow-up and 2,507 were ultimately included in the study. In the second cohort, 1074 cases were followed excluding 758 cases with missing data and duplicates, of which 28 were lost to follow-up during the period, and 1,046 cases were finally included in the study.
This study used data on participants’ habits, medical history, current condition, and medication usage from self-administered questionnaires. A flowchart for enrollment and exclusion criteria is available in a previous study [23]. Follow-up surveys were administered at 19-year intervals for the first cohort and at 7-year intervals for the second cohort. Survival status was ascertained from the Japanese Basic Resident Register. This study analyzed assessment data from both cohorts (N = 3,553). The Institutional Review Board (RIB) of Ehime University Hospital (1903018) reviewed and approved this study. All participants provided written informed consent.
2.2. Evaluation of risk factors
Weight and height were measured as baseline anthropometric indices. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2). Smoking status (in pack-years) was estimated by multiplying the number of years as a smoker by the average number of packs per day. Participants were categorized as non-smokers, ex-smokers, light smokers (< 20 pack-years), and heavy smokers (≥ 20 pack-years). Daily alcohol intake was estimated in units equivalent to one bottle of sake (22.9 g ethanol). Participants were categorized as non-drinkers, occasional drinkers (< 1 unit/day), light daily drinkers (1–2 units/day), and heavy daily drinkers (2–3 units/day). No participant consumed more than 3 units/day. An automated sphygmomanometer was used to measure systolic blood pressure (SBP) and diastolic blood pressure (DBP). Participants were asked to rest for at least 5 min in a seated position before an appropriately sized cuff was placed on their right upper arm. All participants were asked to fast overnight prior to measuring their triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), SUA, blood glucose (BG), alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was modified with a Japanese coefficient as follows to estimate the glomerular filtration ratio (eGFR) in males: Cr ≤ 0.9 mg/dL, 141 × (Cr/0.9) −0.411 × 0.993 age × 0.813; Cr > 0.9 mg/dL, 141 × (Cr/0.9) −1.209 × 0.993 age × 0.813; and in females: Cr ≤ 0.7 mg/dL, 144 × (Cr/0.7) −0.329 × 0.993 age × 0.813; Cr > 0.7 mg/dL, 144 × (Cr/0.7) −1.209 × 0.993 age × 0.813 [24].
Participants who reported a SBP of 140 mmHg or higher, a DBP of 90 mmHg or higher, or were on antihypertensive medication were considered to have hypertension. In addition, participants were classified as having hypertriglyceridemia if their TG levels were 150 mg/dL or higher; as having low HDL cholesterolemia if their HDL-C levels were 39 mg/dL or lower; and as having high LDL-cholesterolemia if their LDL-C levels were 140 mg/dL or higher, or if they were taking antidyslipidemic medication. Those with BG of 126 mg/dL or higher and those on antidiabetic medication were considered diabetic. Males with SUA 8.5 mg/dL or higher and females with SUA 7.5 mg/dL or higher were classified as hyperuricemic [22,[25], [26], [27]]. Those with AST of 40 IU/L or higher or ALT of 45 IU/L or higher were defined as liver dysfunction. eGFR less than 60 mL/min/1.73 m2 or was considered an indicator of CKD. Ischemic heart disease, ischemic stroke, and peripheral vascular disease were all classified as types of CVD.
2.3. Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics (version 28.0; SPSS Inc., Chicago, IL, United States). Continuous variables of a normal distribution were expressed as mean ± standard deviation (SD), and median and interquartile range were reported for variables showing a non-normal distribution (e.g., TG, BG, AST, and ALT). In all analyses, log-transformed values were used for non-normally distributed parameters. Categorical variables were compared by conducting a chi-square analysis, and continuous variables were compared by performing a student's t-test on normally distributed variables. Cox proportional hazards regression was used to examine factors related to all-cause mortality and to model relationships between SUA and all-cause mortality while adjusting for baseline characteristics and using age as the time scale. Adjustments were performed for possible confounding factors: age, BMI, smoking habits, drinking habits, history of CVD, hypertension, hypertriglyceridemia, low HDL-cholesterolemia, high LDL-cholesterolemia, diabetes, liver dysfunction, and CKD. The reference group comprised those with the lowest hazard ratio (HR) for mortality. The observed association between baseline SUA and all-cause mortality was confirmed by performing a sensitivity analysis in groups stratified by age (< 65 years and ≥ 65 years), BMI (< 25 kg/m2 and ≥ 25 kg/m2), CVD (presence and absence), eGFR (< 60 mL/min/1.73 m2 and ≥ 60 mL/min/1.73 m2), SUA-lowering medication (presence and absence), and time to death. The effect variable and all significant confounding variables other than the effect variable were adjusted for in interaction tests. All p-values were two-sided, and p < 0.05 was considered significant.
3. Results
3.1. Baseline characteristics of participants by sex and baseline SUA categories
Of the 3,553 subjects, 1,573 (44.3%) were male. The mean age of the male participants was 63 ± 14 years and the mean age of the female participants was 65 ± 12 years. Table 1a (male) andTable 1b (female) report the baseline characteristics of the participants by their SUA categories. The results indicate that antihypertensive and SUA-lowering medication; higher values of BMI, TG, and SUA; and lower eGFR were associated with higher SUA levels in both males and females. Male subjects with higher SUA levels were younger and had a higher prevalence of CKD, while female subjects were older and had a higher prevalence of CKD.
Table 1a.
