Graphical abstract
Abbreviations: M. oleifera Lam, Moringa oleifera Lam.; MOPs, Moringa oleifera Lam polysaccharides; ABTS, 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2.2-diphenyl-picryl-hydrazyl radical; WAE, Water-assisted extraction; EAE, Enzyme-assisted extraction; MAE, Microwave-assisted extraction; PLE, Pressurized liquid extraction; UAE, Ultrasound-assisted extraction; FTIR, Fourier transform infrared spectroscopy; HPLC, High performance liquid chromatography; MS, Mass spectrometry; NMR, Nuclear magnetic resonance; TOF, Time of flight; GC, Gas chromatography; GC–MS, Gas chromatography-mass spectrometry; HPGPC, High-performance gel permeation chromatography; MW, Molecular weight; Rha, Rhamnose; Xyl, Xylose; Man, Mannose; Glc, Glucose; Gal, Galactose; Ara, Arabinose; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide; BUN, Blood urea nitrogen; Caspase-3, Cysteinyl aspartate specific proteinase 3; Caspase-9, Cysteinyl aspartate specific proteinase 9; COX-2, Cyclooxygenase-2; HDL, High-density Lipoprotein; IL-1β, Interleukin 1β; iNOS, Inducible nitric oxide synthase; LDL, Low-density Lipoprotein; LPS, Lipopolysaccharide; NO, Nitric oxide; TC, Total Cholesterol; TG, Triglycerides; HepG2, Human hepatocellular carcinoma cell line; TNF-α, Tumour necrosis factor-α; IL-2, Interleukin-2; NF-κB, Nuclear factor kappa-B; IL-6, Interleukin-6; IL-10, Interleukin-10; ROS, Reactive oxygen species; MDA, Malondialdehyde; SOD, Superoxide dismutase; GSH-Px, Glutathione peroxidase; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma; NK, Natural killer cell; CCl4, Carbon tetrachloride; AKP, Alkaline phosphatase; AST, Asparate aminotransferase; ALT, Alanine aminotransferase; FRAP, Ferric ion reducing antioxidant power; SCFAs, Short-chain fatty acids; V/C, Ileum crypt and villus length
Keywords: Moringa oleifera Lam, Polysaccharides, Structure characteristics, Biological activities, Structure-biological relationship, Future trends
Highlights
-
•
The extraction, purification, structure characterization and biological activities of M. oleifera Lam polysaccharides from different sources were summarized.
-
•
The relationship between the structure and biological activities of M. oleifera Lam polysaccharides were discussed and compared.
-
•
The future trends and knowledge gaps of M. oleifera Lam polysaccharides were proposed.
Abstract
Moringa oleifera Lam. (M. oleifera Lam) is a perennial tropical deciduous tree that belongs to the Moringaceae family. Polysaccharides are one of the major bioactive compounds in M. oleifera Lam and show immunomodulatory, anticancer, antioxidant, intestinal health protection and antidiabetic activities. At present, the structure and functional activities of M. oleifera Lam polysaccharides (MOPs) have been widespread, but the research data are relatively scattered. Moreover, the relationship between the structure and biological activities of MOPs has not been summarized. In this review, the current research on the extraction, purification, structural characteristics and biological activities of polysaccharides from different sources of M. oleifera Lam were summarized, and the structural characteristics of purified polysaccharides were focused on this review. Meanwhile, the biological activities of MOPs were introduced, and some molecular mechanisms were listed. In addition, the relationship between the structure and biological activities of MOPs was discussed. Furthermore, new perspectives and some future research of M. oleifera Lam polysaccharides were proposed in this review.
1. Introduction
Moringa oleifera Lam. (M. oleifera Lam) is widely distributed in many tropical and subtropical countries. It is well known as the “miracle tree” due to its strong drought resistance, rapid growth and nutritional richness (Abdull Razis et al., 2014, Almatrafi et al., 2017). Almost all parts of M. oleifera Lam can be utilized as a source of edible food and medicinal resources, including the leaves, roots, seeds, flowers and barks (Ma et al., 2020, Rocchetti et al., 2020). Many studies have shown that M. oleifera Lam exhibits different biological activities to improve human health, such as antioxidant, antitumour, antimicrobial, antidiabetic and wound healing activities (Ali et al., 2020, Kaur and Arora, 2020, Munekata et al., 2020, Patriota et al., 2020, Rodríguez et al., 2020, Xie et al., 2018). Therefore, it is often used as a traditional medicine or healthy food in many countries.
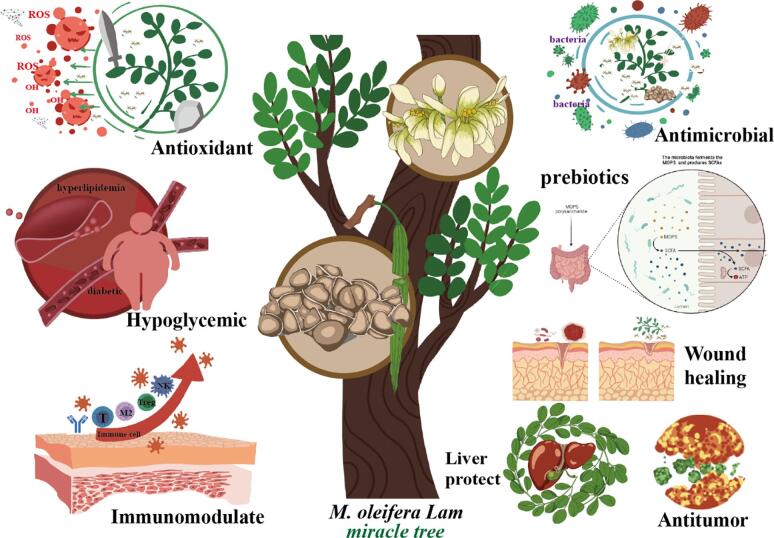
Polysaccharides are biological macromolecules with strong functional activities (Huang et al., 2021, Su and Li, 2020). Polysaccharides are one of the most nutritive and medicinal bioactive components of M. oleifera Lam (Anwar et al., 2007, Saleem et al., 2020, Wang et al., 2021). Thus, polysaccharides from different parts of M. oleifera Lam have received increasing attention. In particular, M. oleifera Lam polysaccharides (MOPs) have a variety of biological activities, including antidiabetic, antioxidant, antitumour, antibacterial and immune regulatory activities (Singhal, Jarald, Showkat, & Daud, 2012). Therefore, MOPs have been widely used in various functional food and medicine fields due to their low toxicity and few obvious side effects (Okuda et al., 2001, Raja et al., 2016). In recent years, many studies have focused on MOPs. These studies mainly focused on the separation, purification, chemical structure characterization, physical properties and biological activities of MOPs. The specific biological activities from different sources of MOPs are shown in Fig. 1.
Fig. 1.
The special biological activities of different source form MOPs.
Moreover, it is worth noting that the bioactivities of MOPs are not only closely related to their extraction, purification methods and structural diversity (Chen, Zhang, Huang, Fu, & Liu, 2017) but also related to their molecular weight, arrangement of glycosidic bonds and chemical modification (Tian et al., 2021). These research data were scattered, and the structure-biological effect relationship was not clear summarized. These two substitutions may affect MOP development and utilization. Based on this, it is urgent to systematically describe the extraction and purification methods, chemical structure and biological activity mechanism of MOPs from various aspects and provide an in-depth summary from a new perspective.
Therefore, this review summarizes the recent advances in the extraction, purification, structural characterization and biological activities of MOPs from different sources of M. oleifera Lam (leaves, seeds, roots, ranks and flowers) and discusses the structure-biological activity relationship between different MOP sources. Furthermore, the challenges and future trends of MOPs are summarized in this review to provide a theoretical basis and reference value for the further development and application of MOPs.
2. Extraction, separation and purification methods of MOPs
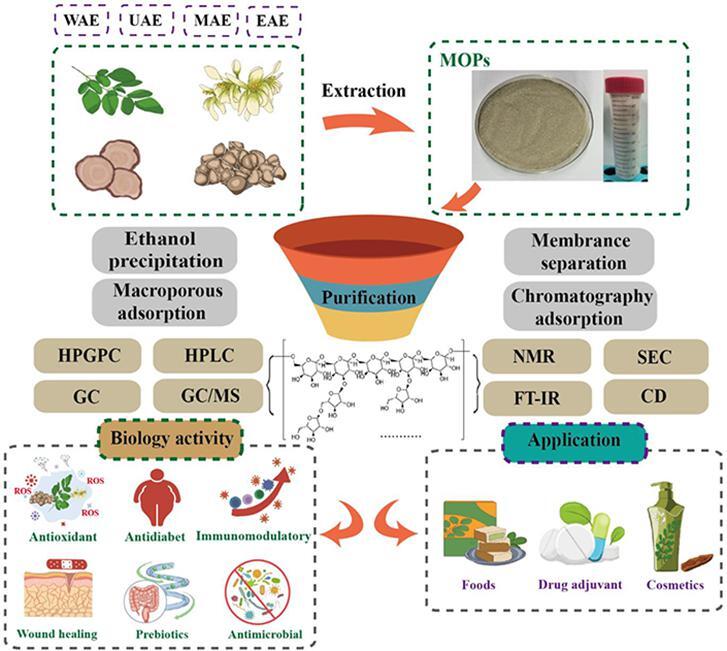
M. oleifera Lam polysaccharides are a polar macromolecule that is generally insoluble in organic solvents and difficult to dissolve in cold water. Therefore, MOPs show different structures, chemical properties and biological activities due to the extraction methods and purification steps and the following steps were used: (1) The raw materials were degreased and decolorized by organic solvents. (2) MOPs were extracted by suitable methods and concentrated for reserve. (3) Organic solvent and macroporous resin were used to deproteinize and ultrafiltration MOPs. The procedures for extraction, purification and identification of MOPs are shown in Fig. 2.
Fig. 2.
Procedures for extraction, purification, and identification of MOPs.
2.1. Extraction of MOPs
Hot water extraction is a common method to extract MOPs (Chen et al., 2020, Zhu et al., 2017). Polysaccharides from M. oleifera Lam leaves were prepared in hot water at an extraction temperature of 90 °C, extraction time of 4 h, and solid-iquid ratio of 1:20 (W/W), and the final MOPs yield was 6.84% (Wang et al., 2018). However, it is worth noting that there are many disadvantages in hot water extraction method, such as a long extraction time, low extraction efficiency and high energy consumption, which may affect the extraction rate and biological activities of MOPs. Therefore, ethanol extraction was also used to extract polysaccharides from M. oleifera Lam seeds. MOPs from seeds were extracted with 80% ethanol combined with 80 ℃ hot water, and the results showed that ethanol at different concentrations could not only remove oil from M. oleifera seeds but also decompose proteins, thus improving the yield of MOPs (Zheng, Hu, Zhou, Liang, Luo, & Xie, 2021). Yu et al., (Yu et al., 2020) compared the difference between hot water extraction and ethanol extraction of MOPs seeds. The results indicated that hot water extraction was better than ethanol extraction. Compared with ethanol extraction, hot water extraction showed safer and economical in industrial application. Polysaccharides extracted from water can be further separated and purified by different concentrations of ethanol. Moreover, ethanol extraction method retains more small molecules or alcohol-soluble substances such as pigments. The solubility of polysaccharides in water was better than ethanol, water extraction showed higher extraction rate. Thus, most studies did not adopt this method to extract MOPs.
