Summary
Background
This study assessed the effectiveness of the NEVERMIND e-health system, consisting of a smart shirt and a mobile application with lifestyle behavioural advice, mindfulness-based therapy, and cognitive behavioural therapy, in reducing depressive symptoms among patients diagnosed with severe somatic conditions. Our hypothesis was that the system would significantly decrease the level of depressive symptoms in the intervention group compared to the control group.
Methods
This pragmatic, randomised controlled trial included 425 patients diagnosed with myocardial infarction, breast cancer, prostate cancer, kidney failure, or lower limb amputation. Participants were recruited from hospitals in Turin and Pisa (Italy), and Lisbon (Portugal), and were randomly assigned to either the NEVERMIND intervention or to the control group. Clinical interviews and structured questionnaires were administered at baseline, 12 weeks, and 24 weeks. The primary outcome was depressive symptoms at 12 weeks measured by the Beck Depression Inventory II (BDI-II). Intention-to-treat analyses included 425 participants, while the per-protocol analyses included 333 participants. This trial is registered in the German Clinical Trials Register, DRKS00013391.
Findings
Patients were recruited between Dec 4, 2017, and Dec 31, 2019, with 213 assigned to the intervention and 212 to the control group. The sample had a mean age of 59·41 years (SD=10·70), with 44·24% women. Those who used the NEVERMIND system had statistically significant lower depressive symptoms at the 12-week follow-up (mean difference=-3·03, p<0·001; 95% CI -4·45 to -1·62) compared with controls, with a clinically relevant effect size (Cohen's d=0·39).
Interpretation
The results of this study show that the NEVERMIND system is superior to standard care in reducing and preventing depressive symptoms among patients with the studied somatic conditions.
Funding
The NEVERMIND project received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No. 689691.
Keywords: Depression, Mental health, e-health, RCT, Somatic conditions, NEVERMIND
Research in context.
Evidence before this study
We searched Google Scholar, PubMed and Web of Science from January 1, 2015 to December 3, 2017 for studies about e-health systems designed to prevent and treat comorbid depressive symptoms in patients with severe somatic conditions. Search terms included “e-mental health” OR “e-health” AND “depression” AND “comorbid”. A systematic review was found that examined the effectiveness of 11 studies involving web-based interventions aimed at treating depression in patients with chronic physical illnesses. Significant reductions in the severity of depression were reported (dw = 0·36, CI = 0·20-0·52, p < 0·01). Furthermore, a systematic review on the effectiveness and cost-effectiveness of e-health systems for patients with somatic conditions was found, which stated that the available evidence is promising but limited.
Added value of this study
To our knowledge, this is the first study evaluating a mobile app-based system designed to reduce and prevent comorbid depressive symptoms in participants with one of five severe somatic diseases. This study adds to the existing research by presenting an e-health system that successfully reduces depressive symptoms in participants with differing somatic conditions who used the system for 12 weeks compared to standard care. It is possible to adjust the NEVERMIND system to include participants with other somatic conditions, as well as primary psychiatric conditions, by including content that is specifically tailored for these groups of patients.
Implications of all evidence available
As depressive disorders are one of the leading causes of burden and disability worldwide, and their prevalence is even higher in patients with severe somatic conditions, offering effective and accessible e-health interventions is of paramount importance in combating them. The results presented in this study are very promising because they show that NEVERMIND is effective in both, reducing depressive symptoms among patients who already developed them, and preventing the onset of depressive symptoms among patients with severe somatic disorders and no symptoms of depression. Further research is needed to confirm the present findings, as well as the possibility to expand the NEVERMIND system to other patient groups.
Alt-text: Unlabelled box
Introduction
Depressive disorders are the leading cause of disability worldwide, with an estimated prevalence of 4·4% (322 million individuals), of which 12% (40 million individuals) are in Europe.1 Compared to the normal population, the risk of developing depression is higher in individuals with a wide range of somatic, i.e., physical, conditions. A few such examples are endometriosis and abdominal pain, epilepsy, multiple sclerosis, breast cancer, myocardial infarction (MI), lower limb amputation, and kidney failure.2, 3, 4, 5, 6 The emergence of depression in so many heterogeneous somatic conditions is likely due to the reduced quality of life and limitations these conditions entail. However, depression itself has notable consequences on morbidity, quality of life, and response to treatment subsequently affecting the prognosis of the primary somatic condition itself.2,5 Several serious consequences have been linked to depression, including the inability to function in daily life, sleep disturbances, and suicide, the latter accounting for 1·5% of global deaths every year.1 Furthermore, depression has shown to result in a significant economic burden, with annual direct treatment costs reaching a mean of €4430 (SD=557) per capita, compared to €2218 (SD=145) for those without depression.7 Even so, most EU healthcare systems currently do not incorporate preventive methods or early diagnosis for the onset of depressive symptoms in patients with severe somatic conditions. The development of holistic care that includes psychological support is needed as years of research have shown the importance of mental health in patients’ long-term health-related quality of life and prognosis.8
E-health interventions to improve mental and somatic well-being have been found to be effective and cost-effective.9 However, systematic reviews of the field highlight that the available evidence is promising but limited.10 More recently, a pilot study conducted on patients with diabetes and hypertension found that a low-intensity app-delivered psychoeducational six-week intervention produced a slight improvement in the reduction of the severity of depressive symptoms in patients who used the app.11 Other studies evaluating web- and mobile-based interventions using cognitive behavioural therapy (CBT) found significantly reduced depressive symptoms and a decreased onset of major depressive symptoms in patients with chronic back pain.12 Even though there is an abundance of e-health systems available that claim to treat and prevent depression, systematic reviews have found that less than 6·2% had published evidence for the efficacy of their frameworks.13
Building on the initial success of the PSYCHE e-health system,14,15 we developed the NEVERMIND system, which stands for NEurobehavioural predictiVE and peRsonalised Modelling of depressIve symptoms duriNg primary somatic Diseases with ICT-enabled self-management procedures. NEVERMIND aims at reducing and preventing depressive symptoms among patients with a heterogeneous set of somatic disorders and disabilities: breast and prostate cancer, kidney failure, MI, and leg amputation. The NEVERMIND system consists of a sensorised shirt and a user interface in the form of a mobile application (app). These wearables are based on the PSYCHE system, for which mood classification accuracies of approximately 97% were found.15 NEVERMIND includes a predictive algorithm (see Christinaki and colleagues16 for details) that was developed to forecast patients’ depressive symptoms based on the biomedical data collected through the shirt (electrocardiogram, respiration dynamics, body movement), and additional information about mental health symptoms, such as depression, anxiety, sleep problems and stress, collected within the app through psychometric questionnaires and specifically developed questions. The above forecasts were used to optimise the frequency with which the app would present questions to the participants, to allow for an optimal amount of information to be collected while at the same time guarding against ‘questionnaire fatigue’.
