Abstract
Resveratrol (RV) is associated with protection against oxidative stress to improve health, however the effect of RV in layers under oxidative stress (OS) is limited. The objective of this experiment was to investigate the negative effect of OS and protective effects of RV against OS in laying hens. 40 Lohmann layers (25-wk-old; BW = 1.44±0.10 kg) were allocated to four treatments in a 2 × 2 factorial arrangement with either RV (0 or 600 mg/kg) or intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW) for 31 days. The results shown that the hens challenged with tBHP presented lower egg-laying rate, feed intake, feed efficiency and higher defective egg rate (P(tBHP)<0.05). The RV were also observed to attenuated egg laying rate and feed intake reduction together with decreased broken egg rate under t-BHP challenge (P(Interaction)≤0.01). The tBHP challenged layer demonstrated lower intestinal morphology (villus height in duodenum and jejunum), lower antioxidant enzymes activities [total superoxidase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC)], and glutathione (GSH) levels and higher malondialdehyde (MDA) level] (P(tBHP)<0.05). Dietary RV increased jejunal SOD, GSH-Px and T-AOC activities, and reduced MDA concentration (P(RV) ≤0.05). Layers under tBHP challenge up-regulated mRNA expression of pro-inflammatory cytokine [interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)] and nuclear factor NF-κB (P(tBHP)<0.05) in jejunum. Dietary RV supplementation down-regulated mRNA gene expression of IL-1β, IL-6, TNF-α and NF-κB (P(RV) ≤0.05). Dietary RV up-regulated mRNA expression of jejunal barrier-related proteins (claudin-1, claudin-2, mucin-1, and occludin) and ovarian reproductive hormone receptor [steroidogenic acute regulatory protein (StAR), androgen receptor (AR), estrogen receptor 1 (ESR1), and activin a receptor type 1 (ACVR1)] (P(RV) ≤0.05). Overall, the results indicate that tBHP induced oxidative stress to result in reducing production performance, intestinal health and induced ovarian inflammation; whereas dietary RV was able to maintain intestinal health and mitigate the negative impact of tBHP challenge on production performance and ovarian function.
Key words: resveratrol, laying hens, egg quality, antioxidant capacity, ovary function
INTRODUCTION
Oxidative stress is a phenomenon caused by an imbalance between production and accumulation of oxygen reactive species (ROS) in cells and tissues. Oxidative stress has been proven to be linked to internal mechanism for many environmental stressors (i.e., UV, ionizing radiations, pollutants, and heat stress), xenobiotics (mycotoxins, heavy metals, pesticides, etc.), health disorders and aging (Pizzino et al., 2017; Liguori et al., 2018; Wang et al., 2019, 2021; Liu et al., 2021a,b, Wang et al., 2021). Moreover, it has been proved that oxidative stress plays a role in follicular atresia to lead to ovarian aging and reproductive disorders (Gupta et al., 2006; Devine et al., 2012; Shen et al., 2012). Serval previous studies has shown that oxidative stress could led to reduction in production performance and intestinal health (Mishra and Jha, 2019; Wang et al., 2019, 2021; Li et al., 2020, Wang et al., 2021).
Resveratrol (trans-3, 5, 40-trihydroxytilbene; RV) is a naturally polyphenol compound found in berries, nuts, skins of grapes and thus in red wine (Jang et al., 1997). RV has antioxidant, antitumor, antiviral, and free radical scavenging properties, that is thought to be an effective compound for the prevention aging and age-related diseases (Li et al., 2018; Nunes et al., 2018; Zhou et al., 2021, Wang et al., 2021). Also, RV is associated with protection against oxidative stress of ovary to increase follicle numbers and prolong the ovary life-extending effect in human and rat model (Liu et al., 2013; Li and Liu, 2018; Ochiai and Kuroda, 2019). Literature has shown that RV improved production performance, egg quality (extend egg shelf life, sensory score, egg quality) and antioxidant capacities of laying hens (Feng et al., 2017; Zhang et al., 2019). However, the response of layers to oxidative stress and protecting effect of RV on production performance, intestinal and ovarian function is not clear.
Therefore, the purpose of the current study was to verify the hypothesis that dietary resveratrol supplementation could attenuate the production performance, egg quality, intestinal health, and ovarian function of laying hens under oxidative stress.
MATERIALS AND METHODS
The experiment was carried out according to the Chinese guidelines for animal Welfare and approved by the Animal Care and Use Committee of Sichuan Agricultural University.
Birds, Experiment Design, and Management
A total of 40 Lohmann gray hens (25 wk of age; initial BW = 1.44 ± 0.10 kg) with similar performance, were randomly assigned to four treatments with 10 replicates of 1 hen. All laying hens were randomly allotted into a 2 by 2 factorial design that were dietary supplementation either resveratrol (0 or 600 mg/kg) or intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW) for 28 d (The dosage of tBHP were set according to the previous study; Wang et al., 2021). Resveratrol (≥99%) and tBHP (≥99%) were purchased from Sigma-Aldrich Corp (St Louis, MO). All diets were formulated to meet nutrient specifications according to the NRC (1994) recommendations for all nutrients in a mash form. Layers were allowed ad libitum access to water and feed. Hens were housed individually in a cage equipped with 2 nipple drinkers and 1 feeder in a temperature-controlled room (maintained at 20–22°C) on a 16L:8D photo-period throughout the duration of study.
Productive Performance and Sample Collection
Egg production, egg weights, feed consumption, and broken eggs (including those with double-yolk, soft-shell, cracked, and broken egg) of were recorded daily. Feed conversion ratio (FCR) was calculated as the ratio of grams of total feed intake to grams of total egg weight. Forty-eight hours post the last tBHP injection (d 28), blood samples were collected from the wing vein (1 layer/replicate, 10 replicates/treatment). Blood samples were then centrifuged at 3,000 × g for 15 min, and then serum was stored at −20°C till analysis. Thereafter, layers were sacrificed by CO2 suffocation, the intestinal (duodenum and jejunum) and ovarian tissues (ovary cortex) were taken and then stored at −80°C till gene expression analysis.
Egg Quality Characteristics
To determine the egg quality indices, egg samples (2 eggs/replicate, 20 eggs/treatment) were collected in 3 continuous days at the end of the supplementation period. The eggshell thickness was measured using a Peacock dial gauge (P-1 Model, Meg Co Ltd., Ozaki, Japan) after removing the shell membrane and it is represented as the average thickness of the upper, middle, and lower end of the shell. Eggshell strength was measured by eggshell force gauge model II (Robotmation Co., Ltd., Tokyo, Japan). Egg internal quality (including Haugh unit [HU], albumen height, and yolk color) were analyzed via an Egg Multi-tester (EMT-7300, Robotmation Co., Ltd.). Eggshell was separated from the albumen and yolk, washed to remove residual albumen, and dried at 65°C for 4 h, then weighed. Albumen, yolk, or eggshell ratio was calculated as 100 × [albumen weight, yolk or eggshell weight (g)/egg weight (g)].