To overcome these shortcomings, some new extraction techniques have been widely used in MOPs. As a new extraction method, ultrasonic extraction (UE) technology uses the mechanical, cavitation and thermal effects to improve the molecular movement rate and solvent penetration ability, finally improving the extraction efficiency of polysaccharides (Leong et al., 2021, Zhu et al., 2020). Response surface methodology (RSM) was used to optimize the ultrasonic-assisted hot water extraction of MOPs. When the power was 100 W, the temperature was 80 °C, and the extraction time was 40 min, the maximum yield of MOPs was 7.36% (Zhang, Yang, Hui, & Sun, 2020). Some studies used ultrahigh pressure technology combined with pressure (400 MPa), solid–liquid ratio (15:1 mL/g), and extraction time (5.5 min) to extract MOPs, and the final yield of polysaccharides was 16.28% (Jin & Zhang, 2020). This method showed a short extraction time and high polysaccharide yield advantages. Moreover, enzyme is one of the commonly biocatalysts, enzyme extraction has attracted more attention in MOPs, and enzyme assisted extraction (EAE) has green environmentally friendly, simple operation and high extraction efficiency. Enzymatic extraction could degrade plant cell wall, enhance cell permeability, and promote the release of polysaccharides in cells, thus improving the extraction efficiency. Many studies have commonly used cellulase, hemi-cellulase, protease and pectinase to isolate MOPs. Moreover, MOPs have been found to have strong antioxidant effects after enzyme hydrolysis (Li, 2019). In addition, some research found that microwave extraction can penetrate the cell medium and break cell components to improve the polysaccharide extraction yield (Chen et al., 2017). For example, Chen et al., (Chen et al., 2017) used microwave (700 W)-assisted hot water (70 °C) to extract MOPs, and the yield of polysaccharides was increased by 2.38% compared with traditional methods. In addition, supercritical fluid extraction (SFE) is a new green separation technology used in MOPs. This method has low temperature and simple postprocessing, but the extraction rate will change with different polar fluids (Gong et al., 2021, Rao et al., 2021).
2.2. Separation and purification of MOPs
MOPs extracted by the above methods should be further purified to evaluate their structural characteristics and biological activities. For the purification process of MOPs, impurities such as protein and pigment in crude polysaccharides were removed in the first step, followed by fractional precipitation, chromatographic column method and membrane separation method, and finally concentration, dialysis and freeze drying of the pure MOPs.
Proteins, which are similar to polysaccharides, belong to polar macromolecules (Chu, 2020, Yuan et al., 2020), thus, deproteinization was the first step of MOP separation. The most common methods of deproteinization were the Sevag method, TCA and enzyme methods. The Sevag method is a classic method that is usually used in MOPs; this method makes glycosidic bonds difficult to break but needs to be repeated more than five times (Sun, Zhang, & Fang, 2020). In addition, the trichloroacetic acid and polysaccharide solution under low-temperature stirring to remove colloidal proteins, but this method degrades a large number of polysaccharides containing furanose residues (Qi et al., 2021, Shi et al., 2019). The enzyme method is also used in purification MOPs. Most of the proteins in MOPs can be removed by adding an appropriate proportion of enzymes (Bogdanova et al., 2019, Wu et al., 2022). However, the disadvantage of this method is that crude polysaccharides with relatively low protein content may not only play the role of deproteinization but also increase the protein content in raw samples. Du et al., (Du, 2015) compared the above three methods of MOPs. The results indicated that the Sevag method could effectively maintain the natural structure of the MOPs, but that the reagent waste was serious and the extraction yield was lower. The TCA method could increase the recovery rate of MOPs but also increased polysaccharide degradation. However, deproteinization with papain could not only preserve the properties of polysaccharides but also improve the recovery of polysaccharides.
There are many pigments in the extraction process that could affect the further separation and purification of MOPs. Ion exchange is a common method for the decolorization of MOPs and the best decolorization method is to use anionic macroporous exchange resin. Macroporous resin could separate polysaccharides with different ionic strengths. AB-8 and HP-20 showed better decolorization of MOPs (Yang, Zhao, & Lin, 2020). Macroporous resin has good decolorization effect and polysaccharide retain high activity after decolorization. However, when adopting this method, it is necessary to choose resin filler with appropriate pore size and clean with solvent before use. The oxidative decolorization method uses hydrogen peroxide as an oxidative decolorization agent to remove pigments. Generally, it is carried out under weakly alkaline conditions, and the pH value should not be too high, otherwise, it will rupture glycosidic bonds and destroy the structure of polysaccharides (Lin et al., 2018, Nie et al., 2021). In addition, the adsorption principle could be used in the separation of MOPs, generally using activated carbon, adsorption resin and diatomite to achieve purification (Bai, Fu, & Lin, 2020). These methods are convenient for the pre-treatment, it showed mild decolorization conditions, low cost and the filler can be used repeatedly. However, the decolorization rate and retention rate of polysaccharide are low. In practice, protein and pigment impurities cannot be removed by a single method. Thus, a combination of methods could be considered for separating MOPs.
The purification of MOPs can be separated according to the molecular structure, molecular size and molecular chemical group (Tang et al., 2020, Yang et al., 2015). According to the different levels of purification, it can be divided into preliminary purification and depth purification. Preliminary purification methods include the fractional precipitation method and ultrafiltration method (Chen et al., 2016, Junter and Lebrun, 2020). Fractional precipitation is the most common method for separating MOPs, and the different solubility properties of MOPs could precipitate in different concentrations of alcohols or ketones. However, a low concentration of ethanol was beneficial to the precipitation of MOPs containing a high molecular weight, while a high concentration of ethanol solution was beneficial to the precipitation of MOPs with a low molecular weight. MOPs precipitated by different concentrations of ethanol also showed different chemical structures (Raja et al., 2016). The ultrafiltration method separates polysaccharides under the applied pressure by using the principle of solute molecular diameter and ultrafiltration membrane pore size (Hu and Goff, 2018, Ngo et al., 2011). For example, an ultrafiltration membrane with a molecular weight of 1000 ∼ 3000 Da could be used to remove low molecular weight compounds of MOPs (Dong et al., 2018, Tian et al., 2021). Ultrafiltration membranes with higher molecular weight (3500–14000 Da) were used to remove MOPs from seeds (Cui et al., 2019, Mehwish et al., 2021).
The polysaccharides obtained in this section will be further purified by different column chromatography methods. Ion-exchange column chromatography is the most common purification method of MOPs. The common ion exchangers are DEAE cellulose, DEAE glucan and DEAE agarose (An et al., 2020, Li et al., 2018). The purified MOPs could then be separated by gel column chromatography, such as dextran gel, agarose gel and propylene dextran gel. In addition, high-performance liquid chromatography separation technology has also been applied to the purification of MOPs (Peluso, Mamane, Dallocchio, Dessi, & Cossu, 2020). The phenol–sulfuric acid method was used to monitor the eluting fractions according to these chromatographic purification processes (Liu et al., 2020, Zhang et al., 2020). The specific extraction and purification methods from different sources of MOPs are shown in Table 1.
Table 1.
Extraction methods and chemical structure characteristics of MOPs.
| Source | Extraction | Purification | Identification | MW (kDa) | Monosaccharide (Molar ratio) | Reference |
|---|---|---|---|---|---|---|
| Leaves | Water (70℃, 70 min), Microwave power (600 W) | 80 % EtoH (4℃, 12 h). | HPGPC, FT-IR, SEM Rheometer | 4.86 × 103 | Xyl, Man, Glu, Gala was 62.55%,8.16%,19.91%,4.74% | (Chen et al., 2017) |
| Leaves | Water (55℃, 2 h), Ultrasound power (800 W) NS37071 enzyme 1% | EtoH (4℃, 12 h), Sevag method. | SEC, HPLC, GC/MS, ACQUITY HPLC | >103 | Man, Rha, Glc, Gal, Ara GlcA, GalA was 5.66%,73.8%,28.17%,32.37%20.04%,1.20%,5.18% | (Yang et al., 2020) |
| Leaves | Water (100℃, 2 h) | EtoH (4℃, 24 h), sevag method, DEAE Sepharose Fast flow ion exchang colum (2.6 cm × 10 cm), NaCl (0.5 mol/L), 500–1000 Da Membranes. | GPC, FT-IR, SEM, UV, AFM, Methylation | 104 × 103 | β-Pyr and Man | (Tian et al., 2021) |
| Leaves | Water (90℃, 4 h) | EtoH (4℃, 24 h), sevag method, DEAE Sepharose Fast flow ion exchange column (1.6 cm × 20 cm), 0.2 mol/L NaCl, 500–1000 Da membranes | HPGPC, UV, Congo red method, GC–MS, 1H and 13C NMR, Methylation | 4.033 × 103 | Ara: Glu: Gla was 47.73:1.00:57.65 | (Li et al., 2020) |
| Leaves | Water (90℃, 4 h) | EtoH (4℃, 12 h), sevag method, D 101, DEAE Sepharose Fast flow ion exchange column (1.6 cm × 20 cm), 0.12 mol/L NaCl 3000 Da membranes | HPGPC, GC–MS, Methylation, NMR | 155.35 | Ara: Glu: Gala was 35.8%, 6.67% and 57.53% | (Dong et al., 2018) |
| Leaves | Water (90℃, 4 h) | Agilent Technologies HPLC, zorbax SB-C18 (250 × 4.6 mm, 5 mm), 5% acetonitrile | HPLC-DAD, HPLC-RID | NA | Raf, Sta, Gra, Xyl, Man, Ara | (Caicedo-Lopez, Luzardo-Ocampo, Cuellar-Nunez, Campos-Vega, Mendoza, & Loarca-Pina, 2019) |
| Flowers | Water (Ice bath 4 h) Ultrasonic (800 W) | EtoH (4℃, 12 h), sevag method, D101, DEAE-52 (2.6 cm × 60 cm), NaCl (0–2 mol/L), DEAE Sepharose 6B (2 × 200 cm), 0.1 mol/L NaCl, 3500 Da membranes. | HPGPC, HPLC-GPC, UV, GC, Methylation, 1H and 13C NMR, IR | 7.65 × 104 | Rha: Ara: Gal was 1:7.32:12.12, d-galacturonic acid (2.5%) | (He et al., 2018) |
| Seeds | Water (90℃, 10 h) | EtoH (4℃, 24 h), 20% acetic acid, Sepharose-6B (2.1 cm × 90 cm) | GLC, GLC-MS, HPLC 1H and 13C NMR (TOCSY, DQF-COSY, NOESY, ROESY, HSQC, and HMBC2D-DQF-COSY) | 1.96 × 102 | d-Gal, 6-O- -Gal, d-Galacid, l-Ara and l-Rha was 1:1:1:1:1. (1 → 4)-linked d-GalpA | (Roy et al., 2007) |
| Seeds | Water (80℃, 4 h) | EtoH (4℃, 24 h), TCA method, 8000–14000 Da membranes. | TLC, HPLC, UV, GPC, 1H and 13C NMR, FT-IR | 3.651 × 103 | Man and Glu (Main subunits), Uronic acid (0.86 ± 3.8%), | (Mehwish et al., 2021) |
| Seeds | Water (80℃, 4 h) | EtoH (4℃, 24 h), DEAE cellulose, sephedx-G100 (2.1 cm × 90 cm) | HPLC, methylation and GLC | NA | α1,4 link d-Glucose | (Mondal et al., 2004) |
| Seeds | Water (80℃, 1.5 h) | EtoH (24 h), sevag method, papain 1%, DEAE-52 (2.6 cm × 60 cm), 0–0.5 mol/L NaCl, Sephadex G-200 (1.7 cm × 70 cm), 3500 Da membranes. | HPLC-GPC, UV GC–MS, 1H and 13C NMR | 127 | l-Rha: d-Ara: d-Xyl: d-Man: d-Glu: d-Gal was 3.33:0.48:0.47:0.39:0.75:1.53 | (Dong, 2016) |
| Roots | Water (90℃, 3 h) | EtoH (4℃, 24 h), sevag method, DEAE Sepharose fast flow ion exchange column, 0.2 mol/L NaCl 3500 Da membranes. | GC–MS, FT-IR, 1H and 13C NMR | NA | Rha: Ara: Fru: Xyl: Man: Gal was 1.5:2.0:3.1:6.0:5.3:1.1 | (Cui et al., 2019) |
| Gum | Water (120℃, 2 h) High pressure (100 kpa) | EtoH (4℃, 24 h), sevag method, Anion exchange chromatography. | GC, FT-IR, GC–MS, Methylation | 190 | Ara: Gal: Xyl: Rha: GlcA was 64: 25:4:3:4 | (Raja et al., 2016) |
NA: information was not available now.