From the combined information of the sensorised shirt and the mobile app, patients received personalised feedback to self-manage their mental health symptoms. On the basis of their symptoms, patients were directed to personalised lifestyle behavioural advice that included sleep hygiene, a healthy diet, physical activity, mindfulness-based therapy, online CBT (Deprexis17) and/or referral to mental-health care. The choice of using e-health interventions based on mindfulness and CBT was based on the available evidence about e-health interventions in the field of mental health. It has been reported that technology-delivered mindfulness can be useful for patients with medical conditions.18 Similarly, online CBT was found to be effective among patients with moderate/severe depression as well as with patients with mild/sub-threshold conditions.19 Another meta-analysis that included 23 controlled trials with either app or web-based interventions in the workplace setting found the greatest effect to be in mindfulness-based interventions followed by CBT based interventions.20 More detailed information about the NEVERMIND system is included in the protocol of the study.21
The aim of this study was to evaluate in a randomised controlled trial (RCT) if the NEVERMIND system was effective in reducing and preventing depressive and other mental-health symptoms in a clinical sample of patients with severe somatic disorders.
Methods
Study design
The effectiveness of the NEVERMIND system was evaluated in a multicentre pragmatic RCT. The study received ethical review by the European Commission as a prerequisite for funding approval for the project. Ethical approval for the NEVERMIND study was submitted and approved by the following local research ethics committees in each of the countries where the intervention was implemented: Pisa Comitato Etico di Area Vasta Nord Ovest (Comitato Etico Sperimentazione Farmaco – CESF); Ethical Committee of Città della Salute e della Scienza di Torino University Hospital and Ethical Committee of San Luigi Gonzaga University Hospital, Orbassano; Ethics Committee of the Medical Academic Centre of the University of Lisbon. Additional ethical approval for the analysis of the pseudoanonymized data was obtained by the Swedish Ethics Review Authority (Etikprövningsmyndigheten).
This procedure, along with the statistical analysis plan, have been described in detail in the previously published protocol of this study.21 The trial has been registered in the German Clinical Trials Register (DRKS00013391). This study did not have a data monitoring committee, as each local principal investigator had the responsibility to oversee their centre.
Participants
Participants diagnosed with breast cancer were recruited by references from oncologists working at the Breast Unit-Oncology Department at Città della Salute e della Scienza di Torino University Hospital (Turin, Italy). Prostate cancer patients were recruited at San Luigi Gonzaga University Hospital (Turin, Italy) and at Città della Salute e della Scienza di Torino University Hospital (Turin, Italy) through referrals from the Urology departments as well as from inpatient registers. Patients with MI were recruited through referrals from cardiologists at the Cardiology department at Città della Salute e della Scienza di Torino University Hospital (Turin, Italy) as well as inpatient registries, and at the Cardiology Department at Santa Maria Hospital (Lisbon, Portugal). Kidney failure patients were recruited at the Centre of Turin at San Luigi Gonzaga University Hospital (Turin, Italy) and at Cisanello Hospital (Pisa, Italy) through referrals from dieticians. Amputees were recruited at Santa Maria Hospital (Lisbon, Portugal). All participants were given detailed information about the procedures and the purpose of the study and signed an informed consent form before the study started.
Inclusion criteria
Participants were eligible to participate if they were 18 years of age or older with a diagnosis of MI, breast cancer, prostate cancer, kidney failure, or leg amputation. Breast and prostate cancer patients not undergoing active treatment were recruited at stage II, III, or IV. Participants with MI were included if they had a type I diagnosis of MI. Kidney failure patients were recruited if they were in a stable clinical condition with chronic kidney failure of stages III, IV, or V as defined by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines,22 whereas amputee patients were recruited if they had a lower limb amputation and were within six months post-surgical intervention.
Exclusion criteria
Participants were excluded if they fell under one of the following categories: patients with a past diagnosis of a major psychiatric disorder other than depression; patients who had started with a new medication for depression less than two months before the start of the study; patients who had had any type of cognitive impairment (e.g., dementia); patients who were evaluated by the Paykel Suicide Scale as actively suicidal; or patients who had followed structured psychological treatments that involved mindfulness-based therapy, CBT, other relaxation techniques, or other structured psychotherapy within three months before enrolment. Furthermore, participants were excluded if they had participated or were participating at the time of enrolment in any clinical trials that could interfere with the study objectives. Finally, those who were unable to participate in the study procedures or could not use smartphone technology were also excluded.
Randomisation and masking
Sequence generation and allocation concealment
Participants were randomly assigned to either the intervention or the control group by block randomisation of size ten, ensuring a balanced allocation of participants to the intervention and control group at each centre. Randomisation was centralised and carried out by an electronic system developed by UPM (Universidad Politécnica de Madrid) in collaboration with KI (Karolinska Institutet) so that the researchers tasked with enrolling and assessing the participants would not know whether the participants were in the intervention or control group.
Eligible participants completed the baseline assessment with a dedicated rater. After this, the treating physician received from the NEVERMIND server a randomly generated code that determined the group (intervention or control) to which the participant was allocated.
Masking
The raters who evaluated the participants at baseline and follow-up were blinded to the group allocation. The researchers responsible for analysing the data were able to determine patients’ group allocation after enrolment.
The researchers who screened the patients according to the inclusion/exclusion criteria, collected informed consent, randomly assigned them to the intervention or control group, and handed out the NEVERMIND system, including instructions on how to use it, were not blind to the group allocation status, making this an open-label trial. No compliance check was performed, as patients were aware of the group they belonged to.
Procedures
Participants in the NEVERMIND intervention group received a sensorised shirt and a mobile app, which collected physiological and psychometric data. Patients were instructed to use the system for 12 consecutive weeks. The first follow-up was done 12 weeks post-baseline, while the second follow-up was done 24 weeks post-baseline. Each centre was instructed to keep a detailed record of any safety events that occurred.
