Seeking to understand the trends in participant accrual and barriers to neuro-oncology clinical trial access, we conducted a comprehensive assessment of past and ongoing neuro-oncology trials in the United States. Data from the ClinicalTrial.gov registry revealed that most closed trials failed to meet their enrollment goals, with the trend worsening over time. We explored factors that may affect trial accrual and access, finding an increasing demand for participant enrollment. Investigating geographic, socioeconomic, and population factors, we found that trial-hosting infrastructure may not exist in large geographically contiguous and sparsely populated areas, and where existent, underserves higher-populated disadvantaged areas.
Barriers to clinical trial participation can lead to low patient accrual contributing to premature trial termination and underpowered or biased inferences, which have significant implications for patient care. In a recent survey, neuro-oncology providers reported the lack of easily accessible trial sites for patients as the primary barrier to study participation.1 Additionally, there is growing evidence that disparities in trial access exist based on socioeconomic status and geographic location among other cancer patient populations and across the general clinical trial population.2,3 However, a detailed understanding of such trends in neuro-oncology and any potential disparities in access is lacking.4 To assess these trial accrual and access questions in neuro-oncology trials across the United States, we conducted a comprehensive study of past and ongoing trials and evaluated trends in trial enrollment, as well as geographic and socioeconomic factors underlying trial availability and access.
We programmatically extracted interventional neuro-oncology trials from the ClinicalTrials.gov5 registry using the R RPostgreSQL6 library and the Aggregate Analysis of ClinicalTrials.gov database,7 by selecting trials related to one or more of 140 search terms relevant to WHO-classified primary malignant CNS tumors. We included trials with sites in the United States and excluded trials targeting pediatric patient populations.
We extracted design characteristics, site zip codes, and enrollment from trials of interest. Enrollment data were supplemented with information from the ClinicalTrials.gov webpage. Zip codes were mapped to 5-digit Zip Code Tabulation Areas (ZCTAs), which were then annotated with the population (log-transformed hereinafter) and geospatial data from the 2019 American Community Survey using the R tidycensus8 library and socioeconomic indicators using the Neighborhood Atlas national-level area deprivation index (ADI).9 The ADI ranks regions from the lowest to the highest level of disadvantage (1-100), using measures of education level, employment/occupation, income, housing, and lifestyle characteristics.9
We used logistic and linear regression to assess factors associated with successful trial accrual, existence of trial-hosting infrastructure, and trial-site prevalence. Spatial lag and autologistic regressions (calculated across median ZCTA span) were conducted to assess geospatial-dependent effects, if Moran’s I revealed geospatial autocorrelations, using the R spdep library.10 All analyses were conducted using R version 3.6.0.
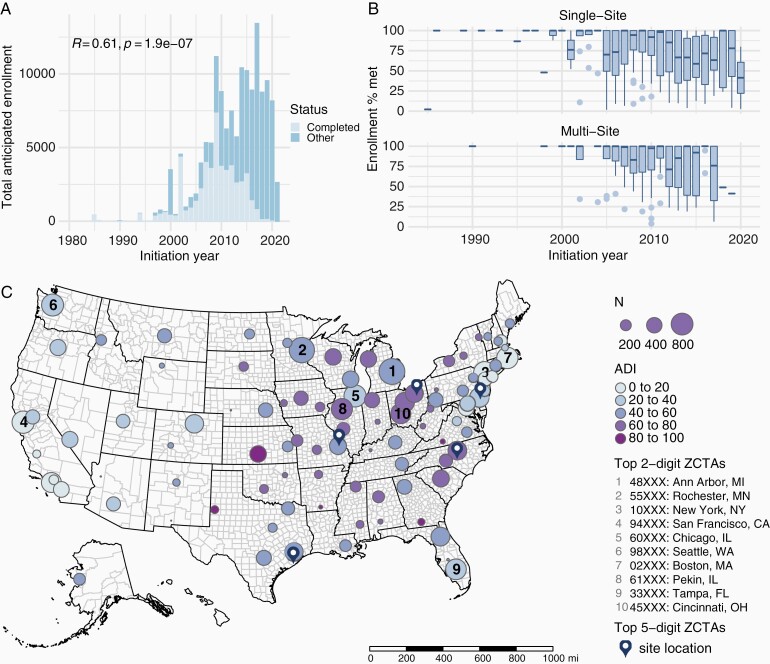
We extracted a total of 2004 interventional neuro-oncology trials initiated between 1966 and 2021 in the United States. Trials were most frequently phase II (41%), completed (46%), unblinded (95%), single-arm (67%), non-randomized (60%) studies of glioblastoma (40%). Excluding withdrawn or terminated trials, 51% of (N = 585) completed trials reporting enrollment data failed to meet their enrollment goal. Logistic regression revealed that the likelihood of trial accrual success decreased annually (year of initiation, odds ratio [OR] = 0.9, P < .00001) but was greater for multi-site compared to single-site studies (OR = 1.49, P = .024), regardless of anticipated enrollment (OR = 1, P = .87). Investigating trends in overall anticipated enrollment, we found that the cumulative demand for trial participation increased annually (Pearson’s R = 0.61, P < .00001, Figure 1A). While this increasing demand is likely a major factor driving worsening accrual-success rates, the wide range of trial enrollment rates (Figure 1B) suggests the existence of additional factors affecting accrual.