However, the different extraction and separation methods will affect the primary structure of polysaccharides, such as the monosaccharide composition and molecular weight, and thus changes will further affect the solubility, stability and helical structure of MOPs.
3. Structural characterization of MOPs
Polysaccharides have complex biological macromolecular structures (Grube et al., 2020), and the structural analysis of MOPs is very complex, not only including molecular weight, monosaccharide composition, the linking of monosaccharide residues, the configuration of glycosidic bonds and other primary structure determination but also including the senior structure. The specific identification methods of the chemical structure from different sources of MOPs are shown in Table 1.
Molecular weight is an important index affecting the activity of MOPs. The osmotic pressure, viscosity, organic reagent deposition and high-performance liquid chromatography (HPLC) methods are usually used to determine the molecular weight of MOPs (Pantsulaia et al., 2020). In addition, in recent years, high-performance gel permeation chromatography (HPGPC) and size exclusion chromatography (SEC) have often been used in the determination of molecular weight, and SEC can be used to calculate the molecular weight (Mw) and dispersion coefficient of polysaccharides (Liu et al., 2020, Zhao et al., 2020).
Complete acid hydrolysis is the first step to identify the monosaccharide composition of MOPs (He, Huang, Huang, Wang, Hu, & Sheng, 2018). Polysaccharides were hydrolysed by sulfuric acid, trifluoroacetic acid and formic acid and then derivatized. The hydrolysed products were separated and analysed by HPLC, GC, GC–MS and high-performance anion exchange chromatography (Omodanisi et al., 2017, Tang et al., 2017). The monosaccharide compositions and the molar ratios of MOPs from different varieties or extraction methods were significantly different. The monosaccharide compositions of MOPs mainly include mannose, rhamnose, glucose, galactose and arabinose (Chen et al., 2017). The functional groups of MOPs are usually determined by infrared radiation (IR) or Fourier transform infrared spectroscopy (FT-IR). A special α-Pyranoside bond occurs at 845 cm−1, and β-Pyranoside bond occurs at 890 cm−1. Pyranoside occurs in three absorption peaks between 1100 cm−1 and 1010 cm−1, while the acetyl group on the glucoside could appear at 1730 cm−1 (Yang et al., 2020).
Some research has used periodate oxidation, smith degradation, and acetylation to estimate the types of MOPs. Polysaccharides were cleaved into oligosaccharides by partial acid hydrolysis and then analysed by the methylation method or GC to determine the composition of the main chain and branched chain. 1D/2D nuclear magnetic resonance (NMR), including 1H and 13C and 2D NMR correlated spectroscopy (COSY), total correlation spectroscopy (TOCSY) and nuclear overhauser effect spectroscopy (NOESY) has been widely used to determine the ectopic configuration, location and linkage sequence of glycoside residues (Guo et al., 2021, Li et al., 2020, Yao et al., 2021). More research has found that the advanced structure of polysaccharides is closely related to biological activity. X-ray derivations, atomic force microscopy and circular dichroism were used to observe and analyse the complex structure of polysaccharides (Mansel et al., 2020, Pieczywek et al., 2020).
At present, a large number of studies have obtained pure MOPs through different separation and purification techniques. Their fine structures are analysed in Table 1 and Table 2. For example, MLP-1 (9.68 × 103 kDa), MLP-2 (6.29 × 103 kDa) and MLP-3 (4.86 × 103 kDa) isolated from M. oleifera Lam leaves were investigated. MLP-1 and MLP-2 showed a triple helix structure and exhibited pseudoplastic flow behaviours. MLP-3 contained Xyl, Man, Glu, Gala, and Ara and showed a non-triple helix structure that may show different biological mechanisms compared with those of MLP-1 and MLP-2 (Chen et al., 2017). A water and enzyme soluble polysaccharide (MOLPF) was isolated from M. oleifera Lam leaves, and its structure was characterized to be GalA) → 3)-Galp-(1→, →6)-Galp-(1→, →6)-Glcp-(1→, →3)-Araf-(1 → and → 2)-Araf- (1→ (Yang et al., 2020). A novel polysaccharide (MRP-1) was isolated from M. oleifera Lam roots, and its structure identification with GC–MS, FT-IR, NMR and methylation showed that MRP-1 contains α-Araf, α-Gly, β-Galp, α-GalpA and β-Gly, and the molar ratio of Rha: Ara: Fru: Xyl: Man and Gal was 1.5:2.0:3.1:6.0:5.3:1.1 (Cui et al., 2019). In addition, a novel MOPs (MOP-2) with a weight of 155.35 kDa, and its structure were composed of the main chain: →3,6)-β-d-Galp (1 → and → 6)-β-d-Galp-(1→, the branch chain: →5)-α-l-Araf-(1 → α-l-Araf-(1→, α-d-Glcp. The Ara: Glu: Gala percentages were 35.8, 6.67 and 57.53 % (Dong et al., 2018). The structure of water-soluble MOPs from M. oleifera Lam seeds were detected by GLC, GLC–MS, HPLC, 1H and 13C NMR (TOCSY, DQF-COSY, NOESY, ROESY, HSQC, and HMBC 2D-DQF-COSY). This polysaccharide has d-GalpA linked, and the molar ratio of d-Gal, 6-O-Gal, d-Galacid, l-Ara and l-Rha was 1:1:1:1:1 (Roy et al., 2007). A novel polysaccharide (MOP-1) was isolated from M. oleifera Lam, which is a neutral polysaccharide (Mw 7.65 × 104 kDa), and its structure was analysed with HPGPC, HPLC-GPC, UV, GC, methylation, NMR, IR and methylation. The result showed that it was composed of 1)-β-d-Galp-(3,4 → ) and → 1)-β-d-Galp-(4→, →1)-α-d-Galp-(2 → Araf-(1 → Galp-(1→, and the main molar ratio of Rha: Ara: Gal was 1:7.32:12.12 (He et al., 2018).
Table 2.
Chemical structure characteristics and the biological activity of MOPs.
| Source | Fraction | Structure | Function | Model | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Leaves | MLP-3 | Non triple helix structure and showed Newtonian fluid. | Hypolipidemic | α-amylase, β-glucosidase (In vitro) | Inhibit α-amylase and β-glucosidase levels. | (Chen et al., 2017) |
| Leaves | MOLPF | GalA) → 3)-Galp-(1→, →6)-Galp-(1→, →6)-Glcp-(1→, →3)-Araf-(1 → and → 2)-Araf-(1 →. | Regulate the acid-binding capacity | Bile (CA, GCA, TCA) acid-binding capacity (In vitro) | Increase the bile acids (CA, GCA, TCA) binding abilities. | (Yang et al., 2020) |
| Leaves | MOP | Triple helix structure, contained a large number of branch chains. | Hypolipidemic | C57BL/6 mice (4 week) (In vivo) | Increase SOD, CAT and V/C levels, Decrease GLU, TC and MDA levels, Change the abundance of microbiome | (Tian et al., 2021) |
| Leaves | MOP-3 | (1 → 3,6)-β-d-Galp (1 → 6)-β-d-Galp (1 → 5)-α-l-Araf T-α-and l-Araf, without Triple helix structure. | Immunomodulate | RAW 264.7 cells (In vitro) | Increase cell viability and pinocytic activities, Increase TNF-α, ROS, NO, IL-6 levels. | (Li et al., 2020) |
| Leaves | MOP-1 | The main chain:1)-β-D- Galp-(3,4 → ) and → 1)-β-D- Galp-(4→, →1)-α-d-Galp-(2 → Araf-(1 → Galp-(1→ | Antioxidant | DPPH radical activity ABTS radical activity FRAP radical activity (In vitro) | Decrease DPPH and ABTS radical activities, increase FRAP levels. | (He et al., 2018) |
| Leaves | MOP-2 | The main chain: →3,6)-β-d-Galp (1 → and → 6)-β-d-Galp-(1→, The branches chain: →5)-α-l-Araf-(1 → α-l-Araf-(1→, α-d-Glcp. | Immunomodulate | RAW 264.7 cells (In vitro) | Increase cell viability and pinocytic activities, increase iNOS, IL-6, TNF-α, ROS, NO levels. | (Dong et al., 2018) |
| Leaves | MOS | NA | Antioxidant | DPPH radical activity ABTS radical activity (In vitro) | Decrease DPPH and ABTS radical activities. | (Caicedo-Lopez et al., 2019) |
| Leaves | MOs-2-a | NA | Prebiotics | ICR mice (6 week-old)(In vivo) | Increase the viscera index (spleen and thymus), increase the V/C, intestinal digestibility, enhance digestive enzymes (Amylase, Lipase, Trypsin) activities, decrease TNF-α, DAO levels, change the abundance of gut microbiome. | (Wang et al., 2019) |
| Leaves | MOP-2 | Does not contain α-(1–4) Glycoside bonds. | Prebiotics | Saliva-gastrointestinal digestion and human fecal fermentation models (In vitro) | The MW of MOP-2 decreased from 155.29 to 145.02 kDa, sugar content increased from 0.159 to 0.234 mg/mL, regulate the structure of the microbial community, increase the SCFAs production | (Wang et al., 2018) |
| Leaves | MO | NA | Antimicrobial | Aureus ATCC25923 (In vitro) | Inhibit S. aureus ATCC25923 growth. | (de Oliveira et al., 2020) |
| Leaves | MOP | NA | Antioxidant | DPPH radical activity ABTS radical activity (In vitro) | Decrease DPPH and ABTS radical activities. | (Nie et al., 2021) |
| Leaves | MLP 100–3 | NA | Immunomodulate | RAW 264.7 cells (In vitro) | Decrease iNOS, COX-2, and TNF-α levels. | (Cui et al., 2018) |
| Leaves | MOP | NA | Hypolipidemic | HepG2 cells STZ-induced SD rats (In vitro and In vivo) | Decrease MDA, iNOS, TG, TC and LDL levels, increase HDL levels. | (Li, 2019) |
| Roots | MRP-1 | α-Araf,α-Gly,β-Galp,α-GalpA and β-Gly. | Immunomodulate | RAW 264.7 cells (In vitro) | Decrease NO, IL-6, IL-1β, TNF-α, iNOS, COX-2 levels. | (Cui et al., 2019) |
| Seeds | MOS-PS | α-Pyranose and β-Pyranose link. | Antimicrobial | E. coli E26, S. aureus G56, P.aeruginosa TL1911 Fibroblast cell lines L929 male rats (6 weeks) (In vitro and in vivo) | Inhibit E. coli E26, S. aureus G56 and P.aeruginosa TL1911 growth, Increase cell viability and migration activities, decrease IL-6, TGF-β and SMAD-3 levels in cells, Reduce the wound healing area of rats, Decrease IL-6, TGF-β and SMAD-3 levels on rat skin. | (Mehwish et al., 2021) |
| Seeds | MOP-D | triple helix structure. | Antioxidant | DPPH radical activity ABTS radical activity (In vitro) | Decrease DPPH and ABTS radical activities. | (Dong, 2016) |
| Seeds | MPG | 1-O-(4-Hydroxymethyl phenyl)-α -LRha. | Protect Liver | Liver cell lines L02 ICR mice (In vitro and In vivo) | Increase cell viability and pinocytic activities, Increase SOD, LDH, CAT and GSH-Px levels, decreased ROS, MDA levels and liver weight. Decrease ALT, AST, and AKP levels in serum, decreased hepatic lipid peroxidation levels. | (Sun et al., 2019) |
| Gum | F1 | 1,6-,1,3- and 1,3,6-linked β-Galp | Antioxidant | DPPH radical activity (In vitro) | Decrease DPPH radical activities. | (Raja et al., 2016) |
NA: information was not available now.
