Abstract
The complexity and the mechanistic role of microbial communities at mucosal surfaces are only now beginning to be understood. Their impact on host metabolism, development, and immune responses to infectious and inert stimuli may be centrally linked to the metabolic functions of these communities within the established microbiome. The structure and function of microbial communities are influenced both early and throughout life by many environmental factors, exposures, diet, and disease. Understanding how the microbiome influences the host during health is likely just as important as understanding how it influences asthmatic disease predisposition and severity.
Introduction
Asthma remains one of the most prevalent chronic respiratory diseases worldwide, afflicting over 300 million children and adults. Asthma also is highly variable in terms of susceptibility to the disease, pathophysiologic mechanisms, and phenotypes [1••]. Despite known risk factors (genetics, environmental exposures) and involvement of specific immune pathways (e.g. type 2 inflammation), the development of asthma still cannot be predicted. Moreover, the clinical spectrum of asthma is broad with individualized differences in disease course and response to treatments. These aspects have long intrigued clinicians and researchers alike and represent critical challenges to improve upon current asthma management strategies.
Given the multifactorial nature of asthma, the potential influence of microbiota on the pathogenesis of asthma and in shaping disease phenotype has attracted great attention. Recent literature has built upon earlier evidence [2–8] of links between asthma and exposure to or colonization by specific bacteria, both in the gut and airway, which suggested mechanistic roles for particular species. Thinking beyond singular species, it is appreciated now that microbiota (the collection of microbes existing together in a given niche) exert both a constant and dynamic pressure on host immune tone and that this occurs not only in the intestinal tract but likely also the respiratory tract [9]. Although lower in overall microbial burden compared to the colon, microbiota found in the respiratory system are topographically distinct in composition and interface with different microenvironments along the respiratory tract [10]. As such, interactions between microbiota and the immune system differ in the two organ systems and likely shape asthma in several ways. Moreover, the impact of these interactions on asthma likely differs by life stage and other external factors. In this review we will highlight recent insights from translational and mechanistic studies on microbiota–immune interactions in asthma that have laid further foundation for the possible development of new treatment or prevention strategies.
Asthma
Environmental microbial exposures: ‘setting the tone’ for asthma susceptibility
Much epidemiologic evidence supports the role of environmental exposures in shaping risk for asthma in early childhood [11]. Population-based studies of individuals with similar genetic backgrounds have clearly shown that differences in lifestyles and therefore opportunities for microbial exposures [12,13,14••] are associated with differences in asthma prevalence. A recently developed metric (FARMI index) attempts to quantitate whether indoor microbiota mimic a farm-like/outdoor profile [14••]. Application of this index was shown to be associated with protection against asthma among children living in non-farm homes. Environmental microbes, however, vary by location and geography, and predictive application of this index would likely need to be tested in specific population contexts, as was pursued in this study. Environment-related differences in asthma risk have been linked to altered innate immune response profiles and T-cell phenotypes [13,14••,15,16]. Among U.S. Amish and Hutterite children, it was shown recently that T-cells profiled from blood displayed different subset phenotypes between the two groups [16]. Activated regulatory CD4+ T-cell phenotypes were increased in Amish children, along with lower levels of co-stimulation and activation markers on conventional CD4 T cells. Amish children also displayed a greater proportion of CD28null CD8 T cells than Hutterite children, which correlated with T-cell IFN-γ production and low serum IgE. Together these observations further link differences in innate responses to altered adaptive immunity and the observed difference in allergic disease between these populations. However, the specific mechanisms by which exposure to an antigenically enriched environment results in these differences in immune phenotype still have yet to be fully elucidated.
