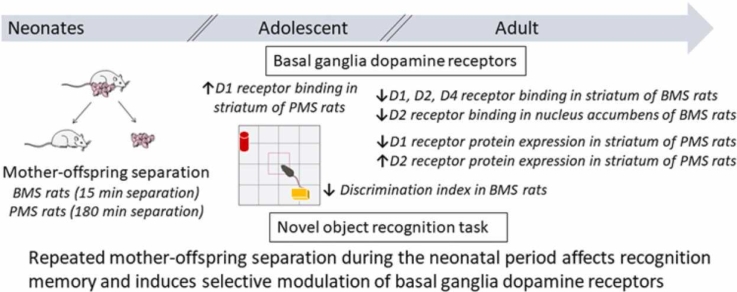
Abstract
Environmental stimuli in early life are recognized to affect brain development and behavior. Mother-pup interaction constitutes a determinant stimulus during this critical period. It is known that the dopaminergic system undergoes significant reorganization during adolescence and that dopamine receptors are involved in recognition memory. Based on the above, we examined the effects of brief and prolonged maternal separation during the neonatal period (15 or 180 min daily) on basal ganglia dopamine receptors and on the behavior in the novel object recognition task of adolescent and adult male rats. Using the NOR task, we observed that the discrimination index (DI) was decreased in rats with brief maternal separations independent of age. Using receptor autoradiography, we observed that brief maternal separation induced decreases in D1, D2 and D4 receptor binding levels in adult basal ganglia nuclei, while prolonged maternal separation induced increases in D1 receptor binding levels in caudate - putamen (CPu) of adolescent rats. With immunoblotting experiments, we found decreases in D1 and increases in D2 total protein levels in CPu of adult rats with prolonged maternal separations. Α positive correlation was observed between DI and D1 binding levels in CPu, internal globus pallidus and substantia nigra, and D2 binding levels in nucleus accumbens core in adult rats, using the Pearson correlation coefficient. Our results indicate that the long-lasting effects of neonatal mother-offspring separation on dopamine receptors depend on the duration of maternal separation and age and that this early life experience impairs recognition memory in adolescent and adult rats. Furthermore, the present results suggest that modulation of striatal dopamine receptors might underlie the reduced recognition memory of adult rats with brief neonatal maternal separations.
Keywords: Neonatal handling, Maternal separation, Dopamine receptors, Nucleus accumbens, Striatum, Novel object recognition
Graphical Abstract
Highlights
-
•
PMS promotes increase in D1 dopamine receptor binding levels in adolescent striatum.
-
•
BMS promotes decreases in D1, D2 and D4 receptor binding levels in adult striatum.
-
•
PMS promotes decrease in D1 and increase in D2 protein expression in adult striatum.
-
•
BMS promotes decrease in discrimination index of NOR task independent of age.
-
•
Striatal D1 receptor binding levels are strongly correlated with discrimination index.
1. Introduction
Environmental stimuli during early life play a pivotal role in modulating the development of the immature brain (Miguel et al., 2019). Positive experiences are linked to increased ability of the organism to respond to, cope with and adapt to stressful input, while negative experiences are highly associated with increased vulnerability to stressors and psychiatric disorders later in life, such as schizophrenia, anxiety related disorders, ADHD, addiction and anhedonia (Heim and Nemeroff, 2001). Adolescence is a critical developmental window and a time of high-risk behavior and increased exploration. It is also a period when the brain is undergoing many complex changes that can exert long-term influences on decision making and cognitive processes (Spear, 2000). In rats, adolescence extents approximately from P35–60 (Larsen and Luna, 2018, Piekarski et al., 2017) and some researchers consider early to mid-adolescence ranging from approximately P28 to P44 and late adolescence extending from approximately P44 to P56 (Bishnoi et al., 2020, Lupien et al., 2009, Spear and Swartzwelder, 2014).
The dopaminergic system undergoes significant reorganization during postnatal development. Several studies indicate that the dopaminergic receptor levels peak during mid-adolescence and then return to adult levels (Gelbard et al., 1989, Giorgi et al., 1987, Tarazi and Baldessarini, 2000, Teicher et al., 1995). Moreover, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC) and dopamine transporter (DAT) levels continue to increase after birth until adulthood (Giorgi et al., 1987, Tarazi et al., 1998b). Five distinct dopamine receptors have been characterized and divided into one of two discrete populations. D1 and D5 receptors belong to the D1-like family, while D2, D3 and D4 receptors belong to D2-like family. D1-like receptors primarily activate adenylyl cyclase and stimulate cAMP accumulation, while D2-like receptors act oppositely. In addition to the regulation of cAMP, several studies have revealed that dopamine receptors can exert their biological effects through alternative signaling pathways, such as the regulation of phospholipace C-mediated pathways or activation of G-protein-independent mechanisms involving β-arrestin 2 (Beaulieu and Gainetdinov, 2011, Beaulieu et al., 2015, Missale et al., 1998). Dopamine receptors are distributed in several brain areas, including caudate-putamen (CPu), nucleus accumbens (NAc), prefrontal cortex, hippocampus, ventral tegmental area, and substantia nigra (SN) (Boyson et al., 1986). In CPu, which corresponds to dorsal striatum, medium spiny neurons (MSNs) of the direct and indirect pathway differ in their expression of dopamine receptors and peptides. Direct pathway striatonigral neurons express D1 dopamine receptors and substance P, while indirect pathway striatopallidal neurons express D2 dopamine receptors and enkephalin (Gerfen et al., 1990, Surmeier et al., 1996). D4 dopamine receptors are located almost exclusively on striatal projection neurons since double labelling immunostaining techniques do not show D4 receptors in striatal interneurons (Rivera et al., 2003). In NAc, which corresponds to ventral striatum, previous studies have described analogous to the dorsal striatum direct and indirect pathways. In NAc, MSNs of the direct pathway express D1 receptors, while MSNs of the indirect pathway express D2 receptors (Heimer et al., 1991, Ikemoto, 2007, Zahm and Heimer, 1990). Subcellular localization of D4 receptors using electron microscopic immnocytochemisty has indicated that the D4 receptors in striatum are primarily postsynaptic (Rivera et al., 2003), while in the shell portion of NAc a major presynaptic localization has been reported (Svingos et al., 2000).
In the present study we used two well established animal models of early life stress, referred in the literature as neonatal handling and maternal separation, which consist of brief or prolonged daily separations of mother and offspring for the first postnatal weeks, correspondingly. The neonatal handling model, originally developed by Levine (1957), consists of brief daily separations (3–15 min) of mother and offspring during the first postnatal weeks. The maternal separation model, originally developed by Plotsky and Meaney (1993), consists of longer mother-offspring separations (1 – 6 h) during the first postnatal weeks. Several neonatal handling and maternal separation paradigms are used, in which the frequency, duration and postnatal period vary. Both paradigms are considered to have long-lasting effects later in life on the hypothalamic-pituitary-adrenal (HPA) axis function and on stress response.
Neonatal handling results in increased number of glucocorticoid receptors in the adult brain as well as reduced corticotropin-releasing hormone, adrenocorticotropin and corticosterone release following exposure to stressful stimuli (Bhatnagar and Meaney, 1995, Chapillon et al., 2002, Meaney et al., 1991, Meaney et al., 1985; Plotsky and Meaney, 1993; Vallée et al., 1997, Vallée et al., 1996). Behavioral studies highlight that neonatally handled animals show reduced emotional responses expressed through increased exploration in new environments and reduced fear and freezing (Chapillon et al., 2002, Fernández-Teruel et al., 1997, Meaney et al., 1991; Meerlo et al., 1999; Tsotsokou et al., 2021; Vallée et al., 1997). Furthermore, it has been reported that neonatal handling improves spatial memory and learning (Kosten et al., 2012, Raineki et al., 2014).
On the other hand, the data on the maternal separation model are not consistent most likely due to the variability in the duration of maternal separation and the extend of the neonatal period during which maternal separation is applied. Several review studies have appeared in the literature addressing the variability of the maternal separation paradigm (Bolton et al., 2017, Kosten et al., 2012, Lehmann and Feldon, 2000, Nishi, 2020, Nylander and Roman, 2013, Pryce and Feldon, 2003, Tractenberg et al., 2016, Wang et al., 2020). Several studies have shown that maternal separation changes the offspring’s neuroendocrine and behavioral stress response in an opposite direction to neonatal handling; maternally separated animals show increased anxiety-like behavior, depressive behavior and impaired cognitive functions (Banqueri et al., 2017, Daniels et al., 2004, de Souza et al., 2022, Huot et al., 2001, Lee et al., 2007, Tsotsokou et al., 2021). Other studies report no significant differences on the HPA axis, on adult anxiety-like behavior, and cognition between maternally separated and control animals (Biagini et al., 1998, Hulshof et al., 2011, Lehmann et al., 1999, Lehmann et al., 1998, Lundberg et al., 2020, Shalev and Kafkafi, 2002).
Previous studies albeit limited have provided evidence for the effects of these neonatal manipulations on dopamine receptors. In male adolescent Sprague-Dawley rats (P44), using immunofluorescence and confocal microscopy, it has been shown that a 4 h per day maternal separation between P2 and P20 heightened the increase of D1 receptor expression while diminished the expression of D2 receptor observed during adolescence on glutamatergic projection neurons of prefrontal cortex (Brenhouse et al., 2013). Moreover, in female adolescent Wistar rats (P39-P42), it has been reported that a 3 h per day maternal separation between P1 and P14 led to a decrease in D5 receptor mRNA levels and an increase in D2 receptor mRNA levels in prelimbic cortex, an increase in D1 and D5 receptor mRNA levels and a decrease in D3 receptor mRNA levels in CPu, as well as a decrease in D2 receptor mRNA levels in NAc (Majcher-Maślanka et al., 2017). Furthermore, using receptor autoradiography in adult male Wistar rats (P70), it has been indicated that 15 min or 360 min separations between P1 and P21 had no effect on D1 and D2 binding sites in CPu and NAc, but an effect was reported on D1 binding sites in hippocampus and D2 binding sites in ventral tegmental area (VTA) of adult rats after 15 min separations (Ploj et al., 2003). Additionally, in adult male Long-Evans rats (P90), it has been reported that a 15 min or a 180 min maternal separation between P1 and P14 led to increases in D1 binding sites but not in D2 binding sites in CPu, and lack of changes in either D1 or D2 receptor binding sites in NAc (Brake et al., 2004).