Figure 1.
Anticipated and actual accrual trends of neuro-oncology trials and distribution of trial sites across the United States. (A) Increase in total anticipated enrollment over the years. (B) Decrease in enrollment rates over the years for completed trials. (C) Geographical distribution of trial sites and median ADI of the aggregated 2-digit ZCTAs hosting the sites. The ten 2-digit ZCTAs hosting the largest number of sites are annotated with their ranking. The five 5-digit ZCTAs hosting the largest number of sites are annotated with map markers. Abbreviations: ADI, area deprivation index; ZCTAs, Zip Code Tabulation Areas.
Thus, we investigated the dual side of accrual:participant access to trial sites. For each ZCTA, we defined the number of trial sites as the number of times the ZCTA hosted a trial site. In total, we identified 29 901 non-unique sites within 1861 unique ZCTAs, covering 6% of all ZCTAs in the United States (N = 33 120). Geospatial and population data were available for all ZCTAs, and ADI was available for 92% of ZCTAs. ZCTAs with large, statistically outlying numbers of sites contained major healthcare institutions and affiliates, including University of Texas MD Anderson Cancer Center (ZCTA 77030), Cleveland Clinic Cancer Center (ZCTA 44106), Duke University (ZCTA 27705), University of Pennsylvania (ZCTA 19104), and Washington University (ZCTA 63110). When aggregated at the larger contiguous 2-digit ZCTAs, areas with the highest number of sites hosted other major healthcare institutions, including University of Michigan (ZCTA 48XXX), Mayo Clinic (ZCTA 55XXX), and Memorial Sloan Kettering Cancer Center (ZCTA 10XXX) (Figure 1C).
Given the small proportion of ZCTAs with at least one trial site, we assessed the impact of geographical and socioeconomic factors on trial access in two steps. First, autologistic regression revealed that trial sites, and therefore trial-hosting infrastructure, were more likely to exist in higher-populated regions (OR = 2.677, P < .00001) neighboring other trial-hosting ZCTAs (OR = 1.905, P < .00001), regardless of ADI (OR = 1.002, P = .147). Second, among ZCTAs with at least one trial site, linear regression revealed areas of lower disadvantage (ADI, β = −0.07, P = .02) and lower population (β = −3.5, P < .00001) were significantly associated with a greater number of sites, independent of geography (Moran’s I for data = 0.023, P = .123; I for residuals = 0.012, P = .354). The number of successfully accruing sites was negatively associated with population (β = −0.292, P = .00006) regardless of ADI (β = −0.003, P = .371), and carried significant geospatial effects from surrounding ZCTAs (ρ = 0.064, P = .00049).
In summary, we conducted a novel comprehensive assessment of past and ongoing neuro-oncology trials, revealing a complex picture of factors affecting worsening accrual rates and trial-access barriers. The increasing and unmet demand for enrollment necessitates measures to improve accrual. The observed relative success of multi-site accrual likely reflects the impact of multi-institutional and consortia efforts, supporting previous guidelines.4 Regarding trial access, trial-hosting infrastructure was disproportionately lacking in sparsely populated, geographically contiguous areas. Where existent, the infrastructure underserved higher-populated, disadvantaged areas, highlighting the crucial need for guidelines4 involving local oncologists and facilities, telemedicine, and reimbursements to reduce the financial burdens of trial participation. Encouragingly, the more equitable distribution, in terms of ADI, of successfully accruing trial sites suggests that reducing socioeconomic disparities in trial access can be achieved hand-in-hand with participant accrual success.
Acknowledgments
We thank the reviewers for their valuable input.
Funding
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Conflict of interest statement. The authors declare no conflict of interest.
Authorship statement. Study design, analysis, and manuscript writing: O.C. and Y.K. Contributions to study design and manuscript writing: M.R.G. and T.S.A. All authors read and approved the manuscript.
References
- 1. Rogers JL, Acquaye A, Vera E, et al. Provider-reported challenges and barriers to referring patients to neuro-oncology clinical trials: a report from the Society for Neuro-Oncology member survey. Neurooncol Pract. 2020;7(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seidler EM, Keshaviah A, Brown C, et al. Geographic distribution of clinical trials may lead to inequities in access. Clin Invest. 2014;4(4):373–380. [Google Scholar]
- 3. Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. [DOI] [PubMed] [Google Scholar]
- 4. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov. Accessed July 28, 2021.
- 6. Conway J, Eddelbuettel D, Nishiyama T, et al. RPostgreSQL: R Interface to the “PostgreSQL” Database System. https://CRAN.R-project.org/package=RPostgreSQL. Accessed July 28, 2021.
- 7. Clinical Trials Transformation Initiative. Aggregate Analysis of ClinicalTrials.gov. http://www.ctti-clinicaltrials.org. Accessed July 28, 2021.
- 8. Walker K, Herman M. tidycensus: Load US Census Boundary and Attribute Data as ‘tidyverse’ and ‘sf’-Ready Data Frames. https://CRAN.R-project.org/package=tidycensus. Accessed July 28, 2021.
- 9. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bivand R, Altman M, Anselin L, et al. Spatial Dependence: Weighting Schemes and Statistics. https://cran.r-project.org/web/packages/spdep/index.html. Accessed November 22, 2021.