Modeling mechanisms of microbiota–immune interactions in asthma
The use of animal models has allowed the development of concepts and assessment of mechanisms that can now be tested in patient populations. Earlier studies [17–20] established that changing the microbiome can protect animals from development of allergic disease. More recent studies have demonstrated that host control of the microbial communities at the mucosal surfaces can alter susceptibility to development of allergic disease, possibly by controlling the community composition that would otherwise predispose hosts to disease phenotypes [21]. Use of an ozone model of pulmonary disease has also demonstrated that even acute disease is regulated by changes in the microbiome [22,23]. In particular, studies have linked different sources of the same inbred mice that exhibit different ozone responses, to the microbiome using passive conventionalization. These latter studies indicated that neonatal male mice were more effectively altered by microbiome conventionalization than female [22]. Other studies using ozone showed that obesity-induced changes in pulmonary disease were abrogated using antibiotic treatment and verified by transfer of obese microbiota to germ-free mice [23]. Probiotic supplementation can alter the overall systemic metabolic profiles, blocking the development of pathogenic immune responses and reducing pulmonary disease [24]. In addition, recent studies have also shown that microbial metabolism of dietary fiber and subsequent increases in systemic short chain fatty acids (SCFA) can lead to a reduction in the development of allergic responses, indicating that diet can influence the microbiome and its products [25,26]. The changes in systemic metabolic profiles appear to have impact on hematopoiesis and bone marrow progenitor cells, therefore having long term effects on subsequent immune responses. Together, these recent studies add to previous work and help frame how the microbiome changes can lead to altered innate and acquired immune responses, resulting in long term immunologic effects that are regulated by microbial-derived metabolic factors.
Clinical studies: microbiota–immune interactions in asthma pathogenesis and phenotype
Several birth cohort studies have demonstrated consistent associations between an altered gut microbiome and risk for childhood allergy or asthma [27–30]. Particular members of the gut microbiota and functional products (e.g. short-chain fatty acids) [26,27] have been mechanistically implicated as mediating the effect, the specifics of which differ between reports and thus suggest several possible avenues of involvement. For example, elevated fecal levels in neonates of the lipokine 12,13-diHOME are associated with increased risk of childhood atopy and asthma [30] with gut bacteria serving as a potential source of it based on metagenomic sequence analysis. It was further shown that administration of 12,13-diHOME to mice or human dendritic cells increased lung inflammation, decreased the number of Treg cells, and altered expression of PPARγ-regulated genes [31]. Thus, similar to protective responses with fiber with SCFA, microbial-derived metabolites may have a primary role in determining detrimental outcomes of the developing immune response.
Outside the gut, alterations in the nasopharyngeal microbiome also have been implicated in risk for allergic asthma in children [32,33•,34]. In an Australian infant cohort [33•], longitudinal analysis of nasopharyngeal swabs collected from birth onward showed that development of a nasal microbiota dominated by Moraxella, Streptococcus or Haemophilus was associated with greater probability of having ‘persistent wheeze’ (pre-asthma phenotype) by age five years. Interestingly, this association was seen only in children who demonstrated evidence of allergic sensitization by age two, but not in those without early allergic sensitization, suggesting an interaction with a primed immune environment. In a Danish cohort [32] differences in nasal microbiota profiles associated with asthma were further linked to differences in mucosal immune mediators measured from nasal epithelial lining fluid. Lastly, looking beyond the preschool years, established nasal microbiota patterns may also be relevant in older children with persistent asthma [35,36]. Among U.S. school-aged children participating in a clinical trial of escalating inhaled corticosteroid use at the first indication of loss of asthma control, children with nasal microbiota high in Corynebacterium/Dolosigranulum experienced a significantly lower rate of these events, compared to those whose nasal microbiota was characterized by other bacterial profiles including Moraxella or Streptococcus-dominant profiles [35]. Together data may be converging on particular bacteria that appear to correlate to the development and/or severity of pulmonary disease.
A growing number of studies focused on adults with asthma have indicated that interactions between the microbiota and immune system also may play a role in different phenotypes of asthma and are associated with measures reflective of such [37,38•,39,40•,41••]. Severe asthma represents a minority of the asthmatic patient population but confers the greatest patient morbidity, costs and health care utilization. Differences in the airway microbiome of asthma patients with moderate to severe disease have been linked to co-morbid obesity, as well as differences in airway immune response patterns [37,38•,39,41••]. A secondary analysis of subjects who participated in an interventional trial of azithromycin for severe asthma found that those with a neutrophilic sputum inflammatory cell phenotype displayed decreased diversity of sputum bacterial microbiota, compared to other inflammatory phenotypes [38•]. This finding was consistent with observations from an earlier smaller study that noted an inverse relationship between type 2-airway inflammation and airway bacterial burden [37]. It also has been demonstrated that airway microbiota differences related to type 2-low inflammatory state are seen in mild asthmatics [40•] and further may involve differences in fungal microbiota [39]. These complex interactions of multiple organisms, including bacteria and fungi, which may be further compounded by different viral infections over the lifespan, will likely all have an impact on the development and severity of asthmatic responses through immune regulation.