MOPs have different types of glycosidic bonds and functional groups. Due to the close relationship between the structure and biological activities of polysaccharides, it is very important to study the spatial structure–activity relationship of MOPs. However, until now, the difference in biological activities caused by chemical structure is still a problem worthy of attention.
4. Biological activities of MOPs
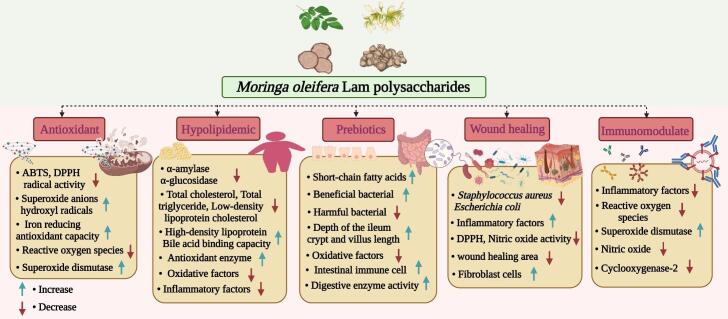
A large number of studies have shown that MOPs are beneficial in improving human health and physiological activities; thus, an increasing number of researchers have begun to pay attention to the biological functions of MOPs. The biological activities of MOPs mainly included antioxidant, antimicrobial, antidiabetic, immunity improvement, glucose and lipid metabolism regulation, wound healing repair, intestinal microorganism regulation and green colloidal food storage materials. According to the reported functional activities and mechanism of MOPs, the present research is summarized in Table 2 and Fig. 3.
Fig. 3.
The biological activity and the regulate mechanisms of MOPs.
4.1. Antioxidant activity
It is well known that oxidative stress can cause many serious diseases, such as cancer, diabetes, arteriosclerosis and nephritis (Ismail Iid, Kumar, Shukla, Kumar, & Sharma, 2020). Many studies have confirmed that MOPs have significant antioxidant activity. Thus, the ways that MOPs may exert their antioxidant effect were included in the following mechanisms: (1) MOPs improve the scavenging ability of ABTS and DPPH free radicals. (2) MOPs increase iron reducing antioxidant capacity. (3) MOPs improve the scavenging ability of superoxide anions and hydroxyl radicals.
For example, a novel MOPs (MOP-1) were purified by using DEAE-52 cellulose and Sepharose 6B. MOP-1 showed scavenging effects on DPPH and ABTS free radicals. Meanwhile, FRAP was increased when MOP-1 was added at 4.0 mg/mL (He et al., 2018). A polysaccharide isolated from M. oleifera Lam leaves scavenges the DPPH (78.91%), superoxide anion scavenging (28.76%) and hydroxyl radical scavenging (29.51%) ability by increasing the reduction capacity of iron ions (Nie et al., 2021). In addition, MOPs (F1) extracted with ultrahigh pressure-assisted water extraction showed a dose-dependent DPPH scavenging activity. The DPPH scavenging rate (86%) was close to that of the standard antioxidant drugs BHA (92%) and BHT (92.3%) at 300 ug/mL (Raja et al., 2016). Not only polysaccharides from M. oleifera Lam leaves but also polysaccharides from seeds showed antioxidant ability. Dong et al., obtained four polysaccharides from M. oleifera seeds (MOP-A, MOP-B, MOP-C, MOP-D) with different concentrations of ethanol. These four homologous polysaccharides showed certain antioxidant activities, but with increasing ethanol concentration, the scavenging ability of ABTS radicals was as follows: MOP-D > MOP-A > MOP-B > MOP-C. The scavenging capacity of hydroxyl free radicals was as follows: MOP-D > MOP-A > MOP-C > MOP-B (Dong, 2016).
4.2. Antidiabetic activity
Type 2 diabetes is a chronic comprehensive disease mainly characterized by glucose metabolism disorder. Type 2 diabetes can be caused by insufficient insulin secretion, insulin resistance, disrupted glucose metabolism, oxidative stress, saturated fat and unhealthy lifestyles (Omodanisi et al., 2017, Tang et al., 2017). Regulating glucose and lipid metabolism is one of the most important activities of MOPs. The mechanisms by which MOPs exert their antidiabetic effects include the following: (1) MOPs inhibit the activity of α-amylase and α-glucosidase. (2) MOPs reduce the contents of total cholesterol, total triglyceride, and low-density lipoprotein cholesterol. (3) MOPs improve the contents of high-density lipoprotein and strengthen the binding of bile acid. (4) MOPs increase antioxidant enzyme levels (SOD, GSH-Px). (5) MOPs reduce the levels of oxidative factors (MDA, CAT). (6) MOPs decrease the levels of inflammatory factors (TNF-α, IL-6 and iNOS).
For example, the hypoglycaemic activity of three MOPs (MLP-1, MLP-2 and MLP-3) isolated from M. oleifera Lam leaves was investigated. Both components (0.01 mg/mL to 0.1 mg/mL) showed a dose-dependent decrease in the α-amylase level. In addition, the inhibitory effect on α-glucosidase was as follows: MLP-1 (89.68%) > MLP-2 (72.19%) > MLP-3 (58.82%) at 0.1 mg/mL. These results indicated that MOPs are a promising method for regulating blood glucose levels after meals (Chen et al., 2017). Bile acids are synthesized from cholesterol and reused in the intestine or liver. Bile acids play an important role in the emulsification and absorption of intestinal lipids. Bile acids are essential for maintaining cholesterol homeostasis and preventing the accumulation of cholesterol and triglycerides. CA, GCA and TCA are three important bile acids in the human body. MOPs (MOLPF-CE) extracted with ultrasound-assisted cellulase had a higher binding ability to bile acids, and the binding abilities were as follows: GCA (80%) and TCA (80%) CA (73%). These results suggest that MOLPF-CE has a potential hypolipidemic effect (Yang et al., 2020). In addition, Tian et al., (Tian et al., 2021) also investigated MOPs (MOPs) for improving antidiabetic effects in high-fat mice. The results showed that both total cholesterol and malondialdehyde in the serum of high fat mice were decreased, while the levels of superoxide dismutase and catalase were increased (P < 0.05) after gavage MOP (60 mg/kg). Moreover, MOP could regulate blood glucose levels by improving antioxidant capacity and intestinal microbes. In addition, research found that streptozotocin (STZ)-induced diabetic mice exhibited cognitive dysfunction and hippocampal nerve cell apoptosis. MOPs significantly reduced neuronal apoptosis and improved cognition in diabetic rats. Furthermore, MOPs reduced the expression levels of Caspase-3, TNF-α, IL-6 and Glut 4 and increased the ratio of Bax/Bcl-2. These results indicated that MOPs improved the cognitive dysfunction induced by STZ in diabetic rats (Sun, Zhao, Liu, Sui, & Zhu, 2021). Many studies have used HepG2 cells as a reliable in vitro model to evaluate insulin resistance and the mechanism of hypoglycaemic drugs. Some research also suggested that MOPs may show hypoglycaemic effects by improving the insulin resistance pathway in HepG2 cells (Wang et al., 2018). Moreover, two kinds of MOPs (Mw > 10 kDa, Mw < 10 kDa) isolated by enzymatic trypsin and purified by membrane ultrafiltration showed great antidiabetic activity in an STZ-induced type 2 diabetic mice model. MOPs significantly decreased the contents of MDA, iNOS, TG, TC and LDL-C (P < 0.05) and increased the contents of GSH-Px and HDL-C (P < 0.05). Furthermore, the number of islet cells in the mouse pancreas was increased. All results revealed that MOPs exhibited excellent antidiabetic activity in type 2 diabetic mice (Li, 2019).
4.3. Immunity activity
Immunomodulatory activity is one of the most important biological activities of natural polysaccharides (Wahab et al., 2020, Wang et al., 2017). The immunomodulatory activity of MOPs mainly occurred through stimulating receptors and activating the macrophage-dependent immune response.
There have been many studies on the immunomodulatory activity of MOPs. For example, Li et al., (Li et al., 2020) evaluated the immunomodulatory effects of MOPs (MOP 3) on RAW 264.7 cells. The results showed that MOP 3 (31.5 ∼ 500 μg/mL) significantly increased the pinocytosis capacity of RAW 264.7 cells (1.33 times vs. control group) and promoted ROS production (P < 0.01). To further clarify its immune mechanism, the mRNA expression levels of iNOS, IL-6 and TNF-α in RAW 264.7 cells were detected by RT–qPCR. The results confirmed that MOPs could improve immunity function by regulating the mRNA expression of iNOS, IL-6 and TNF-α. In addition, ICR mice were used to study the immunomodulatory effects of MOPs. After 4 weeks of treatment with MOPs (MOS-2-A), the intestinal digestive abilities of ICR mice were significantly improved, and the levels of organ indices, digestive enzymes, serum tumour necrosis factor and diamine oxidase were decreased (Wang, Bao, Si, Duan, Weng, & Shen, 2019). A novel polysaccharide (MOP-2, MW 155.35 kDa) isolated from M. oleifera Lam leaves has a significant immunomodulatory effect. The results showed that MOP-2 (31.3, 62.5, 125, 250 and 500 μg/mL) improved the mRNA expression levels of iNOS, IL-6 and TNF-α in RAW 264.7 cells after LPS (50 μg/mL) treatment (Dong et al., 2018). Novel MOPs (MLP 100–3) isolated by DEAE-Sepharose Fast Flow column chromatography showed immunomodulatory effects and anti-inflammatory activity by inhibiting the activation of the NF-κB signalling pathway and decreasing the mRNA expression levels of iNOS, COX-2 and TNF-α (Cui et al., 2019, Cui et al., 2018). In addition, MOPs from other parts also have immunomodulatory activity. The immunomodulatory effect of a novel polysaccharides containing α-ArAF, α-Gly, β-GalP, α-GalPa and β-Gly structures (MRP-1) isolated from M. oleifera Lam roots was investigated. The mRNA expression levels of iNOS, IL-6, IL-1β and TNF-α were decreased after MRP-1 treatment (P < 0.05), which provided a new application prospect for the immunomodulatory effect of M. oleifera Lam roots polysaccharides (Cui et al., 2019). Moreover, MOPs obtained by hot water extraction from M. oleifera Lam seeds significantly inhibited the inflammatory response of J744.1 rat monocytes (Mondal, Chakraborty, Pramanik, Rout, & Islam, 2004).
4.4. As prebiotics to regulate gut microbiota
The gut microbiota as an organ of the human body that has a variety of regulatory functions, such as facilitating digestion in the gastrointestinal tract, improving the absorption rate of nutrients, and even affecting the metabolic mechanism of the human body (Dou et al., 2019, Gao et al., 2017, Jaja-Chimedza et al., 2018). Many studies have shown that MOPs are prebiotics that regulate the composition and function of gut microbes. The mechanisms by which MOPs exert their prebiotic effects include the following: (1) Breakdown by gut bacteria to produce short-chain fatty acids which promote health promotion. (2) Promotion of beneficial bacterial growth and inhibition of harmful bacterial growth by as prebiotics. (3) Reduces oxidative stress in the gut. (4) Improve the depth of the ileum crypt and villus length (V/C) to promote nutrient absorption and maintain the integrity of the intestinal mucosa. (5) Promote intestinal immune cell differentiation and improve intestinal immunity. (6) Improve digestive enzyme activity in the intestinal system.