Baseline characteristics of male participants by baseline serum uric acid categories.
| Men Characteristics N = 1,573 |
SUA < 3.5 mg/dL N = 46 |
3.5 ≤ SUA < 8.5 mg/dL N = 1,475 |
SUA ≥ 8.5 mg/dL N = 52 |
p-value * |
|---|---|---|---|---|
| Age (years) | 68 ± 11 | 63 ± 13 | 60 ± 16 | 0.008 |
| Obesity, n (%) | 5 (10.9) | 404(27.4) | 19 (36.5) | 0.014 |
| Body mass index (kg/m2) | 22.4 ± 2.3 | 23.4 ± 3.0 | 24.7 ± 3.6 | 0.001 |
| Smoking habits (non = 1, ex = 2, light = 3, heavy = 4) (%) | 41.3/41.3/2.2/15.2 | 35.7/48.0/4.9/11.5 | 30.8/59.6/1.9/7.7 | 0.491 |
| Alcohol habits (non = 1, occasional = 2, light = 3, heavy = 4) (%) | 34.8/21.7/23.9/19.6 | 22.7/31.6/26.3/19.4 | 15.4/21.2/28.8/34.6 | 0.038 |
| History of cardiovascular disease, n (%) | 4 (8.7) | 145 (9.8) | 7 (13.5) | 0.664 |
| Hypertension, n (%) | 26 (56.5) | 851 (57.7) | 39 (75.0) | 0.044 |
| Systolic blood pressure (mmHg) | 137 ± 20 | 138 ± 20 | 142 ± 19 | 0.227 |
| Diastolic blood pressure (mmHg) | 80 ± 11 | 82 ± 11 | 87 ± 12 | 0.003 |
| Antihypertensive medication, n (%) | 11 (23.9) | 462 (31.3) | 24 (46.2) | 0.041 |
| Hypertriglyceridemia, n (%) | 7 (15.2) | 317 (21.5) | 19 (36.5) | 0.019 |
| Triglyceride (mg/dL) | 79 (63–105) | 96 (72–138) | 117 (83–186) | 0.001 |
| Low HDL-cholesterolemia, n (%) | 3 (6.5) | 122 (8.3) | 9 (17.3) | 0.064 |
| HDL cholesterol (mg/dL) | 63 ± 17 | 60 ± 16 | 57 ± 16 | 0.133 |
| High LDL-cholesterolemia, n (%) | 10 (21.7) | 341 (23.1) | 13 (25.0) | 0.927 |
| LDL cholesterol (mg/dL) | 114 ± 31 | 110 ± 31 | 110 ± 36 | 0.730 |
| Lipid-lowering medication, n (%) | 0 (0) | 104 (7.1) | 4 (7.7) | 0.172 |
| Diabetes, n (%) | 11 (23.9) | 228 (15.5) | 8 (15.4) | 0.299 |
| Blood glucose (mg/dL) | 99 (91–121) | 103 (93–117) | 103 (94–114) | 0.195 |
| Antidiabetic medication, n (%) | 8 (17.4) | 176 (11.9) | 6 (11.5) | 0.531 |
| Liver dysfunction, n (%) | 4 (8.7) | 155 (10.5) | 11 (21.2) | 0.047 |
| Alanine aminotransferase (IU/L) | 19 (14–24) | 20 (15–27) | 23 (15–38) | 0.016 |
| Aspartate aminotransferase (IU/L) | 24 (19-30) | 24 (20–30) | 26 (21–36) | 0.206 |
| Chronic kidney disease, n (%) | 1 (2.2) | 170 (11.5) | 16 (30.8) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 83.9 ± 13.9 | 77.8 ± 16.2 | 67.5 ± 19.1 | < 0.001 |
| Serum uric acid (mg/dL) | 2.7 ± 0.8 | 5.9 ± 1.1 | 9.2 ± 0.8 | < 0.001 |
| SUA-lowering medication, n (%) | 2 (4.3) | 110 (7.5) | 26 (50.0) | < 0.001 |
Data presented as mean ± standard deviation. Data for triglycerides, blood glucose, alanine aminotransferase, and aspartate aminotransferase were skewed and are thus presented as median (interquartile range) values and were log-transformed for analysis. *P-values are from the Student's t-test for continuous variables or from the χ2-test for categorical variables. Significant values (p < 0.05) are presented in bold.
Table 1b.
Baseline characteristics of female participants by baseline serum uric acid categories.