Morphology of Intestinal Mucosa
Duodenum and jejunum mucosa (1 layer/replicate, 10 replicates/treatment) morphology were analyzed as described previously (Wang et al., 2021). Briefly, the intestinal segments were fixed in 4% paraformaldehyde, then embedded in paraffin, and stained with hematoxylineosin. Villus height and crypt depth were measured in 40 × magnification with a digital camera microscope (BA400Digitl, McAudi Industrial, Group Co., Ltd, Chengdu, China). A total of 10 intact villi and crypts were randomly selected in each sample. Then, the data included villus height, crypt depth and their ratio (V/C) was calculated.
Serum Reproductive Hormones Assay
Serum concentration of estradiol, follicle stimulating hormone (FSH), anti-Müllerian hormone (AMH), and leptin were assessed by Radioimmunoassay Assay kits (RIA; IBL International, Munich, Germany) according to the manufacturer's instructions.
Antioxidant Enzyme Activity of Jejunum
Jejunum tissue samples were homogenized to analyze the antioxidant capacity. Commercial kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) were used to analyze the antioxidant capacity, including activities of superoxide dismutase (SOD), glutathione (GST), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and the content of glutathione (GSH) and malondialdehyde (MDA) according to the manufacturer's instructions.
Real-Time PCR for Jejunum Intestinal Barrier, Inflammatory Cytokine, and Ovarian Function Related mRNA Expression
Total RNA for jejunum mucosa and ovary tissue (1 layer/replicate, 10 replicates/treatment) was prepared using TRIzol reagent (TaKaRa, Dalian, China) and assessed using nucleic acid concentration analyzer NanoDrop 2,000 (Thermo Fisher, Waltham, MA). Complementary DNA (cDNA) was synthesized was synthesized using PrimeScript RT reagent Kit with gDNA Erase (RR047 A, Takara) following the manufacturer's recommended protocol. ABI Prism 7000 Real-time Detection System using SYBR Premix Ex Taq II kit (TaKaRa) was used to conduct the quantitative real-time PCR. The primer sequence for all the genes (interleukin-1β [IL-1β], interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α], zonula occluden-1 [ZO-1], steroidogenic acute regulatory protein [StAR], androgen receptor [AR], estrogen receptor 1 [ESR1], and activin a receptor type 1 [ACVR1]) are presented inTable 2. The house keeping gene (β–actin) was chosen in order to correct for variance in amount of RNA input in the reaction. The relative expression of each gene was calculated by using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Table 1.
Composition and nutrient level of basal diet (as-fed basis).
| Item, % | Amount |
|---|---|
| Corn | 55.90 |
| Soybean meal, 43% | 25.35 |
| Wheat bran | 5.00 |
| Soybean oil | 3.00 |
| Calcium carbonate | 8.15 |
| Calcium hydrophosphate | 1.30 |
| L-Lysine hydrochloride | 0.13 |
| DL-Methionine | 0.20 |
| Threonine | 0.09 |
| NaCl | 0.30 |
| Choline chloride, 50% | 0.10 |
| Vitamin and mineral premix1 | 0.48 |
| Total | 100.00 |
| Analyzed nutrient levels, % | |
| ME2, kcal/kg | 2690.00 |
| Crude protein | 16.54 |
| Calcium | 3.53 |
| Available phosphorus | 0.32 |
| Lysine | 0.85 |
| Methionine | 0.42 |
| Methionine + cysteine | 0.61 |
Provided per kilogram of diet: vitamin A, 10,000 IU; vitamin D3, 3,000 IU; vitamin E, 30 IU; vitamin K3, 4.8 mg; thiamin, 3.0 mg; riboflavin, 9.6 mg; pyridoxine, 6 mg; vitamin B12, 0.3 mg; folic acid, 1.5 mg; niacin, 60 mg; pantothenic acid, 18 mg; biotin,0.6 mg; iron, 60 mg; copper, 8 mg; manganese, 60 mg; zinc, 80 mg; selenium, 0.30 mg; iodine, 0.35 mg.
Calculated according to NRC (1994).
Table 2.
Related gene and primer information.1
| Genes1 | Orientation | Primer sequences (5′-3′) | Product size | Accession number |
|---|---|---|---|---|
| β-actin | Forward | GCTACAGCTTCACCACCACA | 90 | NM_205518.1 |
| Reverse | TCTCCTGCTCGAAATCCAGT | |||
| IL-1β | Forward | ACTGGGCATCAAGGGCTA | 117 | NM_205518.9 |
| Reverse | GGTAGAAGATGAAGCGGGTC | |||
| IL-6 | Forward | CAGGACGAGATGTGCAAGAA | 98 | NM_205518.10 |
| Reverse | GAGAGCTTCGTCAGGCATTT | |||
| TNF-α | Forward | AGATGGGAAGGGAATGAACC | 139 | XM_040647309.1 |
| Reverse | GGAAGGGCAACTCATCTGAA | |||
| NF-κB | Forward | GCTGCTTTGCACAGATGGA | 130 | NM_205134.1 |
| Reverse | CCTTTGCAAAACTGGTTGGT | |||
| Claudin-1 | Forward | GTCTTTGGTGGCGTGATCTT | 117 | NM_001013611.2 |
| Reverse | TCTGGTGTTAACGGGGTGTGA | |||
| Claudin-2 | Forward | CTTTGCTTCATCCCACTGGT | 82 | NM_001277622.1 |
| Reverse | TCAAATTTGGTGCTGTCAGG | |||
| Mucin-1 | Forward | CCGTGGTGAAGAGGATTTGT | 126 | XM_015279045.2 |
| Reverse | TTGGATGCAGTCACACCATT | |||
| Mucin-2 | Forward | ACCAAGCAGAAAAGCTGGAA | 80 | NM_001318434.1 |
| Reverse | AAATGGGCCCTCTGAGTTTT | |||
| ZO-1 | Forward | GGCAAGTTGAAGATGGTGGT | 130 | XM_01527898.2 |
| Reverse | ATGCCAGCGACTGAATTTCT | |||
| StAR | Forward | AGAGGAAGCCCTGCAGAAAT | 92 | NM_204686.2 |
| Reverse | TCAGCACTTTGTCTCCGTTG | |||
| AR | Forward | CCTGAATGAACTTGGGGAGA | 93 | NM_001040090.1 |
| Reverse | ATCTGGTCATCCACATGCAA | |||
| ESR1 | Forward | TTCAAGGGGAGGAATTTGTG | 123 | NM_205138.2 |
| Reverse | TGTCCAGAACACGGTGGATA | |||
| ACVR1 | Forward | ATTCCCAGAGCACAAACCAG | 93 | NM_001110204.1 |
| Reverse | GGATGGTTTCATCGAGCACT |
Abbreviations: ACVR1, activin a receptor type 1; AR, androgen receptor; ESR1, estrogen receptor 1; IL-1β, interleukin-1β; IL-6, interleukin-6; StAR, steroidogenic acute regulatory protein; TNF-α, tumor necrosis factor-α; ZO-1, zonula occluden-1.