Outcomes
The main hypothesis was that the NEVERMIND system would significantly decrease the level of depressive symptoms in the intervention group in comparison to treatment as usual (TAU) in the control group. TAU was defined as the standard treatment practices that were in place at the respective centres before the NEVERMIND trial.21
The primary outcome was depressive symptoms at 12 weeks measured by the Beck Depression Inventory II (BDI-II).23 Secondary outcomes were the prevention of the onset of depressive symptoms measured by the BDI-II at 12 weeks, general interest, mood and vitality measured by the WHO Well-being Scale (WHO-5),24 suicidal ideation and attempt measured by the Paykel Suicide Scale (PSS),25 patient self-efficacy in management of the primary somatic condition measured by the Self-Efficacy for Managing Chronic Disease Scale (SEMCD6),26 level of satisfaction with daily life as measured by the Quality of Life in Neurological Disorders (Neuro-Qol),27 illness perception measured by the Brief Illness Perception Questionnaire (BIPQ- short form),28 self-compassion as measured by the Self-Compassion Scale-short form (SCS-SF),29 level of physical activity measured by the Rapid Assessment of Physical Activity (RAPA),30 perceived stigma measured by the Chronic Illness Anticipated Stigma Scale (CIASS),31 and the sustainability of the effect of the NEVERMIND measured by the BDI-II scale at 24-weeks post-baseline. Sociodemographic variables, such as age, sex, disease type, and living arrangement were collected through an ad-hoc built questionnaire. All outcomes were measured by self-report questionnaires.
Statistical analysis
As this was the first study to evaluate the effectiveness of the NEVERMIND system, the sample size calculation was based on the effectiveness of Deprexis, the online cognitive behavioural therapy component of the NEVERMIND system.17 The sample size was calculated to detect a reduction in depressive symptoms (d=0·54), where a linear model analysis was used with a power of 80%, an intercorrelation coefficient (ICC) of 0·5 and a significance level of 0·05 for the primary outcome. For the proposed linear mixed model analysis, 110 participants were estimated through power calculations. As the primary outcome was to reduce depressive symptoms, the sample size had to be adjusted based on the estimated number of participants that would show depressive symptoms at baseline. We assumed that 1/3 of recruited participants would have at least mild depressive symptoms at baseline as indicated by two meta-analyses on breast cancer and MI patients.2,5 Therefore, a sample of 330 subjects who completed the study was required to achieve adequate power to detect medium effects at the aggregate levels, but not for each disease. According to the protocol,21 our target sample was 330 participants who completed the study; however, the sample size was inflated from 330 to 425 to account for drop-out.
All analyses performed using STATA/MP 15·1 are in accordance with the intention-to-treat (ITT) outcomes. The effects of the NEVERMIND system in reducing depressive symptoms measured by the BDI-II (primary outcome) were tested by means of an analysis of covariance based on intention-to-treat principle with depressive symptoms as baseline adjustment.32,33 Contrary to the protocol,21 which stated that loss to follow-up would be handled through multiple imputation or inverse probability weighting, no procedure for imputation of missing data was conducted as the linear mixed model analysis can handle incomplete data.32 Furthermore, missing data was completely random, was less than 20%, and therefore, a complete case analysis was appropriate.34,35 In the analysis of covariance model, age, sex, living arrangement, intervention, and depressive symptoms at baseline were considered as fixed variables, whereas centre was considered as random effect to detect heterogeneity between centres. The NEVERMIND system was considered effective in reducing depressive symptoms if the intervention coefficient was significant (p<0·05). As a sensitivity analysis, the effectiveness of NEVERMIND in reducing depressive symptoms was also measured with a linear mixed model and compared with the covariance model. For secondary outcomes, namely general interest, mood and vitality, suicidal ideation and attempt, participants’ self-efficacy in management of the primary disease, level of satisfaction with daily life, illness perception, self-compassion, perceived stigma, and the effectiveness of the NEVERMIND system at 24-weeks follow-up were also analysed using a linear mixed model using the same fixed and random effects as the primary outcome. Regarding the effectiveness at the 24-week follow-up, the analysis strayed from the protocol,21 which planned to conduct a last observation carried forward (LOCF) from the 12-week follow-up. Because the LOCF has been highly criticized, we conducted a linear mixed model without the LOCF imputation method. For secondary outcomes, level of physical activity and prevention of depressive symptoms at 12 weeks, a multilevel mixed effect ordered logistic regression and a multilevel mixed effect logistic regression were used, respectively. Statistical significance of the secondary outcomes has been tested for multiple comparisons with the Holm-Bonferroni Sequential Correction.
Intention-to-treat
Following the published protocol, the primary analysis was ITT. Of the 425 participants, 345 participants (81·18%) had data at 12 weeks, while 80 participants (18·82%) were lost to follow-up at 12 weeks. All participants were included in the ITT analysis for the primary outcome and for eight of the ten secondary outcomes. To assess the secondary outcome “prevention of the onset of depressive symptoms”, only participants without moderate or severe depressive symptoms (BDI-II score≤13) at baseline (n=232) were included. To assess the secondary outcome sustainability of the NEVERMIND system at 24 weeks only participants with outcome data available at 24 weeks (n=326) were included.
Per-protocol
According to the protocol, a per-protocol (PP) analysis was also conducted. The PP analysis included only participants who used the NEVERMIND system at least once according to the backend server and who did not have missing data regarding the primary outcome at baseline and 12-weeks follow-up. A total of 333 participants (96·52%) were included for the primary outcome and eight of the ten secondary outcomes. Of the 12 participants who were excluded, eight never used the app and/or shirt while four had missing outcome data. For the other outcomes, 223 (64·64%) were included to assess the prevention of depressive symptoms at 12 weeks whereas 315 participants (91·30%) were included to evaluate the sustainability of the NEVERMIND system at 24-weeks.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All co-authors had access to the dataset. The decision to submit the manuscript for publication was made jointly by all co-authors.
Results
Participants were recruited between December 4th, 2017, and December 31st, 2019, with the last follow-up and data collection ending on June 30th, 2020. Each clinical centre recruited participants and assessed their eligibility. A total of 1161 participants were assessed for eligibility across the different centres.