| Women Characteristics N = 1,980 |
SUA < 3.0 mg/dL N = 105 |
3.0 ≤ SUA < 7.5 mg/dL N = 1857 |
SUA ≥ 7.5 mg/dL N = 18 |
p-value * |
|---|---|---|---|---|
| Age (years) | 64 ± 12 | 64 ± 12 | 74 ± 6 | 0.002 |
| Obesity, n (%) | 13 (12.4) | 508 (27.4) | 10 (55.6) | < 0.001 |
| Body mass index (kg/m2) | 21.4 ± 2.9 | 23.2 ± 3.3 | 24.7 ± 3.8 | < 0.001 |
| Smoking habits (non = 1, ex = 2, light = 3, heavy = 4) (%) | 93.3/5.7/1.0/0.0 | 90.2/7.9/1.3/0.6 | 72.2/27.8/0.0/0.0 | 0.075 |
| Alcohol habits (non = 1, occasional = 2, light = 3, heavy = 4) (%) | 77.1/21.9/1.0/0.0 | 74.3/21.4/3.9/0.4 | 50.0/44.4/5.6/0.0 | 0.181 |
| History of cardiovascular disease, n (%) | 4 (3.8) | 121 (6.5) | 2 (11.1) | 0.391 |
| Hypertension, n (%) | 51 (48.6) | 1,036 (55.8) | 14 (77.8) | 0.057 |
| Systolic blood pressure (mmHg) | 135 ± 23 | 137 ± 22 | 144 ± 23 | 0.232 |
| Diastolic blood pressure (mmHg) | 77 ± 12 | 79 ± 11 | 81 ± 14 | 0.178 |
| Antihypertensive medication, n (%) | 19 (18.1) | 585 (31.5) | 10 (55.6) | 0.001 |
| Hypertriglyceridemia, n (%) | 10 (9.5) | 288 (15.5) | 3 (16.7) | 0.248 |
| Triglyceride (mg/dL) | 82 (57–115) | 90 (68–126) | 107 (91–138) | 0.006 |
| Low HDL-cholesterolemia, n (%) | 2 (1.9) | 59 (3.2) | 0 | 0.572 |
| HDL cholesterol (mg/dL) | 66 ± 14 | 65 ± 16 | 57 ± 12 | 0.072 |
| High LDL-cholesterolemia, n (%) | 24 (22.9) | 727 (39.1) | 8 (44.4) | 0.003 |
| LDL cholesterol (mg/dL) | 114 ± 29 | 125 ± 29 | 133 ± 27 | < 0.001 |
| Lipid-lowering medication, n (%) | 10 (9.5) | 227 (12.2) | 2 (11.1) | 0.705 |
| Diabetes, n (%) | 9 (8.6) | 193 (10.4) | 3 (16.7) | 0.567 |
| Blood glucose (mg/dL) | 97 (88–115) | 98 (90–114) | 106 (94–126) | 0.399 |
| Antidiabetic medication, n (%) | 7 (6.7) | 117 (6.3) | 2 (11.1) | 0.701 |
| Liver dysfunction, n (%) | 3 (2.9) | 81 (4.4) | 1 (5.6) | 0.734 |
| Alanine aminotransferase (IU/L) | 14 (11–19) | 16 (13–20) | 14 (13–17) | 0.037 |
| Aspartate aminotransferase (IU/L) | 21 (18–26) | 21 (19–26) | 21 (18–23) | 0.482 |
| Chronic kidney disease, n (%) | 2 (1.9) | 156 (8.4) | 12 (66.7) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 85.4 ± 16.9 | 78.1 ± 15.6 | 51.6 ± 15.6 | < 0.001 |
| Serum uric acid (mg/dL) | 2.5 ± 0.4 | 4.6 ± 1.0 | 8.2 ± 0.8 | < 0.001 |
| SUA-lowering medication, n (%) | 0 | 14 (0.8) | 2 (11.1) | < 0.001 |
Data presented as mean ± standard deviation. Data for triglycerides, blood glucose, alanine aminotransferase, and aspartate aminotransferase were skewed and are thus presented as median (interquartile range) values and were log-transformed for analysis. *P-values are from the Student's t-test for continuous variables or from the χ2-test for categorical variables. Significant values (p < 0.05) are presented in bold.
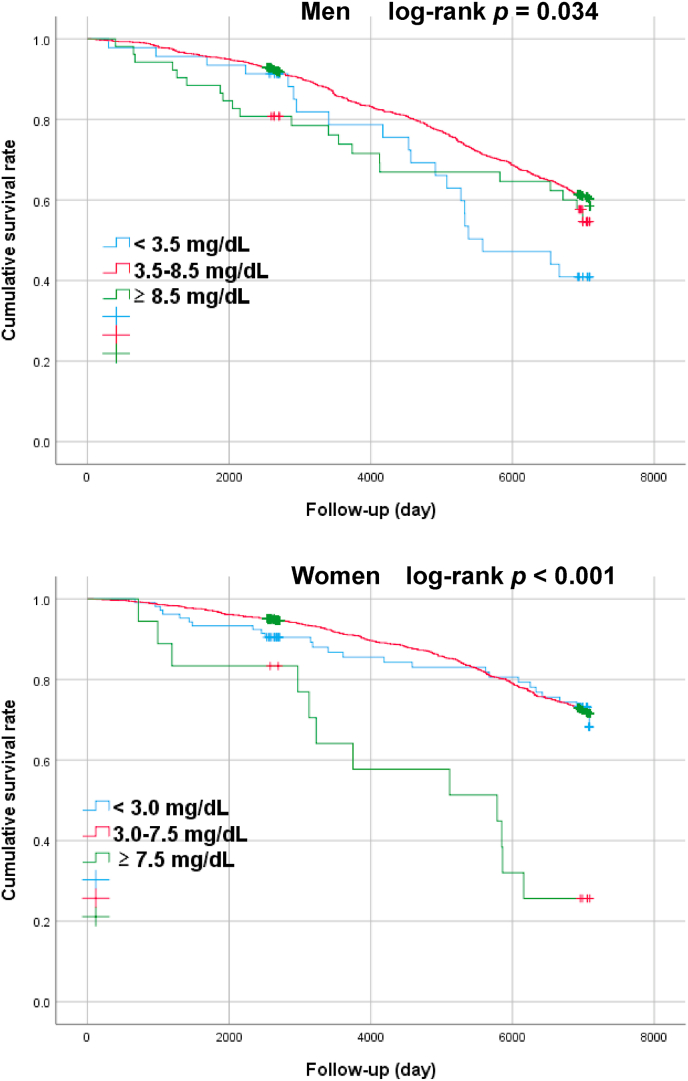
3.2. Kaplan-Meier survival curves for relationships between baseline SUA categories and all-cause mortality by sex
The median (interquartile range) follow-up period was 6,391 (2,694–6,991) days. The follow-up revealed 905 deaths (25.5%), of which 473 were male (30.1% of all males) and 432 were female (21.8% of all females). Kaplan-Meier survival curves were created for survival days and cumulative survival rates to identify patterns in the relationships between the SUA categories and all-cause mortality by sex (Fig. 1).
