Abstract
Sepsis-related acute kidney injury (AKI) is one of the most common complications of sepsis at the intensive care unit (ICU) with more adverse mortality rates. The early diagnosis and reliable monitoring of sepsis-related AKI are essential in achieving a favorable outcome. Novel serum and urinary biomarkers could yield valuable information during this process.
Regarding the widely used Kidney Disease Improving Global Outcomes (KDIGO) classifications, the diagnosis of AKI is still based on the increase of serum creatinine levels and the decrease of urine output; however, these parameters have limitations in reflecting the extent of kidney damage, therefore more sensitive and specific laboratory biomarkers are needed for the early diagnosis and prognosis of sepsis-related AKI. Regarding this, several serum parameters are discussed in this review including presepsin and the most important actin scavenger proteins (gelsolin, Gc-globulin) while other urinary markers are also examined including cell cycle arrest biomarkers, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), Cystatin C and actin.
Novel biomarkers of sepsis-related AKI could facilitate the early diagnosis and monitoring of sepsis-related AKI.
Key words: Sepsis-3, acute kidney injury, presepsin, gelsolin, urinary actin, urinary Gc-globulin, novel biomarker
INTRODUCTION
Sepsis is a complex clinical syndrome with increasing incidence and unfavorable mortality rates which still poses a significant challenge in intensive care despite the availability of advanced treatment methods [1-3]. As stated in the latest Sepsis-3 definitions, sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [4].
CONVENTIONAL SEPSIS BIOMARKERS
Timely diagnosis along with effective causal and supportive treatment of sepsis are essential for achieving adequate recovery. Although not being part of the Sepsis-3 definitions, serum high-sensitivity C-reactive protein (CRP) and procalcitonin (PCT) are still widely used inflammatory markers in the clinical evaluation of sepsis [4, 5]. CRP is a non-specific inflammatory marker; thus, it can be elevated in various acute and chronic diseases (e.g. autoimmune disorders, trauma, malignancies) besides sepsis [6-8]. However, despite CRP being a general inflammatory marker, its mostly inversely proportional relation to albumin – namely the CRP:albumin ratio – has already been investigated in numerous clinical conditions (e.g. sepsis, pancreatitis, coronary artery disease, malignancies) [9-11]. CRP reaches its peak levels 48 hours after the start of the inflammatory process and significantly elevated CRP levels also have a moderate correlation with the severity of sepsis [6, 12]. Compared to CRP, PCT concentrations increase 4-6 hours after the onset of infection while PCT also showed better performance regarding the diagnosis and mortality prediction of sepsis [8, 13, 14]. However, exclusively fungal or viral infections do not elevate PCT, yet other inflammatory conditions (e.g. extensive tissue injury, pancreatitis) besides infection could result in slightly increased PCT levels [14, 15]. Furthermore, the antagonistic changes in PCT and albumin were also investigated in sepsis-related AKI [16].
Besides hs-CRP and PCT, more than 200 novel (mostly serum) sepsis biomarkers have been evaluated, yet no single marker was sensitive or specific enough for accurately diagnosing sepsis [17, 18]. However, a multi-marker approach including various promising sepsis biomarkers (e.g. presepsin, IL-6) may provide useful information regarding the early diagnosis of sepsis.
Presepsin
Presepsin (PSEP) is the soluble N-terminal fragment (MW=13 kDa) of the cluster of differentiation (CD) marker protein CD14 (MW=55 kDa), which is the receptor for lipopolysaccharide (LPS) and LPS-binding protein complexes [19, 20]. Compared to PCT, PSEP has an even more rapid response time of 2-4 hours after the onset of infection, while PSEP was also deemed valuable for the early diagnosis and prognosis of sepsis in contrast to other conditions (e.g. trauma, burn injury, major surgical operations) [21, 22]. PSEP has varying diagnostic cut-off values among 400-600 pg/mL for sepsis, yet there is a concern that PSEP concentrations are affected by kidney function [23-25]. It is presumed that PSEP is filtered by the glomeruli, then reabsorbed and catabolized within proximal tubular cells. Several studies reported increasing PSEP levels as kidney function decreases (e.g. during chronic kidney disease or sepsis-related AKI) [26-28]. However, PSEP – along with hs-CRP and PCT – could be removed from the circulation using different modalities of renal replacement therapy (RRT), therefore potentially causing falsely low inflammatory marker levels [28, 29].