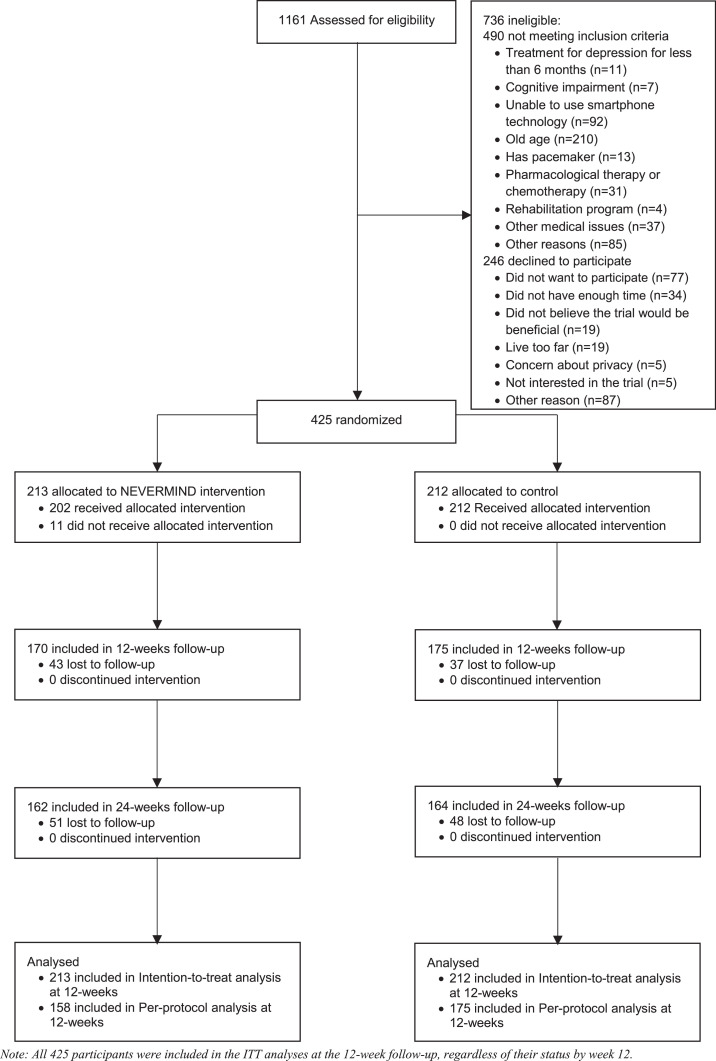
Out of the 1161 participants that were approached, 736 (63·34%) were excluded, as the majority (n=490, 66·58%) did not meet the inclusion criteria and the rest (n=246, 33·42%) declined to participate. Reasons for declining included logistical issues (e.g., not having enough time to devote or living too far), privacy issues, and scepticism about the benefits of the NEVERMIND system (Figure 1).
Figure 1.
CONSORT diagram.
Of the 425 participants enrolled in the study, 80 (18·82%) dropped out by the follow-up at week 12. By the follow-up at week 24, 19 more participants (23·29%) dropped out. At week 24, a total of 51 participants allocated to the intervention group dropped out, while the 49 patients dropped out in the control group. No adverse events were recorded, participants who did not complete the study dropped out due to lack of interest (53·33%), non-compliance (23·33%), medical problems unrelated to the NEVERMIND system (13·33%), family problems (6·67%), and frequent travel (3·33%). Participants who dropped out were not systematically different from those who completed the study regarding BDI-II at baseline (mean=10·82, SD=7·45 and mean=11·4, SD=8·12, respectively; p=0·719) and age (mean=59·76, SD=11·48 and mean=59·33, SD=10·54, respectively; p=0·373). In the total sample, a higher proportion of males dropped out of the study compared to females (21·9% vs 14·4%, p=0·046). However, in the intervention group there were no significant gender differences between patients who completed the study and patients who dropped out.
Baseline sociodemographic characteristics are shown in Table 1. The total sample consisted of 425 participants, with 213 participants allocated to the intervention group and 212 to the control group. The sample had a mean age of 59·41 (SD=10·70) and a slightly higher proportion of males (55·76%, n=237) compared to females (44·24%, n=188). In the control group, participants were more likely to live alone than participants in the intervention group (19·81% and 12·68%, respectively). Participants in both the control and intervention group were predominantly college educated (39·62% and 40·85%, respectively), married (72·64% and 74·18%, respectively), employed full-time or retired (81·6% and 77·94%, respectively) and living with someone (80·19% and 87·32%, respectively).
Table 1.
Baseline Sociodemographic and Clinical characteristics of participants in the NEVERMIND study.
| Intervention | Control | |
|---|---|---|
| Total population (n) | 213 | 212 |
| Sociodemographic characteristics | ||
| Male (%) | 117 (54·93) | 120 (56·60) |
| Female (%) | 96 (45·07) | 92 (43·40) |
| Mean age (SD) | 58·69 (11·13) | 60·12 (10·23) |
| Living arrangement (%) | ·· | ·· |
| Alone | 27 (12·68) | 42 (19·81) |
| With someone | 186 (87·32) | 170 (80·19) |
| Clinical characteristics | ||
| Centre (%) | ·· | ·· |
| Turin | 144 (67·61) | 141 (66·51) |
| Pisa | 15 (7·04) | 18 (8·49) |
| Lisbon | 53 (24·88) | 54 (25·47) |
| Somatic condition (%) | ·· | ·· |
| Breast Cancer | 80 (37·56) | 75 (35·38) |
| Prostate Cancer | 49 (23·00) | 51 (24·07) |
| Myocardial Infarction | 53 (24·88) | 49 (23·11) |
| Kidney failure | 19 (8·92) | 24 (11·32) |
| Leg amputation | 12 (5·63) | 13 (6·13) |
| BDI-II score (SD) | 11·17 (8·38) | 11·41 (7·60) |
| Missing data (%) | 1 (0·005) | 1 (0·005) |
| BDI-II Score (%)* | ·· | ·· |
| Minimal depressive symptoms** | 146 (68·87) | 138 (65·40) |
| Depressive symptoms*** | 66 (16·51) | 73 (20·38) |
| General Well-being (SD) | 61·64 (23·52) | 60·97 (21·08) |
| Missing data (%) | 1 (0·005) | 1 (0·005) |
| Suicidal ideation (SD) | 0·91 (1·99) | 0·73 (2·14) |
| Missing data (%) | 1 (0·005) | 1 (0·005) |
| Illness perception (SD) | 45·08 (11·90) | 45·66 (11·27) |
| Missing data (%) | 1 (0·005) | 2 (0·009) |
| Self-efficacy for managing chronic disease (SD) | 7·00 (2·12) | 6·71 (2·12) |
| Missing data (%) | 1 (0·005) | 2 (0·009) |
| Anticipated stigma (SD) | 1·66 (0·63) | 1·66 (0·66) |
| Missing data (%) | 1 (0·005) | 2 (0·009) |
| Satisfaction with Social Roles & Activities (SD) | 45·98 (4·41) | 45·80 (4·80) |
| Missing data (%) | 1 (0·005) | 2 (0·009) |
| Physical activity (%) | ·· | ·· |
| Sedentary | 77 (36·15) | 75 (35·38) |
| Under-active | 84 (39·44) | 87 (26·89) |
| Active | 52 (24·41) | 50 (37·73) |
| Self-compassion (SD) | 3·36 (0·71) | 3·32 (0·69) |
*Beck Depression Inventory II.