It is known that rodents show an innate preference for novel over familiar objects. Rodents readily approach objects and investigate them physically (Aggleton, 1985). This behavior contains two components, the spatial learning which relies heavily in hippocampal activity and the non-spatial learning of object identity which relies on multiple brain regions involved in procedural memory which relies on the motor centers of the brain. The novel object recognition (NOR) task has become the hallmark method used in assessing non-spatial learning in rodents (Cohen and Stackman, 2015, Denninger et al., 2018). The standard one-trial object recognition task measures spontaneous behavior and involves memory of a familiar object in parallel with the detection and encoding of a novel object and detects disruption and improvement of recognition memory in rodents by measuring their ability to discriminate between familiar and novel objects. The NOR task has been proven very useful to study short-term memory, intermediate-term memory and long-term memory, through manipulation of the retention interval, i.e. the amount of time animals must retain memory of the sample presented during the familiarization phase before the test phase (Antunes and Biala, 2012, Dere et al., 2007, Ennaceur, 2010, Taglialatela et al., 2009).
Previous studies have examined the effects of brief or prolonged maternal separations on novel object recognition in adolescent and adult rats. However, the variability in NOR methodology across the small number of studies complicate the ability to draw clear conclusions. It has been shown that late adolescent female rats (P55) exposed to brief separations exhibit enhanced novel object recognition compared to control (Plescia et al., 2014) and early adolescent rats (P28–33) exposed to brief separations show enhanced novel object recognition compared to adolescent rats exposed to prolonged separations (Frankola et al., 2010). In adult rats, novel object recognition has been explored only after prolonged maternal separations. Using short retention intervals (5 min), Grace et al. (2009) have shown that maternal separation has no effect on novel object recognition at P62, while Hulshof et al. (2011) found that maternally separated rats in combination with an adult stressor have impaired recognition memory. Several studies in adults using longer retention intervals (1 h or 24 h) have revealed either no effect of maternal separation (Makena et al., 2012, Vivinetto et al., 2013), or impaired novel object recognition memory (Aisa et al., 2007, Benetti et al., 2009).
Several studies have shown that modulation of dopamine receptors affects the performance of rodents in the novel object recognition task. The D1-R agonist, SKF 81297, given to rats prior to the test impairs both one-trial object recognition and one-trial object–place recognition at a delay of 15 min, while it enhances both one-trial object recognition and one-trial object–place recognition after a 4-h delay between acquisition and retention trial (Hotte et al., 2005). Moreover, the D1 antagonists SCH23390 and SKF83566, given i.p. prior to the test, induce a deficit in novel object recognition at a delay of 1 min (Horiguchi et al., 2013). Furthermore, Yang et al. (2017) have shown that preventing D1-like receptor activity throughout the brain in knock-out mice or systemically inhibiting D1 receptors with the D1 antagonist SCH23390 immediately after training, effectively blocks the preference for the novel object at a 24 h delay. Moreover the D2 receptor antagonist, raclopride, when administered i.p. impairs one-trial object recognition in rats after a 1-min delay (Woolley et al., 2003). In addition, Besheer et al. (2001) have shown that the dopamine receptor antagonists sulpiride (D2 receptor), U-99194A (D3 receptor), clozapine (D4 receptor) and L-745,870 (D4 receptor), administered prior to the sample trial, have no effect on one-trial object recognition in rats after a 60-min delay.
Attention has been attracted to D4 receptors since several atypical antipsychotic drugs, such as clozapine, have high affinity for these receptors. In humans, polymorphisms of D4 receptor have been associated with novelty-seeking traits (Munafò et al., 2008, Okuyama et al., 2000, Powell et al., 2003) and D4 knock-out mice exhibit reduced novelty-seeking behavior (Dulawa et al., 1999). Moreover, the effects of D4 receptor agonists and antagonists have been investigated on the novel object recognition task. In particular, it has been shown that RO-10–5824, a selective dopamine D4 receptor agonist increases novel object exploration in C57 mice (Powell et al., 2003). Furthermore, it has been shown that the selective dopamine D4 receptor agonist A-412997 improves a temporally induced deficit in the rat novel object recognition task without influencing reward-related behaviour in rat (Woolley et al., 2008). Moreover, when the dopamine D4 receptor agonist, PD168077, has been given at low doses rats failed to discriminate between familiar and novel object, while rats given higher doses explored the novel object more than the familiar object (Sood et al., 2011). A recent study has explored the effect of the D4 agonist, PD168077, and the D4 antagonist, L-745,870, alone, and in combination with clozapine and lurasidone. This study reported that in normal rats, L-745,870 impaired novel object recognition, whereas PD168077 had no effect and further showed that D4 receptor stimulation has a beneficial effect on novel object recognition in sub-chronic phencyclidine- induced novel object recognition deficit in rats (Miyauchi et al., 2017).
In the present study we aimed to examine whether dopamine receptors are affected by early life stress and provide a within study comparison of the effects of brief versus prolonged neonatal maternal separation on basal ganglia dopamine receptors. Based on the involvement of dopamine receptors in novel object recognition, we were interested to examine whether possible changes in basal ganglia dopamine receptors might underlie changes in object recognition memory resulting from neonatal maternal separation. Thus, we examined the effect of brief and prolonged mother-offspring separation on D1, D2 and D4 dopamine receptors (protein expression and biding levels) and on the novel object recognition task in male rats, as well as the correlation between the novel object recognition task and dopamine receptor binding and protein levels in the brain regions examined, using the Pearson correlation analysis. Furthermore, we were interested to examine whether the long-lasting effects of early life stress are differently manifested on dopamine receptors and NOR between adolescence and adulthood.
2. Materials and methods
2.1. Experimental animals and neonatal manipulations
Male Wistar rats were raised in the Animal Facility of the School of Medicine of the University of Patras under standard conditions (21 ± 1 °C; 12 h light/dark cycle, lights on at 08:00 h) and received food and water ad libitum. Two or three virgin females were housed with one stud male rat for 10 days. Females were caged separately, and litters were randomly assigned to one of the three experimental groups: control (C), brief maternally separated (BMS), and prolonged maternally separated (PMS) animals. The day of birth was defined as postnatal day 0 (P0). At weaning day (P22), three to four male animals of the same litter and group were placed per cage and kept under standard housing conditions in the same room. Experiments were approved by the Veterinary Administration of the Prefecture of Achaia, Greece (license number: EL-13-BIOexp04) and carried out in agreement with the ethical recommendation of the European Communities Council Directives of November 24, 1986 (86/609/EEC) and of September 22, 2010 (2010/63/EU). All efforts were made to minimize the number of animals used and their suffering.
The neonatal handling and maternal separation protocols were employed in the present study. Every day the mother was removed to an adjacent cage (always the same throughout the handling period). Subsequently, all offspring of the litter were placed together in a plastic cage (always the same throughout the handling period) lined with bedding material and transferred in another room maintained at 30–32 °C either for 15 min (brief maternally separated , BMS group) or 180 min (prolonged maternally separated, PMS group). After the appropriate period, pups and then their mothers were returned to their home cages. The neonatal manipulations were employed from P1 until P21. Control litters (C group) were left undisturbed until weaning.
2.2. Tissue preparation
For the in vitro receptor binding experiments, adolescent and adult male rats were deeply anesthetized with isoflurane, decapitated and the brains were isolated and flash-frozen in − 50 °C isopentane. Brain tissue was kept at − 80 °C, until use. Coronal 15 µm sections were collected using a cryostat (Leica CM1500, Germany) at − 20 °C, and thaw mounted onto slides (StarFrost, Knittel Gläser, Germany). Sections were air-dried at RT and stored at − 80 °C until the day of experimentation. Three coronal sections were collected non-consecutively on each slide. Sections were collected according to the rat brain atlas (Paxinos and Watson, 2007) and included caudate-putamen (CPu), NAc (AP 2.28–AP 1.56), internal globus pallidus (GPi) (AP −1.92 – AP −2.76) and SN (AP −4.44 – AP −4.92). For the Western blot experiments, brain tissue of C, BMS and PMS adult male rats were used. For striatal tissue, brains were isolated as described above and right and left striata were dissected out, frozen rapidly in liquid nitrogen, and stored at − 80°C, until the day of homogenization. For NAc tissue, coronal 50 µm sections at AP 2.28 – AP 1.56 were collected and stored at –80 °C. Nucleus accumbens was anatomically defined according to the rat brain atlas (Paxinos and Watson, 2007) using a stereoscope, isolated from the surrounding brain tissue by scrapping and stored in eppendorf microtubes until the day of homogenization.
2.3. In vitro receptor binding
Control (C), brief maternally separated (BMS) and prolonged maternally separated (PMS) adolescent (P39–41) (n = 6–8 per group) and adult rats (P113–115 for D1 and D2 receptors and P209–212 for D4 receptors) (n = 8–10 per group) were used in the present study to identify D1, D2 and D4 dopamine receptors using in vitro receptor binding. The same cohort of adolescent animals was used for D1, D2 and D4 receptor binding assays and a different cohort of adult animals was used for D1, D2 and D4 receptor binding assays. Experimental assays were performed according to the previously established protocols (Fanarioti et al., 2015, Tarazi et al., 1998a, Tarazi and Baldessarini, 2000). D1 receptor binding was performed using 2.1–3.0 nM [3H] SCH23390 (83.2 Ci/mmol) (PerkinElmer Life Science, Belgium) and 40 nM ketanserin (Tocris, UK). Non-specific binding was determined in the presence of 1 μM cis-flupenthixol (RBI, UK). D2 receptor binding was performed using 3.5–3.7 nM [3H] raclopride (73.8 and 80.4 Ci/mmol) (PerkinElmer Life Science, Belgium). Non-specific binding was determined in the presence of 1 μM cis-flupenthixol. D4 receptor binding was performed using 2.7–2.8 nM [3H] YM-09151–2 (82.4 Ci/mmol) (PerkinElmer Life Science, Belgium), 0.5 mM DTG (Tocris, UK), 300 nM raclopride (EMD Millipore, USA) and 0.1 mM pindolol (Tocris, UK). Non-specific binding was determined in the presence of 50 μΜ (S)-(-) sulpiride (Tocris, UK).
2.4. Autoradiography and quantification
The slides were opposed to BioMax MR film (Sigma Aldrich) in Χ-ray film cassettes along with [3H] microscales (ARC, St. Louis, MO, USA) for 5 – 11.5 months, depending on the receptor. After exposure, films were developed and fixed in Fuji developer and fixer. Autoradiograms were scanned and relative optical densities (ROD) were measured with the MCID 7.0 software (Imaging Research Inc, St. Catharines, ON, Canada). ROD measurements were converted into fmoles of tritiated ligand per mg tissue equivalent according to the calibration obtained from the tritium standards (ARC). The anatomical structures of CPu, NAc, SN and GPi were defined according to the rat brain atlas (Paxinos and Watson, 2007). NAc was divided in core (NAcC) and shell (NAcS). The total as well as the non-specific binding levels were determined for each animal. Specific binding was calculated by subtracting non-specific from total binding. For each brain area, 6–9 sections for total and 2–3 sections for non-specific binding in each animal were quantified.