Summary and future directions
Evidence is accumulating linking mucosal microbial communities and their products with the nature of the immune responses that regulate the development and severity of asthma. Ongoing and future studies will be able to better understand these links through both improved disease models and rapidly evolving molecular and analytic tools to interrogate the interactions between host and microbiota. The latter include metagenomic-based analyses and their integration with other ‘omics’ data to obtain more comprehensive functional insights. It seems likely that the relationships uncovered will differ by life stage and asthma phenotype (Figure 1), which varies greatly between patients. Critical knowledge has been gained in recent years, but additional mechanistic links remain elusive. Despite similar known risk factors or exposures in a given group of individuals, it is difficult to predict with certainty who will develop asthma or will exhibit a more treatable versus severe clinical course. Related questions include what mechanisms drive adult-onset asthma and does this differ in those with or without a history of atopy. Likewise, sex is an important variable with males having more susceptibility early in life and females with increasing asthma incidence post-puberty. How the microbiome contributes to these sex-associated differences in asthma prevalence is unclear. Importantly, whether targeting of specific microbes that predominate during asthma development, or manipulation of dietary intake or supplements [42,43], has any therapeutic potential will be interesting to determine. Despite the numerous questions that remain, many dynamic factors have been identified that contribute to differences in asthma incidence and phenotype. These have plausible mechanistic links to differences in microbiome functions both in the intestinal and respiratory tract, which may prove to have a significant role in the heterogeneity of asthma.
Figure 1.
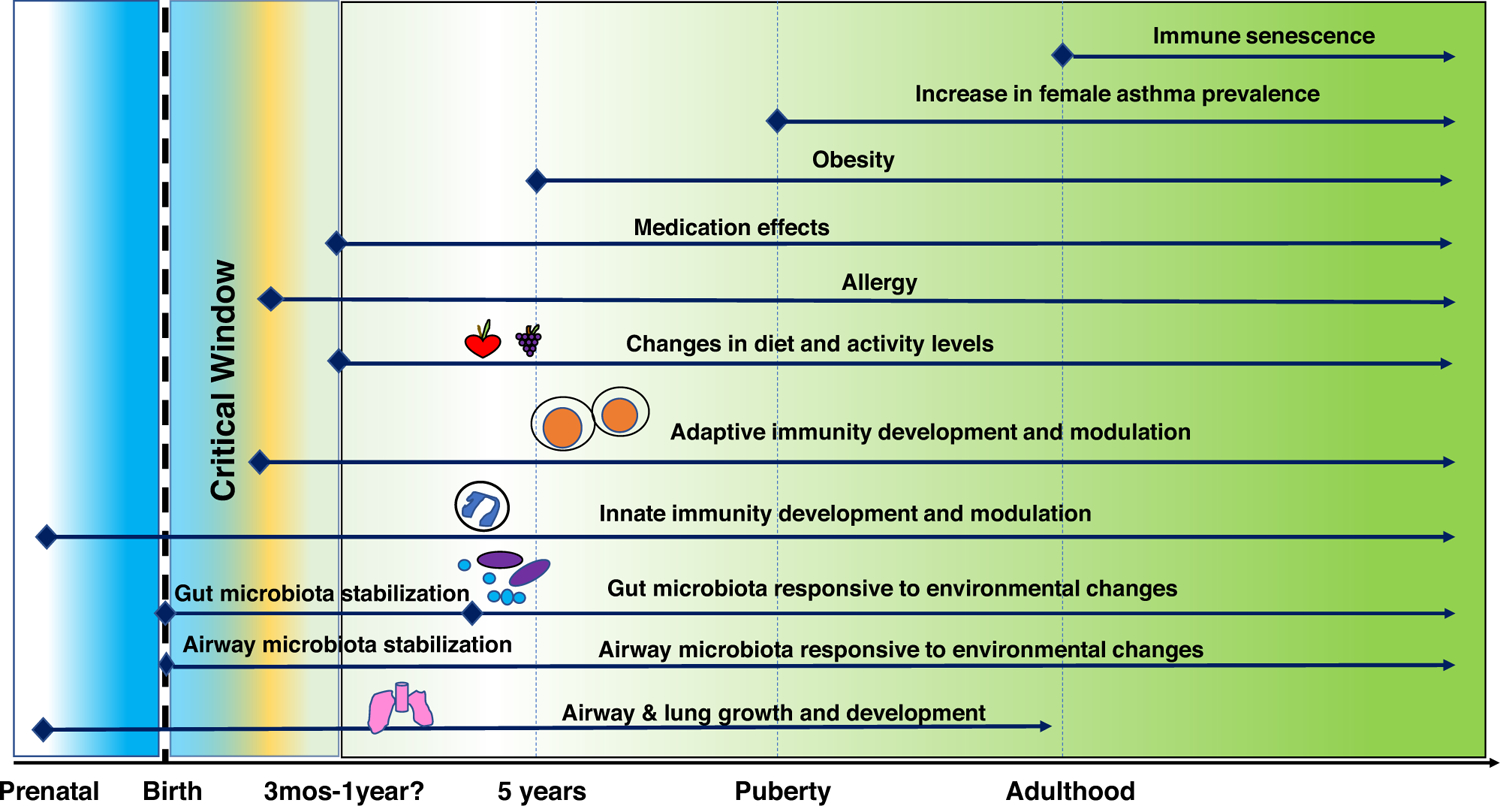
Dynamic factors with plausible links to the host microbiota that are associated with asthma pathogenesis or asthmatic phenotype. Mechanisms are understood for some but not all interactions.