**BDI-II score≤13.
***BDI-II score>14.
The centre in Turin enrolled more than half of the total sample (n=285) whereas the centre in Lisbon enrolled 107 participants, and the centre in Pisa recruited 33 participants (Table 1). A higher proportion of the participants were diagnosed with breast cancer (n=155) followed by prostate cancer (n=100). Baseline BDI-II showed that a larger proportion of participants in both the intervention and control group had minimal depressive symptoms where the intervention and control group had a mean BDI-II score of 11·17 (SD=8·38) and 11·41 (SD=7·60) respectively (Table 1). Participants in the intervention and control group did not statistically differ in baseline depressive symptoms or in any of the other clinical characteristics measured at baseline (Table 1).
Primary outcome
The ITT analysis showed that participants in the NEVERMIND group had significantly lower depressive symptoms at 12 weeks compared to participants in the control group (mean difference=-3·03; 95% CI -4·45 to -1·62; p<0·001), with a mean BDI-II score of 6·88 (SD=7·42) and 10·21 (SD=8·62) respectively (Table 2). The marginal mean scores of the two groups at baseline, 12 weeks and 24 weeks are shown in Supplementary Figure 1. The difference in reduction in depressive symptoms equals to a standardised mean difference (SMD) or Cohen's d of 0·39 which is almost double of what is considered a clinically relevant effect.36 Men had significantly benefited more from the intervention at 12 weeks compared to women (mean difference=2·28; CI 0·65 to 3·92; p=0·006). The crude and adjusted model of the primary outcome (BDI-II), without the effect of the intervention, are presented in Supplementary Tables 1 and 2, respectively. The results showed a significant effect of time (mean difference=-2·73; 95% CI -3·88 to -1·58; p<0·001 and mean difference=-2·90; 95% CI -3·93 to -1·86; p<0·001, respectively) and gender (mean difference=2·42; 95% CI 0·54 to 4·30; p=0·012). As a sensitivity analysis the reduction in depressive symptoms at 12 weeks was also evaluated with a repeated measure analysis without treatment variable as main effect but with the interaction between the treatment variable and time, as recommended in Twisk et al.37 The sensitivity analysis showed similar results (mean difference=-3·15; 95% CI -4·52 to -1·78; p<0·001) as the principal analysis.
Table 2.
ITT covariance analysis of the primary outcome Beck Depression Inventory II (BDI-II) at 12 weeks, with centre as random effect (N=425).
| Coefficient | 95% CI | p value | |
|---|---|---|---|
| Intervention | -3·03 | -4·45 to -1·62 | <0·001 |
| BDI baseline | 0·49 | 0·40 to 0·59 | <0·001 |
| Living alone | -0·61 | -2·57 to 1·34 | 0·538 |
| Female | 2·28 | 0·65 to 3·92 | 0·006 |
| Age | -0·001 | -0·08 to 0·07 | 0·910 |
N.B The coefficient of Intervention represents the effectiveness of the NEVERMIND system in reducing depressive symptoms at 12- weeks. The variable centre included 3 centres: 2 in Italy and 1 in Portugal.
The PP analysis showed that participants in the intervention group had significantly lower depressive symptoms at 12 weeks than the control group (mean difference=-2·86; 95% CI -4·23 to -1·48; p<0·001), with a mean of 7·20 (SD=7·57) and 10·21 (SD=8·62), respectively.
Secondary outcomes
The ITT analysis for the secondary outcomes (Table 3) showed that participants in the NEVERMIND group had significantly lower suicidal ideation and attempt at 12 weeks (mean difference=-0·61; 95% CI -1·13 to -0·10; p=0·020) compared to the control group. A large effect size was also found for incidence of depressive symptoms, with those in the intervention group being 67% less likely to develop depressive symptoms compared to those in the control group (OR=0·43; 95% CI 0·22 to 0·87; p=0·019). Lastly, participants in the intervention group had significantly lower depressive symptoms 24 weeks post-baseline, with a mean of 7·39 (SD=7·35), compared to the control group, which had a mean of 10·20 (SD=9·06) (mean difference=-1·34; 95% CI -2·41 to -0·26; p=0·015), and older patients had lower depressive symptoms at 24 weeks (mean difference=-0·06; 95% CI -0·11 to -0·01; p=0·037). No significant differences were found for other secondary outcomes.
Table 3.
ITT covariance analyses of secondary outcomes (N=425).
| Coefficient | 95% CI | p value* | |
|---|---|---|---|
| WHO-5 | 5·46 | -0·24 to 11·16 | 0·060 |
| Self-Efficacy | 0·05 | -0·48 to 0·58 | 0·850 |
| CIASS | -0·06 | -0·23 to 0·11 | 0·470 |
| PSS | -0·61 | -1·13 to -0·10 | 0·020 |
| BIPQ | 0·00 | -2·48 to 2·48 | 0·999 |
| Neuro-QoL | -0·42 | -1·83 to 0·98 | 0·556 |
| SCS-SF | 0·11 | -0·08 to 0·29 | 0·255 |
| Sustainability | -1·34 | -2·41 to -0·26 | 0·015 |
| OR | 95% CI | p value* | |
| RAPA | 0·85 | 0·49 to 1·46 | 0·548 |
| Incidence of depressive symptoms | 0·43 | 0·22 to 0·87 | 0·019 |
Note: WHO-5: World Health Organization Well-Being Questionnaire-5; Self-Efficacy for Managing Chronic Disease Scale; CIASS: Chronic Illness Anticipated Stigma Scale; PSS: Paykel Suicide Scale; BIPQ: Body Illness Perception Questionnaire; Neuro-QoL: Quality of Neurological Disorders; SCS-SF: Self-Compassion Scale (Short Form); RAPA: Rapid Assessment of Physical Activity; Incidence of depressive symptoms: prevention of the onset of depressive symptoms measured by the BDI-II at 12 weeks; Sustainability: the sustainability of the effect of the NEVERMIND measured by the BDI-II scale at 24-weeks post-baseline.