CONVENTIONAL MARKERS OF SEPSIS-RELATED AKI
AKI refers to an abrupt decrease in kidney function resulting in the retention of numerous waste products and the dysregulation of extracellular volume [30, 31]. The Kidney Disease Improving Global Outcomes (KDIGO) classification is widely used for the diagnosis of AKI based on the increase in serum creatinine (sCr) levels and the decrease of urine output, both of which are kidney function markers [32]. Urine output often decreases before the elevation of sCr concentration could be detected, yet not all reductions in urine output indicate AKI. Unfortunately, due to the non-linear relationship between glomerular filtration rate (GFR) and sCr, the increase of creatinine could be overlooked in the early phase of AKI. Therefore, the changes in these parameters do not reflect the extent of kidney damage, hence more sensitive and specific laboratory biomarkers are needed for the early diagnosis and prediction of AKI [33, 34].
So far, several novel biomarkers have been investigated to improve early diagnosis and prognosis of sepsis-related AKI [34, 35]. However, the clinical use of the studied biomarkers remains unclear due to heterogeneity of AKI itself and the limitations of novel AKI biomarkers. Regarding this, several biomarkers are discussed including tissue inhibitor of metalloproteinases-2 (TIMP-2), insulin-like growth factor-binding protein 7 (IGFBP7), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1) and Cystatin C (CysC) while urinary actin and the most important proteins of the actin scavenger system (gelsolin, Gc-globulin) are also presented in this review.
NOVEL DAMAGE BIOMARKERS IN SEPSIS-RELATED AKI
Pre-injury phase stress markers
It has been proposed that the development of AKI is mostly preceded by a so-called acute kidney stress (AKS) phase which can occur due to several sources of renal insults (e.g. hypoperfusion, nephrotoxic drugs, cytokines, reactive oxygen species). The expression of several cell growth regulating proteins including TIMP-2 (MW=21 kDa) and IGFBP7 (MW=29 kDa) could be upregulated in the tubular system as a consequence of kidney stress, thus leading to G1 cell cycle arrest through the induction of several apoptotic pathways. In the case of prolonged kidney stress, the persistent urinary increase of these biomarkers could also indicate apoptotic tubular injury, thereby signaling the early development of AKI. Urine measurements of TIMP-2 and IGFBP7 were found useful in the early diagnosis and prognosis of AKI according to several multicentric studies. However, these biomarkers did not prove to be beneficial in patients with more severe stages of AKI while false positive results could also occur frequently in low-risk patients [36-38].
Tubular markers of AKI progression
NGAL is a glycoprotein (MW=25 kDa) of the lipid carrier protein superfamily expressed mostly on the surface of neutrophils while also being scarcely present in other cell types (e.g. nephrocytes, hepatocytes). NGAL is filtered through the glomeruli and reabsorbed in the proximal tubules under normal circumstances. NGAL expression is rapidly upregulated if the tubular system is affected during ischemic or nephrotoxic renal injury, hence NGAL is detectable in the urine as early as 3 hours after the onset of kidney damage. Plasma NGAL levels seem to have a stronger correlation with absolute neutrophil count than proinflammatory cytokines, therefore lower plasma NGAL levels could still occur due to neutropenia in patients with systemic inflammation [39]. However, urine NGAL levels may also be significantly increased in AKI patients with neutropenia if the tubular system is affected. As NGAL also has an antimicrobial effect by binding siderophores, it was also found valuable along with hepcidin – a main regulator of iron homeostasis – in the prognosis of sepsisrelated AKI. All in all, urinary NGAL elevation could reflect the decreased reabsorption of filtered NGAL due to the dysfunction or injury of the proximal tubules while its expression could also be upregulated in the tubular system during sepsis-related AKI [40-43].
KIM-1 is a transmembrane glycoprotein (MW=39 kDa) containing extracellular immunoglobulin and mucin domains having a low expression in the kidney under physiological conditions. However, it is upregulated after ischemia-reperfusion injury, especially in proliferating de-differentiated proximal tubular epithelial cells 48 hours after injury. KIM-1 appears to be a highly sensitive marker of AKI in non-cardiac surgical patients as well as after cardiac surgery. Persistent serum KIM-1 elevation indicates ongoing tubular injury, potentially increasing the risk for the development of chronic kidney disease, while urinary KIM-1 also shows a similar correlation to kidney injury [44-47].