2.5. Immunoblotting
Groups of control (C) brief maternally separated (BMS) and prolonged maternally separated (PMS) adult rats (P95–115) were used. Striatum (n = 6–8 per group) and nucleus accumbens (n = 4–6 per group) were homogenized in cold 1% SDS, including 1% phosphatase and 1% protease inhibitors (Sigma-Aldrich). The homogenates were left on ice for 1 h and centrifuged at 6000 rpm for 10 min at 4 ° C to remove insoluble materials. The pellet was resuspended in the same buffer and recentrifuged under the same conditions. The supernatants were harvested, separated in aliquots, and stored at − 80°C. A small number of homogenates was used to determine protein concentrations using the NanoDrop™ 2000/2000c Spectrophotometer (Thermo Fisher Scientific, USA). Equal amounts of protein (35 μg) were subjected to SDS-PAGE electrophoresis on 10% SDS-polyacrylamide gel. Proteins were then transferred to 0.45 µm PVDF membranes (Amersham) for 90 min at 0.4 A. To block non-specific sites the blots were incubated with 5% nonfat powdered milk in TBS-T (50 mM Trizma-Base, 150 mM NaCl, 0.05% Tween-20, pH 7.6) for 1 h at RT. Blots were then incubated overnight at 4 °C with anti- dopamine D1 (1:1000, ab20066, abcam) or D2 (1:1000, ab-85367, abcam) receptor antibodies. After three washes with TBST, the membranes were incubated for 1 h at RT with specific anti-IgG horseradish peroxidase (HRP)-conjugated antibody (1:25000, AP132P, Millipore). Bands were visualized on Fuji HR-U films (Kisker) by enhanced chemiluminescence (WBKLS0500, Millipore) according to the manufacturer’s instructions. Blots were blocked again, probed with anti- β-actin antibody (1:1000, sc-47778, Santa Cruz Technologies) and incubated with specific anti-IgG HRP-conjugated antibody (1:25000, A16084, ThermoFischer). The exposure time of films was for 30 s to 5 min to ensure that we were operating within the linear range of the film. Molecular weights were determined by comparison with pre-stained protein molecular weight marker standards (MPW04, Nippon Genetics). ECL-exposed films were scanned and quantified using the NIH ImageJ software (National Institutes of Health). Optical density measurements of each band were normalized against to the corresponding measurement of β-actin which serves as a gel loading control. Each gel contained samples from the three experimental groups placed randomly. Values are representative of two or more independent experiments and expressed as the percentage of the mean of the control samples included in the same blot.
2.6. Behavioral task
The behavioral tests were carried out during the light cycle and performed at mid-adolescent and adult animals. Different cohorts from the receptor binding experiments were used for the behavioral tasks. All rats were gently handled for 4–5 consecutive days prior to testing for 1 min/day and accustomed to the experimental room for 1 h prior to the experiment. All behavioral tests were performed between 10:00 a.m. - 17:00 p.m. and recorded with a video camera placed above the apparatus. Further analysis was conducted by two blinded, unbiased observers.
2.6.1. Novel object recognition task
Recognition memory was tested in a novel object recognition task by exploiting the rat’s natural tendency to explore novel objects. The novel object discrimination test used in the present study was a modification of that described by Ennaceur and Delacour (1988). This task takes advantage of the spontaneous preference of rodents for novelty and does not require reinforcement of behavior. Adolescent male rats (P41–46, n = 9–10 per group), and adult male rats (P90–93, n = 11 per group) were subjected to the novel object recognition (NOR) behavioral task. Each session comprised of three trials with 1 min delay between them and was performed in an open field apparatus at room temperature (21 ± 1 °C) and under natural light conditions with about equal illumination across the arena (80 ± 20 lux). Two square, semi-transparent plexiglass arenas were used, with dimensions of 40×40×30 cm and 100×100×60 cm (L x W x H) for mid-adolescent and adult animals, respectively. It was found necessary to scale the arenas and objects to the size of the animals within each age group to maximize exploratory behavior while minimizing incidental contact with testing objects, as pointed out in the literature (Reger et al., 2009). The objects used for testing were four differently shaped and textured, easy to clean items that could be fixed to the bottom of the arena and their size was chosen so that they could not be climbed by the animals. The choice of the items was based on preliminary observations that indicated equal preference for each item used in the adolescent and adult group. The objects were positioned at 6 cm or 15 cm from the borders of the arena for adolescent or adult rats, respectively.
In the first trial (T1), the rat was placed in the arena for 3 min. During the second trial (T2), two identical objects were placed at the corners of the central square and the animal could explore for 3 min. During the third trial (T3) the animal could explore two non-identical objects for 3 min; one object was used in the T2 trial cleaned with 70% ethanol and placed at its original location (F-familiar) and the other object was new (N-novel object). Between the trials, each animal was transferred to its home cage for 1 min, and the arena was cleaned thoroughly with 70% ethanol. At the beginning of T1 rats were placed in the center of the arena, whereas at T2 and T3, the rats were placed in the corner of the arena facing out. The locations of novel objects were counterbalanced across animals to reduce object and place preference effects. Exploration time was defined as attending the object from 2 cm or less. The differential exploration of the objects during T3 was quantified by calculating the discrimination index (DI), expressed as the ratio (N − F)/(N + F), where N is time spent exploring the novel object and F is time spent exploring the familiar object. A positive score indicated more time spent with the novel object, whereas a negative score indicated more time spent with the familiar object.
2.7. Statistical analysis
In vitro receptor binding data and novel object recognition data were analyzed by two-way analysis of variance (two-way ANOVA) with manipulation and age as independent factors. Separate one-way ANOVA followed by LSD post hoc tests were performed to detect significant differences between groups. Western blot data were analyzed by one-way ANOVA, followed by LSD post hoc tests. The existence of a possible statistical correlation between the degree of change in dopamine receptor binding levels or protein levels, and the extent of change in the behavior was investigated using the Pearson correlation analysis. The level of statistical significance was set at 0.05 (P < 0.05). All tests were performed with the SPSS software (Release 10.0.1, SPSS, USA). The data used in the bivariate Pearson correlation of SPSS were the mean values of dopamine or protein levels in different brain regions for adult or adolescent animals for the three experimental groups. Since the two-way ANOVA analysis of the data from the novel object recognition task did not reveal a significant manipulation-by-age interaction, as described below in Section 3.1 of Results, the data used in the bivariate Pearson correlation of SPSS were the mean values of the discrimination indexes of both adult and adolescent animals for each experimental group.
3. Results
3.1. Effects of neonatal manipulations on behavior
To investigate whether the long-term effects of neonatal manipulations are different on novel object recognition of adolescent and adult rats and whether these effects depend on the duration of the maternal separation employed, we performed the novel objection recognition (NOR) task in male adolescent and adult rats that had experienced either brief (15 min) or prolonged (180 min) daily maternal separations, referred as brief maternally separated (BMS) and prolonged maternally separated (PMS) animals, respectively.
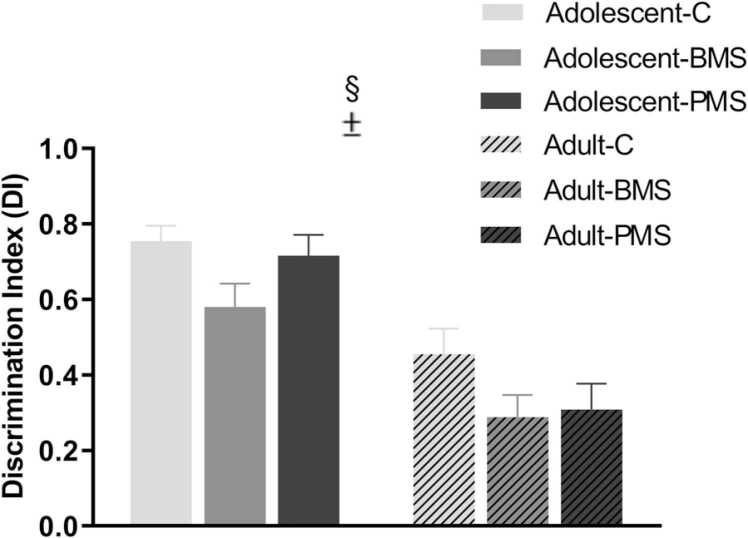
The discrimination index (DI) was calculated as mentioned in methodology and a positive score indicated more time spent in the novel object, whereas a negative score indicated more time spent in the familiar object. While a two-way ANOVA analysis did not reveal a significant manipulation-by-age interaction (F2,50 =0.43, P = 0.65), it revealed a significant effect of manipulation (F1,50 =3.79, P < 0.03) and a significant effect of age (F1,50 =42.34, P < 0.001) (Fig. 1). Post hoc tests showed that DI was decreased by 27% (P = 0.01) in BMS compared to control animals independent of age. Moreover, DI was decreased from adolescence to adulthood in all three groups. In control, BMS and PMS animals, these decreases were 40% (P < 0.01), 50% (P < 0.01) and 55% (P < 0.001), respectively. Collectively, these data indicate that adolescent rats spent more time with the novel object than adults. Also, these data indicate that rats subjected to brief, but not prolonged, neonatal maternal separations spent significantly less time with the novel object as adolescent or adults, compared to control animals.
Fig. 1.
Effects of neonatal manipulations on adolescent and adult rat behavior as assessed in the novel object recognition task. The discrimination index (DI), calculated as described in methodology, was measured in control, BMS, and PMS adolescent (P41–46) and adult (P90–93) rats. Statistical analysis revealed that DI is decreased in BMS rats compared to control rats independent of age, and adolescent rats display increased DI compared to adult rats. Data are expressed as mean ± SEM of discrimination indices; n = 9–10 adolescent and 11 adult rats/group; ± statistically significant manipulation effect (P < 0.03); § statistically significant age effect (P < 0.001; two-way ANOVA with manipulation and age as independent factors). C: control; BMS: brief maternally separated; PMS: prolonged maternally separated animals.
3.2. Effects of neonatal manipulations on dopamine receptors
To investigate the long-term effects of neonatal manipulations on dopamine receptors of basal ganglia nuclei in adolescent and adult rat brain and examine whether these effects depend on the duration of mother-offspring separation employed, we performed in vitro autoradiography experiments using radioligands for dopamine receptor subtypes D1, D2 and D4. We measured binding levels in CPu and NAc (NAcC and NAcS) for all DA receptor subtypes. We also measured binding levels in GPi and SN for D1, but not D2 and D4 receptors, since specific binding levels of these two receptors were undetected in these regions (Fig. 4, Fig. 5, Fig. 7). In Fig. 2 and Fig. 3 representative autoradiograms demonstrate binding levels of dopamine receptor subtypes D1, D2 and D4 in basal ganglia nuclei of adolescent and adult rats, respectively. D1, D2 and D4 receptor binding levels are higher in CPu than NAc and are distributed rather uniformly in the medio-lateral and dorso-ventral extent of CPu. We also assessed total protein levels for D1 and D2 receptors in CPu and NAc, using Western blot analysis (Fig. 6).