Statistical Analysis
Data were analyzed two-way analysis of variance (ANOVA) using GLM procedure of SAS 9.2 (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Inc., La Jolla, CA). The model included the main effects of tBHP injection and addition of resveratrol, as well as their interaction. The results are presented as mean ± SEM. Contrasts between treatments were evaluated by Tukey's range test at a significance level of 0.05.
RESULTS
Production Performance
The hens challenged with tBHP presented lower egg-laying rate, feed intake, feed efficiency, and higher broken egg rate compared no tBHP challenge group (Table 3; P(tBHP) < 0.05). Dietary supplementation with RV significantly increased egg laying rate and decreased broken egg rate of layers irrespective of tBHP challenge (P(RV) = 0.01). The RV were also observed to attenuated reduction in egg laying rate and feed intake, and decreased broken egg rate under t-BHP challenge (P(Interaction) ≤ 0.01). No difference in average egg weight was noted among treatments (P > 0.05).
Table 3.
Effect of resveratrol on production performance of laying hens under oxidative stress
| Item1 | Laying rate, % | Egg weight, g | ADFI, g | FCR | Defective egg rate, % | |
|---|---|---|---|---|---|---|
| tBHP | RV | |||||
| - | - | 96.20b | 55.85 | 107.43 | 1.91b | 1.21b |
| - | + | 98.45a | 55.32 | 105.74 | 1.92b | 0.89b |
| + | - | 69.73d | 53.71 | 94.69 | 2.55a | 4.78a |
| + | + | 87.45c | 55.01 | 104.26 | 2.15b | 1.47b |
| SEM | 1.21 | 0.94 | 2.51 | 0.03 | 0.11 | |
| P-value | <0.01 | 0.19 | <0.01 | 0.02 | 0.01 | |
| P-value of main factor | ||||||
| tBHP | <0.01 | 0.41 | <0.01 | 0.02 | <0.01 | |
| RV | 0.01 | 0.29 | 0.11 | 0.51 | 0.01 | |
| tBHP × RV | <0.01 | 0.89 | 0.02 | 0.16 | 0.01 | |
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 1 layer/replicate, 10 replicates/treatment. Abbreviations: ADFI, average daily feed intake; FCR, feed conversation ratio; RV, 600 mg/kg resveratrol; tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW).
Egg Quality
Eggshell color (higher lightness, lower redness, and yellowness values), strength, and thickness were lower in tBHP challenge (Table 4; P(tBHP) < 0.05). A decease in the albumen height, Haugh unit, albumen relative weight, and yolk color were observed in the tBHP challenge group compared to the no tBHP challenge group (P(tBHP) < 0.05). The effect of RV was found to enhanced the eggshell quality (color, shell thickness, and strength), albumen height, and Haugh unit compared to no RV addition irrespective of tBHP challenge (P(RV) < 0.05). The RV were also observed to increase eggshell color (lower lightness, higher redness and yellowness values), yolk color, and albumen relative weight under tBHP challenge (P(Interaction) < 0.05).
Table 4.
Effect of resveratrol on egg quality of laying hens under oxidative stress.
| Item1 | Eggshell color |
Eggshell strength, kg/cm3 | Eggshell thickness, mm−2 | Albumen height, mm | Haugh unit | York color | Albumen relative weight, % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||
| tBHP | RV | |||||||||
| - | - | 73.81b | 7.71b | 22.24b | 4.23 | 0.404 | 7.94b | 91.27b | 6.31a | 59.76a |
| - | + | 73.65b | 9.05a | 24.37a | 4.84 | 0.421 | 8.32a | 95.05a | 6.19a | 58.47a |
| + | - | 75.65a | 6.56c | 16.65c | 2.73 | 0.359 | 6.59c | 79.11c | 5.53b | 54.86b |
| + | + | 72.04b | 9.12a | 23.52a | 4.56 | 0.41 | 7.92b | 91.32b | 6.44a | 56.71a |
| SEM | 0.75 | 0.35 | 1.16 | 0.32 | 0.02 | 0.50 | 2.45 | 0.23 | 0.78 | |
| P-value | <0.01 | 0.02 | 0.04 | 0.01 | 0.01 | 0.02 | 0.04 | 0.41 | 0.01 | |
| P-value of main factor | ||||||||||
| tBHP | 0.02 | 0.03 | <0.01 | 0.03 | 0.01 | 0.03 | 0.01 | 0.05 | 0.01 | |
| RV | 0.34 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.01 | 0.57 | 0.39 | |
| tBHP × RV | 0.02 | 0.05 | 0.01 | 0.14 | 0.23 | 0.11 | 0.14 | <0.01 | 0.01 | |
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 1 layer/replicate, 10 replicates/treatment. Abbreviations: RV, 600 mg/kg resveratrol; tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW). L* = lightness; a* = redness; b* = yellowness.
Serum Reproductive Hormone
A decrease in the serum concentration of estradiol, FSH, AMH, and leptin were observed in the tBHP challenged group compared to the control one (Table 5; P(tBHP) < 0.05). Feeding RV were found to increase FSH levels irrespective of tBHP challenge (P(RV) < 0.05), while the increase in serum estradiol, AMH and leptin concentration were more pronounced when layers received RV supplemented diet under tBHP challenge (P(Interaction) < 0.05).
Table 5.