CysC (MW=13 kDa) is constantly produced by all nucleated cells, filtered by the glomeruli, then mostly reabsorbed and catabolized in the proximal tubular cells. The measurement of serum CysC was found to be preferable to sCr in patients after non-traumatic and traumatic amputation while also being superior in predicting cardiovascular events and mortality in elderly patients [48-50]. However, as CysC is a more reliable kidney function marker compared with sCr, it seems to be influenced by old age, large doses of corticosteroids, conditions affecting the thyroid gland, inflammation and malignancies. As the changes in sCr concentrations have some limitations, especially late in the clinical course of ICU patients, urine CysC seems to be unaffected by several non-renal factors affecting creatinine levels in sepsis-related AKI [50-53].
RELEVANCE OF ACTIN AND THE ACTIN SCAVENGER SYSTEM
Actin
Actin is a multifunctional globular protein (MW=42 kDa) existing in monomeric/globular (G-actin) and in polymeric/filamentous (F-actin) forms. In acute tissue injuries the released extracellular actin is found to be highly toxic in the circulation due to its spontaneous polymerizing tendency causing unfavorable effects on the coagulation system. Gelsolin and Gc-globulin are the most important proteins of the so-called actin scavenger system which is responsible for binding and depolymerizing extracellular actin in the circulation, thus making the urinary appearance of these protein complexes unlikely [54-57]. However, an earlier study indicates that actin could be detected in the urine of kidney transplant patients with sustained AKI [58]. Recently published data suggest that urinary actin (u-actin) could be a complementary diagnostic biomarker to sCr in sepsis-related AKI while higher u-actin levels also seemed to reflect the severity of AKI [59]. Significantly different admission u-actin levels were found between 24 control and 60 septic patients (median: 0.78 vs. 3.98 μg/L, p<0.001), while samples from 17 septic non-AKI and 43 sepsis-related AKI patients also showed differences (median: 1.27 vs. 9.52 μg/L, p<0.001) (Fig 1). Admission u-actin levels were even more elevated in 43 patients with sepsis-related AKI and were in good agreement with the severity of AKI stages (median: 3.16 vs. 10.78 vs. 11.55 μg/L, p<0.05) (Fig 2). This tendency remained the same when referring u-actin to urine creatinine. Parameters of first-day septic patient samples could discriminate AKI from non-AKI state (AUC ROC, p<0.001): u-actin: 0.876; se-creatinine: 0.875. Derived cutoff value for u-actin was 2.63 μg/L (sensitivity: 86.0%, specificity: 82.4%). Although this study has several limitations, u-actin showed moderate correlation with other urinary parameters. Furthermore, extremely elevated u-actin levels were found in sepsis-related AKI patients with RRT requirement. Despite actin being bound to the actin scavenger proteins (gelsolin, Gc-globulin) in the circulation, u-actin could appear in the urine due to both severe glomerular and/or tubular injury, so it seems that the elevation of u-actin indicates severe kidney injury (Figure 1, Figure 2).
Figure 1.
![Time points: T1: within 24h after admission; T2: second day morning; T3: third day morning; n: sample count. **p<0.01; ***p<0.001. Reprinted with permission from Ragán et al. (2021) (CC BY 4.0) [59].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3155/9092722/b64fb1d5fc0a/ejifcc-33-011-g001.jpg)
Urinary actin in sepsis. U-actin levels of control and septic patients (A) along with sepsis and sepsis-related AKI patients (B) during follow-up
Figure 2.
![Time points: T1: within 24h after admission; T2: second day morning; T3: third day morning; n: sample count. *p<0.05; **p<0.01. Reprinted with permission from Ragán et al. (2021) (CC BY 4.0) [59].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3155/9092722/9735f407cba3/ejifcc-33-011-g002.jpg)
U-actin (A) and u-actin/u-creatinine (B) levels of the individual sepsis-related AKI stages during follow-up
Gelsolin
Gelsolin (GSN) is a multifunctional protein existing in three different isoforms. Secreted or plasma GSN (MW=83 kDa) is an essential component of the extracellular actin scavenger system [55, 60, 61]. Besides actin, GSN may also bind to pro-inflammatory mediators and bacterial wall components (lipoteichoic acid, LPS). Since GSN seems to have a protective role in the body (e.g. by depolymerizing actin filaments in the circulation), a growing body of evidence indicates decreasing GSN levels in various clinical conditions (e.g. severe sepsis, multiple organ dysfunction syndrome (MODS), extensive trauma, acute liver failure, myocardial infarction) [62-64]. Our previous study showed as well that the increase of serum actin was inversely proportional to the amounts of serum GSN which was associated with increased mortality rate [65]. Furthermore, the simultaneous measurement of other inflammatory parameters and GSN levels may provide valuable information in the management of critically ill patients.