Acknowledgements
Writing support provided by National Institutes of Health grants AI129958 (Y.J.H.), HL150682 and AI138348 (N.L.)
Footnotes
Declaration of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.••.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, Cullinan P, Custovic A, Ducharme FM, Fahy JV et al. : After asthma: redefining airways diseases. Lancet 2018, 391:350–400 [DOI] [PubMed] [Google Scholar]; A thorough authoritative and transformative discussion on approaches to asthma diagnosis, management and scientific research, that calls for a radical shift in the conceptualization of asthma to advance therapeutic or preventative strategies.
- 2.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F et al. : Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007, 56:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP et al. : Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 1998, 158:998–1001. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K et al. : Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007, 357:1487–1495. [DOI] [PubMed] [Google Scholar]
- 5.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD et al. : Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 2014, 133:1301–1307 1307.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L et al. : Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 2014, 9:e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J et al. : Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011, 184:198–205. [DOI] [PubMed] [Google Scholar]
- 8.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD et al. : Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med 2014, 20:642–647. [DOI] [PubMed] [Google Scholar]
- 9.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, Li Y, Shen N, Ghedin E, Morris A et al. : Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016, 1:16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man W, de Steenhuijsen Piters W, Bogaert D: The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 2017, 15:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FD: Childhood asthma inception and progression: role of microbial exposures, susceptibility to viruses and early allergic sensitization. Immunol Allergy Clin North Am 2019, 39:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C et al. : Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011, 364:701–709. [DOI] [PubMed] [Google Scholar]
- 13.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques Dos Santos M, Anderson RL, Metwali N et al. : Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med 2016, 375:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.••.Kirjavainen PV, Karvonen AM, Adams RI, Täubel M, Roponen M, Tuoresmäki P, Loss G, Jayaprakash B, Depner M, Ege MJ et al. : Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med 2019, 25:1089–1095 FARMI index [DOI] [PubMed] [Google Scholar]; This study describes the development and validation of an index to predict the ‘farm-like’ nature of microbial communities found in indoor environmental samples from non-farm households and its association with a protective effect on asthma prevalence in the test populations.
- 15.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A et al. : Microbial exposure during early life has persistent effects on natural killer T function. Science 2012, 336:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrusch CL, Stein MM, Gozdz J, Holbreich M, von Mutius E, Vercelli D, Ober C, Sperling AI: T-cell phenotypes are associated with serum IgE levels in Amish and Hutterite children. J Allergy Clin Immunol 2019, 144:1391–1401.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B et al. : Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy 2010, 40:811–819. [DOI] [PubMed] [Google Scholar]
- 18.Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, McCoy K, Marsland BJ, Harris NL: Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011, 184:198–205. [DOI] [PubMed] [Google Scholar]
- 19.Obieglo K, van Wijck Y, de Kleijn S, Smits HH, Taube C: Microorganism-induced suppression of allergic airway disease: novel therapies on the horizon? Expert Rev Respir Med 2014, 8:717–730. [DOI] [PubMed] [Google Scholar]
- 20.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, Marsland BJ: Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med 2014, 20:642–647. [DOI] [PubMed] [Google Scholar]
- 21.Banskar S, Detzner AA, Juarez-Rodriguez MD, Hozo I, Gupta D, Dziarski R: The Pglyrp1-regulated microbiome enhances experimental allergic asthma. J Immunol 2019, 203:3113–3125. [DOI] [PubMed] [Google Scholar]
- 22.Brown TA, Tashiro H, Kasahara DI, Cho Y, Shore SA: Early life microbiome perturbation alters pulmonary responses to ozone in male mice. Physiol Rep 2020, 8:e14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashiro H, Cho Y, Kasahara DI, Brand JD, Bry L, Yeliseyev V, Abu-Ali G, Huttenhower C, Shore SA: Microbiota contribute to obesity-related increases in the pulmonary response to ozone. Am J Respir Cell Mol Biol 2019, 61:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonseca W, Lucey K, Jang S, Fujimura KE, Rasky A, Ting HA, Petersen J, Johnson CC, Boushey HA, Zoratti E et al. : Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol 2017, 10:1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ: Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014, 20:159–166. [DOI] [PubMed] [Google Scholar]