Effect of resveratrol on serum hormone of laying hens under oxidative stress.
| Item1 | Estradiol, ng/L | FSH, mmol/mL | AMH, ng/L | Leptin, ng/mL | |
|---|---|---|---|---|---|
| tBHP | RV | ||||
| - | - | 1317.56a | 65.08a | 9.30b | 10.06 |
| - | + | 1452.05a | 67.42a | 9.81a | 9.26 |
| + | - | 950.86b | 43.89b | 7.97c | 9.03 |
| + | + | 1411.15a | 57.07b | 10.01a | 9.30 |
| SEM | 187.27 | 3.85 | 0.22 | 0.22 | |
| P-value | <0.01 | 0.01 | 0.03 | 0.69 | |
| P-value of main factor | |||||
| tBHP | 0.02 | <0.01 | <0.01 | <0.01 | |
| RV | 0.23 | 0.01 | <0.01 | <0.01 | |
| tBHP × RV | <0.01 | 0.24 | <0.01 | <0.01 | |
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 1 layer/replicate, 10 replicates/treatment. Abbreviations: tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW); RV, 600 mg/kg resveratrol; FSH, follicle-stimulating hormone; AMH, anti-Müllerian hormone.
Jejunal Morphology
No effect of tBHP challenge and RV supplementation were observed on crypt depth measured in this study (Table 6; P > 0.05); However, the tBHP challenged layer demonstrated decreased villus height in duodenum and jejunum compared to layers in the control group (P(tBHP) < 0.05). The duodenum villus height was enhanced and jejunum V/C ratio was reduced by dietary supplementation with RV irrespective of tBHP challenge (P(RV) < 0.01). The increase in villus height and V/C ratio was more pronounced when layers received RV supplemented diet under tBHP challenge (P(Interaction) < 0.01).
Table 6.
Effect of resveratrol on egg quality of laying hens under oxidative stress
| Item1 | Duodenum |
Jejunum |
|||||
|---|---|---|---|---|---|---|---|
| Villus height | Crypt depth | V/C | Villus height | Crypt depth | V/C | ||
| tBHP | RV | ||||||
| - | - | 1175.24b | 137.14 | 9.09a | 817.65a | 106.36 | 8.30a |
| - | + | 1103.18b | 126.29 | 7.09b | 790.29a | 95.22 | 7.21b |
| + | - | 897.69c | 120.78 | 7.73b | 726.74b | 99.58 | 7.83a |
| + | + | 1325.79a | 121.19 | 9.29a | 728.22b | 97.41 | 6.99b |
| SEM | 39.84 | 7.89 | 0.34 | 25.10 | 5.88 | 0.33 | |
| P-value | <0.01 | 0.10 | <0.01 | <0.01 | 0.22 | <0.01 | |
| P-value of main factor | |||||||
| tBHP | 0.04 | 0.32 | 0.07 | <0.01 | 0.61 | 0.09 | |
| RV | <0.01 | 0.34 | 0.35 | 0.41 | 0.06 | <0.01 | |
| tBHP × RV | <0.01 | 0.29 | <0.01 | 0.38 | 0.25 | 0.52 | |
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 1 layer/replicate, 10 replicates/treatment. Abbreviations : RV, 600 mg/kg resveratrol; tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW); V/C, villus height to crypt depth ratio.
Jejunal Antioxidant Capacities
The activities of antioxidant enzymes (SOD, GSH-Px, T-AOC) and GSH levels in the jejunum were lower, while MDA level was higher in layers under tBHP challenge (Table 7; P(tBHP) < 0.05). Dietary RV treatment increased SOD, GSH-Px, and T-AOC activities, and reduced the concentration of MDA (P(RV) ≤ 0.05). The GST activity was not affected by either tBHP challenge or dietary RV supplementation (P > 0.05).
Table 7.
Effect of resveratrol on antioxidant capacity in jejunum of laying hens under oxidative stress.
| Item1 | SOD, U/mg prot | GSH, μmol/g prot | GSH-Px, U/mg prot | GST, U/mg prot | T-AOC, nmol/mg prot | MDA, nmol/mg | |
|---|---|---|---|---|---|---|---|
| tBHP | RV | ||||||
| - | - | 110.12 | 98.36 | 210.11 | 88.79 | 2.14 | 4.11 |
| - | + | 134.28 | 107.43 | 244.26 | 92.41 | 2.44 | 3.21 |
| + | - | 78.19 | 34.33 | 107.14 | 78.77 | 1.14 | 7.24 |
| + | + | 124.79 | 88.18 | 200.91 | 77.24 | 2.69 | 4.01 |
| SEM | 12.24 | 14.24 | 21.41 | 13.79 | <0.01 | 0.47 | |
| P-value | <0.01 | 0.04 | 0.01 | 0.48 | 0.01 | ||
| P-value of main factor | |||||||
| tBHP | <0.01 | 0.01 | 0.02 | 0.14 | 0.01 | 0.02 | |
| RV | 0.04 | 0.01 | 0.05 | 0.27 | 0.04 | 0.03 | |
| tBHP × RV | 0.21 | 0.34 | 0.47 | 0.39 | 0.45 | 0.47 | |
Abbreviations: AR, average egg laying rate; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; MDA, malondialdehyde; RV, 600 mg/kg resveratrol; SOD, total superoxide dismutase; T-AOC, total antioxidant capacity; tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW).
Each mean represents 1 layer/replicate, 10 replicates/treatment.
Jejunal Inflammatory Cytokine and Barrier Integrity Related Gene Expression
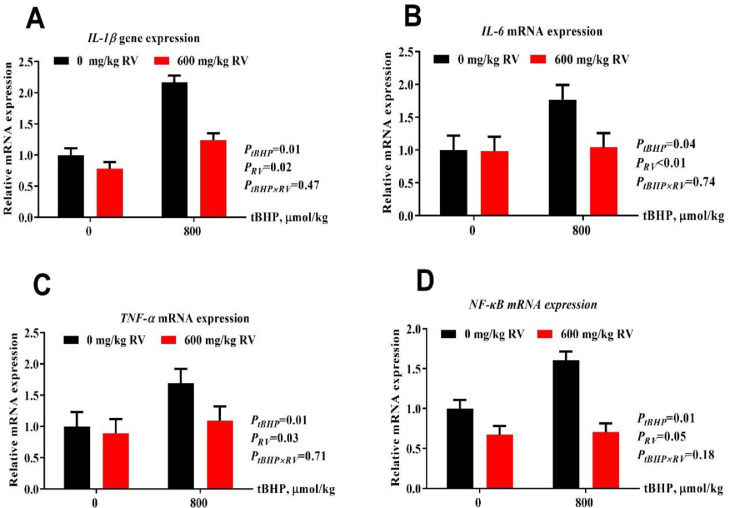
Layers under tBHP challenge up-regulated mRNA expression of proinflammatory cytokine (including IL-1β, IL-6, and TNF-α) and its upstreaming nuclear factor NF-κB (Figure 1; P(tBHP) < 0.05). Dietary RV supplementation down-regulated mRNA gene expression of IL-1β, IL-6, TNF-α, and NF-κB (P(RV) ≤ 0.05).