Fig. 4.
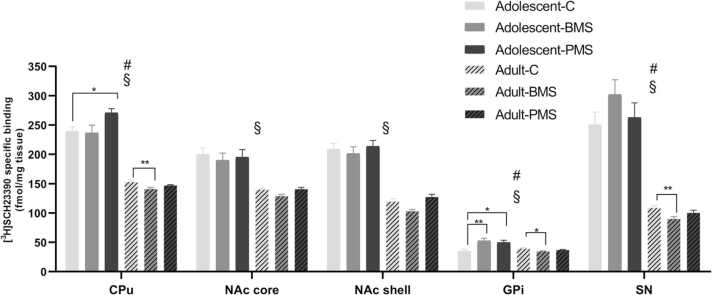
Effects of neonatal manipulations on D1 receptors labelled with [3H] SCH23390 in basal ganglia nuclei of adolescent and adult male rats. Bar graphs depict specific [3H] SCH23390 binding levels in brain regions of male adolescent (P39–41) and adult (P113–115) control, BMS and PMS rats. Data are presented as mean ± SEM of specific binding expressed in fmol/mg tissue (n = 6–8 adolescent and 8–10 adult rats/group). # Statistically significant manipulation x age interaction (P < 0.05); § statistically significant age effect (P < 0.001; two-way ANOVA with manipulation and age as independent factors). C: control; BMS: brief maternally separated; PMS: prolonged maternally separated rats. CPu: caudate-putamen; NAcC: nucleus accumbens core; NAcS: nucleus accumbens shell; GPi: internal globus pallidus; SN: substantia nigra; * P < 0.05; * *P < 0.01.
Fig. 5.
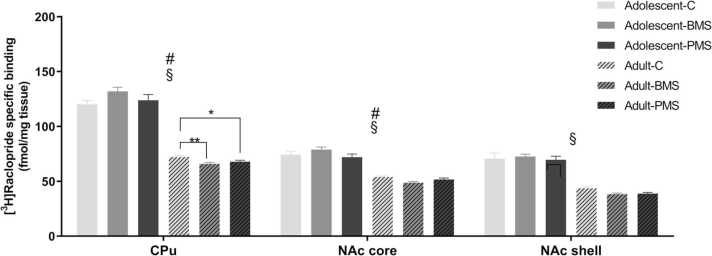
Effects of neonatal manipulations on D2 receptors labelled with [3H] raclopride in basal ganglia nuclei of adolescent and adult male rats. Bar graphs depict specific [3H] raclopride binding levels in brain regions of male adolescent (P39–41) and adult (P113–115) control, BMS and PMS rats. Data are presented as mean ± SEM of specific binding expressed in fmol/mg tissue (n = 6–8 adolescent and 8–10 adult rats/group). # Statistically significant manipulation x age interaction (P < 0.05); § statistically significant age effect (P < 0.001; two-way ANOVA with manipulation and age as independent factors). C: control; BMS: brief maternally separated; PMS: prolonged maternally separated rats. CPu: caudate-putamen; NAcC: nucleus accumbens core; NAcS: nucleus accumbens shell. * P < 0.05; * *P < 0.01.
Fig. 7.
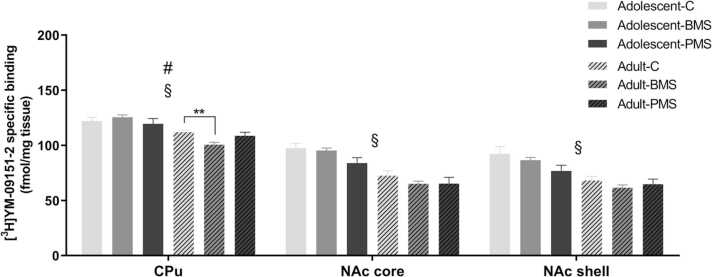
Effects of neonatal manipulations on D4 binding levels labeled with [3H] YM-09151–2 in basal ganglia nuclei of adolescent and adult male rats. Bar graphs depict specific [3H] YM-09151–2 binding levels in brain regions of male adolescent (P39–41) and adult (P209–212) control, BMS and PMS rats. Data are presented as mean ± SEM of specific binding expressed in fmol/mg tissue (n = 6–8 adolescent and 8–10 adult rats/group). # Statistically significant manipulation x age interaction (P < 0.05); § statistically significant age effect (P < 0.001; two-way ANOVA with manipulation and age as independent factors). C: control; BMS: brief maternally separated; PMS: prolonged maternally separated rats. CPu: caudate-putamen; NAcC: nucleus accumbens core; NAcS: nucleus accumbens shell. * *P < 0.01.
Fig. 2.
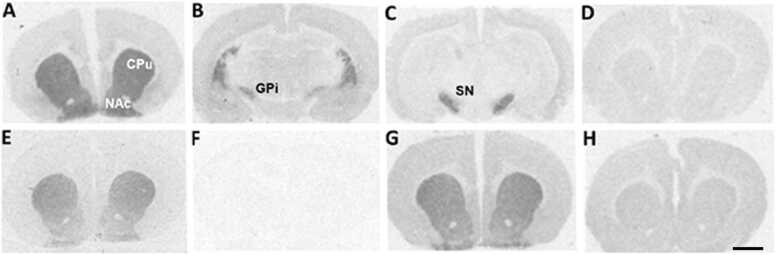
Representative autoradiograms of D1, D2 and D4 receptor binding levels in coronal brain sections from adolescent male rats. Top row: D1 dopamine receptors labelled with [3H] SCH23390 at the level of CPu and NAc (A), GPi (B), and SN (C); non-specific [3H] SCH23390 binding (D). Bottom row: D2 dopamine receptors labelled with [3H] raclopride at the level of CPu and NAc (E); non-specific [3H] raclopride binding (F); D4 dopamine receptors labelled with [3H] YM-09151–2 at the level of CPu and NAc (G); non-specific [3H] YM-09151–2binding (H). The scale bar corresponds to 2 mm. CPu: caudate-putamen; NAc: nucleus accumbens; GPi: internal globus pallidus; SN: substantia nigra.
Fig. 3.
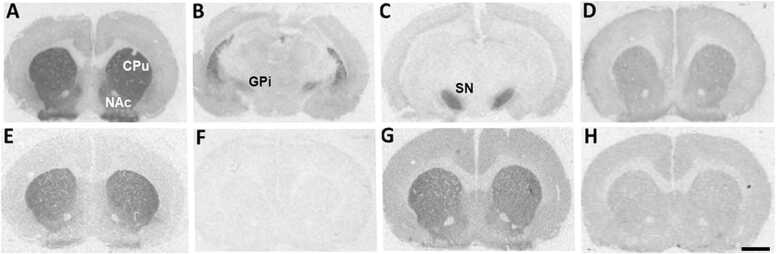
Representative autoradiograms of D1, D2 and D4 receptor binding levels in coronal brain sections from adult male rats. Top row: D1 dopamine receptors labelled with [3H] SCH23390 at the level of CPu and NAc (A), GPi (B), and SN (C); non-specific [3H] SCH23390 binding (D). Bottom row: D2 dopamine receptors labelled with [3H] raclopride at the level of CPu and NAc (E); non-specific [3H] raclopride binding (F); D4 dopamine receptors labelled with [3H] YM-09151–2 at the level of CPu and NAc (G); non-specific [3H] YM-09151–2 binding (H). CPu: caudate-putamen; The scale bar corresponds to 2 mm. NAc: nucleus accumbens; GPi: internal globus pallidus; SN: substantia nigra.
Fig. 6.
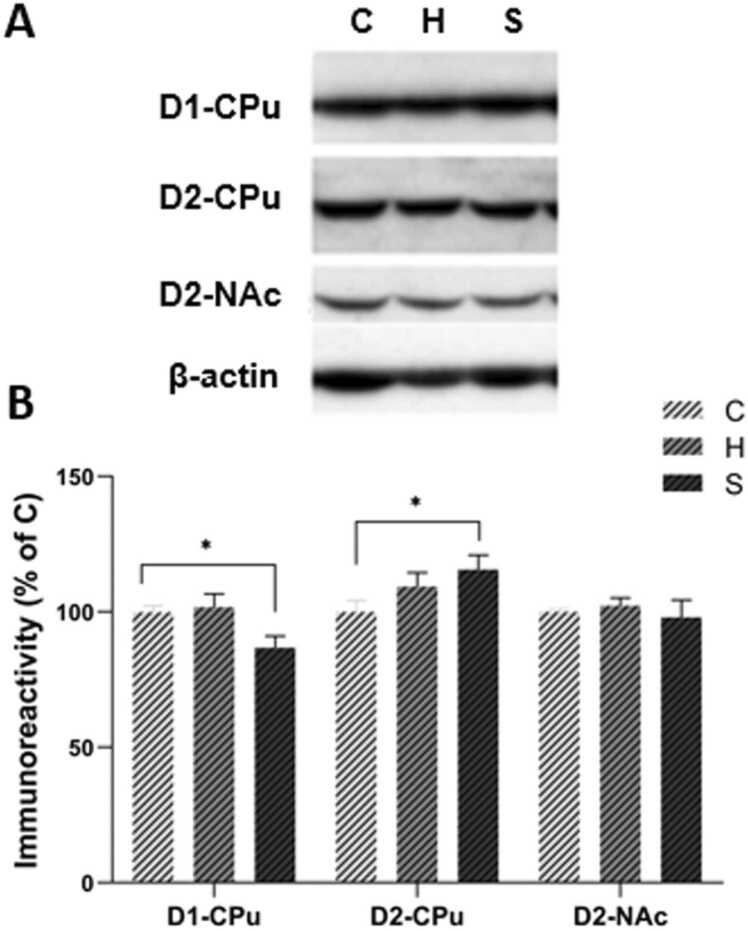
Effects of neonatal manipulations on D1 and D2 dopamine receptor protein levels in basal ganglia nuclei of adult male rats. A. Representative immunoblots corresponding to D1, D2 receptors and β-actin (used as internal control) from total homogenates of caudate-putamen (CPu) and nucleus accumbens (NAc). B. Bar graphs depicting D1 and D2 protein levels in CPu and NAc (expressed as percent of control). Data are presented as mean ± SEM; n = 4–8 rats/group; * statistically significant manipulation effect (P < 0.05, one-way ANOVA followed by post-hoc analysis); C: control; BMS: brief maternally separated; PMS: prolonged maternally separated rats.