*None of the p-values meet significance when corrected for multiple testing.
The PP analysis showed significantly lower suicide ideation and attempt for the intervention group compared to the control group (mean difference=-0·67; 95% CI -1·22 to -0·11; p=0·018). However, unlike the ITT analysis, no significant effect was found regarding the prevention of the onset of depressive symptoms at 12 weeks or for the sustainability of the effect of the system 24 weeks post-baseline.
Discussion
This study is a pragmatic multicentre RCT that examined the effects of the NEVERMIND system, a newly developed digital tool for the self-management of mental health, on depressive symptoms in a group of patients with severe somatic conditions. The results show that the NEVERMIND system was significantly more effective in treating depressive symptoms in comparison with TAU while also showing significant positive results for reducing suicidal ideation and preventing the onset of depressive symptoms. After 12 weeks, patients who had used the NEVERMIND system had a clinically relevant reduction of 37·36% in depressive symptoms on the basis of Cohen's d, while patients who received TAU had a lower reduction of 12·90%. This reduction is greater than the minimal clinically important difference (MCID), equal to a 17·50% reduction in scores at baseline, which was found to correspond to patients' reports of significant improvement.38 Importantly, patients with no baseline depressive symptoms in the intervention group showed a lower incidence of depressive symptoms at 12 weeks follow-up than the equivalent group of patients in the TAU. This result indicates that NEVERMIND had preventive effects on the onset of depressive symptoms in the studied population. Regarding gender, an exploration of the contents of the NEVERMIND system can help identify if certain modules were more appealing and thus more used by men than women. For example, it could be that men preferred to use the module that focussed on physical exercises, while women preferred to use the mindfulness module. This could explain why men benefited more from the intervention than women.
The effectiveness of the NEVERMIND system was sustained at 24 weeks follow-up (12 weeks after the end of the study) though to a lower extent than at 12 weeks follow-up. These findings are in line with previous studies on other interventions suggesting that identifying strategies aimed at improving the sustainability of treatment effects is an important direction in future research.39
Lastly, patients who used the system showed a significant decrease in suicide ideation after 12 weeks compared to TAU. This finding may be a consequence of the treatment and preventive effects on depression, but it is also possible that certain modules had a direct effect on suicide ideation. The next step would be to investigate, in a study with a larger sample size, whether the NEVERMIND system could be used to reduce suicide attempts or death by suicide in a similar clinical population.
Other secondary outcomes (general interest, mood and vitality, illness perception, self-efficacy of managing a chronic disease, perceived stigma, and self-compassion) did not show a significant improvement after 12 weeks of using the NEVERMIND system. This might be related to ceiling/floor effects as the scores were within the normal ranges at baseline. However, the interventions proposed might not have focused enough on these outcomes, leaving room for future improvements of NEVERMIND.
The NEVERMIND system showed significant results according to the ITT but not to the PP analyses with regard to lowering the incidence of depressive symptoms and the sustainability of the system's effectiveness. The lack of significance of the PP analyses might be due to the smaller sample size. The ITT analysis had missing data, but the linear mixed-effects model used handles missing data using maximum likelihood estimation, which is superior to any ad-hoc imputation for the percentage of missing data we had in our study.35
The NEVERMIND system was developed specifically for patients with either breast or prostate cancer, recent lower-limb amputation, severe MI, or kidney failure. It can, therefore, not be expected to achieve similar efficiency without adaptation in patients with other types of somatic conditions or even in patients with the same conditions but different severity. However, it is possible to adjust the system for a broader public at risk of developing comorbid depressive symptoms by including additional content. Future research is needed to confirm these findings and to test the viability of the NEVERMIND approach to the treatment and prevention of depression among patients with a broader range of somatic conditions, as well as within the context of primary psychiatric disorders.
This study had several limitations. Firstly, it was not possible to ensure complete blinding because the NEVERMIND system included physical items (an app and a shirt). As the participants knew which group they belonged to, a compliance check could not be performed. Moreover, fixed instead of random block sizes were used for the block randomization, which could have enabled the investigators to be aware of the randomization allocation. However, the raters who administered the questionnaires to patients at baseline and follow-up were different from the recruiters who randomised the patients and provided them with the system. The recruiters were also blinded to the size of the block as well as the total number of patients, thereby making it more difficult to predict group allocation. Secondly, the TAU was not standardised across centres or the different disease groups, which is a drawback of pragmatic trials. On the other hand, pragmatic RCTs give an estimate on how the treatment works in a real-life clinical scenario.40 A third limitation was that patients’ ability to use a Smartphone does not necessarily reflect the competency needed to navigate the NEVERMIND system. A fourth limitation was that, with regards to the prevention hypothesis using the PP-design, the study may have been underpowered. There was a large difference in group size between amputees and patients with kidney failure and the other somatic diseases. This could be because of difficulties collecting a large enough sample due to patients’ age and digital literacy. Even if this study has only one primary outcome, ten secondary outcomes have been analysed. The results for the secondary outcomes do not meet significance after being adjusted for multiple testing, and they can only have an exploratory rather than a confirmatory interpretation. A fifth limitation is the substantial amount of missing data, which should be explored further. Furthermore, the selection of patients with specific diagnoses, depending on the hospitals participating in the research project, limits the generalizability of the results to other somatic conditions. Finally, this study did not analyze which components of the intervention were used but analyzed the effectiveness of the NEVERMIND system as a whole. Further studies are needed to understand the effectiveness of the different modules that were included in the system.
The NEVERMIND study also had several strengths. A large enough sample size was obtained, yielding adequate statistical power to evaluate the effectiveness of the NEVERMIND system in treating depressive symptoms. The 12 weeks follow-up of patients is also in line with the National Institute for Health and Care Excellence (NICE) guideline, where between nine and 12 weeks of follow-up is recommended for low and medium-intensity psychosocial interventions for depression in adults.41 Also, patients who dropped out from the study did not systematically differ from those who completed the study with regard to age and baseline depressive symptoms.