Figure 1.
Effect of dietary resveratrol supplementation on inflammatory cytokine mRNA gene expression in jejunum. (A) IL-1β, (B) IL-6, (C) TNF-α, and (D) NF-κB mRNA expression. Abbreviations: IL-1β, interleukin-1 β; IL-6, interleukin-1; NF-κB, nuclear factor-kappa B; RV, 600 mg/kg resveratrol; tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW); TNF-α, tumor necrosis factor alpha.
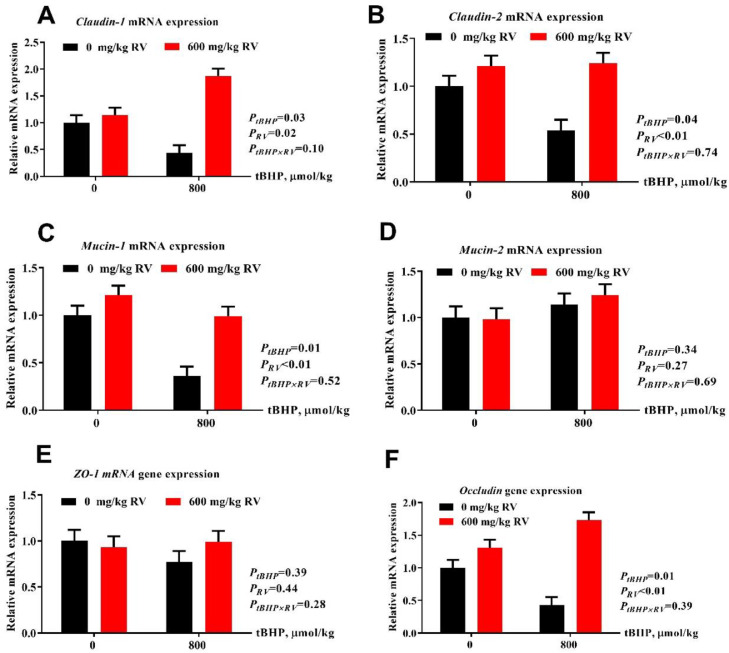
Moreover, compared with the layers receive no challenge, layers under t-BHP challenge had lower mRNA expression of barrier-related proteins (claudin-1, claudin-2, Mucin-1, and Occludin; Figure 2; P(tBHP) < 0.05) in jejunum mucosa. As expected, dietary RV upregulated mRNA expression of intestinal barrier related protein (claudin-1, claudin-2, Mucin-1, and Occludin) (P(RV) ≤0.05). Mucin-2 and ZO-1 were not affected by either tBHP or RV addition (P > 0.05).
Figure 2.
Effect of dietary resveratrol supplementation on intestinal barrier-related mRNA gene expression in jejunum. (A) Claudin-1 (B) Claudin-2, (C) Mucin-1, (D) Mucin-2, (E) ZO-1, and (F) Occludin mRNA expression. Abbreviations: RV, 600 mg/kg resveratrol, tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW); ZO-1, zonula occluden-1.
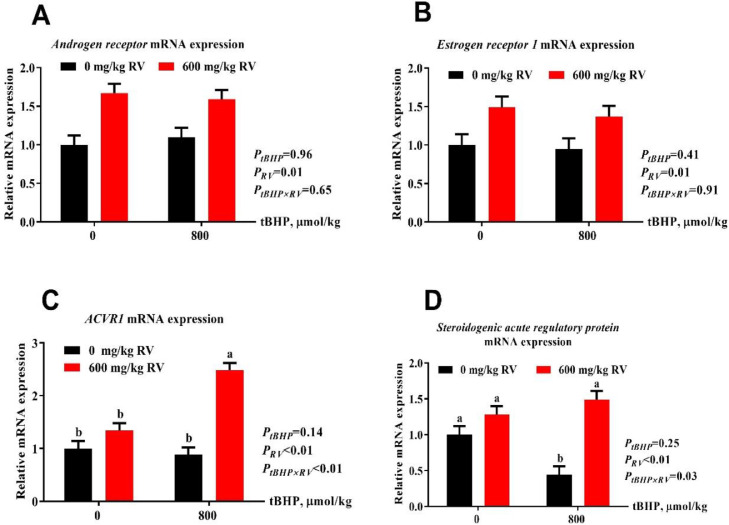
Ovarian Reproductive Receptor Gene Expression
As shown in Figure 3, dietary supplementation with RV increased reproductive receptor mRNA expression, including AR, ESR1, ACVR1 and StAR compared with no addition (P(RV) ≤ 0.01). No effects of tBHP were found on reproductive receptor gene expression (P(tBHP) > 0.05).
Figure 3.
Effect of dietary resveratrol supplementation on ovarian reproduction related hormone receptor mRNA gene expression. (A) Androgen receptor, (B) Estrogen receptor 1, (C) Activin a receptor type 1, and (D) Steroidogenic acute regulatory protein mRNA expression. Abbreviations: ACVR1, activin a receptor type 1; RV, 600 mg/kg resveratrol, tBHP, intraperitoneal injection of tert-butyl hydroperoxide (tBHP) (0 or 800 μmol/kg BW).