3.2.1. D1 dopamine receptors
Statistical analysis of data for [3H] SCH23390 specific binding levels by two-way ANOVA revealed a significant manipulation-by-age interaction (Fig. 4). An effect was observed in CPu (F2,44 =3.99, P = 0.03), GPi (F2,45 =10.48, P < 0.001) and SN (F2,39 =3.33, P = 0.05). Post-hoc analysis showed increased D1 receptor binding levels in CPu of adolescent PMS rats by 13% (P = 0.04), as well as in GPi of adolescent BMS and PMS rats by 51% ( < 0.01) and 43% (P = 0.01), respectively, compared to respective controls. On the contrary, D1 receptor binding levels were decreased in CPu, GPi and SN of adult BMS animals by approximately 8% ( < 0.01), 12% (P = 0.01) and 17% (P < 0.01), respectively, compared to respective controls. These findings indicate that early-life maternal separations have a different impact on D1 receptor binding levels in adolescence and adulthood, depending on their duration; in CPu prolonged separations increase D1 binding levels in adolescents, while brief separations decrease D1 binding levels in adults; in GPi, prolonged and brief separations increase D1 binding levels in adolescents, while brief separations decrease D1 binding levels in adults; in SN brief separations have an impact only on adult animals.
Moreover, in all regions studied, two-way ANOVA analysis of data revealed a significant effect of age (Fig. 4). D1 receptor binding levels decreased from adolescence to adulthood in CPu (F1,44 =334.51, P < 0.001), NAcC (F1,46 =81.27, P < 0.001), NAcS (F1,47 =228.79, P < 0.001), GPi (F1,45 =16.66, P < 0.001), and SN (F1,39 =226.63, P < 0.001). Post-hoc analysis showed that D1 binding levels decreased from adolescent to adult animals by an average of 39%.
Furthermore, D1 receptor total protein levels were assessed in CPu of adult rats with Western blotting. Fig. 6 shows bar graphs depicting D1 protein levels and representative immunoblots from CPu of control, BMS and PMS rats. Statistical analysis of immunoblotting data by one-way ANOVA revealed a significant effect of neonatal manipulation on D1 receptor protein expression (F2,19 =4.32, P = 0.03). Specifically, D1 protein levels of PMS rats were lower by 13% (P = 0.02) compared to control animals (Fig. 6).
3.2.2. D2 dopamine receptors
Statistical analysis of data for [3H] raclopride specific binding levels by two-way ANOVA showed a significant manipulation-by-age interaction (Fig. 5). While no significant changes were observed in adolescent animals, an effect was observed in adult CPu (F2,43 =4.75, P = 0.01), and NAcC (F2,43 =3.85, P = 0.03). Further post-hoc analysis revealed decreased D2 receptor binding levels in CPu and NAcC of adult BMS animals by approximately 9% (P < 0.01) and 10% (P < 0.01), respectively, compared to respective control animals. The D2 binding levels were also decreased in CPu of adult PMS animals by 6% (P = 0.03) compared to respective control. These findings indicate that the effects of early-life maternal separations on D2 receptor binding levels in CPu and NAc are not displayed early in adolescence but later in adulthood; brief as well as prolonged separations decrease D2 receptor binding levels in adult CPu, while only brief separations decrease D2 receptors in adult NAcC.
Moreover, two-way ANOVA analysis of data revealed a significant effect of age (Fig. 5). D2 receptor binding levels decreased from adolescence to adulthood in CPu (F1,43 =593.83, P < 0.001), NAcC (F1,43 =228.79, P < 0.001) and NAcS (F1,43 =217.94, P < 0.001). Post-hoc analysis showed that D2 binding levels are decreased from adolescent to adult animals by an average of 40%.
Furthermore, D2 receptor total protein levels were assessed in CPu and NAc of adult rats with Western blotting. Fig. 6 shows bar graphs depicting D2 protein levels and representative immunoblots from CPu and NAc of control, BMS and PMS adult rats. Statistical analysis of immunoblotting data by one-way ANOVA showed a significant effect of neonatal manipulation on D2 protein expression in CPu (F2,19 =3.60, P = 0.05), while no effect was observed on D2 receptor protein expression in NAc (F2,13 = 0.39, P = 0.69). Specifically, D2 receptor protein levels of PMS animals were higher by 16% (P = 0.02) compared to control (Fig. 6).
3.2.3. D4 dopamine receptors
Statistical analysis of [3H] YM-09151–2 binding levels by two-way ANOVA showed a significant manipulation-by-age interaction in CPu (F2,39 =3.45, P = 0.04). D4 receptor binding levels were decreased in CPu of adult BMS rats by 10% (P < 0.01), as compared to the corresponding control, while there was no effect observed in adolescent animals (Fig. 7). These findings indicate that the effects of brief maternal separations during the neonatal period on D4 receptor binding levels are limited to CPu and are not displayed early in adolescence but later in adulthood.
Moreover, two-way ANOVA analysis of data revealed a significant effect of age. D4 receptor binding levels decreased from adolescence to adulthood in CPu (F1,39 =33.99, P < 0.001), NAcC (F1,39 =54.64, P < 0.001) and NAcS (F1,39 =33.06, P < 0.001). Post-hoc analysis showed that D4 binding levels are decreased from adolescent to adult animals by an average of 22%.
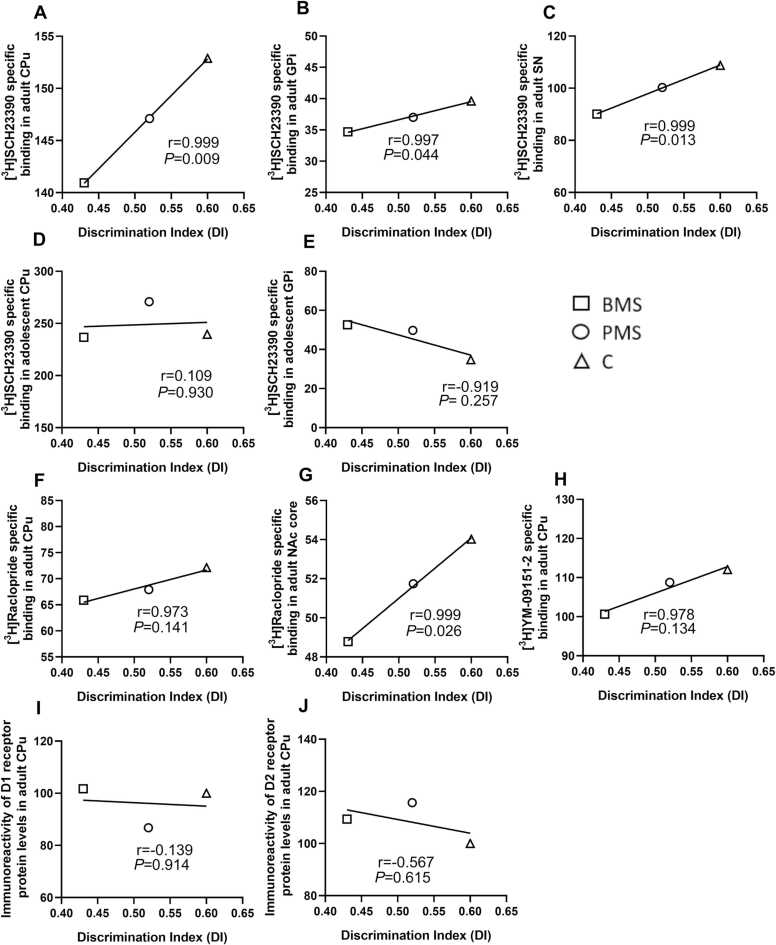
3.3. Correlation of changes in dopamine receptors with changes in NOR
Novel object recognition was correlated with dopamine receptor binding and protein levels in the brain regions where changes were statistically significant, as described above. Pearson correlation coefficient revealed positive statistically significant correlations between the discrimination index (DI) measured in the novel object recognition task and D1 binding levels in CPu (r = 0.999, P = 0.009), GPi (r = 0.997, P = 0.044), and SN (r = 0.999, P = 0.013), as well as between the discrimination index and D2 binding levels in NAcC (r = 0.999, P = 0.026) of adult rats. However, no statistically significant correlation was revealed between the discrimination index and D2 (r = 0.973, P = 0.141) or D4 (r = 0.978, P = 0.134) binding levels in CPu of adult rats or between the discrimination index and D1 (r = - 0.139, P = 0.914) and D2 (r = - 0.567, P = 0.615) protein levels in CPu of adult rats. Moreover, no statistically significant correlation was found between DI and D1 binding levels in CPu (r = 0.109, P = 0.930) and GPi (r = - 0.919, P = 0.257) in adolescent rats (Fig. 8).
Fig. 8.
Scatterplots of the correlations between the discrimination index (DI) assessed in the novel object recognition task, and dopamine receptors. A – E: Correlation between DI and D1 receptors labelled with [3H] SCH23390 in adult CPu (A), adult GPi (B), adult SN (C), adolescent CPu (D), and adolescent GPi (E). F, G: Correlation between DI and D2 receptors labelled with [3H] Raclopride in adult CPu (F) and adult NAc core (G). H. Correlation between DI and D4 receptors labeled with [3H] YM-09151–2 in adult CPu. I: Correlation between DI and D1 receptor protein levels in adult CPu.J. Correlation between DI and D2 receptor protein levels in adult CPu. Data are the mean values of dopamine receptor binding or protein levels of adult or adolescent animals in different brain regions and the mean values of the discrimination indexes of both adult and adolescent animals for C, BMS and PMS rats (see Materials and methods). C: control; BMS: brief maternally separated; PMS: prolonged maternally separated rats. CPu: caudate-putamen; NAc core: nucleus accumbens core.
4. Discussion
The results of the present study demonstrate the effects of brief and prolonged mother-offspring separations during the neonatal period on object recognition memory and on basal ganglia dopamine receptors in male adolescent and adult rats.
To examine recognition memory in adolescent and adult rats, the experimental animals were subjected to a novel object recognition task using three consecutive 3-min trials with interstitial intervals of 1 min. In the present study, the short 1 min retention interval between the familiarization and test trial allowed us to study object recognition and short-term memory which depend on pre-existing substrates and is independent of protein synthesis and gene transcription (Goelet et al., 1986, Huang, 1998, Marx and Gilon, 2012). According to our data, it is apparent that brief or prolonged mother-offspring separations did not impair novel object recognition at either adolescence or adulthood, since BMS and PMS animals spent significantly longer time with the novel object, similar to control animals. However, depending on the duration of the separation, mother-offspring separations affected the performance in novel object recognition in adolescent and adult rats. It is interesting that rats exposed to brief separations had a weaker performance in the novel object recognition task, based on the decreased discrimination index (DI) compared to control, while novel object recognition was not affected in rats exposed to prolonged separations.