Gc-globulin
Gc-globulin (MW=52-59 kDa) has 3 major isoforms (Gc1f, Gc1s, Gc2) while also being a member of the albuminoid superfamily, which consists of several transport proteins in the circulation including albumin, alpha-fetoprotein and afamin [66]. Albumin is the most abundant human serum protein acting as a transporter of endogenous and exogenous substances including thyroxine, fatty acids and drugs. Gc-globulin is mainly produced by the liver with a reference range of 300-600 mg/L, yet severely decreased levels of 50-120 mg/L were observed in acute injury or sepsis. Gc-globulin is involved in the vitamin D biosynthesis process by binding and transporting vitamin D metabolites while it also plays an important role in modulating cells of the adaptive immune response [67, 68]. Under physiological conditions, Gc-globulin is filtered freely through the glomeruli, then reabsorbed and catabolized by proximal tubular epithelial cells resulting only in a trace urinary excretion. Therefore, tubular kidney damage is expected to result in increased urinary Gc-globulin concentrations [69, 70]. There is only scarce data on urinary appearance of Gc-globulin in sepsis, yet this marker was already investigated in other conditions including endometriosis, diabetic nephropathy and contrast-induced nephropathy as well [67, 71]. Recently, our research group started investigating urinary Gc-globulin/urine creatinine (u-Gc-globulin/u-Cr) levels in sepsis-related AKI by conducting a small sample size pilot study. Compared to 6 control patients, significantly elevated admission u-Gc-globulin/u-Cr levels were found in 9 septic and 12 sepsis-related AKI patients (median: 1.8 vs. 21.5 vs. 136.7 μg/mmol, p<0.001) (Figure 3).
Figure 3.
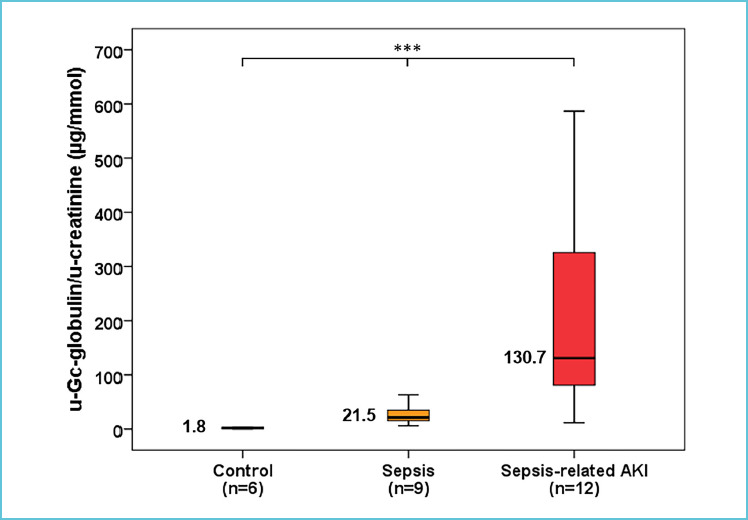
Urinary Gc-globulin in sepsis and in sepsis-related AKI. Admission u-Gc-globulin/u-creatinine levels of control, septic and sepsis-related AKI patients.
SUMMARY OF NOVEL BIOMARKERS IN SEPSIS-RELATED AKI
Despite their limitations and the heterogeneity of AKI itself, the discussed laboratory markers yield valuable information regarding the early diagnosis and effective prognosis of sepsis-related AKI. Most widely known laboratory markers including TIMP-2xIGFBP-7, NGAL, KIM-1 and Cystatin C can be measured using commercially available diagnostic assays, thereby providing accurate results with a short turnaround time (less than 1 hour). Serum GSN was measured using an automated immune turbidimetric assay developed in our laboratory with a short turnaround time (less than 1 hour) as well, yet this measurement method is not yet commercially available.