As depressive symptoms are highly prevalent in patients with somatic conditions, with prevalence rates reaching up to 40%, and few digital interventions having been developed to address this, the NEVERMIND system proved to be one of the few effective e-health tools, to our knowledge, in combating the development and increase of depressive symptoms in these populations. As an all-in-one solution containing mindfulness-based therapy, CBT, and behavioural advice, the NEVERMIND system allows for screening, monitoring, and early intervention in psychiatric comorbidities such as depression, even in the absence of a large team of specialists. As patients can rely on the NEVERMIND system for support for minimally to moderately severe depressive symptoms, the healthcare system can focus resources on patients in need of professional psychiatric help. Future research should focus on evaluating the system in other patient groups and on the impact of the usability of the system on its effectiveness.
Contributors
VC led the development of the study design and methodology, wrote several sections of the manuscript and supervised all phases of the study and analyses. NGP wrote the first draft of the manuscript and conducted the statistical analysis, revised the manuscript after the input of other co-authors, and verified the underlying data. GH participated in the conception of the study and methodology, contributed to the statistical analysis and critically revised the manuscript. TV wrote some sections of the manuscript, participated in data management and analysis, critically revised the manuscript, and verified the underlying data. LC, CG, BM, LO, MO, RPo, IR, GV, and EPS are the principal investigators for the NEVERMIND project in their respective countries and critically revised all parts of the manuscript. GV, SC, SGM, SO, RPa, EC, TP are the site coordinators for the NEVERMIND project and contributed to the revision of the manuscript. PB critically revised the statistical analysis and the manuscript, and verified the underlying data. SD and VG conducted local data collection and project management assistance in their respective country. CS and LG contributed to the recruitment of patients in the university hospitals in Turin, Italy. SB contributed to the development of the mindfulness intervention and critically reviewed the manuscript. EPS is the principal investigator of the NEVERMIND project, participated in the design of the study and the NEVERMIND system, and critically revised the manuscript. GV contributed to the coordination of all scientific aspects of the NEVERMIND project, participated in the design of the study and the NEVERMIND system, contributed to data collection and processing, contributed to the statistical data analysis, verified the underlying data, and critically revised the manuscript. All authors had access to the data and critically revised the draft of the manuscript and approved the final manuscript. All authors confirm that they accept responsibility to submit for publication.
Data sharing statement
Individual participant data for this study is not available due to lack of ethical committee approval for data sharing and lack of consent from the recruited patients. Aggregated level data, with investigator support, is available for systematic reviews and meta-analyses after approval of a proposal by the NEVERMIND research consortium.
Funding
The NEVERMIND project received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No. 689691.
Declaration of interests
BM declares that he is employed by Gaia AG, the developer and owner of Deprexis, an internet-based intervention for depression, which is offered selectively to patients within the context of the NEVERMIND system. Other authors declare that they have no competing interests.
Acknowledgments
The authors wish to thank all researchers and affiliated staff participating in the implementation of the NEVERMIND project. The authors are thankful to Professor Francisco Sampaio, Professor Dulce Brito, Professor Fausto Pinto, Dr Ricardo Pinheiro, Dr Liana Shvachiy, Dr Angela Leal, Dr Fernanda Gabriel, Dr Pedro Morais and Sara Pereira from the department of Rehabilitation and Cardiology of the Santa Maria Hospital (CHULN) and the Cardiovascular Centre of the University of Lisbon for the screening, recruitment and follow-up of patients with lower limb amputation and myocardial infarction. The authors also wish to thank Professor Francesco Oliva, Francesca Malandrone, Alessia Testa, Irene Bovi, and all the staff of the San Luigi Gonzaga University Hospital and the Città della Salute e della Scienza di Torino University Hospital for the screening, recruitment and follow up of patients with breast and prostate cancer, myocardial infarction and kidney failure. Finally, the authors would like to thank Dr Maria Francesca Egidi and Elena Lucchetti of the Cisanello Hospital for recruiting and following up patients with kidney failure.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101423.
Supplementary materials
References
- 1.World Health Organisation . World Health Organisation; 2017. Depression and Other Common Mental Disorders.https://www.who.int/publications/i/item/depression-global-health-estimates Accessed 3 July 2021. [Google Scholar]
- 2.Feng L., Li L., Liu W., et al. Prevalence of depression in myocardial infarction: a PRISMA-compliant meta-analysis. Medicine (Baltim) 2019;98(8) doi: 10.1097/MD.0000000000014596. e14596-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambadauro P., Carli V., Hadlaczky G. Depressive symptoms among women with endometriosis: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;220(3):230–241. doi: 10.1016/j.ajog.2018.11.123. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S., Vecchio M., Craig J.C., et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 5.Pilevarzadeh M., Amirshahi M., Afsargharehbagh R., Rafiemanesh H., Hashemi S.M., Balouchi A. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(3):519–533. doi: 10.1007/s10549-019-05271-3. [DOI] [PubMed] [Google Scholar]
- 6.Zaheer A., Sharif F., Khan Z., Batool S., Iqbal H. Quality of life and depression among lower limb amputees. Ann King Edw Med Univ. 2020;26(2):364–368. [Google Scholar]
- 7.König H., Rommel A., Thom J., et al. The excess costs of depression and the influence of sociodemographic and socioeconomic factors: results from the German health interview and examination survey for adults (DEGS) Pharmacoeconomics. 2021;39(6):667–680. doi: 10.1007/s40273-021-01000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megari K. Quality of life in chronic disease patients. Health Psychol Res. 2013;1(3):e27. doi: 10.4081/hpr.2013.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbert N.J., van Os-Medendorp H., van Renselaar W., et al. Effectiveness and cost-effectiveness of ehealth interventions in somatic diseases: a systematic review of systematic reviews and meta-analyses. J Med Internet Res. 2014;16(4):e110. doi: 10.2196/jmir.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charova E., Dorstyn D., Tully P., Mittag O. Web-based interventions for comorbid depression and chronic illness: a systematic review. J Telemed Telecare. 2015;21(4):189–201. doi: 10.1177/1357633X15571997. [DOI] [PubMed] [Google Scholar]