Fig. 1.
Analysis on association between SUA categories and all-cause mortality by sex during the follow-up period using a survival function. P-values were obtained through a log-rank test of equality across various strata.
The cumulative survival rate was significantly lower in female subject with hyperuricemia (females: ≥ 7.5 mg/dL) compared with the reference group (SUA ≥ 3.0 and SUA < 7.5 mg/dL) (log-rank p < 0.001). In male subject, on the other hand, hypouricemia group (SUA ≤ 3.5 mg/dL) showed lower cumulative survival rate compared to reference group (SUA ≥ 3.5 and SUA < 8.5 mg/dL) (log-rank P = 0.034).
3.3. Hazard ratios for all-cause mortality per 1.0 mg/dL increase in baseline SUA levels by sex
Table 2 reports the relationship between a 1.0 mg/dL increase in baseline SUA levels and all-cause mortality. As per the unadjusted and multivariable-adjusted model, for each 1.0 mg/dL increase in SUA level, the table shows the HR for all-cause mortality in both males with baseline SUA of ≥ 6.0 mg/dL (HR: 1.25; 95% CI: 1.07–1.47) and females with baseline SUA of ≥ 5.0 mg/dL (HR: 1.27; 95% CI: 1.03–1.58).
Table 2.
Hazard ratios for all-cause mortality per 1.0 mg/dL increase in baseline serum uric acid levels by sex.
| Men Characteristics N = 1,573 |
SUA < 6.0 mg/dL N = 823 |
p-value | SUA ≥ 6.0 mg/dL N = 750 |
p-value |
|---|---|---|---|---|
| Unadjusted HR (95% CI) | 0.90 (0.79–1.02) | 0.108 | 1.28 (1.10–1.49) | 0.002 |
| Age-adjusted HR (95% CI) | 1.07 (0.93–1.23) | 0.379 | 1.27 (1.09–1.48) | 0.002 |
| Multivariable-adjusted HR * (95% CI) |
1.00 (0.87–1.16) |
0.960 |
1.25 (1.07–1.47) |
0.007 |
| Women Characteristics N = 1,980 |
SUA < 5.0 mg/dL N = 1,353 |
p-value |
SUA ≥ 5.0 mg/dL N = 627 |
p-value |
| Unadjusted HR (95% CI) | 1.06 (0.89–1.27) | 0.509 | 1.53 (1.27–1.85) | < 0.001 |
| Age-adjusted HR (95% CI) | 1.04 (0.88–1.24) | 0.636 | 1.23 (1.01–1.50) | 0.040 |
| Multivariable-adjusted HR * (95% CI) | 1.03 (0.86–1.24) | 0.769 | 1.27 (1.03–1.58) | 0.028 |
HR, hazard ratio; CI, confidence interval. * Multivariable-adjusted for age, obesity, smoking habits, drinking habits, history of cardiovascular disease, hypertension, hypertriglyceridemia, low HDL-cholesterolemia, high LDL-cholesterolemia, diabetes, liver dysfunction, and chronic kidney disease.
3.4. Relationship between baseline SUA categories and all-cause mortality by sex
Table 3 shows the results for the unadjusted model. The hyperuricemia group for female and the hypouricemia group for male subjects reported a higher HR for all-cause mortality when compared with the reference group. In the multivariate model, however, the hyperuricemia group for both male and female subjects showed a higher HR for all-cause mortality than reference group. The results also show that adjusted HR for all-cause mortality significantly increased in male subjects reporting a SUA ≥ 8.5 mg/dL (HR: 1.67; 95% CI: 1.06–2.63) and in female subjects reporting a SUA ≥ 7.5 mg/dL (HR: 2.17; 95% CI: 1.20–3.94).
Table 3.
Hazard ratios of baseline serum uric acid categories for all-cause mortality by sex.
| Men Characteristics N = 1,573 |
SUA < 3.5 mg/dL N = 46 |
p-value | 3.5 ≤ SUA < 8.5 mg/dL N = 1,475 |
SUA ≥ 8.5 mg/dL N = 52 |
p-value | p for trend |
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | 1.70 (1.09–2.67) | 0.020 | Reference | 1.31 (0.85–2.04) | 0.222 | 0.036 |
| Age-adjusted HR (95% CI) | 1.17 (0.75–1.84) | 0.491 | Reference | 1.82 (1.18–2.83) | 0.007 | 0.023 |
| Multivariable HR * (95% CI) |
1.22 (0.78–1.93) |
0.388 |
Reference |
1.67 (1.06–2.63) |
0.026 |
0.061 |
| Women Characteristics N = 1,980 |
SUA < 3.0 mg/dL N = 105 |
p-value |
3.0 ≤ SUA < 7.5 mg/dL N = 1,857 |
SUA ≥ 7.5 mg/dL N = 18 |
p-value |
p for trend |
| Unadjusted HR (95% CI) | 1.07 (0.72–1.61) | 0.739 | Reference | 4.24 (2.39–7.53) | <0.001 | < 0.001 |
| Age-adjusted HR (95% CI) | 1.03 (0.68–1.54) | 0.901 | Reference | 2.27 (1.28–4.04) | 0.005 | 0.020 |
| Multivariable HR * (95% CI) |
1.00 (0.66–1.51) |
0.998 |
Reference |
2.17 (1.20–3.94) |
0.010 |
0.038 |
| Total Men Women Characteristics N = 3,553 |
SUA < 3.5 mg/dL SUA < 3.0 mg/dL N = 151 |
p-value |
3.5 ≤ SUA < 8.5 mg/dL 3.0 ≤ SUA < 7.5 mg/dL N = 3,332 |
SUA ≥ 8.5 mg/dL SUA ≥ 7.5 mg/dL N = 70 |
p-value |
p for trend |
| Unadjusted HR (95% CI) | 1.19 (0.88–1.61) | 0.249 | Reference | 2.03 (1.43–2.87) | < 0.001 | < 0.001 |
| Age-adjusted HR (95% CI) | 1.03 (0.76–1.39) | 0.859 | Reference | 2.19 (1.54–3.10) | < 0.001 | < 0.001 |
| Multivariable HR ** (95% CI) | 1.11 (0.82–1.50) | 0.515 | Reference | 1.87 (1.31–2.67) | 0.001 | 0.002 |
HR, hazard ratio; CI, confidence interval. * All-adjusted for age, obesity, smoking habits, drinking habits, history of cardiovascular disease, hypertension, hypertriglyceridemia, low HDL-cholesterolemia, hyper LDL-cholesterolemia, diabetes, liver dysfunction, and chronic kidney disease. ** All-adjusted for sex, age, body mass index, smoking habits, drinking habits, history of cardiovascular disease, hypertension, hypertriglyceridemia, low HDL-cholesterolemia, high LDL-cholesterolemia, diabetes, liver dysfunction, and chronic kidney disease. Significant values (p < 0.05) are presented in bold.