For example, Tian et al., (Tian et al., 2021) found a novel polysaccharide (MOP) with an MW of 104,031 Da. The results showed that MOP (0, 20, 40, 60 mg/kg) significantly decreased the GLU, TC, BUN and MDA levels of C57BL/6 mice (P < 0.05), while the contents of SOD and CAT were significantly increased (P < 0.05). At the same time, the crypt depth and villus length of the ileum (V/C) in the MOP group were significantly higher than those in the control group (P < 0.05), indicating that MOP may improve the absorption capacity of nutrients in the intestine and show antioxidant effects. Moreover, LC/MS analysis showed that MOP increased the proportions of Firmicutes, Bacteroides and Lachnospiracea, Lachnospiracea is a beneficial bacterium in the gut that can produce short-chain fatty acids and antimicrobial peptides to resist pathogens. Conversely, MOP decreased the proportions of Helicobacter, Oscillibacter and D Ruminiclostridium, which may produce toxin factors that damage human health. Therefore, MOP could improve the abundance of beneficial bacteria and reduce the abundance of harmful bacteria to regulate the intestinal microecological balance. The effects of a homogenous polysaccharide (MOs-2-a) on gut microbiota were investigated. ICR mice were gavage with MOs-2-a (10 mg/kg, 50 mg/kg,) every day. Furthermore, the immunological and intestinal digestive abilities of mice were significantly improved. Moreover, the villus height, mucosal thickness and V/C value of the ileum were significantly increased, indicating that MOs-2-a could improve the differentiation abilities of immune organs and enhance immune function. In addition, the activities of digestive enzymes (amylase, lipase, alkaline phosphatase, and trypsin) were enhanced after treatment with MOs-2-a. Moreover, the proportions of Lachnospiraceae, Helicobacteraceae, Deferribacteraceae, Lactobacillus, Helicobacter and Peptostreptococcaceae showed a significant positive correlation, and these beneficial bacteria could participate in producing vitamins and proteins in the intestine. In conclusion, MOs-2-a may play a prebiotic effect by maintaining the integrity of intestinal mucosa, regulating the composition of intestinal microflora and enhancing the activities of various digestive enzymes to degrade starch, protein and lipids of food (Li et al., 2021, Wang et al., 2019). Other research investigated the digestion and fermentation characteristics of MOPs (MOP-2) by using a simulated saliva-gastrointestinal digestion model and a human faecal fermentation model. The results showed that the molecular weight of MOP-2 decreased from 155.29 kDa to 145.02 kDa during intestinal digestion, and the sugar content of MOP-2 increased from 0.159 mg/mL to 0.234 mg/mL, indicating that MOP-2 was partially degraded during intestinal digestion. Moreover, MOP-2 increased the proportions of Phascolarctobacterium, Coprococcus, Roseburia and Bacteroides, which could increase the production of short-chain fatty acids, especially butyric acid, acetic acid, propionic acid and valeric acid. Moreover, the total SCFAs concentration increased significantly from 2161.8 ± 17.5 mg/kg to 3979.4 ± 21.38 mg/kg. Therefore, MOP-2 may act as a prebiotic to regulate microbial community structure and promote intestinal health.
4.5. Antimicrobial and wound healing activity
Research has found that MOPs show good antibacterial properties that promote wound healing (Arora and Kaur, 2019, Jayawardana et al., 2015).
The sponge scaffold of MOPs had better scavenging ability of DPPH and NO, MOPs could prevent wound suppuration by inhibiting the growth of Staphylococcus aureus and Escherichia coli (Parwani et al., 2016, Rubio-Elizalde et al., 2019). In addition, Oliveira et al., (de Oliveira, de Abreu Filho, de Jesus Bassetti, Bergamasco, & Gomes, 2020) evaluated the antibacterial activity of M. oleifera Lam leaves polysaccharide (WMOL) on Staphylococcus aureus ATCC 25,923 isolated from milk. The results showed that WMOL (125–1000 μg/mL) had a significant inhibitory effect on ATCC2592 by destroying the mature biofilm. In addition, Mehwish et al., (Mehwish et al., 2021) used MOPs (MOS-PS) from M. oleifera Lam seeds combined with silver ions to prepare a polysaccharide silver ion composite nanomaterial (MOS-Ps-AGNPs). The antibacterial activity and wound healing ability of the MOS-Ps-AGNPs were evaluated in vitro and in vivo. The results showed that MOS-Ps-AGNPs had stronger antibacterial activity against gram-negative bacteria, including E. coli. Then, a scratch test was used to determine the migration healing ability of MOS-PS-AGNPs on L929 cells. The migration rate of MOS-PS-AGNPs (6.5 μg/mL) was significantly increased (91%). Finally, a mouse wound healing model was constructed to evaluate the wound healing ability of MOS-PS-AGNPs. The results showed that the skin wounds of mice treated with MOS-PS-AGNPs (25, 50 and 100 mg/kg) were smooth. HE analysis showed that new epidermis and granulation tissue were formed on the surface of the skin. Furthermore, MOS-Ps-AGNPs, as an alternative wound dressing material, may regulate the levels of IL-6, IL-10, TGF-β and SMAD-3 to reveal a strong wound repair ability.
4.6. New green colloid material
MOPs could not only be used to inhibit bacterial growth but could also be used as a green colloidal material containing antibacterial drugs to improve drug utilization. Meanwhile, MOPs could also be used as a new food packaging material for food storage and preservation.
Natural gums mainly composed of polysaccharides are green feedstocks for synthesizing a variety of functional materials. Ranote et al., (Ranote, Kumar, Kumari, Kumar, Chauhan, & Joshi, 2019) used MOPs (MOG) to synthesize a new biobased polyurethane foam (MOGPUF). These new foams showed better antibacterial ability. Moreover, Singh et al., (Singh & Kumar, 2020) used MOPs to develop a hydrogel dressing as a slow drug carrier for enhanced wound healing and discussed the exploration of Levofloxacin. In addition, etherified MOPs can be used as dye sorbents in waste treatment (Ranote, Chauhan, & Joshi, 2020). Moreover, Lee et al., developed puffer materials that contain MOPs. These materials are a new food packaging method that exhibits antimicrobial activity against Listeria monocytogenes. During storage, cheese packaging with these materials effectively inhibited microbial growth and retarded lipid oxidation compared with the control sample (Lee, Yang, & Song, 2016). Furthermore, the gum based on MOPs seeds was prepared via radiation-induced graft copolymerization of N vinylole. Then, mucoadhesion and gel strength were determined and showed that the polymers had nonhemolytic mucoadhesive and antioxidant activities (Singh & Kumar, 2018). In addition, the present study evaluated MOPs as carriers for colon-specific drug delivery in vitro drug release studies, and MOPs-containing formulations showed better drug release of nearly 90.46% (Singhal et al., 2012).
4.7. Other activities
Current studies have found that MOPs can protect against liver damage and alleviate cancer. Sun et al., (Sun et al., 2019) purified the phenolic glycoside 1-O-(4-hydroxymethylphenyl)-α-l-rhamnopyranoside (MPG) from M. oleifera Lam seeds. MPG reduced the hepatotoxicity of CCl4-induced L02 cells and ICR mouse models. The results showed that MPG (10, 50, 100 and 200 μg/mL) significantly increased cell viability and SOD activity by regulating inflammatory cytokine (TNF-α, IL-1β and MCP-1) levels and reduced the hepatotoxicity of CCl4-treated mice. Therefore, MOPs could be used as a valuable functional food for treating liver injury. Moreover, MOPs showed an inhibitory effect on human colon cancer cells (HCT116 cells and HeLa cells), and the inhibitory rates of HCT116 cells and HeLa cells were 59.32% and 47.57%, respectively. The inhibitory effect of MOPs with different molecular weights on HCT116 cells was also different, and MOPs with molecular weights (Mw > 300 kDa) showed a strong antitumour effect (Ma, Yang, Xie, Yan, Li, & Tie, 2020).
5. Relationship between the structure and biological activity of MOPs
The molecular structure of polysaccharides is closely related to their biological activity. The molecular weight, monosaccharide composition, uronic acid content, glycosidic bond type, branched chain structure, structural modification and structural conformation will affect the biological activities of MOPs. However, due to the complex structures of MOPs, there are few reports on the relationship between MOP structures and biological activities. Therefore, we attempted to elaborate the relationship between MOPs structures and biological activities. The specific chemical structure and biological activities from different sources of MOPs are shown in Table 2.
5.1. Influence of the molecular weight (Mw)
First, molecular weight is the most important factor affecting the biological activities of MOPs. Chen et al., (Chen et al., 2017) found that MOPs (MLP-3) with low molecular weight (Mw 4.86 × 103 kDa) showed stronger α-glucosidase and α-amylase inhibition effects than those with high molecular weight (MLP-1 MLP-2), suggesting that MLP-3 has the potential for antidiabetic. At the same time, MLP-3 showed a lower viscosity and better Newtonian fluid characteristics, which could be easily utilized by intestinal microorganisms. In addition, MOPs with high and medium molecular weights may have high immunomodulatory activity. Dong et al., (Dong et al., 2018) found that MOPs with higher molecular weight (Mw 155.35 kDa) could stimulate macrophage activation, thereby activating immunomodulatory activity. However, previous studies also confirmed that MOPs with high molecular weight showed better antioxidant activities than MOPs with low molecular weight (Tian et al., 2021). MOPs with a molecular weight of 7.65 × 104 kDa showed better radical cationic scavenging capacity and iron reduction antioxidant capacity (He et al., 2018).
5.2. Influence of monosaccharide composition and uronic acid
In addition, the composition types of monosaccharides also affect the biological activity of MOPs. For example, MOPs containing more lactose improve its antioxidant capacity. MOPs with more rhamnose content could be better utilized by intestinal microbes. A new polysaccharide with a molar ratio of mannose, rhamnose, glucose and galactose (0.49:3.65:0.63:1.27) was isolated from M. oleifera Lam leaves. This polysaccharide not only improved intestinal mucosal barrier function and enhanced digestive enzyme activity but also maintained the integrity of the intestinal mucosa and regulated the composition of the gut microbiota as prebiotics (Wang et al., 2019). In addition, the galactose structure also affected the antioxidant capacity of MOPs, and MOPs with a higher content of galactose structure could remove the accumulation of peroxides (He et al., 2018, Raja et al., 2016). In addition, MOPs including more rhamnose structures could reduce oxidative damage in the liver (Sun et al., 2019). Interestingly, some studies also investigated the activity of MOPs by detecting the composition of uronic acids. MOPs with uronic acid structures had better antioxidant capacity. In particular, the galacturonic acid structure and arabinuronic acid are the main functional structures that exert antioxidant activity in MOPs (He et al., 2018). This may because the carbon atoms of polysaccharides containing aldehyde acids have a large positive charge, and the steric resistance of free radical attack was small, so they showed strong radical scavenging activity. Dong et al., (Dong et al., 2018) also found that MOPs could promote the levels of ROS, NO, IL-6 and TNF-α when the glucuronic acid content was 0.81 ± 0.12% and the galacturonic acid content was 1.49 ± 0.10%.