DISCUSSION
Oxidative stress induces apoptosis and affects cellular homeostasis, which was demonstrated to decrease ovarian function by inducing follicle atresia (Shen et al., 2017; Cao et al., 2018). In this study, we confirmed that the oxidative stress induced by injection of tBHP significantly reduced egg production rate and feed intake of laying hens; moreover, the administration of RV diet improved the egg production performance of laying hens in present study. Oxidative stress resulted from environmental toxicant and other stressors were reported to damage the health and production performance of layers (Devine et al., 2012; Wang et al., 2020b; Abbas et al., 2020). t-BHP known to cause peroxidation of membrane lipids and deplete cellular GSH and is commonly used as a model substance for evaluation of mechanisms of cellular alterations resulting from oxidative stress in cells and tissues (Kučera et al., 2014). The tBHP were found to successfully induce oxidative damage and decreased laying performance in our previous study (Wang et al., 2021). In accordance with our current study, oxidative stress induced by high levels of molybdenum and vanadium were also led to egg production reduction and the addition of antioxidants (tea polyphenols) was able to reverse this effect by improving the antioxidant capacity and intestinal health in layers (Yuan et al., 2016; Wang et al., 2018, 2021). Studies in mammals have found that oxidative stress can reduce the number of follicles in each stage of the ovarian cycle and impair ovarian function (Miyamoto et al., 2010; Abou-Seif and Youssef, 2001; Brannian and Hansen, 2002). Additionally, previous studies have also reported that oxidative stress decreases hormone secretion (including lower estradiol, FSH, LH, and IGF-1) and impairs the glutathione redox cycle (Miyamoto et al., 2010; Yu et al., 2019). Leptin were reported to exert an important role in the regulation of ovarian folliculogenesis indirectly by controlling of luteinizing hormone and FSH secretion (Brannian and Hansen, 2002). Similarly, the OS decreased estradiol, FSH, AMH, and leptin levels in the current study, which explain the reduction in egg laying rate. However, the literature about oxidative stress on the ovary function of poultry is not well elucidated, which needs to be explored in future studies. Plant polyphenols were chosen in laying hens’ diet to increase production performance recently due to its antioxidative, antiaging, reducing antiinflammation, and antimicrobial properties. Zhang et al. (2019) found that 200 mg/kg RV supplementation improved feed intake, egg laying rate and feed efficiency of layers. Liu et al. (2014) also reported that RV improves growth performance and reduces oxidative stress in heat-stressed blacked-boned chickens (Liu et al., 2014). This may because the RV can increase the health status of laying hens by improving the health status of intestine, ovary and oviduct (enhance antioxidant capacities, decreasing inflammation; Reis et al., 2019).
In the current study, tBHP challenge was found to result in reduction in eggshell quality (including pale shell color, lower thickness, and strength) and albumen quality. Moreover, the RV treatment resulted in a higher shell color in OS-challenged birds, which is in agreement with previous observation where the decrease in oxidative stress-induced shell color was reversed by antioxidant supplementation studies (Wang et al., 2016; Yuan et al., 2016).The eggshell pigmentation has been widely used as a potential indicator for stress and disease condition in commercial laying hens (Samiullah et al., 2015). It is evident that stress stimuli, including environmental stressors (heat stress, crowding, fear, etc.) and dietary hazards (heavy metals, mycotoxins, toxicants, etc.) led to pale coloration eggshell eggs (Wang et al., 2016, 2018, Wang et al., 2020b; Yuan et al., 2016). Ma et al. (2021) also reported that dietary arsenic supplementation (40.67 mg/kg) would lead to oxidative stress to reduce egg laying rate, albumen height, Haugh unit, and eggshell strength of laying hens. The green tea polyphenol epigallocatechin-3-gallate were found to increase eggshell color by increasing morphology and antioxidant capacity of uterus in layers exposed to oxidative stress (induced by 5 or 10 mg/kg vanadium) in previous studies (Yuan et al., 2016; Wang et al., 2018). Resveratrol also can affect calcium metabolism (Sahin et al., 2010). Liu et al. (2005) showed that resveratrol (0.7 mg/kg) supplementation increased bone mineral density and inhibited femur calcium loss associated with estrogen deficiency in ovariectomized rats. Zhang et al. (2019) found that 400 mg/kg RV extent the egg shelf lives (increase albumen quality) during storage. Moreover, we found that egg yolk color was decreased by tBHP challenge and recovered by RV supplementation. Egg yolk color is produced by oxycarotenoids, commonly known as xanthophyll pigments, derived from the hen's feed. It has been reported that the oxycarotenoids can be oxidized by agents such as peroxides and trace minerals. Previous studies have suggested that antioxidants, such as vitamin E, quercetin, and selenium were found to increase the magnitude of the yolk color and antioxidant capacities of eggs (Surai, 2000; Amevor et al., 2021). However, Sahin et al. (2010) didn't observe any effect of RV (200 or 400 mg/kg) on feed intake, egg production, inner and outer egg quality indicators in quails. The discrepancy may rely on the difference poultry strains, physiology stage and the stress model they are exposed to.
The intestinal epithelium, composed of single layer of cells, sits at the interface between an organism and its luminal environment, and as such is prone to oxidative stress (Circu and Aw, 2012). Intestinal morphology determines the nutrient absorption capacity and villus height is generally recognized as a good indicator for intestinal health. An increase in villus height indicates an increased absorption surface and higher capability of absorption available nutrients, whereas deeper crypt depth indicates that the villi in the small intestinal mucosa are atrophied (Celi et al., 2019). ROS, such as hydrogen peroxide and nitric oxide, disrupt tight junctions (TJs), and elevate paracellular permeability in vitro. TJs (such as ZO-1, occludin, and claudin-1 and -4) regulate the paracellular transport of ions, molecules, and water, and it determined the integrity of epithelia cell and mucus gel layer (Moretó and Pérez-Bosque, 2009; Ulluwishewa et al., 2011). In this study, we found that OS led to downregulation of intestinal barrier associated protein (claudin-1, claudin-2, mucin-1, occludin). Also, OS induced inflammation (indicated by higher mRNA expression of pro-inflammatory cytokine, such as IL-1β, IL-6, and TNF-α) and its upstreaming nuclear factor NF-κB), decreased the villus height and antioxidant capacities of small intestine, as demonstrated by lower activities of antioxidant enzymes, SOD, GSH-Px, and T-AOC, and higher corresponding levels of MDA. Wang et al., 2020a also reported that layers exposed to high stocking density stress also led to deceases in villus height of small intestine. RV has been evident to restrain oxidative stress and alleviate inflammatory responses in a rat model of polycystic ovary syndrome (Sadi et al., 2015). It also plays several roles in antioxidative and anti-inflammatory pathways and ameliorates metabolic syndrome (Javkhedkar et al., 2015). Xing et al. (2019) and Wang et al. (2021) reported that RV (400 mg/kg) supplementation decreased oxidative stress in fatty liver hemorrhagic syndrome by enhancing antioxidant enzymes (Nrf2, SOD-1, and HO-1) and decreasing inflammatory cytokines (NF-κB, IL-1β, IL-6, and TNF-α) mRNA expression in ovary. Intestinal oxidative stress produces proinflammatory cytokines, which increase mucus layer permeability, leading to intestinal and systemic inflammation. Mucosal oxidative stress would result from the disruption of these unique redox control nodes, and subsequent contribute to the development of inflammation and other intestinal pathology. Yang et al. (2021) also reported that dietary supplementation with 400 mg/kg RV protect against oxidative damage and inflammation injury (induced by heat stress) indicated by improving tight junction protein (claudin 1, occludin) mRNA expression, antioxidant enzyme activities (SOD and CAT), and reducing the secretion of IL-1β in duck jejunum.