Our behavioral data using the novel object recognition task indicate that brief mother-offspring separations impair recognition memory in both adolescent and adult rats. To our knowledge, there are no studies using the 1 min interval in the NOR task after brief or prolonged mother-offspring separations to study short-term recognition memory, as performed in the present study. Our results are not in agreement with previous studies in adolescent rats which have indicated that adolescent rats exposed to brief separations showed enhanced novel object recognition (Frankola et al., 2010, Plescia et al., 2014). However, these studies used longer retention intervals (1 h) between the familiarization and test trial than the 1 min used in the present study and different stages of adolescence, which may explain the discrepancy with our results. In adult rats, novel object recognition has been explored only after prolonged maternal separations and only two studies used short retention intervals (5 min), close to the 1 min used in our study. In accordance with our results, Grace et al. (2009) have shown that maternal separation had no effect on novel object recognition at P62. On the contrary, Hulshof et al. ( 2011) examined the effects of maternal separation in combination with an adult stressor and thus their results indicating an impairment in the object recognition task are not comparable to ours.
Moreover, the NOR data in the present study indicated a difference in novel object recognition performance depending on age; the discrimination index in adolescents was approximately twice fold higher than adults suggesting that adolescent rats perform better than adults in this recognition memory task. Our results agree with the observations of Stansfield and Kirstein (2006) showing that adolescent rats (P34) spent more time with a novel object relative to young adults (P59), using 1 min retention interval. On the contrary, Reger et al. (2009) using longer retention intervals (15 min up to 48 h) found no difference between juvenile (P29-P40) and adult (P50) recognition abilities.
To examine the effects of mother-offspring separation on D1, D2 and D4 dopamine receptors of basal ganglia in adolescence and adulthood, we performed receptor binding and immunoblotting experiments. In adult dorsal striatum (CPu), D1, D2 and D4 binding sites were down regulated mostly in response to brief mother-offspring separations, while in adolescent dorsal striatum, D1 binding sites, but not D2 and D4, were up-regulated in response to prolonged separations. A different effect on D1 binding sites was observed in GPi and SN; D1 binding levels were down regulated in response to brief mother-offspring separations. Knowing that dendrites of SNc neurons release dopamine onto the GABAergic neurons of SNr and that D1 receptors have been localized in SNr (Cheramy et al., 1981, Yung et al., 1995), we assume that the D1 binding sites detected here are most likely localized in SNr. Moreover, changes in D1 receptor binding in GPi, most likely involve terminals of axon collaterals of the nigrostriatal dopaminergic fibers arising from SNc and innervating dorsal striatum (Lindvall and Björklund, 1979). Overall, our results indicate that the effects of neonatal manipulations on dopamine receptors are different in adolescent than adults and depend on the duration of mother-offspring separation.
Prior studies have examined age-dependent changes in dopamine receptor binding and showed a peak of D1 and D2 binding sites around P40 and a subsequent reduction in striatum of male rats (Andersen et al., 1997, Gelbard et al., 1989, Tarazi and Baldessarini, 2000, Teicher et al., 1995). This developmental pattern has been attributed to the overproduction of synapses and receptors from infancy to onset of puberty and subsequent pruning during transition from adolescence to adulthood. The observed upregulation of D1 receptor binding induced by prolonged maternal separation in adolescent striatum could be indicative of a selective effect of this early life stress on synapse elimination.
There is growing consensus that early life stress may be altering brain development thus promoting risk for addiction and psychiatric disorders (Andersen and Teicher, 2009, Heim et al., 2019). It is also known that adolescence is a developmental period characterized by heightened vulnerability to illicit drug use (Johnston et al., 2005) and the onset of neuropsychiatric disorders (Lewis et al., 2004). Moreover, the developmental changes in the dopaminergic system have been suggested as potential substrates for adolescent-onset drug abuse and psychopathologies. The dopamine system undergoes striking maturation during adolescence (Wahlstrom et al., 2010) and rodent behavioral and imaging studies suggest that adolescent D1 receptors are hypofunctional and D2 receptors are hyperfunctional (Chen et al., 2010, Frantz and Van Hartesveldt, 1999). Furthermore, a recent study suggested that the functional consequences of D1 and D2 receptor activation are immature during adolescence. In the above study, significant differences between adolescent and adult rats were observed in behavioral responses to D1 and D2-like agonists, and in the behavioral interactions between D1 and D2 receptors (Dwyer and Leslie, 2016). The results of the present study showing that D1 dopamine receptors are selectively affected in adolescence by prolonged neonatal maternal separations suggest that an early life stress may alter the developmental trajectory of dopamine receptors, and this may have functional consequences on behavior of adolescents.
As already mentioned in the introduction, D1 receptors are located on the MSNs of the direct CPu output pathway, while the indirect pathway consists of MSNs that express D2 receptors. It has been proposed that the direct pathway facilitates the initiation and execution of voluntary movements, timing of motor actions, gating of working memory and motor responses to specific stimuli, while the indirect pathway results in inhibition of all motor activities and termination of responses (Nambu, 2004, Schroll and Hamker, 2013). The observed upregulation of D1 receptor binding induced by prolonged maternal separation in adolescent striatum disturbs the balance between the two systems in favor of the direct pathway which may underlie specific deficits in social interactions and especially in goal-directed behaviors observed in adolescence. Indeed, a recent study in animal models indicated that deficits in goal directed behavior, which in humans may lead to clinical symptoms of inattention, impulsivity, and hyperactivity, are due to an imbalance between activation of dopamine D1 and D2 pathways that govern action control in favor of D1 over D2 pathways (Natsheh and Shiflett, 2018).
In the present study, knowing that NAc is the target of the mesolimbic dopaminergic pathway and is part of the reward system, we were interested to assess the effects of brief and prolonged mother-offspring separations on dopamine receptors D1, D2 and D4 in both core and shell of NAc in adolescence and adulthood. We detected changes restricted to NAcC of adults in response to brief mother-offspring separations, where a down-regulation in D2 binding sites was observed. In the ventral striatum, previous studies have described analogous to the dorsal striatum direct and indirect pathways. In NAc, the medium spiny neurons (MSN) of the direct pathway express D1 receptors, while MSNs of the indirect pathway express D2 receptors (Heimer et al., 1991, Ikemoto, 2007, Zahm and Heimer, 1990). In recent studies researchers have developed a reversible neurotransmission blocking technique to block selectively the transmission in each pathway and revealed that the direct pathway is critical in reward and the indirect pathway is critical in aversive behavior (Hikida et al., 2010). By applying a CPP task and using chocolate food as reward, the above study has shown impaired learning ability in mice in which the direct pathway was blocked. Yawata et al. (2012) have further indicated that activation of D1 receptors and inactivation of D2 receptors postsynaptically in the NAc control reward learning and aversive learning in a direct pathway and indirect pathway-specific manner, respectively. Considering the above, the lack of change in D1 receptors and the down regulation of D2 receptors observed in NAcC of adult rats suggests that brief mother-offspring separation affects preferentially the NAc indirect output pathway.
Previous studies have indicated that maternal separation affects the number of tyrosine hydroxylase-expressing neurons, but not total TH expression, in SN and VTA of adolescent and adult male and female rats (Chocyk et al., 2011). Furthermore, it has been shown that maternal separation affects the density of tyrosine hydroxylase immunoreactive fibers in prefrontal cortex and in the core and shell of nucleus accumbens in adolescent female rats (Majcher-Maślanka et al., 2017). Based on the above, we can suggest that the observed changes in D1, D2 and D4 receptor binding levels could result from changes in dopamine synthesis and release in the nigrostriatal pathway caused by brief or prolonged neonatal maternal separations.
To further examine whether brief or prolonged mother-offspring separations might influence D1 and D2 receptor protein expression, we performed immunoblotting experiments. Using specific antibodies for D1 and D2 receptors in homogenate extracts from CPu and NAc we found that total D1 and D2 protein levels in CPu and NAc of adult rats were altered in PMS but not in BMS rats. Initially, these data showed that the observed decreases in D1 and D2 binding levels after brief separations are not accompanied by changes in their respective total protein expression levels. Thus, the reduced D1 and D2 binding levels, which reflect functional cell surface receptors, are not due to decreased receptor translation but may rather result from increased internalization and subsequent sequestration to endosomal compartments due to altered recycling rates. Moreover, the immunoblotting data indicated that prolonged mother-offspring separations resulted in an imbalance between D1 and D2 dopamine receptors in adult striatum; D1 receptor protein expression is decreased, while D2 receptor protein expression is increased in CPu of PMS rats. Knowing that direct pathway striatonigral neurons express D1 dopamine receptors, while indirect pathway striatopallidal neurons express D2 dopamine receptors, we could assume that this imbalance between D1 and D2 protein expression favors the CPu indirect vs the direct output pathway. However, since these changes in dopamine receptor protein levels are not followed by changes in receptor binding levels, these changes in protein levels are most likely not reflected on the functions of CPu direct and indirect output pathways or on the behavior of adult animals with prolonged mother-offspring separations.
An interesting question that arises from our data is whether the observed changes in dopamine receptor binding levels of striatum, which reflect functional cell surface receptors, could underlie the impairment of recognition memory of rats experiencing brief mother-offspring separation. Previous studies have reported that recognition memory relies on a neuronal network including prefrontal cortex, hippocampus and perirhinal cortex; recognition of a novel object depends on perirhinal cortex, while the location and recency recognition memory results from interactions between prefrontal cortex and hippocampus (Barker et al., 2007, Barker and Warburton, 2011, Cohen and Stackman, 2015, Winters et al., 2008). The role of dopamine in recognition memory has been demonstrated in many studies using either knock-out mice or systemic administration of dopamine receptor antagonists by showing that modulation of dopamine receptors affects the performance of rodents in the novel object recognition task (Besheer et al., 2001, Horiguchi et al., 2013, Hotte et al., 2005, Wei et al., 2018, Woolley et al., 2008). It is interesting to note that Yang et al. (2017) have indicated that systemic administration of the D1 antagonist SCH23390 prevents increases in the AMPA/NMDA receptor ratio and suggested a role for D1 receptor activity in hippocampal synaptic plasticity associated with novel object recognition. It is important to note that a recent study has demonstrated that intra-hippocampal infusions of either dopamine, or a D1/D5 receptor agonist, as well as systematic administration of L-Dopa reverses the impairment of recognition memory of rats with prolonged mother-offspring separations (Neves et al., 2020).