5.3. Influence of glycosyl linkage type and branching structures
Moreover, MOPs with branching structures also showed better antioxidant activity. MOPs with a high branching structure and galactose arabinoid structure could effectively decrease DPPH levels. In addition, MOPs with more branches could combine with each other to form a cross-linked network structure, which could improve the moisture absorption and water retention capacity of polysaccharides, increase the elasticity and viscosity, and be used as auxiliary materials for preparing natural polymer wall materials (Mehwish et al., 2021). In contrast, MOPs with less branching and a lower galactose content showed a weaker binding ability of bile acids, which may be detrimental to the regulation of lipid balance (Li et al., 2020, Yang et al., 2020). The type of glycosidic bond also significantly affected the activity of MOPs. Li et al., (Li et al., 2020) found that MOPs with arabinose, glucose and galactose structures and the glycosidic link of (1 → 3,6)-β-d-Galp, (1 → 6)-β-d-Galp, (1 → 5)-α-l-Araf, and T-α-l-Araf could enhance the immunity of RAW 264.7 cells and enhance pinocytosis ability. Cui et al., (Cui et al., 2019) also indicated that MOPs containing typical α-glycosidic bonds with α-ArAF, α-Gly, β-GalP, α-GalPa and β-Gly could enhance immune activity. In addition, the polysaccharide contains fewer α-(1–4) glycosidic bonds, which have good digestion and decomposition ability during simulated digestion in vitro and are significantly utilized by intestinal flora to increase SCFAs (Li et al., 2021). Furthermore, MOPs with the main stem contain a → 1)-β-d-Galp-(3,4 → ) structure and have a highly differentiated branch chain at the O-4 position, branching from → 1)-β-d-Galp-(4→, →1)-α-d-Galp-(2 → Araf-(1 → Galp-(1→, which could improve the reduction ability of iron and further improve the scavenging ability of ABTS and DPPH (He et al., 2018).
5.4. Influence of functional structure and helix structure
It is worth noting that the biological activities of MOPs could also be improved by chemical modification, but the activities of MOPs modified by different modification methods is also different. Mehwish et al., (Mehwish et al., 2021) used MOPs embedded silver ions (MOS-PS) to make wound dressing. MOS-PS mainly consisted of α-structured pyranose and β-pyranose and showed better antibacterial activity and wound healing ability. Moreover, sulphated MOPs also showed high antioxidant activity (Hernández, García, López, Puls, Parajó, & Martín, 2013). Sulphated polysaccharides refer to polysaccharide containing sulphate groups on the sugar units, sulphated modification could significantly improve structure characteristics, promote bioactivities and even add new bioactivities to polysaccharides. Thus, sulphated modification polysaccharides are increasingly causing more attention (Huang et al., 2019, Wang et al., 2018). After added sulphate groups into MOPs, it was found that MOPs with Sulphated modification showed better antioxidant activity than normal MOPS. The hydroxyl radical scavenging rate up to 79.8% and the DPPH scavenging rate up to 65.79% (Choudhary, Bodakhe, & Gupta, 2013). In addition, the molecular chain conformation is also an important factor in the biological activities of MOPs. For example, MOPs with triple helix structures could increase beneficial bacteria and decrease harmful bacteria in vivo, thus affecting microbial community function and regulating the balance of glucose and lipid metabolism (Tian et al., 2021). However, polysaccharides without triple helix structures had better oxidative regulation ability on glycolipid metabolism (Chen et al., 2017). The two homogeneous polysaccharides (MOP-D-a-1 and MOP-D-a-2) were isolated by gel chromatography purification. MOP-D-a-1 did not have a triple helix structure, while MOP-D-a-2 had an obvious triple helix structure. Compared with MOP-D-a-2, MOP-D-a-1 showed better scavenging ability of ABTS and hydroxyl radicals (Dong, 2016).
In conclusion, the biological activities of MOPs is closely related to their chemical structures. However, current studies only establish the associations between single structures and specific activities of MOPs. Therefore, it is necessary to further study the detailed structures of MOPs and establish the multidimensional correlation to clarify the structure-biological activity relationship of MOPs.
6. Final conclusions and future perspectives
M. oleifera Lam, as an edible plant and herbal medicine, is rich in nutrients and has various biological activities. Polysaccharides are one of the main functional factors in M. oleifera Lam (MOPs). Therefore, in recent years, many studies have been carried out on the separation, purification, structure identification and biological activities evaluation of MOPs. However, due to the complexity of the structure of MOPs and the differences in structure in different parts, it is difficult to determine the correlation between structure and biological activities.
Current research has summarized some results of MOPs. While the purification and characterization of MOPs, the relationship between structure-biological activity and the future application of MOPs still need to be researched in the future. (1) First, due to the complexity of polysaccharides structure, the detection of MOPs structure can be combined with a variety of separation and detection technologies to improve the utilization rate of MOPs. (2) In addition, functional research on MOPs is relatively basic at present, and the relationship between polysaccharides structure and biological activities has not been deeply studied. Studies on the in-depth biological molecular mechanisms of MOPs should be explicit in the future. Moreover, to improve the biological activities, it is necessary to carry out research on the structural modification of MOPs. (3) Molecular docking simulation can also be used to establish a polysaccharides and biological activities target database of MOPs, and big database can be used to determine the functional activities and targeted therapy of MOPs. (4) MOPs are mostly combined with proteins and phenols to exert their effects. Future research could focus on developing drug homologous functional foods based on polysaccharides-protein structures, polysaccharides-polyphenol structures and other polysaccharide complexes of MOPs. (5) In addition, MOPs can be used not only in food but also in cosmetics such as soaps and creams. (6) Finally, M. oleifera Lam leaves are a new resource for foods. Thus, many polysaccharides research studies have focused on leaves. However, all parts of the M. oleifera Lam polysaccharide contains different biological activities. Therefore, the other parts of the polysaccharides of M. oleifera Lam may be targeted as raw materials for food safety evaluation and to increase the application of MOPs. The future developments and trends of MOPs are shown in Fig. 4.
Fig. 4.
The Future trends and applications of MOPs.
In conclusion, this review summarizes the research progress in the extraction, isolation, purification, structural characterization and biological activities of polysaccharides from different parts of M. oleifera Lam and enumerates the biological activities and possible molecular mechanisms of MOPs. Furthermore, the relationship between the structure and biological activities of MOPs was discussed in this review, to further understand the structural diversity, to understand the structure–biological relationship of MOPs and to finally provide some theoretical reference for future MOPs research.
Funding
This work was supported by the “Major Project of Science and Technology Department of Yunnan Province” (202002AA100005 and 202102AE090027-2), the “Cassava Industrial Technology System of China” (CARS-11-YNSJ), and the “Yunnan Province Ten Thousand Plan Industrial Technology Talents project” (YNWR-CYJS-2020–010).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jun Sheng, Email: shengjun_ynau@163.com.
Yang Tian, Email: tianyang1208@163.com.
References
- Abdull Razis A.F., Ibrahim M.D., Kntayya S.B. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev. 2014;15(20):8571–8576. doi: 10.7314/apjcp.2014.15.20.8571. [DOI] [PubMed] [Google Scholar]
- Ali A., Garg P., Goyal R., Kaur G., Li X., Negi P.…Kulshrestha S. A Novel Herbal Hydrogel Formulation of Moringa oleifera for Wound Healing. Plants (Basel) 2020;10(1):25. doi: 10.3390/plants10010025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Almatrafi M., Vergara-Jimenez M., Murillo A., Norris G., Blesso C., Fernandez M. Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism. Int J Mol Sci. 2017;18(7):1330. doi: 10.3390/ijms18071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Q.i., Ye X., Han Y.i., Zhao M., Chen S.i., Liu X.…Wang W. Structure analysis of polysaccharides purified from Cyclocarya paliurus with DEAE-Cellulose and its antioxidant activity in RAW264.7 cells. Int J Biol Macromol. 2020;157:604–615. doi: 10.1016/j.ijbiomac.2019.11.212. [DOI] [PubMed] [Google Scholar]
- Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21(1):17–25. doi: 10.1002/(ISSN)1099-157310.1002/ptr.v21:110.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Arora D.S., Kaur N. Antimicrobial Potential of Fungal Endophytes from Moringa oleifera. Appl Biochem Biotechnol. 2019;187(2):628–648. doi: 10.1007/s12010-018-2770-y. [DOI] [PubMed] [Google Scholar]
- Bai C.H., Fu Z., Lin Y. 辣木叶多糖对益生菌生长及耐受性的影响 [Effect of Moringa Leaf Polysaccharide on Proliferation and Tolerance Capability of Probiotics] FOOD SCIENCE AND TECHNOLOGY. 2020;45(09):7–13. doi: 10.13684/j.cnki.spkj.2020.09.002. [DOI] [Google Scholar]
- Bogdanova L.R., Rogov A.M., Zueva O.S., Zuev Y.F. Lipase enzymatic microreactor in polysaccharide hydrogel: Structure and properties. Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 2019;68(2):400–404. doi: 10.1007/s11172-019-2399-1. [DOI] [Google Scholar]
- Caicedo-Lopez L.H., Luzardo-Ocampo I., Cuellar-Nunez M.L., Campos-Vega R., Mendoza S., Loarca-Pina G. Effect of the in vitro gastrointestinal digestion on free-phenolic compounds and mono/oligosaccharides from Moringa oleifera leaves: Bioaccessibility, intestinal permeability and antioxidant capacity. Food Res Int. 2019;120:631–642. doi: 10.1016/j.foodres.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang B., Huang Q., Fu X., Liu R.H. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Industrial Crops and Products. 2017;100:1–11. doi: 10.1016/j.indcrop.2017.01.042. [DOI] [Google Scholar]
- Chen G., Yuan Q., Saeeduddin M., Ou S., Zeng X., Ye H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr Polym. 2016;153:663–678. doi: 10.1016/j.carbpol.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Chen S., Liu C., Huang X., Hu L., Huang Y., Chen H.…Nie S. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts. Journal of Functional Foods. 2020;66:103791. doi: 10.1016/j.jff.2020.103791. [DOI] [Google Scholar]
- Choudhary M.K., Bodakhe S.H., Gupta S.K. Assessment of the antiulcer potential of Moringa oleifera root-bark extract in rats. J Acupunct Meridian Stud. 2013;6(4):214–220. doi: 10.1016/j.jams.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Chu Y.J. 辣木多糖除蛋白的工艺技术 [Process of Removing Protein from Spicewood Polysaccharide] Process Technology. 2020;19:86–89. doi: 10.16736/j.cnki.cn41-1434/ts.2020.19.028. [DOI] [Google Scholar]
- Cui C., Chen S., Wang X., Yuan G., Jiang F., Chen X., Wang L. Characterization of Moringa oleifera roots polysaccharide MRP-1 with anti-inflammatory effect. Int J Biol Macromol. 2019;132:844–851. doi: 10.1016/j.ijbiomac.2019.03.210. [DOI] [PubMed] [Google Scholar]
- Cui, C., Wang, X. Y., Yuan, G. W., Liu, Z. P., Chen, X. Y., & Wang, L. (2018). 辣木叶多糖MLP100-3的提取纯化与体外抗炎活性研究 [Isolation, purification and anti-inflammatory activity of MLP100-3 polysaccharides from Moringa oleifera leaves]. Journal of Jinan University(Natural Science & Medicine Edition), 39(05), 410-418+425.
