Abstract
Objectives:
Myocardial ischemia is a lack of blood supply to myocardial tissue. Rapid reperfusion therapy is required to prevent myocardial infarction. However, ischemia and reperfusion contribute to myocardial and endothelial injury or ischemia-reperfusion injury (IRI). A pro-inflammatory cytokine interleukin-8 (IL-8/CXCL8) plays an important role in the activation of neutrophil accumulation and promotes endothelial dysfunction. Therefore, inhibition of IRI through the regulation of inflammation using a CXCL8 receptor inhibitor reparixin is an attractive target. The aim of this study is to evaluate the effect of reparixin on endothelial cell viability after IRI.
Methods:
Human vascular endothelial cells (EA.hy926) were cultured and pretreated with reparixin at concentrations of 0−1 mg/ml. To simulate ischemia, the cells were exposed to simulated ischemia solution for 60 min. Then, the cells were given complete medium as reperfusion followed by treatment with reparixin and incubated for 24 h. Cell viability was tested using MTT assay.
Results:
Percentages of cell viability of reparixin-treated groups of 0.0625 mg/mL (67.88 ± 7.82% control) and 0.125 mg/mL (84.28 ± 4.68% control) were significantly higher than that of the IR group (44.31 ± 4.64% control) at P < 0.05. The percentage of cell viability in the 0.125 mg/mL reparixin-treated group was not significantly different compared to the control.
Conclusion:
Pretreatment and treatment of endothelial cells with reparixin, which is a CXCL-8 receptor inhibitor, demonstrated a protective effect on cell viability after simulated ischemia-reperfusion. However, further studies to investigate the underlying mechanisms are needed.
Keywords: Endothelial cells, ischemia-reperfusion injury, reparixin
Introduction
Acute myocardial infarction (AMI) is a leading cause of death globally.[1] Restoration of myocardial blood flow is a critical treatment for myocardial protection.[2] There are several myocardial reperfusion techniques including percutaneous coronary intervention, antithrombotic therapy, and coronary artery bypass grafting that have been intensively developed for coronary blood flow restoration.[3] Unfortunately, myocardial reperfusion after the ischemic episode usually contributes to the detrimental consequences of acute myocardial ischemia-reperfusion injury (IRI).[4] Myocardial IRI induces coronary endothelial dysfunction that in turn promotes myocardial injury.[5] Compared to myocardial cells, coronary endothelial cells (ECs) are more susceptible to IRI.[6] Exaggerated inflammatory reactions following EC activation are closely associated with oxidative stress during ischemia.[7,8] Anti-inflammatory and antioxidant agents may have therapeutic potential for the treatment of endothelial and myocardial injury. Therefore, new targeted therapies for protecting early endothelial injury have been explored.
Reparixin (repertaxin) is a therapeutic inhibitor of CXC-chemokine receptor types 1 (CXCR1) and 2 (CXCR2) or an interleukin-8 (IL-8/CXCL8) receptor inhibitor. It has been shown to reduce polymorphonuclear (PMN) infiltrates and vascular permeability.[9] A recent study has reported that reparixin was able to mitigate the postoperative adverse outcomes of cardiac surgery such as hemodynamic instability.[10] Moreover, several reports have been shown that reparixin minimized the impact of IRI on graft rejection after organ transplantation including liver, kidney, pancreatic islet, and lung transplantation.[9,11-13] However, the role of reparixin in EC dysfunction in myocardial IRI is unknown. The investigation of potential therapeutic of reparixin against EC injury-related IRI by reducing leukocyte accumulation and vascular leakage is still required. There is currently limited data on the effects of reparixin and its mechanisms of action on the endothelium during IRI. Therefore, this study aims to examine the effect of reparixin on EC viability after simulated IR. This study will help better understand the roles of ECs in IRI and to extend the knowledge in clinically applicable strategies.
Materials and Methods
Chemical and reagents
Reparixin was purchased from APExBIO Technology LLC (Texas, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (Missouri, USA).
Cell culture
Human EC line EA.hy926 (ATCC® CRL-2922Ô) was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were incubated with 5% CO2 at 37°C and were passaged when 80% confluence was reached. The cells at density of 40,000 cells were plated in 96-well plates overnight before performing experiments.