However, the measurement of u-actin and u-Gc-globulin/u-creatinine was carried out using quantitative Western blot techniques, hence the routine clinical utility of these biomarkers is questionable. Therefore, our research group is currently working on the development of more rapid and efficient ELISA methods for measuring u-actin and u-Gc-globulin/u-creatinine. All of the discussed laboratory markers are shown in Table 1.
Table 1.
Classification of novel AKI biomarkers
| Diagnostic utility based on renal injury site | Measurement method | |
|---|---|---|
| Novel sepsis-related AKI biomarkers | ||
| Urine TIMP-2xIGFBP-7 (MW=21 kDa)x(MW=29 kDa) | Tubular injury | Point of Care test |
| Urine NGAL (MW=25 kDa) | Tubular injury | Automated immune turbidimetric assay |
| Urine KIM-1 (MW=39 kDa) | Tubular injury | ELISA |
| Urine Cystatin C (MW=13 kDa) | Tubular injury | Automated immune turbidimetric assay |
| Actin scavenger system proteins | ||
| Urinary actin (MW=42 kDa) | Glomerular and tubular injury | Western blot |
| Serum Gelsolin (MW=83 kDa) | Glomerular injury | Automated immune turbidimetric assay |
| Urine Gc-globulin (MW=52-59 kDa) | Glomerular and tubular injury | Western blot |
Abbreviations: TIMP-2: tissue inhibitor of metalloproteinases-2; IGFBP7: insulin-like growth factor-binding protein 7; NGAL: neutrophil gelatinase-associated lipocalin; KIM-1: kidney injury molecule-1; MW: molecular weight; ELISA: enzyme-linked immunosorbent assay.
CONCLUSION
The early diagnosis and effective therapy of sepsis and sepsis-related AKI are essential for a successful recovery. However, the currently used biomarkers (sepsis: PCT, hs-CRP; AKI: secreatinine, urine output) show several limitations regarding the diagnosis and prognosis of sepsis and AKI, hence investigating novel laboratory markers may prove to be beneficial in achieving a more favorable outcome. Most of the discussed AKI biomarkers provide valuable information regarding the injury of the tubular system, yet the monitoring of serum and urinary actin levels along with measuring the proteins of the actin-scavenger system (GSN, Gc-globulin) could yield more complex information for the assessment of overall renal damage involving both glomerular and tubular sources of kidney injury. Moreover, the increase of these markers could yield valuable information regarding the need for the early initiation of RRT, thus potentially attenuating renal injury and improving the outcome of sepsis-related AKI. Urinary markers provide a non-invasive tool for monitoring of inflammatory conditions. Since sepsis-related AKI is a heterogeneous clinical syndrome, it seems that the measurement of a single marker alone would be insufficient for accurate diagnostic and monitoring purposes. Therefore, a multi-marker approach involving various serum and urinary biomarkers and the complex evaluation of the clinical parameters should improve patient management at the ICU.
Acknowledgements
The present research was funded by the 2020-4.1.1.-TKP2020 project (Thematic Excellence Program 2020 - National Excellence Subprogram of the Hungarian Ministry for Innovation and Technology) and partially by the Human Reproduction National Laboratory as part of the “Establishment of National Laboratories 2020” program. Zoltán Horváth-Szalai was supported by the University of Pécs, Medical School, Hungary grant (KA-2019-36). We express our special thanks for the invaluable help of the nurses and the colleagues from every participating institute.
Footnotes
Ethical statement
The authors state that they have obtained institutional review board approval from the Regional Research Ethics Committee of the University of Pécs (no. 4327.316-2900/KK15/2011) conforming to the 7th revision of the Helsinki Declarations (2013) for the research described. Verbal and written informed consent were obtained from the patients for the inclusion of their medical and treatment history within this work.
Competing interests: The authors have declared that no competing interests exist.