In the current study, we found that dietary supplementation with RV increased mRNA expression of AR, ESR1, ACVR1, and StAR. Androgen is an important regulator of prostate gland development and function, including proliferation, differentiation, maintenance (Chang et al., 1988). The androgen receptor (AR) is a crucial mediator of androgen action and a ligand-dependent transcription factor that belongs to the nuclear steroid hormone receptor super-family. Resveratrol has been reported to exert estrogenic effects, increasing uterine and ovarian wet weight (Henry and Witt, 2002; Wang et al., 2021). Han et al. (2021) reported that RV increased estrogen receptor 2 (ESR2) expression. This may also explain the increasing effect of RV on egg production performance. Also, Morita et al. (2012) found that resveratrol increased the expression of LH receptor and StAR mRNA expression in rat GCs, suggesting a possibility that resveratrol may promote steroidogenesis and luteinization (a process of terminal differentiation of granulosa cells) in the ovary of rat. StAR is known to govern the rate-limiting step in steroidogenesis, which is the translocation of cholesterol from the outer to the inner mitochondrial membrane (Kiriakidou et al., 1996; Devoto et al., 2002). However, other studies demonstrated that RV depressed the AR transcriptional activity in cancer cells and in ovarian follicle (Bowers et al., 2000; Ozatik et al., 2020). The discrepancies may due to the different animal type, stress model, and RV levels used the study. As the oxidative stress is the general mechanism for aging and hazardous toxicant in animals, further study is needed to elucidate the mechanism within the response of RV in oxidative stress.
CONCLUSIONS
These results indicated that tBHP (intraperitoneal injection of 800 μmol/kg BW of tBHP) induced oxidative stress in layers, which may lead to reduction in production performance, egg quality, intestinal health, and induced ovarian inflammation. Dietary supplementation with 600 mg/kg RV was able to maintain intestinal and ovarian function and mitigate the negative impact of tBHP challenge on production performance and egg quality.
Acknowledgments
Acknowledgments
We are grateful for the following projects, National Natural Science Foundation of China (Grant No. 31872792), the National Key Research and Development Program of China (Grant No. 2021YFD1300204) and Sichuan Provincial Science and Technology Projects (Grant No. 2022YFH0070) for financial support.
DISCLOSURES
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have been approved the manuscript that is enclosed.
References
- Abbas A.O., Alaqil A.A., El-Beltagi H.S., El-Atty H.K.A., Kamel N.N. Modulating laying hens productivity and immune performance in response to oxidative stress induced by E. Coli challenge using dietary propolis supplementation. Antioxidants (Basel) 2020;9:893. doi: 10.3390/antiox9090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Seif M.A., Youssef A.A. Oxidative stress and male IGF-1, gonadotropin and related hormones in diabetic patients. Clin. Chem. Lab. Med. 2001;39:618–623. doi: 10.1515/CCLM.2001.099. [DOI] [PubMed] [Google Scholar]
- Amevor F.K., Cui Z., Ning Z., Du X., Jin N., Shu G., Deng X., Zhu Q., Tian Y., Li D., Wang Y., Zhang Z., Zhao X. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. 2021;100 doi: 10.1016/j.psj.2021.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers J.L., Tyulmenkov W., Jernigan S.C., Klinge C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Brannian J.D., Hansen K.A. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin. Reprod. Med. 2002;20:103–112. doi: 10.1055/s-2002-32501. [DOI] [PubMed] [Google Scholar]
- Cao Y., Shen M., Jiang Y., Sun S.C., Liu H. Melatonin reduces oxidative damage in mouse granulosa cells via restraining JNK-dependent autophagy. Reproduction. 2018;155:307–319. doi: 10.1530/REP-18-0002. [DOI] [PubMed] [Google Scholar]
- Celi P., Verlhac V., PerezCalvo E., Schmeisser J., Kluenter A.M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed. Sci. Technol. 2019;250:9–31. [Google Scholar]
- Chang C.S., Kokontis J., Liao S.T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Circu M.L., Aw T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012;23:729–737. doi: 10.1016/j.semcdb.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto L., Kohen P., Vega M., Castro. R. R. Gonzalez O., Retamales I., Carvallo P., Christenson L.K., Strauss J.F. Control of human luteal steroidogenesis. Mol. Cell Endocrinol. 2002;186:137–141. doi: 10.1016/s0303-7207(01)00654-2. [DOI] [PubMed] [Google Scholar]
- Feng Z.H., Gong J.G., Zhao G.X., Lin X., Liu Y.C., Ma K.W. Effects of dietary supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Br. Poult. Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Miller K.P., Babus J.K., A J. Flaws methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006;93:382–392. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Han S., Cicek A.F., Tokmak A., Ustun T.Y., Gokay N.E., Uludag M.O., Demirel M.A. Effect of resveratrol on receptor expression and serum levels of estrogen and progesterone in the rat endometritis model. Reproduct. Sci. 2021;28:2610–2622. doi: 10.1007/s43032-021-00586-3. [DOI] [PubMed] [Google Scholar]
- Henry L.A., Witt D.M. Resveratrol: phytoestrogen effects on reproductive physiology and behavior in female rats. Horm. Behav. 2002;41:220–228. doi: 10.1006/hbeh.2001.1754. [DOI] [PubMed] [Google Scholar]
- Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Javkhedkar A.A., Quiroz Y., Rodriguez-Iturbe B., Vaziri N.D., Lokhandwala M.F., Banday A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R840–R846. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M., McAllister J.M., Sugawara T., Strauss J.F. Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J. Clin. Endocrinol. Metab. 1996;81:4122–4128. doi: 10.1210/jcem.81.11.8923870. [DOI] [PubMed] [Google Scholar]
- Kučera O., Endlicher R., Roušar T., Lotková T.H., Garnol T., Drahota Z., Cervinková Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell Longev. 2014;2014 doi: 10.1155/2014/752506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.M., Liu L.P., Yin B., Liu Y.Y., Dong W.W., Gong S., Zhang J., Tan J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems. Poult. Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 2018;44:1431–1438. doi: 10.1111/jog.13680. [DOI] [PubMed] [Google Scholar]