Evaluation of reparixin on cell viability
To determine the toxicity of reparixin on ECs, the cells were treated with the medium supplemented with various concentrations of reparixin (0-1 μg/ml) for 24 h. MTT assay was used to analyze cell viability as previously described.[14] Briefly, MTT solution was added to the medium to achieve a final concentration of 0.5 mg/ml and incubated at 37°C for 2 h. After that, the supernatant was gently removed and dimethyl sulfoxide was used to solubilize the purple formazan crystals. The absorbance of the samples was measured using a microplate reader at a wavelength of 570 nm. Cells cultured in the medium were used as the control group.
Simulating ischemia-reperfusion injury
To simulate ischemia, the cells were incubated with a simulated ischemia solution containing 140 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5mM HEPES, 10 mM 2-deoxy-d-glucose, and 10 mM sodium dithionite.[15] To optimize the duration of simulated ischemia, the cells were exposed to the ischemic solution at 37°C in an atmosphere with 5% CO2 for different durations (0, 15, 30, 60, and 90 min). Cells cultured in complete medium were used as the control group.
To simulate IRI, after simulating ischemia, the ischemic solution was removed and replaced by fresh complete medium to simulate reperfusion. The cells were then incubated at 37°C in an atmosphere with 5% CO2 for 24 h and 48 h. Following completed simulating IR protocol, cell viability was performed using the MTT assay. After the test, a simulated ischemia duration of 60 min and reperfusion duration of 24 h was selected to use in subsequent experiments.
Evaluation of reparixin on cell viability after simulating ischemia-reperfusion
To determine the effect of reparixin, after 1 h of serum deprivation, the cells were pretreated with various concentrations of reparixin (0−1 μg/ml) for 60 min. Simulated ischemia was then induced by incubating the cells with a simulated ischemia solution at 37°C in an atmosphere with 5% CO2 for 60 min. After removal of the ischemic solution and washing with phosphate-buffered saline, the cells were given complete medium to simulate reperfusion and reparixin was added at the same concentration as the pretreatment; the cells were then incubated at 37°C in an atmosphere with 5% CO2 for 24 h. Cells cultured in complete medium were used as the control group. The MTT assay was then performed to determine cell viability.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). All comparisons were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The statistical tests were performed using GraphPad Prism version 5 software (GraphPad Software, Inc., La Jolla, CA, USA). A P-value less than 0.05 was considered statistically significant.
Results
Effect of reparixin on cell viability
To investigate the toxicity of reparixin, the EA.hy926 ECs were treated with various concentrations of reparixin (0−1 μg/ml) for 24 h before measuring the percentage cell viability using the MTT assay. Figure 1 shows the percentages of cell viability of the treatment and control groups. The result shows that there were no significant differences between the percentages of cell viability of these groups. Therefore, the reparixin concentrations for subsequent experiments were chosen.
Figure 1.
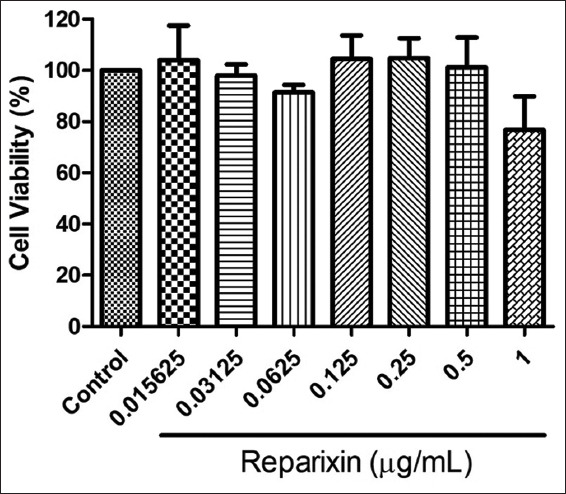
Determination of EA.hy926 cell viability after treatment with different concentrations of reparixin. The results are expressed as mean ± standard deviation. Three experiments with independent cell preparations were performed
Effect of simulated ischemia and reperfusion in different durations on cell viability
Figure 2 shows percentages of cell viability after simulated ischemia (0, 15, 30, 60, and 90 min) and simulated IR at 24 h and 48 h. After 15, 30, 60, and 90 min of simulated ischemia, the percentages of cell viability of the ischemia groups were significantly lower than that of the control group. According to simulated IR, after simulated ischemia and 24 h of replenishment with complete medium to simulate reperfusion, the percentages of cell viability of the IR groups were significantly lower than that of the control group. The percentages of cell viability of 60- and 90-min IR were significantly lower than those of the 15- and 30-min IR groups. For simulated ischemia and 48 h reperfusion, only 60- and 90-min IR were significantly lower than that of the control group and there were increases in the percentage of cell viability of all groups. Therefore, the optimal simulated ischemia duration of 60 min with reperfusion at 24 h was selected for subsequent experiments.