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. Jama. 2017;318(13):1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. Jama Network Open. 2019;2(2):e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. 2016;315(8):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Medicine. 2017;43(3):304-377. [DOI] [PubMed] [Google Scholar]
- 6.Póvoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragão A, et al. C-reactive protein as an indicator of sepsis. Intensive Care Medicine. 1998;24(10):1052-1056. [DOI] [PubMed] [Google Scholar]
- 7.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, et al. High-sensitivity C-reactive protein and risk of sepsis. PloS One. 2013;8(7):e69232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. Journal of Cellular Biochemistry. 2019;120(4):5852-5859. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary & Pancreatic Diseases International: HBPD INT. 2017;16(4):424-430. [DOI] [PubMed] [Google Scholar]
- 10.Karabag Y, Cagdas M, Rencuzogullari I, Karakoyun S, Artac I, Ilis D, et al. Relationship between C-reactive protein/albumin ratio and coronary artery disease severity in patients with stable angina pectoris. Journal of Clinical Laboratory Analysis. 2018;32(7):e22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto T, Fujitani M, Fukuyama H, Hatanaka S, Koizumi Y, Kawabata A. The C-Reactive Protein/Albumin Ratio Is Useful for Predicting Short-Term Survival in Cancer and Noncancer Patients. Journal of Palliative Medicine. 2019;22(5):532-537. [DOI] [PubMed] [Google Scholar]
- 12.Sierra R, Rello J, Bailén MA, Benítez E, Gordillo A, León C, et al. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensive Care Medicine. 2004;30(11):2038-2045. [DOI] [PubMed] [Google Scholar]
- 13.Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clinica Chimica Acta. 2002;323(1):17-29. [DOI] [PubMed] [Google Scholar]
- 14.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2013;13(5):426-435. [DOI] [PubMed] [Google Scholar]
- 15.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret G-Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Critical Care Medicine. 2006;34(7):1996-2003. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Wu X, Qin H, Zhu H. The PCT to Albumin Ratio Predicts Mortality in Patients With Acute Kidney Injury Caused by Abdominal Infection-Evoked Sepsis. Frontiers in Nutrition. 2021;8(179). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannakopoulos K, Hoffmann U, Ansari U, Bertsch T, Borggrefe M, Akin I, et al. The Use of Biomarkers in Sepsis: A Systematic Review. Current Pharmaceutical Biotechnology. 2017;18(6):499-507. [DOI] [PubMed] [Google Scholar]
- 18.Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Critical Care. 2020;24(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirakawa K, Naitou K, Hirose J, Takahashi T, Furusako S. Presepsin (sCD14-ST): development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clinical Chemistry and Laboratory Medicine. 2011;49(5):937-939. [DOI] [PubMed] [Google Scholar]
- 20.Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. Journal of Infection and Chemotherapy. 2005;11(5):234-238. [DOI] [PubMed] [Google Scholar]
- 21.Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy. 2012;18(6):891-897. [DOI] [PubMed] [Google Scholar]
- 22.Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. Journal of Infection and Chemotherapy. 2011;17(6):764-769. [DOI] [PubMed] [Google Scholar]
- 23.Velissaris D, Zareifopoulos N, Karamouzos V, Karanikolas E, Pierrakos C, Koniari I, et al. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus. 2021;13(5):e15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CC, Lan HM, Han ST, Chaou CH, Yeh CF, Liu SH, et al. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: a systematic review and meta-analysis. Annals of Intensive Care. 2017;7(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Liu D, Liu Y-N, Wang R, Xie L-X. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Critical Care. 2015;19(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata T, Yasuda Y, Ando M, Abe T, Katsuno T, Kato S, et al. Clinical impact of kidney function on presepsin levels. PloS One. 2015;10(6):e0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Hoshino K, Kiyomi F, Kawano Y, Mizunuma M, Tanaka J, et al. Comparison of accuracy of presepsin and procalcitonin concentrations in diagnosing sepsis in patients with and without acute kidney injury. Clinica Chimica Acta. 2019;490:200-206. [DOI] [PubMed] [Google Scholar]