3.5. Relationship between baseline SUA categories and all-cause mortality by sub-analysis
Finally, Table 4 reports the results for participants stratified by age (< 65 years and ≥ 65 years), BMI (< 25 and ≥ 25 kg/m2), History of CVD, CKD (eGFR < 60 mL/min/1.73 m2 and eGFR ≥ 60 mL/min/1.73 m2), SUA-lowering medication (presence and absence), and time to death. The findings confirmed a significant association between hyperuricemia and all-cause mortality in the group that survived for more than three years. This association was present, regardless of the presence of CKD or SUA-lowering medication. The findings also showed the association between hypouricemia with SUA-lowering medication and all-cause mortality. There was no interaction between hypo-/hyper-uricemia and these factors.
Table 4.
Hazard ratios of baseline serum uric acid for all-cause mortality by sub-analysis.
| Total Men Women Characteristics N = 3,553 |
SUA < 3.5 mg/dL SUA < 3.0 mg/dL N = 151 |
p-value | 3.5 ≤ SUA < 8.5 mg/dL 3.0 ≤ SUA < 7.5 mg/dL N = 3,332 |
SUA ≥ 8.5 mg/dL SUA ≥ 7.5 mg/dL N = 70 |
p-value | p for interaction |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| Age | ||||||
| < 65 years (N = 1,522) | 1.51 (0.69–3.31) | 0.299 | Reference | 2.46 (1.01–6.00) | 0.048 | 0.559 |
| ≥ 65 years (N = 2,031) | 1.07 (0.77–1.48) | 0.699 | Reference | 1.81 (1.22–2.69) | 0.003 | |
| Body mass index | ||||||
| < 25.0 kg/m2 (N = 2,594) | 1.00 (0.72–1.40) | 0.983 | Reference | 2.21 (1.41–3.46) | 0.001 | 0.099 |
| ≥ 25.0 kg/m2 (N = 959) | 2.19 (1.01–4.73) | 0.047 | Reference | 1.48 (0.79–2.79) | 0.223 | |
| History of cardiovascular disease | ||||||
| No (N = 3270) | 1.10 (0.79–1.53) | 0.566 | Reference | 2.07 (1.38–3.11) | < 0.001 | 0.278 |
| Yes (N = 283) | 1.31 (0.56–3.06) | 0.535 | Reference | 1.09 (0.45–2.60) | 0.855 | |
| CKD (eGFR) | ||||||
| < 60 mL/min/1.73 m2 (N = 357) | 2.41 (0.72–8.00) | 0.152 | Reference | 2.09 (1.16–3.77) | 0.014 | 0.229 |
| ≥ 60 mL/min/1.73 m2 (N = 3,196) | 1.06 (0.77–1.45) | 0.725 | Reference | 1.81 (1.14–2.89) | 0.012 | |
| Serum uric acid-lowering medication | ||||||
| No (N = 3,399) | 1.08 (0.79–1.47) | 0.628 | Reference | 1.86 (1.17–2.95) | 0.009 | 0.511 |
| Yes (N = 154) | 7.61 (1.12–51.6) | 0.038 | Reference | 2.18 (1.13–4.22) | 0.021 | |
| Time to death | ||||||
| < 1,095 days (N = 73) | – | – | – | – | – | – |
| ≥ 1,095 days (N = 3,480) | 1.04 (0.75–1.44) | 0.810 | Reference | 1.73 (1.18–2.55) | 0.005 | |
HR, hazard ratio; CI, confidence interval. Multivariable-adjusted for sex, age, obesity, smoking habits, drinking habits, history of cardiovascular disease, hypertension, hypertriglyceridemia, low HDL-cholesterolemia, high LDL-cholesterolemia, diabetes, liver dysfunction, and chronic kidney disease. Significant values (p < 0.05) are presented in bold.
4. Discussion
This study presented a long-term prospective cohort study on the relationship between baseline SUA and all-cause mortality risk among a community-based population sample. Drawing on findings from the median 17.5-year follow-up, this cohort study mainly highlighted that a significant and independent predictor of all-cause mortality is baseline hyperuricemia (males: SUA ≥ 8.5 mg/dL; females: SUA ≥ 7.5 mg/dL) after adjusting for confounders in community-dwelling individuals. The sensitivity analysis showed a similar trend, although there was a U-shaped relationship between baseline SUA levels and all-cause mortality in the group comprising subjects with SUAlowering medication who had been determined to be in need of treatment.