- 11.Menezes P., Quayle J., Garcia Claro H., et al. Use of a mobile phone app to treat depression comorbid with hypertension or diabetes: a pilot study in Brazil and Peru. JMIR Ment Health. 2019;6(4):e11698. doi: 10.2196/11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sander L.B., Paganini S., Terhorst Y., et al. Effectiveness of a guided web-based self-help intervention to prevent depression in patients with persistent back pain: the PROD-BP randomized clinical trial. JAMA Psychiatry. 2020;77(10):1001–1011. doi: 10.1001/jamapsychiatry.2020.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall J.M., Dunstan D.A., Bartik W. Apps with maps-anxiety and depression mobile apps with evidence-based frameworks: systematic search of major app stores. JMIR Ment Health. 2020;7(6):e16525. doi: 10.2196/16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanata A., Valenza G., Nardelli M., Gentili C., Scilingo E.P. Complexity index from a personalized wearable monitoring system for assessing remission in mental health. IEEE J Biomed Health Inform. 2015;19(1):132–139. doi: 10.1109/JBHI.2014.2360711. [DOI] [PubMed] [Google Scholar]
- 15.Valenza G., Gentili C., Lanatà A., Scilingo E.P. Mood recognition in bipolar patients through the PSYCHE platform: preliminary evaluations and perspectives. Artif Intell Med. 2013;57(1):49–58. doi: 10.1016/j.artmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Christinaki E., Papastylianou T., Carletto S., et al. Well-being forecasting using a parametric transfer-learning method based on the fisher divergence and hamiltonian monte carlo. EAI Endorsed Trans Bioeng Bioinform. 2020;1(1):e6. [Google Scholar]
- 17.Twomey C., O'Reilly G., Bültmann O., Meyer B. Effectiveness of a tailored, integrative Internet intervention (deprexis) for depression: updated meta-analysis. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0228100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikolasek M., Berg J., Witt C.M., Barth J. Effectiveness of mindfulness- and relaxation-based ehealth interventions for patients with medical conditions: a systematic review and synthesis. Int J Behav Med. 2018;25(1):1–16. doi: 10.1007/s12529-017-9679-7. [DOI] [PubMed] [Google Scholar]
- 19.Karyotaki E., Efthimiou O., Miguel C., et al. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry. 2021;78(4):361–371. doi: 10.1001/jamapsychiatry.2020.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratton E., Lampit A., Choi I., Calvo R.A., Harvey S.B., Glozier N. Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PloS one. 2017;12(12) doi: 10.1371/journal.pone.0189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carli V., Wasserman D., Hadlaczky G., et al. A protocol for a multicentre, parallel-group, pragmatic randomised controlled trial to evaluate the NEVERMIND system in preventing and treating depression in patients with severe somatic conditions. BMC Psychiatry. 2020;20(1):93. doi: 10.1186/s12888-020-02494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daugirdas J.T., Depner T.A., Inrig J., et al. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Beck A.T., Steer R.A., Brown G.K. Manual for the Beck Depression Inventory (BDI-II) 2nd Edition. Psychological Corporation; San Antonio: 1996. Manual for The Beck Depression Inventory Second Edition (BDI-II) [Google Scholar]
- 24.WHO Organisation . WHO Organisation; 1998. Wellbeing Measures in Primary Health Care/The Depcare Project.https://www.euro.who.int/__data/assets/pdf_file/0016/130750/E60246.pdf Accessed 3 July 2021. [Google Scholar]
- 25.Paykel E.S., Hallowell C., Dressler D.M., Shapiro D.L., Weissman M.M. Treatment of suicide attempters: a descriptive study. Arch Gen Psychiatry. 1974;31(4):487–491. doi: 10.1001/archpsyc.1974.01760160039009. [DOI] [PubMed] [Google Scholar]
- 26.Lorig K.R., Sobel D.S., Ritter P.L., Laurent D., Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 27.Gershon R.C., Lai J.S., Bode R., et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012;21(3):475–486. doi: 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Raes F., Pommier E., Neff K.D., Van Gucht D. Construction and factorial validation of a short form of the self-compassion scale. Clin Psychol Psychother. 2011;18(3):250–255. doi: 10.1002/cpp.702. [DOI] [PubMed] [Google Scholar]
- 30.Topolski T.D., LoGerfo J., Patrick D.L., Williams B., Walwick J., Patrick M.B. The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. -A. [PMC free article] [PubMed] [Google Scholar]
- 31.Earnshaw V.A., Quinn D.M., Kalichman S.C., Park C.L. Development and psychometric evaluation of the chronic illness anticipated stigma scale. J Behav Med. 2013;36(3):270–282. doi: 10.1007/s10865-012-9422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman A., Hill J. Cambridge University Press; Cambridge: 2006. Data Analysis Using Regression and Multilevel/Hierarchical Models. [Google Scholar]
- 33.Schielzeth H., Dingemanse N.J., Nakagawa S., et al. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol Evol. 2020;11(9):1141–1152. [Google Scholar]
- 34.Armijo-Olivo S., Warren S., Magee D. Intention to treat analysis, compliance, drop-outs and how to deal with missing data in clinical research: a review. Phys Ther Rev. 2009;14(1):36–49. [Google Scholar]
- 35.Jakobsen J.C., Gluud C., Wetterslev J., Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials-a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuijpers P., Turner E.H., Koole S.L., van Dijke A., Smit F. What is the threshold for a clinically relevant effect? The case of major depressive disorders. Depress Anxiety. 2014;31(5):374–378. doi: 10.1002/da.22249. [DOI] [PubMed] [Google Scholar]
- 37.Twisk J., Bosman L., Hoekstra T., Rijnhart J., Welten M., Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. 2018;10:80–85. doi: 10.1016/j.conctc.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Button K.S., Kounali D., Thomas L., et al. Minimal clinically important difference on the beck depression inventory-II according to the patient's perspective. Psychol Med. 2015;45(15):3269–3279. doi: 10.1017/S0033291715001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chien C.H., Liu K.L., Chien H.T., Liu H.E. The effects of psychosocial strategies on anxiety and depression of patients diagnosed with prostate cancer: a systematic review. Int J Nurs Stud. 2014;51(1):28–38. doi: 10.1016/j.ijnurstu.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Gamerman V., Cai T., Elsäßer A. Pragmatic randomized clinical trials: best practices and statistical guidance. Health Serv Outcomes Res Methodol. 2019;19(1):23–35. [Google Scholar]
- 41.National Collaborating Centre for Mental Health (UK) British Psychological Society; Leicester (UK): 2010. Depression: The Treatment and Management of Depression in Adults (Updated Edition) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.