- 28.Shimoyama Y, Umegaki O, Kadono N, Minami T. Presepsin and prognostic nutritional index are predictors of septic acute kidney injury, renal replacement therapy initiation in sepsis patients, and prognosis in septic acute kidney injury patients: a pilot study. BMC Nephrology. 2021;22(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. Presepsin and sepsis-induced acute kidney injury treated with continuous renal replacement therapy: will another promising biomarker bite the dust? Critical Care. 2015;19:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813-818. [DOI] [PubMed] [Google Scholar]
- 31.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nature Reviews Nephrology. 2018;14(10):607-625. [DOI] [PubMed] [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2(1):1-138. [Google Scholar]
- 33.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrology, Dialysis, Transplantation. 2009;24(11):3263-3265. [DOI] [PubMed] [Google Scholar]
- 34.Oh DJ. A long journey for acute kidney injury biomarkers. Renal Failure. 2020;42(1):154-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Medicine. 2017;43(6):816-828. [DOI] [PubMed] [Google Scholar]
- 36.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical Care. 2013;17(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Y, Gong Z, Wu Y, Tian Y, Liao X. Diagnostic Value of Urine Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Acute Kidney Injury: A Meta-Analysis. PloS One. 2017;12(1):e0170214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellum JA, Artigas A, Gunnerson KJ, Honore PM, Kampf JP, Kwan T, et al. Use of Biomarkers to Identify Acute Kidney Injury to Help Detect Sepsis in Patients With Infection. Critical Care Medicine. 2021;49(4):e360-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JW, Fujii T. The Prevalence of Low Plasma Neutrophil Gelatinase-Associated Lipocalin Level in Systemic Inflammation and its Relationship with Proinflammatory Cytokines, Procalcitonin, Nutritional Status, and Leukocyte Profiles. Clinical Laboratory. 2019;65(6). [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Xie S, Xiao K, Yan P, He W, Xie L. Biomarkers of Sepsis-Induced Acute Kidney Injury. BioMed Research International. 2018;2018:6937947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert C, Zapf A, Haase M, Rover C, Pickering JW, Albert A, et al. Neutrophil Gelatinase-Associated Lipocalin Measured on Clinical Laboratory Platforms for the Prediction of Acute Kidney Injury and the Associated Need for Dialysis Therapy: A Systematic Review and Meta-analysis. American Journal of Kidney Diseases. 2020;76(6):826-841e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JJ, Lee TH, Lee CC, Chang CH. Using lipocalin as a prognostic biomarker in acute kidney injury. Expert Review of Molecular Diagnostics. 2021;21(5):455-464. [DOI] [PubMed] [Google Scholar]
- 43.Qiu Z-L, Yan B-Q, Zhao R, Xu D-W, Shen K, Deng X-q, et al. Combination of hepcidin with neutrophil gelatinase-associated lipocalin for prediction of the development of sepsis-induced acute kidney injury. Clinica Chimica Acta. 2021;523:38-44. [DOI] [PubMed] [Google Scholar]
- 44.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney International. 2002;62(1):237-244. [DOI] [PubMed] [Google Scholar]
- 45.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. American Journal of Physiology Renal physiology. 2004;286(3):F552-F563. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Don-Wauchope AC. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: a systematic review. Inflammation & Allergy Drug Targets. 2011;10(4):260-271. [DOI] [PubMed] [Google Scholar]
- 47.Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, et al. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PloS One. 2014;9(1):e84131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurlow JS, Abbott KC, Linberg A, Little D, Fenderson J, Olson SW. SCr and SCysC Concentrations Before and After Traumatic Amputation in Male Soldiers: A Case-Control Study. American Journal of Kidney Diseases. 2014;63(1):167-170. [DOI] [PubMed] [Google Scholar]
- 49.Aakjær M, Houlind MB, Treldal C, Ankarfeldt MZ, S Jensen P, Andersen O, et al. Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing. Journal of Clinical Medicine. 2019;8(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the Risk of Death and Cardiovascular Events among Elderly Persons. New England Journal of Medicine. 2005;352(20):2049-2060. [DOI] [PubMed] [Google Scholar]