To the best of the authors’ knowledge, few studies have reported SUA levels associated with optimal patient survival. This study is the first to show a critical association between increased SUA levels and reduced survival and the first to establish a significant reduction in survival associated with values above a defined threshold range [17,27]. However, it remains yet to be proven if SUA is an independent risk factor for mortality and whether there exists a specific threshold associated with increased risk.
In Japan, hyperuricemia is generally defined as SUA > 7.0 mg/dL, irrespective of sex [28]. This study showed that baseline SUA levels of ≥ 8.5 mg/dL for males and ≥ 7.5 mg/dL for females were associated with increased mortality risks, only after adjustment for confounding risk factors. Virdis et al. [29] recently reported a linear association between SUA and mortality for a sample of 22,714 Italian subjects. The thresholds were 321 μmol/L (5.4 mg/dL) for males and 279 μmol/L (4.7 mg/dL) for females, and these values were significantly lower than those reported in this study. Further to this, a large-scale cohort study comprising 500,511 Japanese subjects (40–74 years) noted a significant increase in HR for all-cause mortality in males with SUA levels ≥ 7.0 mg/dL and in females with SUA levels ≥ 5.0 mg/dL [27]. By contrast, reports have shown differences in the relationship between SUA and all-cause mortality across both sexes, with some indicating a relationship only in men [7,8,16] or women [11,17,30]. The present study defined hyperuricemia as SUA ≥ 8.5 mg/dL for males and SUA ≥ 7.5 mg/dL for females. The results revealed that hyperuricemia was associated with an increased risk in all-cause mortality in both Japanese males and females. Furthermore, for each 1 mg/dL increase in SUA level, a significant increase in the HR for all-cause mortality in males with baseline SUA ≥ 6.0 mg/dL and in females with baseline SUA ≥ 5.0 mg/dL was reported. The differences in SUA thresholds for all-cause mortality reported in this study may reflect inherent differences in the populations of interest and the different degrees of adjustment for confounding factors [22].
Previous studies on the relationship between SUA and all-cause mortality have yielded conflicting results, sparking debate over whether low or high levels of SUA have a negative health impact [[20], [21], [22],31]. A retrospective cohort study performed on 26,525 Irish participants showed that the trend for the association between SUA and mortality differed between men and women. That is, mortality risk significantly increased in men at extreme thresholds of SUA < 304 μmol/L (3.1 mg/dL) and SUA > 454 μmol/L (7.6 mg/dL), and this relationship was U-shaped. On the other hand, mortality risk increased for women with SUA > 409 μmol/L (6.8 mg/dL), and this association was J-shaped [22]. Hu et al. [31] used data from the National Health and Nutrition Examination Survey involving 9,118 adults in the United States and demonstrated a U-shaped association for both sexes. The inflection point for SUA was 6 mg/dL in males and 4 mg/dL in females. Cho et al.‘s [19] study was based on 375,163 males and females in South Korea. Compared with the sex-specific reference category, they reported that the multivariable-adjusted HRs for all-cause mortality in the lowest SUA categories (< 3.5 mg/dl for males and < 2.5 mg/dl for females) were 1.58 (95% CI: 1.18–2.10) and 1.80 (95% CI: 1.10–2.93) for males and females, respectively. Corresponding HRs in the highest SUA categories (males: ≥ 9.5 mg/dL; females: ≥ 8.5 mg/dL) were 2.39 (95% CI: 1.57–3.66) and 3.77 (95% CI: 1.17–12.17) for males and females, respectively.
The mechanisms behind an increased all-cause mortality in individuals with hyperuricemia are yet to be fully understood. This may be explained by the relative contribution of the antioxidant effects of UA and its role in endothelial dysfunction. To this effect, studies have reported an association between elevated SUA levels and increased antioxidant capacity [32]. However, the protective antioxidant effect may be lost if it is overwhelmed by other detrimental effects that increase in association with higher UA levels. SUA alters the proliferation and migration of human vascular smooth muscle cells, as well as their release of nitric oxide (NO), via the expression of C-reactive protein (CRP) [33,34], stimulating cell proliferation, angiotensin II production, and oxidative stress via the tissue renin-angiotensin system [35]. NO increases blood flow to skeletal muscles, promotes glucose uptake, and is strongly associated with the action of insulin [36]. Thus, hyperuricemia induces endothelial dysfunction. This may allude to the pathogenic mechanism through which SUA induces vascular disease [37]. In a previous study, SUA was shown to be an independent determinant of insulin resistance and that a combined assessment of SUA and high-sensitivity CRP levels provides additional information on the risk stratification of community-dwelling females with metabolic syndrome [38]. Therefore, high SUA levels may contribute to high mortality. Recent studies suggested that pre-menopausal women reported lower SUA levels than post-menopausal women because estrogenic compounds increased renal UA clearance [39] and were strongly associated with other cardiovascular risk factors such as age, sex, BMI, SBP, DBP, total cholesterol, TG, and BG [32]. Thus, the association between SUA and all-cause mortality largely reflects the predominance of metabolic risk factors such as aging, insulin resistance, dyslipidemia, and renal dysfunction, which may exclude the effect of SUA on males exhibiting the aforementioned factors.