The role of striatal dopamine in recognition memory has been addressed by generating a dopamine deficient mouse and assessing the behavioral effects of region-specific restoration of dopamine signaling (Darvas and Palmiter, 2009, Darvas and Palmiter, 2010). Darvas and Palmite (2009, 2010), using several behavioral tasks, such as the object recognition and the Morris water maze task, have shown that the restoration of dopamine to the dorsolateral or medial striatum was sufficient to rescue behaviors such as spatial learning, and spatial and object memory. In the object recognition task, they found that dopamine deficient mice did not explore any of the objects, while mice with virally rescued dopamine in the dorsolateral striatum spent significantly more time with the novel object on the test day. However, object exploration was not restored in mice with virally rescued dopamine in the ventromedial striatum. Their findings support that dopaminergic signaling in dorsal striatum is sufficient for mice to learn several cognitive tasks. Based on the above studies showing that striatal dopamine participates in object recognition, we could suggest that the observed downregulation of dopamine receptors in CPu might participate in the weaker performance in the novel object recognition task of adult rats exposed to brief mother-offspring separations. This suggestion is supported by the statistically significant positive correlation between the change in D1 binding levels in CPu and the discrimination index of the NOR task. However, our data on adolescent rats suggest that different mechanisms may underlie recognition memory during development, since dopamine receptors in CPu of adolescents exposed to brief mother-offspring separation were not altered.
In conclusion, our results indicate that neonatal mother-offspring separation causes long-lasting effects on basal ganglia dopamine receptors which depend on the duration of neonatal maternal separation and are different between adolescent and adult rats. Our results further suggest that the dopamine receptor changes observed in adult striatum could underlie the impairment of recognition memory of rats experiencing brief mother-offspring separation.
CRediT authorship contribution statement
Ada Sinani: Investigation, Methodology, Writing – original draft, Visualization. Andriana Vassi: Investigation, Methodology, Writing – original draft, Visualization. Giota Tsotsokou: Investigation, Methodology. Maria Nikolakopoulou: Investigation. Elias D. Kouvelas: Conceptualization, Writing – review and editing. Ada Mitsacos: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review and editing.
Acknowledgements
This work was supported by the Polembros Shipping Limited (grant 27720000, Research Committee, University of Patras). The authors are grateful to Panagiotis Giompres for his experimental/technical assistance and advice and to Antonis Stamatakis for his assistance in statistical analysis.
Declaration of interest statement
Declaration of interest: none.
Contributor Information
Ada Sinani, Email: antigonisinani@gmail.com.
Andriana Vassi, Email: andrianavassi@gmail.com.
Giota Tsotsokou, Email: giotatsotsokou@gmail.com.
Maria Nikolakopoulou, Email: maria.nikolakopulu8@gmail.com.
Elias D. Kouvelas, Email: kouvelas@upatras.gr.
Ada Mitsacos, Email: mitsacos@upatras.gr.
References
- Aggleton J.P. One-trial object recognition by rats. Q. J. Exp. Psychol. Sect. B. 1985;37:279–294. doi: 10.1080/14640748508401171. [DOI] [Google Scholar]
- Aisa B., Tordera R., Lasheras B., Del Río J., Ramírez M.J. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Rutstein M., Benzo J.M., Hostetter J.C., Teicher M.H. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banqueri M., Méndez M., Arias J.L. Behavioral effects in adolescence and early adulthood in two length models of maternal separation in male rats. Behav. Brain Res. 2017;324:77–86. doi: 10.1016/j.bbr.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Barker G.R.I., Bird F., Alexander V., Warburton E.C. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G.R.I., Warburton E.C. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.-M., Gainetdinov R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642.182. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.M., Espinoza S., Gainetdinov R.R. Dopamine receptors - IUPHAR review 13. Br. J. Pharmacol. 2015;172:1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti F., Mello P.B., Bonini J.S., Monteiro S., Cammarota M., Izquierdo I. Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. Int. J. Dev. Neurosci. 2009;27:59–64. doi: 10.1016/j.ijdevneu.2008.09.200. [DOI] [PubMed] [Google Scholar]
- Besheer J., Short K.R., Bevins R.A. Dopaminergic and cholinergic antagonism in a novel-object detection task with rats. Behav. Brain Res. 2001;126:211–217. doi: 10.1016/S0166-4328(01)00245-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Meaney M.J. Hypothalamic‐pituitary‐adrenal function in chronic intermittently cold‐stressed neonatally handled and non handled rats. J. Neuroendocrinol. 1995;7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Biagini G., Pich E.M., Carani C., Marrama P., Agnati L.F. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int. J. Dev. Neurosci. 1998;16:187–197. doi: 10.1016/S0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Bishnoi I.R., Ossenkopp K.P., Kavaliers M. Sex and age differences in locomotor and anxiety-like behaviors in rats: from adolescence to adulthood. Dev. Psychobiol. 2020;56:1–16. doi: 10.1002/dev.22037. [DOI] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Ivy A., Baram T.Z. New insights into early-life stress and behavioral outcomes. Curr. Opin. Behav. Sci. 2017;14:133–139. doi: 10.1016/j.cobeha.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson S.J., McGonigle P., Molinoff P.B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. 1986;6:3177–3188. doi: 10.1523/jneurosci.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake W.G., Zhang T.Y., Diorio J., Meaney M.J., Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Lukkes J.L., Andersen S.L. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapillon P., Patin V., Roy V., Vincent A., Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev. Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Chen Y.I., Choi J.K., Xu H., Ren J., Andersen S.L., Jenkins B.G. Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev. Neurosci. 2010;32:125–138. doi: 10.1159/000286215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A., Leviel V., Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Chocyk A., Przyborowska A., Dudys D., Majcher I., Maćkowiak M., Wedzony K. The impact of maternal separation on the number of tyrosine hydroxylase-expressing midbrain neurons during different stages of ontogenesis. Neuroscience. 2011;182:43–61. doi: 10.1016/j.neuroscience.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Cohen S.J., Stackman R.W. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels W.M.U., Pietersen C.Y., Carstens M.E., Stein D.J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis. 2004;19:3–14. doi: 10.1023/B:MEBR.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Darvas M., Palmiter R.D. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J. Neurosci. 2010;30:1158–1165. doi: 10.1523/JNEUROSCI.4576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M., Palmiter R.D. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza J.A., da Silva M.C., de Souza Ferraz Junior J.C., de Souza F.L., de Souza S.L. Maternal separation in the light or dark phase of the circadian cycle has different effects on the corticosterone levels and anxiety-like behavior in male adult rats. Physiol. Behav. 2022:247. doi: 10.1016/j.physbeh.2022.113725. [DOI] [PubMed] [Google Scholar]
- Denninger J.K., Smith B.M., Kirby E.D. Novel object recognition and object location behavioral testing in mice on a budget. J. Vis. Exp. 2018;2018:1–10. doi: 10.3791/58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E., Huston J.P., De Souza Silva M.A. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dulawa S., Grandy D., Low M., Paulus M., Geyer M. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J.B., Leslie F.M. Adolescent maturation of dopamine D1 and D2 receptor function and interactions in rodents. PLoS One. 2016;11:1–21. doi: 10.1371/journal.pone.0146966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Fanarioti E., Mavrikaki M., Panagis G., Mitsacos A., Nomikos G.G., Giompres P. Behavioral and neurochemical changes in mesostriatal dopaminergic regions of the rat after chronic administration of the cannabinoid receptor agonist WIN55,212-2. Int. J. Neuropsychopharmacol. 2015;18:1–17. doi: 10.1093/ijnp/pyu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Teruel A., Escorihuela R.M., Castellano B., González B., Tobeña A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the roman rat lines. Behav. Genet. 1997;27:513–526. doi: 10.1023/A:1021400830503. [DOI] [PubMed] [Google Scholar]
- Frankola K.A., Flora A.L., Torres A.K., Grissom E.M., Overstreet S., Dohanich G.P. Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiol. Learn. Mem. 2010;94:91–99. doi: 10.1016/j.nlm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Frantz K.J., Van Hartesveldt C. The locomotor effects of quinpirole in rats depend on age and gender. Pharmacol. Biochem. Behav. 1999;64:821–826. doi: 10.1016/S0091-3057(99)00162-8. [DOI] [PubMed] [Google Scholar]
- Gelbard H.A., Teicher M.H., Faedda G., Baldessarini R.J. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Dev. Brain Res. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Engber T.M., Mahan L.C., Susel Z., Chase T.N., Monsma F.J., Sibley D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giorgi O., De Montis G., Porceddu M.L., Mele S., Calderini G., Toffano G., Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Dev. Brain Res. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V.F., Schacher S., Kandel E.R. The long and the short of long-term memory - A molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Grace L., Hescham S., Kellaway L.A., Bugarith K., Russell V.A. Effect of exercise on learning and memory in a rat model of developmental stress. Metab. Brain Dis. 2009;24:643–657. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Heim C.M., Entringer S., Buss C. Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psycho. 2019;105:127–137. doi: 10.1016/j.psyneuen.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L., Zahm D.S., Churchill L., Kalivas P.W., Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-Y. [DOI] [PubMed] [Google Scholar]
- Hikida T., Kimura K., Wada N., Funabiki K., Nakanishi Shigetada S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Horiguchi M., Hannaway K.E., Adelekun A.E., Huang M., Jayathilake K., Meltzer H.Y. D1 receptor agonists reverse the subchronic phencyclidine (PCP)-induced novel object recognition (NOR) deficit in female rats. Behav. Brain Res. 2013;238:36–43. doi: 10.1016/j.bbr.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Hotte M., Naudon L., Jay T.M. Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol. Learn. Mem. 2005;84:85–92. doi: 10.1016/j.nlm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Huang E.P. Synaptic plasticity: going through phases with LTP. Curr. Biol. 1998;8:350–352. doi: 10.1016/s0960-9822(98)70219-2. [DOI] [PubMed] [Google Scholar]
- Hulshof H.J., Novati A., Sgoifo A., Luiten P.G.M., Den Boer J.A., Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav. Brain Res. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Thrivikraman K.V., Meaney M.J., Plotsky P.M. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology ((Berl)) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complexctory tubercle complex. Brain Res. Rev. 2007;56:27–28. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L., Bachman J., Schulenberg J. Volume II. NIH; 2005. Monitoring the Future National Survey Results on Drug Use, 1975–2005; pp. 19–45. (College Students and Adults Ages). [Google Scholar]