- 51.Séronie-Vivien S, Delanaye P, Piéroni L, Mariat C, Froissart M, Cristol JP. Cystatin C: current position and future prospects. Clinical Chemistry and Laboratory Medicine. 2008;46(12):1664-1686. [DOI] [PubMed] [Google Scholar]
- 52.Martensson J, Martling CR, Oldner A, Bell M. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrology, Dialysis, Transplantation. 2012;27(2):576-581. [DOI] [PubMed] [Google Scholar]
- 53.Szirmay B, Kustan P, Horvath-Szalai Z, Ludany A, Lakatos A, Muhl D, et al. Novel automated immune turbidimetric assay for routine urinary cystatin-C determinations. Bioanalysis. 2018;10(6):377-384. [DOI] [PubMed] [Google Scholar]
- 54.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW. Angiopathic consequences of saturating the plasma scavenger system for actin. Proceedings of the National Academy of Sciences of the United States of America. 1990; 87(4):1381-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. The New England Journal of Medicine. 1992;326(20):1335-1341. [DOI] [PubMed] [Google Scholar]
- 56.Belsky JB, Morris DC, Bouchebl R, Filbin MR, Bobbitt KR, Jaehne AK, et al. Plasma levels of F-actin and F:G-actin ratio as potential new biomarkers in patients with septic shock. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals. 2016;21(2):180-185. [DOI] [PubMed] [Google Scholar]
- 57.Sudakov NP, Klimenkov IV, Byvaltsev VA, Nikiforov SB, Konstantinov YM. Extracellular Actin in Health and Disease. Biochemistry Biokhimiia. 2017;82(1):1-12. [DOI] [PubMed] [Google Scholar]
- 58.Kwon O, Molitoris BA, Pescovitz M, Kelly KJ. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. American Journal of Kidney Diseases. 2003;41(5):1074-1087. [DOI] [PubMed] [Google Scholar]
- 59.Ragan D, Kustan P, Horvath-Szalai Z, Szirmay B, Bugyi B, Ludany A, et al. Urinary actin, as a potential marker of sepsisrelated acute kidney injury: A pilot study. PloS One. 2021; 16(7):e0255266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maiti S, Bamburg JR. Actin-Capping and -Severing Proteins. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry (Second Edition). Waltham: Academic Press; 2013. p. 18-26. [Google Scholar]
- 61.Krebs J. Calcium | Calcium-Binding Proteins: Cytosolic (Annexins, Gelsolins, and C2-Domain Proteins)*. In: Jez J, editor. Encyclopedia of Biological Chemistry III (Third Edition). Oxford: Elsevier; 2021. p. 621-629. [Google Scholar]
- 62.Suhler E, Lin W, Yin HL, Lee WM. Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Critical Care Medicine. 1997;25(4):594-598. [DOI] [PubMed] [Google Scholar]
- 63.Dahl B, Schiodt FV, Ott P, Gvozdenovic R, Yin HL, Lee WM. Plasma gelsolin is reduced in trauma patients. Shock. 1999;12(2):102-104. [DOI] [PubMed] [Google Scholar]
- 64.Lee PS, Patel SR, Christiani DC, Bajwa E, Stossel TP, Waxman AB. Plasma gelsolin depletion and circulating actin in sepsis: a pilot study. PloS One. 2008;3(11):e3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horvath-Szalai Z, Kustan P, Muhl D, Ludany A, Bugyi B, Koszegi T. Antagonistic sepsis markers: Serum gelsolin and actin/gelsolin ratio. Clinical Biochemistry. 2017;50(3):127-133. [DOI] [PubMed] [Google Scholar]
- 66.Mozzi A, Forni D, Cagliani R, Pozzoli U, Vertemara J, Bresolin N, et al. Albuminoid genes: evolving at the interface of dispensability and selection. Genome Biology and Evolution. 2014;6(11):2983-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delanghe JR, Speeckaert R, Speeckaert MM. Behind the scenes of vitamin D binding protein: more than vitamin D binding. Best Practice & Research Clinical Endocrinology & Metabolism. 2015;29(5):773-786. [DOI] [PubMed] [Google Scholar]
- 68.Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D Binding Protein: A Historic Overview. Frontiers in Endocrinology. 2020;10(910). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahl B, Schiødt FV, Ott P, Wians F, Lee WM, Balko J, et al. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Critical Care Medicine. 2003;31(1):152-156. [DOI] [PubMed] [Google Scholar]
- 70.Horvath-Szalai Z, Kustan P, Szirmay B, Lakatos A, Christensen PH, Huber T, et al. Predictive value of serum gelsolin and Gc globulin in sepsis - a pilot study. Clinical Chemistry and Laboratory Medicine. 2018;56(8):1373-1382. [DOI] [PubMed] [Google Scholar]
- 71.Chaykovska L, Heunisch F, von Einem G, Alter ML, Hocher C-F, Tsuprykov O, et al. Urinary Vitamin D Binding Protein and KIM-1 Are Potent New Biomarkers of Major Adverse Renal Events in Patients Undergoing Coronary Angiography. PloS One. 2016;11(1):e0145723. [DOI] [PMC free article] [PubMed] [Google Scholar]