4.1. Limitation of the study
The key strengths of this study include its accuracy, which was only possible through a long-term follow-up study with a given number of subjects, as well as its adjustment for many possible confounders. However, this study is not free from limitations. Firstly, the sample focused on relatively healthy middle-aged and older adults (mean age: 64 ± 13 years) who participated in a health checkup and who lived in rural Japan, a country with a significant aging population. The sample cannot be considered representative of the general population. Secondly, the survey covered those whose deaths were registered in the Basic Resident Ledger. It did not account for those who moved away from the region during the survey period. Thirdly, the possible effects of medication (e.g., antihypertensive, lipid-lowering, antidiabetic, and SUA-lowering medication), underlying disease, metabolic syndrome, and lifestyle modification (e.g., dietary practice that may play an important role in SUA production) at baseline and during follow-up cannot be overlooked. Fourthly, renal function assessment was performed only by eGFR without urinary albumin data. Fifth, the threshold for the low SUA group may have been too low to evaluate the U-shaped relationship between SUA levels and all-cause mortality. Finally, it is possible that the relatively low number of participants and deaths could have weakened the causal relationship between SUA levels and all-cause mortality.
5. Conclusions
The follow-up study on subjects aged 22 years and above found a strong association between hyperuricemia (male: SUA ≥ 8.5 mg/dL; female: SUA ≥ 7.5 mg/dL) and increased all-cause mortality on Japanese community-dwelling individuals, after adjusting for potential confounders such as body composition index and metabolic factors.
Ethics approval
The study was approved by the Ethics Committee of the Ehime University Graduate School of Medicine (IRB: no. 1903018). Informed consent was obtained from all participating subjects in the study.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research from the Foundation for Development of Community (2021). No additional external funding was received. The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Declaration of conflicting interests
The authors declare that they have no competing interests.
Authors’ contributions
AK and RK participated in the study design, performed the statistical analysis, and drafted the manuscript. AK, RK, DN, and TK contributed to data acquisition and interpretation. AK and RK contributed to the conception and design of the statistical analysis. RK conceived the study, participated in its design, coordinated and helped with drafting the manuscript. All authors have read and approved the manuscript.
Acknowledgements
We thank Uni-edit (https://uni-edit.net/) for editing and proofreading this manuscript.
Contributor Information
Asuka Kikuchi, Email: kikuchi.asuka.xo@ehime-u.ac.jp.
Ryuichi Kawamoto, Email: rykawamo@m.ehime-u.ac.jp.
Daisuke Ninomiya, Email: 98065dn@jichi.ac.jp.
Teru Kumagi, Email: terukuma@m.ehime-u.ac.jp.
List of abbreviations
- SUA
serum uric acid
- CVD
cardiovascular diseases
- IRB
Institutional Review Board
- HR
hazard ratio
- CI
confidence interval
- BMI
body mass index
- UA
uric acid
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- TG
triglyceride
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- BG
blood glucose
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
estimate the glomerular filtration ratio
- SD
standard deviation
- NO
nitric oxide
- CRP
C-reactive protein
References
- 1.Kushiyama A., Nakatsu Y., Matsunaga Y., Yamamotoya T., Mori K., Ueda K., et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediat Inflamm. 2016;2016:8603164. doi: 10.1155/2016/8603164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyngdoh T., Marques-Vidal P., Paccaud F., Preisig M., Waeber G., Bochud M., et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaubert M., Marlinge M., Alessandrini M., Laine M., Bonello L., Fromonot J., et al. Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signal. 2018;14:191–199. doi: 10.1007/s11302-018-9604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali N., Mahmood S., Islam F., Rahman S., Haque T., Islam S., et al. Relationship between serum uric acid and hypertension: a cross-sectional study in Bangladeshi adults. Sci Rep. 2019;9:9061. doi: 10.1038/s41598-019-45680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodama S., Saito K., Yachi Y., Asumi M., Sugawara A., Totsuka K., et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman D.S., Williamson D.F., Gunter E.W., Byers T. Relation of serum uric acid to mortality and ischemic heart disease: the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 7.Niskanen L.K., Laaksonen D.E., Nyyssönen K., Alfthan G., Lakka H.-M., Lakka T.A., et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 8.Zhao G., Huang L., Song M., Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231:61–68. doi: 10.1016/j.atherosclerosis.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Kleber M.E., Delgado G., Grammer T.B., Silbernagel G., Huang J., Krämer B.K., et al. Uric acid and cardiovascular events: a mendelian randomization study. J Am Soc Nephrol. 2015;26:2831–2838. doi: 10.1681/ASN.2014070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo T., Liu X., Jiang L., Mao S., Yin X., Guo L. Hyperuricemia and coronary heart disease mortality: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2016;16:207. doi: 10.1186/s12872-016-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M., Hu X., Fan Y., Li K., Zhang X., Hou W., et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. 2016;6:19520. doi: 10.1038/srep19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konta T., Ichikawa K., Kawasaki R., Fujimoto S., Iseki K., Moriyama T., et al. Association between serum uric acid levels and mortality: a nationwide community-based cohort study. Sci Rep. 2020;10:6066. doi: 10.1038/s41598-020-63134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying H., Yuan H., Tang X., Guo W., Jiang R., Jiang C. Impact of serum uric acid lowering and contemporary uric acid-lowering therapies on cardiovascular outcomes: a systematic review and meta-analysis. Front. Cardiovasc. Med. 2021;8:641062. doi: 10.3389/fcvm.2021.641062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang M.W., Chin H.J., Joo K.W., Na K.Y., Kim S., Han S.S. Hyperuricemia is associated with acute kidney injury and all-cause mortality in hospitalized patients. Nephrology. 2019;24:718–724. doi: 10.1111/nep.13559. [DOI] [PubMed] [Google Scholar]