- Li Y.R., Li S., Lin C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cac-ciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.L., He J.H., Xie H.B., Yang Y.S., Li J.C., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu M., Yin Y., Ye X., Zeng M., Zhao Q., Keefe D.L., Liu L. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013;28:707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- Liu Z.P., Li W.X., Yu B., Huang J., Sun J., Huo J.S., Liu C.X. Effects of trans resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. J. Med. Food. 2005;8:14–19. doi: 10.1089/jmf.2005.8.14. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhu Y.R., Zhao Z.H., Jiang P., Yin F.Q. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity and immune response of duodenum in broilers under heat stress. Animals. 2021;11:2279. doi: 10.3390/ani11082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta C(T) method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Y., Shi Y., Wu Q., Ma W. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult. Sci. 2021;100:982–992. doi: 10.1016/j.psj.2020.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Sato F.E., Kasahara E., Jikumaru M., Hiramoto K., Tabata H., Katsuragi M., Odo S., Utsumi K., Indoue M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010;49:674–681. doi: 10.1016/j.freeradbiomed.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Moretó M., Pérez-Bosque A. Dietary plasma protein, the intestinal immune system, and the barrier functions of the intestinal mucosal. J. Anim. Sci. 2009;87:E92–E100. doi: 10.2527/jas.2008-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Wada-Hiraike O., Yano T., Shirane A., Hirano M., Hiraike H., Koyama S., Oishi H., Ylshino O., Miyamoto Y., Sone K., Oda K., Nakagawa S., Tsutsui K., Taketani Y. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: an implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012;10:14. doi: 10.1186/1477-7827-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC, Nutrient Requirements of Poultry, 1994, Nat. Acad. Press; Washington, D. C
- Nunes S., Danesi F., Rio D.D., Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr. Res. Rev. 2018;31:85–97. doi: 10.1017/S095442241700021X. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Kuroda K. Preconception resveratrol intake against infertility: friend or foe? Reprod. Med. Biol. 2019;19:107–113. doi: 10.1002/rmb2.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozatik F.Y., Ozatik O., Yigitaslan S., Kaygisiz B., Erol K. Do resveratrol and dehydroepiandrosterone increase diminished ovarian reserve? Eurasian J. Med. 2020;52:6–11. doi: 10.5152/eurasianjmed.2019.19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017;2017 doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J.H., Gebert R.R., Barreta M., Boiago M.M.M., Souza C.F., Baldissera M.D., Santos I.D., Wagner R., Laporta L.V., Stefani L.M., Da Silva A.S. Addition of grape pomace flour in the diet on laying hens in heat stress: impacts on health and performance as well as the fatty acid profile and total antioxidant capacity in the egg. J. Thermal Biol. 2019;80:141–149. doi: 10.1016/j.jtherbio.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Sadi G., Pektas M.B., Koca H.B., Tosun M., Koca T. Resveratrol improves hepatic insulin signaling and reduces the inflammatory response in streptozotocin-induced diabetes. Gene. 2015;270:213–230. doi: 10.1016/j.gene.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Sahin K., Akdemir F., Orhan C., Tuzcu M., Hayirli A., Sahin N. Effect of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 2010;89:1190–1198. doi: 10.3382/ps.2010-00635. [DOI] [PubMed] [Google Scholar]
- Samiullah S., Roberts J.R., Chousalkar K. Eggshell color in brown -egg laying hens-a review. Poult. Sci. 2015;94:2566–2575. doi: 10.3382/ps/pev202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Jiang Y., Guan Z., Cao Y., Li L., Liu H., Sun S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy. 2017;13:1364–1385. doi: 10.1080/15548627.2017.1327941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Lin F., Zhang J., Tang Y., Chen W.K., Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012;287:25727–25740. doi: 10.1074/jbc.M112.349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000;41:235–243. doi: 10.1080/713654909. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia R., Gong H., Celi P., Zhuo Y., Ding X., Bai S., Zen Q., Yin H., Xu S., Liu J., Mao X., Zhang K. The effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants. 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang Z.Q., Celi P., Yan L., Ding X.M., Bai S.P., Zeng Q.F., Mao X.B., Feng B., Xu S.Y., Zhang K.Y. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants (Basel) 2019;8:503. doi: 10.3390/antiox8100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yuan Z., Zhang K., Ding X., Bai S., Zeng Q., Peng H., Celi P. Epigallocatechin-3-gallate protected vanadium-induced eggshell depigmentation via P38-Nrf/HO-1 signaling pathway in laying hens. Poult. Sci. 2018;97:3109–3118. doi: 10.3382/ps/pey165. [DOI] [PubMed] [Google Scholar]
- Wang J.P., He K.R., Ding X.M., Luo Y.H., Bai S.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary vanadium and vitamin C on egg quality and antioxidant status in laying hens. J. Anim. Physiol. Anim. Nutr. 2016;100:440–447. doi: 10.1111/jpn.12377. [DOI] [PubMed] [Google Scholar]
- Wang J., Qiu L., Gong H., Celi P., Yan L., Ding X., Bai S., Zeng Q., Mao X., Xu S., Wu C., Zhang K. Effect of dietary 25-dydroxycholecalciferol supplementation and high stocking density on performance, egg quality, and tibia quality in laying hens. Poult. Sci. 2020;99:2608–2615. doi: 10.1016/j.psj.2019.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xing C., Yang F., Zhou S., Li G., Zhang C., Cao H., Hu G. Abnormal expression of liver autophagy and apoptosis-related mRNA in fatty liver haemorrhagic syndrome and improvement function of resveratrol in laying hens. Poult. Sci. 2020;49:171–178. doi: 10.1080/03079457.2019.1698712. [DOI] [PubMed] [Google Scholar]
- Xing C., Wang Y., Dai X., Fang F., Luo J., Liu P., Zhang C., Cao H., Hu G. The protective effects of resveratrol on antioxidant function and the mRNA expression of inflammatory cytokines in the ovaries of hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2019;99:1019–1027. doi: 10.1016/j.psj.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Luo P., Chen S.J., Deng Z.C., Fu X.L., Xu D.N., Tian Y.B., Huang Y.M. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Huang M., Wang Y., Rodeni S., Wei Q., Wang W., Mao D. Sodium arsenite injection induces ovarian oxidative stress and affects steroidogenesis in rats. Biol. Trace Elem. Res. 2019;189:186–193. doi: 10.1007/s12011-018-1467-y. [DOI] [PubMed] [Google Scholar]
- Yuan Z.H., Zhang K.Y., Ding X.M., Luo Y.H., Bai S.P., Zeng Q.F., Wang J.P. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult. Sci. 2016;95:1709–1717. doi: 10.3382/ps/pew097. [DOI] [PubMed] [Google Scholar]
- Zhang C., Kang X., Zhang T., Huang J. Positive effects of resveratrol on egg-laying ability, egg quality, and antioxidant activity in hens. J. Appl. Poult. Res. 2019;28:1099–1105. [Google Scholar]
- Zhou D.D., Luo M., Huang S.Y., Saimaiti A., Shang A., Gan R.Y., Li H.B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021;2021 doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]