- 26.Lewis G, Wang B, Shafiei Jahani P, Hurrell BP, Banie H, Aleman Muench GR, Maazi H, Helou DG, Howard E, Galle-Treger L et al. : Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front Immunol 2019, 10:2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL et al. : Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015, 7:307ra. [DOI] [PubMed] [Google Scholar]
- 28.Arrieta MC, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, Vaca M, Boutin RCT, Morien E, Jin M et al. : Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol 2018, 142:424–434.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AM, Kunøe A, Fink NR, Chawes BL et al. : Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 2018, 9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW et al. : Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016, 22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM et al. : Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat Microbiol 2019, 4:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorsen J, Rasmussen MA, Waage J, Mortensen M, Brejnrod A, Bønnelykke K, Chawes BL, Brix S, Sørensen SJ, Stokholm J, Bisgaard H: Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat Commun 2019, 10:5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.•.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, Holt BJ, Kusel M, Serralha M, Troy N et al. : Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe 2018, 24:341–352.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights how different nasopharyngeal microbiota communities predominate early in life and may differentially contribute to diverse pulmonary responses. It further suggests that colonization with pathogens predicts chronic wheeze in allergen-sensitized children.
- 34.Mansbach JM, Luna PN, Shaw CA, Hasegawa K, Petrosino JF, Piedra PA, Sullivan AF, Espinola JA, Stewart CJ, Camargo CA Jr: Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing. J Allergy Clin Immunol 2020, 145:518–527.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Jackson D, Bacharier LB, Mauger D, Boushey H, Castro M, Durack J, Huang Y, Lemanske RF Jr, Storch GA et al. : The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun 2019, 10:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCauley K, Durack J, Valladares R, Fadrosh DW, Lin DL, Calatroni A, LeBeau PK, Tran HT, Fujimura KE, LaMere B et al. : Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J Allergy Clin Immunol 2019, 144:1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H: The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 2015, 136:874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.•.Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C et al. : Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol 2018, 141:94–103.e15 [DOI] [PubMed] [Google Scholar]; This study identified differences in sputum microbial community structure between severe asthma patients with neutrophilic versus non-neutrophilic inflammatory phenotypes, suggestive that lower airway microbiome features may be useful markers for individualization of asthma treatments.
- 39.Sharma A, Laxman B, Naureckas ET, Hogarth DK, Sperling AI, Solway J, Ober C, Gilbert JA, White SR: Associations between fungal and bacterial microbiota of airways and asthma endotypes. J Allergy Clin Immunol 2019, 144:1214–1227.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•.Durack J, Lynch SV, Nariya S, Bhakta NR, Beigelman A, Castro M, Dyer AM, Israel E, Kraft M, Martin RJ et al. : Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol 2017, 140:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed previously unrecognized associations between atopic sensitization and changes in the bronchial microbiome irrespective of asthma status, and of microbiome differences related to corticosteroid response.
- 41.••.Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, Van Horn S, Sokolowska M, Altunbulakli C, Eljaszewicz A et al. : Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun 2019, 10:5711. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that obesity in addition to asthmatic status associates with differences in both gut and respiratory tract microbiota and that deficiency in specific gut microbes associates with asthma severity.
- 42.Hjelmsø MH, Shah SA, Thorsen J, Rasmussen M, Vestergaard G, Mortensen MS, Brejnrod A, Brix S, Chawes B, Bønnelykke K et al. : Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat Commun 2020, 11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O’Connor GT, Sandel M, Beigelman A, Bacharier LB, Zeiger RS et al. : Six-year follow-up of a trial of antenatal vitamin d for asthma reduction. N Engl J Med 2020, 382:25–533. [DOI] [PMC free article] [PubMed] [Google Scholar]