- 15.Takae K., Nagata M., Hata J., Mukai N., Hirakawa Y., Yoshida D., et al. Serum uric acid as a risk factor for chronic kidney disease in a Japanese community - the hisayama study. Circ J : Off. J. Jpn. Circ. Soc. 2016;80:1857–1862. doi: 10.1253/circj.CJ-16-0030. [DOI] [PubMed] [Google Scholar]
- 16.Otaki Y., Konta T., Ichikawa K., Fujimoto S., Iseki K., Moriyama T., et al. Possible burden of hyperuricaemia on mortality in a community-based population: a large-scale cohort study. Sci Rep. 2021;11:8999. doi: 10.1038/s41598-021-88631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamei K., Konta T., Ichikawa K., Sato H., Suzuki N., Kabasawa A., et al. Serum uric acid levels and mortality in the Japanese population: the Yamagata (Takahata) study. Clin Exp Nephrol. 2016;20:904–909. doi: 10.1007/s10157-016-1228-1. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Iso H., Murakami Y., Miura K., Nagai M., Sugiyama D., et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-Japan study. J Atherosclerosis Thromb. 2016;23:692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho S.K., Chang Y., Kim I., Ryu S. U-shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. 2018;70:1122–1132. doi: 10.1002/art.40472. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C.-F., See L.-C., Yu K.-H., Chou I.-J., Chiou M.-J., Luo S.-F. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology. 2012;52:127–134. doi: 10.1093/rheumatology/kes223. [DOI] [PubMed] [Google Scholar]
- 21.Tseng W.C., Chen Y.T., Ou S.M., Shih C.J., Tarng D.C. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: the role of malnourishment. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browne L.D., Jaouimaa F.Z., Walsh C., Perez-Ruiz F., Richette P., Burke K., et al. Serum uric acid and mortality thresholds among men and women in the Irish health system: a cohort study. Eur J Intern Med. 2021;84:46–55. doi: 10.1016/j.ejim.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto R., Kikuchi A., Akase T., Ninomiya D., Kumagi T. Low density lipoprotein cholesterol and all-cause mortality rate: findings from a study on Japanese community-dwelling persons. Lipids Health Dis. 2021;20:105. doi: 10.1186/s12944-021-01533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horio M., Imai E., Yasuda Y., Watanabe T., Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis : Off. Natl. J. Kidney. Found. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 25.Tomita M., Mizuno S., Yamanaka H., Hosoda Y., Sakuma K., Matuoka Y., et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–409. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 26.Kamei K., Konta T., Ichikawa K., Sato H., Suzuki N., Kabasawa A., et al. Serum uric acid levels and mortality in the Japanese population: the Yamagata (Takahata) study. Clin Exp Nephrol. 2016;20:904–909. doi: 10.1007/s10157-016-1228-1. [DOI] [PubMed] [Google Scholar]
- 27.Konta T., Ichikawa K., Kawasaki R., Fujimoto S., Iseki K., Moriyama T., et al. Association between serum uric acid levels and mortality: a nationwide community-based cohort study. Sci Rep. 2020;10:6066. doi: 10.1038/s41598-020-63134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hisatome I., Ichida K., Mineo I., Ohtahara A., Ogino K. third ed. Gout and Uric & Nucleic Acids; 2019. Kuwabara, M. And Japanese Society of out and Nucleic Acids Guidelines for Management of hyperuricemia and Gout; pp. 1–40. [Google Scholar]
- 29.Virdis A., Masi S., Casiglia E., Tikhonoff V., Cicero A.F.G., Ungar A., et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension (Dallas) 2020;75:302–308. doi: 10.1161/HYPERTENSIONAHA.119.13643. [DOI] [PubMed] [Google Scholar]
- 30.Juraschek S.P., Tunstall-Pedoe H., Woodward M. Serum uric acid and the risk of mortality during 23 years follow-up in the scottish heart health extended cohort study. Atherosclerosis. 2014;233:623–629. doi: 10.1016/j.atherosclerosis.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L., Hu G., Xu B.P., Zhu L., Zhou W., Wang T., et al. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: a cohort study. J Clin Endocrinol Metabol. 2020;105 doi: 10.1210/clinem/dgz068. [DOI] [PubMed] [Google Scholar]
- 32.Chu N.F., Wang D.J., Liou S.H., Shieh S.M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol. 2000;16:13–17. doi: 10.1023/a:1007654507054. [DOI] [PubMed] [Google Scholar]
- 33.Kang D.H., Park S.K., Lee I.K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol : JASN (J Am Soc Nephrol) 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 34.Gersch C., Palii S.P., Kim K.M., Angerhofer A., Johnson R.J., Henderson G.N. Inactivation of nitric oxide by uric acid. Nucleos Nucleot Nucleic Acids. 2008;27:967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corry D.B., Eslami P., Yamamoto K., Nyby M.D., Makino H., Tuck M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.A., Montagnani M., Koh K.K., Quon M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 37.Jin M., Yang F., Yang I., Yin Y., Luo J.J., Wang H., et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci. 2012;17:656–669. doi: 10.2741/3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamoto R., Tabara Y., Kohara K., Miki T., Kusunoki T., Takayama S., et al. Usefulness of combining serum uric acid and high-sensitivity C-reactive protein for risk stratification of patients with metabolic syndrome in community-dwelling women. Endocrine. 2013;44:132–139. doi: 10.1007/s12020-013-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stöckl D., Döring A., Thorand B., Heier M., Belcredi P., Meisinger C. Reproductive factors and serum uric acid levels in females from the general population: the KORA F4 study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032668. [DOI] [PMC free article] [PubMed] [Google Scholar]