Figure 2.
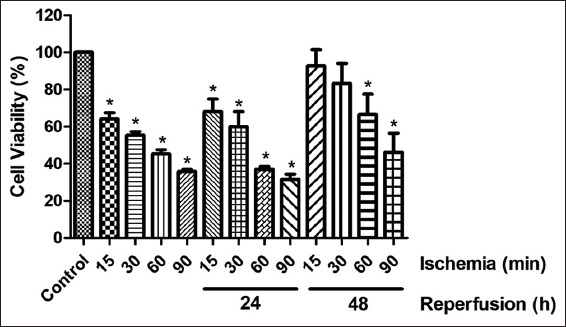
Determination of EA.hy926 cell viability after simulated ischemia of variable duration (15, 30, 60, or 90 min) and simulated ischemia with 24 and 48 h of reperfusion. The results are expressed as mean ± standard deviation. Three experiments with independent cell preparations were performed. *P < 0.05 versus control group
Effect of reparixin on cell viability after simulating ischemia reperfusion
Figure 3 shows percentages of cell viability of the EA.hy926 cells after simulating IR with various concentrations of reparixin (0−1 μg/ml). The percentages of cell viability in the 0.0625 and 0.125 μg/mL reparixin-treated groups were 67.88 ± 7.82 and 84.28 ± 4.68% of control, respectively. Comparing to the IR group, they were significantly higher than that of the IR group (44.31 ± 4.64% of control). The percentage of cell viability in the 0.125 μg/mL reparixin-treated group was the only group that was not significantly different compared to the control group.
Figure 3.
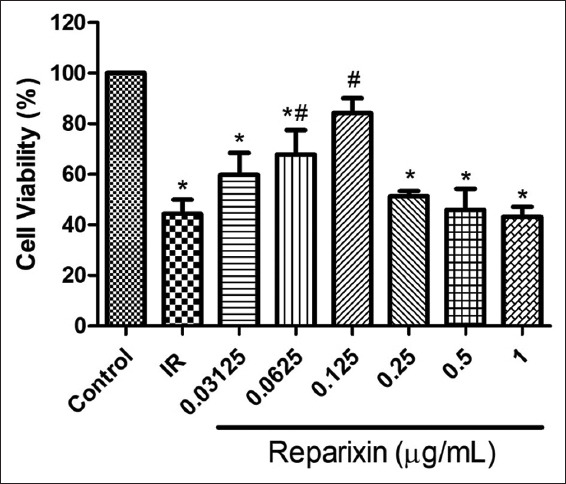
Effect of reparixin on EA.hy926 cell viability after simulated ischemic-reperfusion. The results are expressed as mean ± standard deviation. Three experiments with independent cell preparations were performed. *P < 0.05 vs. control group. # P < 0.05 versuss IR group. IR, ischemic-reperfusion
Discussion
Vascular endothelial dysfunction in myocardial IRI is critical in the management of myocardial damage. Therefore, attenuating the impairment of endothelial-myocardial interactions in IRI can preserve EC and myocyte viability. Oxidative stress and the inflammatory response are important in the pathological process of the acute endothelial and myocardial interactive response to IRI. A variety of CXC chemokines are involved in IRI. CXCL8 or IL-8 plays a role in neutrophil-induced myocardial IRI. Coronary EC dysfunction is a consequence of myocardial IRI. Several candidate therapeutic targets for myocardial and endothelial protection have been developed. CXCL8 receptor inhibitors are a therapeutic target for the prevention of reperfusion injury by targeting CXCL8 activity.