- Kosten T.A., Kim J.J., Lee H.J. Early life manipulations alter learning and memory in rats. Neurosci. Biobehav. Rev. 2012;36:1985–2006. doi: 10.1016/j.neubiorev.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B., Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim H.J., Kim J.G., Ryu V., Kim B.T., Kang D.W., Jahng J.W. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci. Res. 2007;58:32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Rev. Neurosci. 2000;11:383–408. doi: 10.1515/REVNEURO.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Pryce C.R., Bettschen D., Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol. Biochem. Behav. 1999;64:705–715. doi: 10.1016/S0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Stöhr T., Schuller J., Domeney A., Heidbreder C., Feldon J. Long-term effects of repeated maternal separation on three different latent inhibition paradigms. Pharmacol. Biochem. Behav. 1998;59:873–882. doi: 10.1016/S0091-3057(97)00529-7. [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Cruz D., Eggan S., Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann. N. Y. Acad. Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. Dopaminergic innervation of the globus pallidus by collaterals from the nigrostriatal pathway. Brain Res. 1979;172:169–173. doi: 10.1016/0006-8993(79)90907-7. [DOI] [PubMed] [Google Scholar]
- Lundberg S., Nylander I., Roman E. Behavioral profiling in early adolescence and early adulthood of male wistar rats after short and prolonged maternal separation. Front. Behav. Neurosci. 2020;14:1–10. doi: 10.3389/fnbeh.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Majcher-Maślanka I., Solarz A., Wędzony K., Chocyk A. The effects of early-life stress on dopamine system function in adolescent female rats. Int. J. Dev. Neurosci. 2017;57:24–33. doi: 10.1016/j.ijdevneu.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Makena N., Bugarith K., Vivienne R. Maternal separation enhances object location memory and prevents exercise-induced MAPK/ERK signalling in adult Sprague–Dawley rats. Metab. Brain Dis. 2012;27:377–385. doi: 10.1007/s11011-012-9298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx G., Gilon C. The molecular basis of memory. ACS Chem. Neurosci. 2012;3:633–642. doi: 10.1021/cn300097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H., Bodnoff S.R., Iny L.J., Sapolsky R.M. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1985;9:731–734. doi: 10.1016/0278-5846(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Mitchell J.B., Aitken D.H., Bhatnagar S., Bodnoff S.R., Iny L.J., Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Horvath K.M., Nagy G.M., Bohus B., Koolhaas J.M. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J. Neuroendocrinol. 1999;12:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Miguel P.M., Pereira L.O., Silveira P.P., Meaney M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019;61:1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- Missale C., Russel Nash S., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyauchi M., Neugebauer N.M., Meltzer H.Y. Dopamine D4 receptor stimulation contributes to novel object recognition: relevance to cognitive impairment in schizophrenia. J. Psychopharmacol. 2017;31:442–452. doi: 10.1177/0269881117693746. [DOI] [PubMed] [Google Scholar]
- Munafò M.R., Yalcin B., Willis-Owen S.A., Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol. Psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog. Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Natsheh J.Y., Shiflett M.W. Dopaminergic modulation of goal-directed behavior in a rodent model of attention-deficit/hyperactivity disorder. Front. Integr. Neurosci. 2018;12:1–13. doi: 10.3389/fnint.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves B.H.S., Barbosa G.P.D.R., Rosa A.C., de S., Picua S.S., Gomes G.M., Sosa P.M., Mello-Carpes P.B. On the role of the dopaminergic system in the memory deficits induced by maternal deprivation. Neurobiol. Learn. Mem. 2020:173. doi: 10.1016/j.nlm.2020.107272. [DOI] [PubMed] [Google Scholar]
- Nishi M. Effects of early-life stress on the brain and behaviors: implications of early maternal separation in rodents. Int. J. Mol. Sci. 2020:21. doi: 10.3390/ijms21197212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I., Roman E. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology. 2013;229:555–569. doi: 10.1007/s00213-013-3217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama Y., Ishiguro H., Nankai M., Shibuya H., Watanabe A., Arinami T. Identification of a polymorphism in the promoter region of DRD4 associated with the human novelty seeking personality trait. Mol. Psychiatry. 2000;5:64–69. doi: 10.1038/sj.mp.4000563. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- Piekarski D.J., Johnson C.M., Boivin J.R., Thomas A.W., Lin W.C., Delevich K., Galarce E.M., Wilbrecht L. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 2017;1654:123–144. doi: 10.1016/j.brainres.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plescia F., Marino R.A.M., Navarra M., Gambino G., Brancato A., Sardo P., Cannizzaro C. Early handling effect on female rat spatial and non-spatial learning and memory. Behav. Processes. 2014;103:9–16. doi: 10.1016/j.beproc.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Ploj K., Roman E., Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/S0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Powell S.B., Paulus M.P., Hartman D.S., Godel T., Geyer M.A. RO-10-5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacology. 2003;44:473–481. doi: 10.1016/S0028-3908(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 2003;27:57–71. doi: 10.1016/S0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Raineki C., Lucion A.B., Weinberg J. Neonatal handling: an overview of the positive and negative effects. Dev. Psychobiol. 2014;56:1613–1625. doi: 10.1002/dev.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M., Hovda D., Giza C. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev. Psychobiol. 2009;51:672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A., Trías S., Peñafiel A., Narváez J.Á., Díaz-Cabiale Z., Moratalla R., De La Calle A. Expression of D4 dopamine receptors in striatonigral and striatopallidal neurons in the rat striatum. Brain Res. 2003;989:35–41. doi: 10.1016/S0006-8993(03)03328-6. [DOI] [PubMed] [Google Scholar]
- Schroll H., Hamker F.H. Computational models of basal-ganglia pathway functions: focus on functional neuroanatomy. Front. Syst. Neurosci. 2013;7:1–18. doi: 10.3389/fnsys.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U., Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol. Biochem. Behav. 2002;73:115–122. doi: 10.1016/S0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- Sood P., Idris N.F., Cole S., Grayson B., Neill J.C., Young A.M. PD168077, a D4 receptor agonist, reverses object recognition deficits in rats: Potential role for D4 receptor mechanisms in improving cognitive dysfunction in schizophrenia. J. Psychopharmacol. 2011;25:792–800. doi: 10.1177/0269881110387840. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Neurobehavioral changes in adolescence. Curr. Dir. Psychol. Sci. 2000;9:111–114. doi: 10.1111/1467-8721.00072. [DOI] [Google Scholar]
- Spear L.P., Swartzwelder H.S. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci. Biobehav. Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield K.H., Kirstein C.L. Effects of novelty on behavior in the adolescent and adult rat. Dev. Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Surmeier D.J., Song W.J., Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/jneurosci.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos A.L., Periasamy S., Pickel V.M. Presynaptic dopamine D4 receptor localization in the rat nucleus accumbens shell. Synapse. 2000;36:222–232. doi: 10.1002/(SICI)1098-2396(20000601)36:3<222::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Taglialatela G., Hogan D., Zhang W.-R., Dineley K. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi F.I., Baldessarini R.J. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int. J. Dev. Neurosci. 2000;18:29–37. doi: 10.1016/S0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi F.I., Campbell A., Yeghiayan S.K., Baldessarini R.J. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: comparison of D1-, D2- and D4-like receptors. Neuroscience. 1998;83:169–176. doi: 10.1016/S0306-4522(97)00386-2. [DOI] [PubMed] [Google Scholar]
- Tarazi F.I., Tomasini E.C., Baldessarini R.J. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci. Lett. 1998;254:21–24. doi: 10.1016/S0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Hostetter J.C. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev. Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- Tractenberg S.G., Levandowski M.L., de Azeredo L.A., Orso R., Roithmann L.G., Hoffmann E.S., Brenhouse H., Grassi-Oliveira R. An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 2016;68:489–503. doi: 10.1016/j.neubiorev.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Tsotsokou G., Nikolakopoulou M., Kouvelas E.D., Mitsacos A. Neonatal maternal separation affects metabotropic glutamate receptor 5 expression and anxiety-related behavior of adult rats. Eur. J. Neurosci. 2021;54:4550–4564. doi: 10.1111/ejn.15358. [DOI] [PubMed] [Google Scholar]
- Vallée M., Mayo W., Dellu F., Moal M., Le, Simon H., Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. J. Neurosci. 1997;17:2626–2636. doi: 10.1523/jneurosci.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M., Mayo W., Maccari S., Le Moal M., Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: Relationship to corticosterone secretion response. Brain Res. 1996;712:287–292. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- Vivinetto A.L., Suárez M.M., Rivarola M.A. Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behav. Brain Res. 2013;240:110–118. doi: 10.1016/j.bbr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D., White T., Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci. Biobehav. Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Levine J.L.S., Avila-Quintero V., Bloch M., Kaffman A. Systematic review and meta-analysis: effects of maternal separation on anxiety-like behavior in rodents. Transl. Psychiatry. 2020:10. doi: 10.1038/s41398-020-0856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Wang G., Wang H., He J., Zhang N., Wu Z., Xiao L., Yang C. Sex-dependent impact of different degrees of maternal separation experience on OFT behavioral performances after adult chronic unpredictable mild stress exposure in rats. Physiol. Behav. 2018;194:153–161. doi: 10.1016/j.physbeh.2018.04.034. [DOI] [PubMed] [Google Scholar]
- Winters B.D., Saksida L.M., Bussey T.J. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Woolley M.L., Marsden C.A., Sleight A.J., Fone K.C.F. Reversal of a cholinergic-induced deficit in a rodent model of recognition memory by the selective 5-HT6 receptor antagonist, Ro 04-6790. Psychopharmacology. 2003;170:358–367. doi: 10.1007/s00213-003-1552-5. [DOI] [PubMed] [Google Scholar]
- Woolley M.L., Waters K.A., Reavill C., Bull S., Lacroix L.P., Martyn A.J., Hutcheson D.M., Valerio E., Bate S., Jones D.N.C., Dawson L.A. Selective dopamine D4 receptor agonist (A-412997) improves cognitive performance and stimulates motor activity without influencing reward-related behaviour in rat. Behav. Pharmacol. 2008;19:765–776. doi: 10.1097/FBP.0b013e32831c3b06. [DOI] [PubMed] [Google Scholar]
- Yang K., Broussard J.I., Levine T.A., Jenson D., Arenkiel B., Dani A.J. Dopamine Receptor Activity Participates in Hippocampal Synaptic Plasticity Associated with Novel Object Recognition. Eur. J. Neurosci. 2017;45:138–146. doi: 10.1111/ejn.13406.Dopamine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata S., Yamaguchi T., Danjo T., Hikida T., Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 2012;109:12764–12769. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung K.K.L., Bolam J.P., Smith A.D., Hersch S.M., Ciliax B.J., Levey A.I. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-E. [DOI] [PubMed] [Google Scholar]
- Zahm D.A., Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J. Comp. Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]