- de Oliveira A.M., de Abreu Filho B.A., de Jesus Bassetti F., Bergamasco R., Gomes R.G. Natural Extract of Moringa oleifera Leaves Promoting Control of Staphylococcus aureus strains biofilm on PVC surface. Food and Bioprocess Technology. 2020;13(10):1817–1832. doi: 10.1007/s11947-020-02521-x. [DOI] [Google Scholar]
- Dong C.G. Master diss; Harbin Institute of Technology: 2016. 辣木籽水溶性多糖的分离纯化、结构表征及其抗氧化活性研究 [Study on isolation, purification, structure and antioxidant activity of water-soluble polysaccharide from moringa seed] [Google Scholar]
- Dong Z., Li C., Huang Q., Zhang B., Fu X., Liu R.H. Characterization of a novel polysaccharide from the leaves of Moringa oleifera and its immunostimulatory activity. Journal of Functional Foods. 2018;49:391–400. doi: 10.1016/j.jff.2018.09.002. [DOI] [Google Scholar]
- Dou Z., Chen C., Fu X. Bioaccessibility, antioxidant activity and modulation effect on gut microbiota of bioactive compounds from Moringa oleifera Lam. leaves during digestion and fermentation in vitro. Food Funct. 2019;10(8):5070–5079. doi: 10.1039/c9fo00793h. [DOI] [PubMed] [Google Scholar]
- Du Y. Sichuan Agricultural University; Master diss: 2015. 辣木多糖的提取与体外抗丙肝病毒活性的研究 [Study on extraction and anti HCV activity in vitro of Moringa polysaccharide] [Google Scholar]
- Gao X., Xie Q., Liu L., Kong P., Sheng J., Xiang H. Metabolic adaptation to the aqueous leaf extract of Moringa oleifera Lam.-supplemented diet is related to the modulation of gut microbiota in mice. Appl Microbiol Biotechnol. 2017;101(12):5115–5130. doi: 10.1007/s00253-017-8233-5. [DOI] [PubMed] [Google Scholar]
- Gong T., Liu S., Wang H., Zhang M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Bioscience. 2021;42:101153. doi: 10.1016/j.fbio.2021.101153. [DOI] [Google Scholar]
- Grube M., Dinu V., Lindemann H., Pielenz F., Festag G., Schubert U.S.…Nischang I. Polysaccharide valproates: Structure - property relationships in solution. Carbohydr Polym. 2020;246:116652. doi: 10.1016/j.carbpol.2020.116652. [DOI] [PubMed] [Google Scholar]
- Guo F., Han M., Lin S., Ye H., Chen J., Zhu H., Lin W. Enteromorpha prolifera polysaccharide prevents high- fat diet-induced obesity in hamsters: A NMR-based metabolomic evaluation. J Food Sci. 2021;86(8):3672–3685. doi: 10.1111/jfds.v86.810.1111/1750-3841.15818. [DOI] [PubMed] [Google Scholar]
- He T.B., Huang Y.P., Huang Y., Wang X.J., Hu J.M., Sheng J. Structural elucidation and antioxidant activity of an arabinogalactan from the leaves of Moringa oleifera. Int J Biol Macromol. 2018;112:126–133. doi: 10.1016/j.ijbiomac.2018.01.110. [DOI] [PubMed] [Google Scholar]
- Hernández E., García A., López M., Puls J., Parajó J.C., Martín C. Dilute sulphuric acid pretreatment and enzymatic hydrolysis of Moringa oleifera empty pods. Industrial Crops and Products. 2013;44:227–231. doi: 10.1016/j.indcrop.2012.11.001. [DOI] [Google Scholar]
- Hu X., Goff H.D. Fractionation of polysaccharides by gradient non-solvent precipitation: A review. Trends in Food Science & Technology. 2018;81:108–115. doi: 10.1016/j.tifs.2018.09.011. [DOI] [Google Scholar]
- Huang G., Chen F., Yang W., Huang H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends in Food Science & Technology. 2021;109:564–568. doi: 10.1016/j.tifs.2021.01.038. [DOI] [Google Scholar]
- Huang L., Shen M., Morris G.A., Xie J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends in Food Science & Technology. 2019;92:1–11. doi: 10.1016/j.tifs.2019.08.008. [DOI] [Google Scholar]
- Ismail Iid I., Kumar S., Shukla S., Kumar V., Sharma R. Putative antidiabetic herbal food ingredients: Nutra/functional properties, bioavailability and effect on metabolic pathways. Trends in Food Science & Technology. 2020;97:317–340. doi: 10.1016/j.tifs.2020.01.017. [DOI] [Google Scholar]
- Jaja-Chimedza A., Zhang L.i., Wolff K., Graf B.L., Kuhn P., Moskal K.…Raskin I. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J Funct Foods. 2018;47:376–385. doi: 10.1016/j.jff.2018.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardana B.C., Liyanage R., Lalantha N., Iddamalgoda S., Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT - Food Science and Technology. 2015;64(2):1204–1208. doi: 10.1016/j.lwt.2015.07.028. [DOI] [Google Scholar]
- Jin X.Y., Zhang P.Q. 超高压提取辣木叶多糖工艺条件的优化 [Optimization polysaccharide extraction conditions from moringa oleifera by ultra high pressure method] Food engineering. 2020;03:32–34. [Google Scholar]
- Junter G.A., Lebrun L. Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance. J Pharm Anal. 2020;10(4):291–312. doi: 10.1016/j.jpha.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N., Arora D.S. Prospecting the antimicrobial and antibiofilm potential of Chaetomium globosum an endophytic fungus from Moringa oleifera. AMB Express. 2020;10(1):206. doi: 10.1186/s13568-020-01143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y., Yang H.J., Song K.B. Application of a puffer fish skin gelatin film containing Moringa oleifera Lam. leaf extract to the packaging of Gouda cheese. J Food Sci Technol. 2016;53(11):3876–3883. doi: 10.1007/s13197-016-2367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong Y.K., Yang F.-C., Chang J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr Polym. 2021;251:117006. doi: 10.1016/j.carbpol.2020.117006. [DOI] [PubMed] [Google Scholar]
- Li C., Dong Z., Zhang B., Huang Q., Liu G., Fu X. Structural characterization and immune enhancement activity of a novel polysaccharide from Moringa oleifera leaves. Carbohydr Polym. 2020;234:115897. doi: 10.1016/j.carbpol.2020.115897. [DOI] [PubMed] [Google Scholar]
- Li C., Zhou S., Fu X., Huang Q., Chen Q. In vitro digestibility and prebiotic activities of a bioactive polysaccharide from Moringa oleifera leaves. J Food Biochem. 2021;45(11) doi: 10.1111/jfbc.13944. [DOI] [PubMed] [Google Scholar]
- Li Q.L. South China University of Technology; Master diss: 2019. 辣木叶提取物的制备、降血糖活性评价及降糖功效因子结构解析 [Preparation of Moringa oleifera leaf extract, evaluation of its hypoglycemic effect and structure characterization of the main hypoglycemic ingredient] [Google Scholar]
- Li T., Xu S., Bi J., Huang S., Fan B., Qian C. Metabolomics study of polysaccharide extracts from Polygonatum sibiricum in mice based on (1) H NMR technology. J Sci Food Agric. 2020;100(12):4627–4635. doi: 10.1002/jsfa.10523. [DOI] [PubMed] [Google Scholar]
- Li X., Xiong F., Liu Y., Liu F., Hao Z., Chen H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Enteromorpha intestinalis. Int J Biol Macromol. 2018;111:319–325. doi: 10.1016/j.ijbiomac.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Lin X., Xu Y.J., Xiao G.S., Zou B., Tang D.B., Yu Y.S., Wu J.J. 干燥方式对辣木叶营养活性成分、抗氧化活性及色泽的影响 [Effects of Drying Methods on Bioactive Components, Antioxidant Capacity and Color of the Leaves of Moringa oleifera Lam] Chinese Journal of Tropical Crops. 2018;39(12):8. [Google Scholar]
- Liu G., Liu Y., Yan S., Li J. Acetic acid reducing the softening of lotus rhizome during heating by regulating the chelate-soluble polysaccharides. Carbohydr Polym. 2020;240:116209. doi: 10.1016/j.carbpol.2020.116209. [DOI] [PubMed] [Google Scholar]
- Liu X.-X., Liu H.-M., Yan Y.-Y., Fan L.-Y., Yang J.-N., Wang X.-D., Qin G.-Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lwt. 2020;117:108645. doi: 10.1016/j.lwt.2019.108645. [DOI] [Google Scholar]
- Ma L., Yang M., Xie J., Yan L., Li L.F., Tie Y. 辣木叶多糖对五种肿瘤细胞的抑制效果 [Inhibitory Effect of Polysaccharides from Moringa oleifera on the Cancer Cells of Five Cancers] CHINESE JOURNAL OF TROPICAL AGRICULTURE. 2020;40(04):49–55. [Google Scholar]
- Ma Z.F., Ahmad J., Zhang H., Khan I., Muhammad S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South African Journal of Botany. 2020;129:40–46. doi: 10.1016/j.sajb.2018.12.002. [DOI] [Google Scholar]
- Mansel B.W., Ryan T.M., Chen H.-L., Lundin L., Williams M.A.K. Polysaccharide conformations measured by solution state X-ray scattering. Chemical Physics Letters. 2020;739:136951. doi: 10.1016/j.cplett.2019.136951. [DOI] [Google Scholar]
- Mehwish H.M., Liu G.e., Rajoka M.S.R., Cai H., Zhong J., Song X.…He Z. Therapeutic potential of Moringa oleifera seed polysaccharide embedded silver nanoparticles in wound healing. Int J Biol Macromol. 2021;184:144–158. doi: 10.1016/j.ijbiomac.2021.05.202. [DOI] [PubMed] [Google Scholar]
- Mondal S., Chakraborty I., Pramanik M., Rout D., Islam S.S. STRUCTURAL STUDIES OF AN IMMUNOENHANCING POLYSACCHARIDE ISOLATED FROM MATURE PODS (FRUITS) OF MORINGA OLEIFERA (SAJINA) Medicinal Chemistry Research. 2004;13(6-7):390–400. [Google Scholar]
- Munekata P.E.S., Rocchetti G., Pateiro M., Lucini L., Domínguez R., Lorenzo J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Current Opinion in Food Science. 2020;31:81–87. doi: 10.1016/j.cofs.2020.03.003. [DOI] [Google Scholar]
- Ngo D.-H., Wijesekara I., Vo T.-S., Van Ta Q., Kim S.-K. Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Research International. 2011;44(2):523–529. doi: 10.1016/j.foodres.2010.12.030. [DOI] [Google Scholar]
- Nie S., Wu J.Y., Cheng N.X., Wen Z.S., Wang W.C. 辣木叶粗多糖的制备单糖组成及抗氧化活性研究 [Study on Preparation, Monosaccharide Composition and Antioxidant Activity of Crude Polysaccharide from Moringa oleifera Leaves] Journal of Anhui Agricultural Sciences. 2021;49(12):4. [Google Scholar]
- Okuda T., Baes A.U., Nishijima W., Okada M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001;35(2):405–410. doi: 10.1016/s0043-1354(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Omodanisi E., Aboua Y., Oguntibeju O. Assessment of the Anti-Hyperglycaemic, Anti-Inflammatory and Antioxidant Activities of the Methanol Extract of Moringa Oleifera in Diabetes-Induced Nephrotoxic Male Wistar Rats. Molecules. 2017;22(4):439. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantsulaia S., Targamadze K., Khundadze N., Kharaishvili Q., Volonterio A., Chitty M.…Chankvetadze B. Potential and current limitations of superficially porous silica as a carrier for polysaccharide-based chiral selectors in separation of enantiomers in high-performance liquid chromatography. J Chromatogr A. 2020;1625:461297. doi: 10.1016/j.chroma.2020.461297. [DOI] [PubMed] [Google Scholar]
- Parwani L., Bhatnagar M., Bhatnagar A., Sharma V., Sharma V. Evaluation of Moringa oleifera seed biopolymer-PVA composite hydrogel in wound healing dressing. Iranian Polymer Journal. 2016;25(11):919–931. doi: 10.1007/s13726-016-0479-8. [DOI] [Google Scholar]
- Patriota L.L.d.S., Ramos D.d.B.M., dos Santos A.C.L.A., Silva Y.A., Gama e Silva M., Torres D.J.L.…Napoleão T.H. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem Toxicol. 2020;145:111691. doi: 10.1016/j.fct.2020.111691. [DOI] [PubMed] [Google Scholar]