In the present study, the efficacy of reparixin on ECs following IRI was investigated. We assessed vascular EC viability using an in vitro model of simulated IRI. The results demonstrated no toxicity of reparixin to ECs in the micromolar range (0−1 μg). Consistently, there was a significant increase in the percentages of cell viability following simulated IRI of 0.0625 and 0.125 μg/mL reparixin-treated groups compared to that of the control group. The data indicated that 0.125 μg/ml of reparixin can minimize EC death following simulation of Ea.hy926 cells with intermittent hypoxia/reoxygenation. The percentage of cell viability did not differ between 0.125 μg/mL reparixin-treated groups and that of the control group. In addition, higher doses did not provide an additional increase in cell viability. The results are consistent with previous findings in both animal studies and clinical trials. Reparixin or repertaxin has been shown to mitigate tissue damage following transient brain ischemia in rats.[16] In addition, an inhibitory effect of repertaxin on inflammation following intestinal ischemia and reperfusion injury in rats has been found.[17] In the setting of patients undergoing on-pump coronary artery bypass graft surgery, intravenous reparixin was able to reduce the recruitment of neutrophils and hemodynamic alterations.[10]
Reparixin, a noncompetitive allosteric inhibitor of the chemokine receptors CXCR1/2, has attracted attention in targeted drug therapy for some types of cancer and multiple diseases associated with inflammation.[18,19] The CXC chemokine receptors CXCR1 and CXCR2, known as CXCL8 receptors, are both expressed on ECs.[18] The mechanism of CXCL8-CXCR1/2 signaling in IRI has been explored previously. CXCL8 is initially produced by endothelial and EA.hy926 cells in response to several stimulatory factors.[20,21] A previous study found that CXCL8-induced neutrophil activation was involved in acute myocardial IRI.[22] Under myocardial ischemia-reperfusion conditions, neutrophils migrate and accumulate in the coronary vascular endothelium, arterial wall, and around cardiomyocytes in the ischemic and reperfused myocardium. Neutrophils produce toxic agents such as free radicals and proteases and also promote the production and release of inflammatory factors.[23] de Oliveira et al. revealed that reparixin, as antineutrophil therapeutic strategy, not only reduced neutrophil influx into the liver but also suppressed the increase in serum concentrations of TNF-α, IL-6, and CCL3 in a mouse model of liver IRI.[11] Similarly, Bertini et al. found that repertaxin protected organs against reperfusion injury by inhibiting PMN infiltration.[24] Although the present study did not demonstrate the effect of CXCR1/2 inhibitor on reperfusion injury mediated by neutrophils, it is possible that reparixin is able to increase the viability of ECs through blocking CXCR1/2 using an in vitro model of IRI. It was previously demonstrated that increased expression of CXCR2 occurs in aortic ECs under hypoxic conditions.[25] In addition, blocking CXCR1/2 could inhibit the increase of intracellular free calcium during the reperfusion period.[26]
Although it has been reported that CXCL8-induced stimulation of neutrophil is associated with IRI in many cell types, our study was the first showing the role of CXCL8 receptor inhibitor in the mitigation of endothelial injury using the in vitro model of IRI. Our result may imply, at least in part, that endothelial IRI occurs through the receptor of CXCL8 on ECs directly. However, further investigation of the expression and roles of CXCL8-CXCR1/2 on ECs as well as the relative contribution of the ligand and their receptors to normal and pathological conditions is necessary. Exploring whether the CXCL8 plays a major role in chemotaxis of neutrophils into the ECs is also concerned. These findings will be fundamental for pharmacological development of selective CXCR1/2 blockers for the prevention of endothelial IRI in various situations, for example, in AMI reperfusion therapy.
Conclusion
The present study demonstrates that reparixin can protect ECs against IRI using the in vitro cell viability model.
Authors’ Declaration Statements
Ethics approval and consent to participate
Not applicable.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Competing interests
The authors declare that they have no conflicts of interest with the contents of this article.
Funding statement
This study was supported by the Naresuan University Research Grant under the grant number R2561B038.
Authors’ Contributions
Conceptualization: TN, PT; Methodology: TN, PT, DS; Validation: PT, TN; Formal analysis: PT, TT, DS, TN; Investigation: TT, TP, SM, PT, TN; Resources: TN, PT; Data curation: PT, TP, DS; Writing-Original Draft: TN, PT; Writing-Review & Editing: PT, TN, DS; Visualization: PT, TN; Supervision: TN; Project administration: TN; Funding acquisition: TN.
References
- 1.World Health Statistics 2021:Monitoring Health for the SDGs, Sustainable Development Goals. Geneva: World Health Organization; 2021. [Last accessed on 2022 Jan 13]. Available from: https://apps.who.int/iris/bitstream/handle/10665/342703/9789240027053-eng.pdf . [Google Scholar]
- 2.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 3.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–71. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Neri M, Riezzo I. Ischemia/reperfusion injury following acute myocardial infarction:A critical issue for clinicians and forensic pathologists. Mediat Inflamm. 2017;2017:7018393. doi: 10.1155/2017/7018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle EM, Jr, Pohlman TH, Cornejo CJ, Verrier ED. Endothelial cell injury in cardiovascular surgery:Ischemia-reperfusion. Ann Thorac Surg. 1996;62:1868–75. doi: 10.1016/s0003-4975(96)00950-2. [DOI] [PubMed] [Google Scholar]
- 6.Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT. Role of endothelial cells in myocardial ischemia-reperfusion injury. Vasc Dis Prev. 2010;7:1–14. doi: 10.2174/1874120701007010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, He GW, Underwood MJ, Yu CM. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury:Perspectives and implications for postischemic myocardial protection. Am J Transl Res. 2016;8:765–77. [PMC free article] [PubMed] [Google Scholar]
- 8.Awooda HA, Lutfi MF, Sharara GG, Saeed AM. Oxidative/nitrosative stress in rats subjected to focal cerebral ischemia/reperfusion. Int J Health Sci. 2015;9:17–24. doi: 10.12816/0024679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawlick RL, Wink J, Pepper AR, Bruni A, Abualhassen N, Rafiei Y, et al. Reparixin, a CXCR1/2 inhibitor in islet allotransplantation. Islets. 2016;8:115–24. doi: 10.1080/19382014.2016.1199303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opfermann P, Derhaschnig U, Felli A, Wenisch J, Santer D, Zuckermann A, et al. A pilot study on reparixin, a CXCR1/2 antagonist, to assess safety and efficacy in attenuating ischaemia-reperfusion injury and inflammation after on-pump coronary artery bypass graft surgery. Clin Exp Immunol. 2015;180:131–42. doi: 10.1111/cei.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Oliveira TH, Marques PE, Poosti F, Ruytinx P, Amaral FA, Brandolini L, et al. Intravital microscopic evaluation of the effects of a CXCR2 antagonist in a model of liver ischemia reperfusion injury in mice. Front Immunol. 2017;8:1917. doi: 10.3389/fimmu.2017.01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, et al. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67:1753–61. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 13.Zarbock A, Allegretti M, Ley K. Therapeutic inhibition of CXCR2 by reparixin attenuates acute lung injury in mice. Br J Pharmacol. 2008;155:357–64. doi: 10.1038/bjp.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Assay Guidance Manual. Bethesda, MD: Eli Lilly and Company and the National Center for Advancing Translational Sciences; 2013. [Last accessed on 2022 Jan 14]. Cell viability assays. 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK144065 . [Google Scholar]
- 15.Yu YP, Huang XM, Fu YF. Curcumin protects H9c2 cardiomyocyte against ischemia/reperfusion injury through inactivation of glycogen synthase kinase-3. Int J Clin Exp Pathol. 2016;9:3226–32. [Google Scholar]
- 16.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–31. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–42. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7:1543–88. doi: 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes AH, Brandolini L, Aramini A, Bianchini G, Silva RL, Zaperlon AC, et al. DF2755A, a novel non-competitive allosteric inhibitor of CXCR1/2, reduces inflammatory and post-operative pain. Pharmacol Res. 2016;103:69–79. doi: 10.1016/j.phrs.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Uruski P, Mikuła-Pietrasik J, Drzewiecki M, Budkiewicz S, Gładki M, Kurmanalina G, et al. Diverse functional responses to high glucose by primary and permanent hybrid endothelial cells in vitro. J Mol Cell Cardiol. 2021;156:1–6. doi: 10.1016/j.yjmcc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, et al. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635–42. doi: 10.1136/gut.42.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Hernández CD, Torres-Alarcón LA, González-Cortés A, Peón AN. Ischemia/reperfusion injury:Pathophysiology, current clinical management, and potential preventive approaches. Mediat Inflamm. 2020;2020:8405370. doi: 10.1155/2020/8405370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–97. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2:Prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791–6. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldobaeva A, Wagner EM. Difference in proangiogenic potential of systemic and pulmonary endothelium:Role of CXCR2. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1117–23. doi: 10.1152/ajplung.00370.2004. [DOI] [PubMed] [Google Scholar]
- 26.Stadtmann A, Zarbock A. CXCR2:From bench to bedside. Front Immunol. 2012;3:263. doi: 10.3389/fimmu.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