- Peluso P., Mamane V., Dallocchio R., Dessì A., Cossu S. Noncovalent interactions in high-performance liquid chromatography enantioseparations on polysaccharide-based chiral selectors. J Chromatogr A. 2020;1623:461202. doi: 10.1016/j.chroma.2020.461202. [DOI] [PubMed] [Google Scholar]
- Pieczywek P.M., Kozioł A., Płaziński W., Cybulska J., Zdunek A. Resolving the nanostructure of sodium carbonate extracted pectins (DASP) from apple cell walls with atomic force microscopy and molecular dynamics. Food Hydrocolloids. 2020;104:105726. doi: 10.1016/j.foodhyd.2020.105726. [DOI] [Google Scholar]
- Qi Y., Ren W., Zhang H., Chen G., Huang W., Li X.…Zhao W. Optimization of Extraction and Purification of Polysaccharides from Veronicastrum axillare, and Evaluation of Their Biological Activities. Chem Biodivers. 2021;18(3) doi: 10.1002/cbdv.v18.310.1002/cbdv.202000864. [DOI] [PubMed] [Google Scholar]
- Raja W., Bera K., Ray B. Polysaccharides from Moringa oleifera gum: Structural elements, interaction with β-lactoglobulin and antioxidative activity. RSC Advances. 2016;6(79):75699–75706. doi: 10.1039/c6ra13279k. [DOI] [Google Scholar]
- Ranote S., Chauhan G.S., Joshi V. Etherified Moringa oleifera gum as rapid and effective dye adsorbents. Chemical Engineering Journal. 2020;387:124055. doi: 10.1016/j.cej.2020.124055. [DOI] [Google Scholar]
- Ranote S., Kumar D., Kumari S., Kumar R., Chauhan G.S., Joshi V. Green synthesis of Moringa oleifera gum-based bifunctional polyurethane foam braced with ash for rapid and efficient dye removal. Chemical Engineering Journal. 2019;361:1586–1596. doi: 10.1016/j.cej.2018.10.194. [DOI] [Google Scholar]
- Rao M.V., Sengar A.S., C K S., Rawson A. Ultrasonication-A green technology extraction technique for spices: A review. Trends in Food Science & Technology. 2021;116:975–991. doi: 10.1016/j.tifs.2021.09.006. [DOI] [Google Scholar]
- Rocchetti G., Pagnossa J.P., Blasi F., Cossignani L., Hilsdorf Piccoli R., Zengin G.…Lucini L. Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res Int. 2020;127:108712. doi: 10.1016/j.foodres.2019.108712. [DOI] [PubMed] [Google Scholar]
- Rodríguez G.M., Sibaja J.C., Espitia P.J.P., Otoni C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocolloids. 2020;103:105630. doi: 10.1016/j.foodhyd.2019.105630. [DOI] [Google Scholar]
- Roy S.K., Chandra K., Ghosh K., Mondal S., Maiti D., Ojha A.K.…Islam S.S. Structural investigation of a heteropolysaccharide isolated from the pods (fruits) of Moringa oleifera (Sajina) Carbohydr Res. 2007;342(16):2380–2389. doi: 10.1016/j.carres.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Rubio-Elizalde I., Bernaldez-Sarabia J., Moreno-Ulloa A., Vilanova C., Juarez P., Licea-Navarro A., Castro-Cesena A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr Polym. 2019;206:455–467. doi: 10.1016/j.carbpol.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Saleem A., Saleem M., Akhtar M.F. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. South African Journal of Botany. 2020;128:246–256. doi: 10.1016/j.sajb.2019.11.023. [DOI] [Google Scholar]
- Shi S., Zhang W., Ren X., Li M., Sun J., Li G.…Wang J. An advanced and universal method to high-efficiently deproteinize plant polysaccharides by dual-functional tannic acid-fe(III) complex. Carbohydr Polym. 2019;226:115283. doi: 10.1016/j.carbpol.2019.115283. [DOI] [PubMed] [Google Scholar]
- Singh B., Kumar A. Radiation-induced graft copolymerization of Nvinyl imidazole onto moringa gum polysaccharide for making hydrogels for biomedical applications. Int J Biol Macromol. 2018;120(Pt B):1369–1378. doi: 10.1016/j.ijbiomac.2018.09.148. [DOI] [PubMed] [Google Scholar]
- Singh B., Kumar A. Graft and crosslinked polymerization of polysaccharide gum to form hydrogel wound dressings for drug delivery applications. Carbohydr Res. 2020;489:107949. doi: 10.1016/j.carres.2020.107949. [DOI] [PubMed] [Google Scholar]
- Singhal A.K., Jarald E.E., Showkat A., Daud A. In vitro evaluation of Moringa oleifera gum for colon-specific drug delivery. Int J Pharm Investig. 2012;2(1):48–51. doi: 10.4103/2230-973X.96926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Li L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr Polym. 2020;229:115407. doi: 10.1016/j.carbpol.2019.115407. [DOI] [PubMed] [Google Scholar]
- Sun C., Li W., Liu Y., Deng W., Adu-Frimpong M., Zhang H.…Xu X. In vitro/in vivo hepatoprotective properties of 1-O-(4-hydroxymethylphenyl)-alpha-L-rhamnopyranoside from Moringa oleifera seeds against carbon tetrachloride-induced hepatic injury. Food Chem Toxicol. 2019;131 doi: 10.1016/j.fct.2019.05.039. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhang M., Fang Z. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: A review. Trends in Food Science & Technology. 2020;105:468–482. doi: 10.1016/j.tifs.2019.02.026. [DOI] [Google Scholar]
- Sun Y., Zhao C., Liu Y.Y., Sui Y.L., Zhu J.Z. 辣木叶对糖尿病大鼠认知功能及海马神经细胞凋亡的影响 [Effects of spicy leaves on cognitive function and apoptosis of hippocampal neurons in diabetic rats] Chin. J Appl Phys. 2021;37(06):638–643. doi: 10.12047/j.cjap.6123.2021.070. [DOI] [PubMed] [Google Scholar]
- Tang W., Liu D., Yin J.-Y., Nie S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends in Food Science & Technology. 2020;99:76–87. doi: 10.1016/j.tifs.2020.02.015. [DOI] [Google Scholar]
- Tang Y., Choi E.-J., Han W.C., Oh M., Kim J., Hwang J.-Y.…Kim E.-K. Moringa oleifera from Cambodia Ameliorates Oxidative Stress, Hyperglycemia, and Kidney Dysfunction in Type 2 Diabetic Mice. Journal of Medicinal Food. 2017;20(5):502–510. doi: 10.1089/jmf.2016.3792. [DOI] [PubMed] [Google Scholar]
- Tian H., Liang Y., Liu G., Li Y., Deng M., Liu D.…Sun B. Moringa oleifera polysaccharides regulates caecal microbiota and small intestinal metabolic profile in C57BL/6 mice. Int J Biol Macromol. 2021;182:595–611. doi: 10.1016/j.ijbiomac.2021.03.144. [DOI] [PubMed] [Google Scholar]
- Wahab O., Sobhy H.M., Badr A., Ghazalah A.A. Effect of Moringa oleifera seeds powder on performance and immunity of broiler chicks. AIMS Agriculture and Food. 2020;5(4):896–910. doi: 10.3934/agrfood.2020.4.896. [DOI] [Google Scholar]
- Wang F., Bao Y., Zhang C., Zhan L., Khan W., Siddiqua S.…Xiao J. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit Rev Food Sci Nutr. 2021;6:1–25. doi: 10.1080/10408398.2020.1870099. [DOI] [PubMed] [Google Scholar]
- Wang F., Bao Y.F., Si J.J., Duan Y., Weng Z.B., Shen X.C. The Beneficial Effects of a Polysaccharide from Moringa oleifera Leaf on Gut Microecology in Mice. J Med Food. 2019;22(9):907–918. doi: 10.1089/jmf.2018.4382. [DOI] [PubMed] [Google Scholar]
- Wang F., Bao Y.F., Zhang Y., Weng Z.B., Zhou Z.J., Shen X.C. 辣木叶多糖的分离纯化及其体外降糖活性研究 [Isolation and purification of polysaccharides from Moringa oleifera leaves and in vitro hypoglycemic activity] Journal of Food Safety and Quality. 2018;9(07):1592–1598. [Google Scholar]
- Wang F., Zhong H.H., Chen W.K., Liu Q.P., Li C.Y., Zheng Y.F., Peng G.P. Potential hypoglycaemic activity phenolic glycosides from Moringa oleifera seeds. Nat Prod Res. 2017;31(16):1869–1874. doi: 10.1080/14786419.2016.1263846. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xie J., Shen M., Nie S., Xie M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends in Food Science & Technology. 2018;74:147–157. doi: 10.1016/j.tifs.2018.02.010. [DOI] [Google Scholar]
- Wu T., Liu C., Hu X. Enzymatic synthesis, characterization and properties of the protein-polysaccharide conjugate: A review. Food Chem. 2022;372:131332. doi: 10.1016/j.foodchem.2021.131332. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang Y., Jiang W.-W., Luo X.-F., Dai T.-Y., Peng L.…Sheng J. Moringa oleifera Leaf Petroleum Ether Extract Inhibits Lipogenesis by Activating the AMPK Signaling Pathway. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Wang Y., Li X., Yu P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr Polym. 2015;117:1021–1027. doi: 10.1016/j.carbpol.2014.09.082. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhao M., Lin L. Effects of extraction methods on structural characteristics and bile acid-binding capacities of Moringa oleifera leaf polysaccharide fractions. International Journal of Food Science & Technology. 2020;55(4):1539–1546. doi: 10.1111/ijfs.v55.410.1111/ijfs.14430. [DOI] [Google Scholar]
- Yao H.-Y.-Y., Wang J.-Q., Yin J.-Y., Nie S.-P., Xie M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res Int. 2021;143:110290. doi: 10.1016/j.foodres.2021.110290. [DOI] [PubMed] [Google Scholar]
- Yu F., Yang A.P., Shi X., p., Ge, W. L., Wang, J., & Jiang, C. Y 辣木籽营养成分测定及多糖提取工艺 [The Determination of Nutritional Components and Polysaccharide Extraction Technology of Moringa Seeds] Food Industry. 2020;41(11):82–85. [Google Scholar]
- Yuan L., Zhong Z.C., Liu Y. Structural characterisation and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int J Biol Macromol. 2020;154:1400–1407. doi: 10.1016/j.ijbiomac.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Zhang S.Q., Yang J., Hui Y.H., Sun J.Y. 响应面法优化超声波辅助提取辣木叶多糖工艺 [Optimization of Ultrasound-assisted Extraction of Polysaccharide from Moringa Oleifera Leaf by Response Surface Methodology] Food Research And Development. 2020;41(16):69–74. [Google Scholar]
- Zhang Wen-Hui, Wu Jian, Weng Liangyu, Zhang Hongjie, Zhang Jian, Wu Anbo. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr Polym. 2020;227:115332. doi: 10.1016/j.carbpol.2019.115332. [DOI] [PubMed] [Google Scholar]
- Zhao Ping, Li Xia, Wang Ying, Yan Lanyi, Guo Lanping, Huang Luqi, Gao Wenyuan. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr Polym. 2020;233:115836. doi: 10.1016/j.carbpol.2020.115836. [DOI] [PubMed] [Google Scholar]
- Zheng Y.Y., Hu H.J., Zhou Y., Liang M.L., Luo M.Y., Xie Y.Q. 辣木籽硒多糖的提取工艺研究 [Study on Extraction Technology for Se Polysaccharide from Moringa Oleifera Seeds] Guangzhou Chemical Industry. 2021;49(18):48–51. [Google Scholar]
- Zhu A., Liu S., Wu K., Ren C., Xu M. Comparing of hot water and acid extraction of polysaccharides from proso millet. Chemical Industry and Chemical Engineering Quarterly. 2017;23(1):141–150. doi: 10.2298/ciceq151011026z. [DOI] [Google Scholar]
- Zhu Lina, Lu Yang, Sun Zhuo, Han Juan, Tan Zhenjiang. The application of an aqueous two-phase system combined with ultrasonic cell disruption extraction and HPLC in the simultaneous separation and analysis of solanine and Solanum nigrum polysaccharide from Solanum nigrum unripe fruit. Food Chem. 2020;304:125383. doi: 10.1016/j.foodchem.2019.125383. [DOI] [PubMed] [Google Scholar]