ABSTRACT
Background
As Parkinson's disease (PD) progresses, response to oral medications decreases and motor complications appear. Timely intervention has been demonstrated as effective in reducing symptoms. However, current instruments for the identification of these patients are often complicated and inadequate. It has been suggested that anti‐PD intensified therapy (IT) can serve as a proxy for increased burden of disease.
Objective
To explore whether IT aligns with events reflecting advanced PD (APD) burden.
Methods
This was a retrospective analysis of PD beneficiaries in the second‐largest healthcare provider in Israel. Patients with PD diagnosed between January 2000 and June 2018 and treated with levodopa (l‐dopa) ≥5 times/day and/or ≥1000 mg l‐dopa equivalent daily dose were defined as the IT cohort (n = 2037). Treated patients with PD not fulfilling this criterion were defined as the nonintensified therapy (NIT) cohort (n = 3402). Point prevalence and 5‐ and 10‐year cumulative incidence of IT were assessed. Baseline demographic and comorbidities, 1‐year healthcare resource use, health costs, and time to clinical events were assessed and compared between cohorts.
Results
IT was associated with significantly (P < 0.05) higher healthcare resource use compared with NIT. In turn, IT patients incurred higher healthcare costs (P < 0.001) and were at greater risk for mortality, hospitalization, disability, and device‐aided therapy use (P < 0.001, for all comparisons).
Conclusions
Treatment intensity can serve as an objective and robust indicator of more APD. This readily extractable marker can be easily integrated into electronic medical record alerts to actively target more advanced patients and to guide risk‐appropriate care.
Keywords: Parkinson's disease, levodopa, antiparkinson agents, dyskinesias, cost of illness
Parkinson's disease (PD) is a progressive neurodegenerative disorder affecting 100 to 150 individuals per 100,000 population, with a greater likelihood among men and individuals older than age 65 years. 1 Despite the effectiveness of symptomatic therapies for the motor features of PD in the early stages of the disease, with time, response to oral dopaminergic preparations, primarily levodopa (l‐dopa)‐based drugs, begins to fluctuate, with both dyskinesia and on/off phenomena. This stage is paralleled by exacerbation of both motor deficits and nonmotor complications, significantly interfering with patient mobility, safety, productivity, independence, emotional well‐being, and quality of life. 2 At this stage, device‐assisted therapies (DAT), such as deep brain stimulation, continuous subcutaneous apomorphine infusion, and l‐dopa–carbidopa intestinal gel, may be helpful. 3
Timely intervention in patients with progressing PD has been demonstrated as effective in reducing symptoms, maintaining quality of life, 4 , 5 , 6 , 7 and reducing direct healthcare costs. 8 Yet, the existing instruments for the identification of patients with advanced PD (APD) 9 are complicated, involve multiple subjective and objective factors, and generally fail to comprehensively assess the full range of PD manifestations. 10 , 11 , 12 This shortcoming is generally attributed to the highly pathophysiological, genetic, and clinical heterogeneity of the disease and the limited generalizability of existing tools in diverse patient populations. One of these mentioned instruments is the recently developed Delphi panel of clinical indicators of APD, which relies heavily on movement disorder specialist evaluations 13 , 14 ; however, more than half of the patients diagnosed with PD in the United States were seen only by primary care physicians in 2011. 15 Such gaps in the clinical profiling of patients with PD were shown to result in suboptimal quality of care. 16 Sensitive and reliable indicators for the advanced stage of the disease remain to be identified 17 , 18 to facilitate standardized, objective, and robust diagnosis, surveillance, and screening for the clinical progression of PD with the ultimate aim of referring patients to a specialist for a detailed assessment. This study exploited real‐world data from a large healthcare organization to explore the utility of intensified drug regimens as a proxy for APD.
Methods
Patient Population
This retrospective cohort analysis was carried out at Maccabi Health Service (MHS), a 2.5‐million‐enrollee, state‐mandated health organization in Israel, servicing 26% of the total population. In accordance with the National Health Insurance Act, membership in MHS is open to all Israeli citizens. The organization's electronic medical records (EMRs) database integrates data from the MHS central laboratory, medication prescriptions, records of purchases within the MHS pharmacy network, consultations, hospitalizations, procedures, and sociodemographic information. The study was approved by the Institutional Ethics Committee of MHS at Bait Ba'lev Hospital. All data were anonymously analyzed, and patient consent was therefore not required.
For the purpose of the study, patients with PD were defined as MHS members with idiopathic PD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9]: 332.0) diagnosed by a neurologist and with at least 1 anti‐PD medication purchase between January 1, 2000, and June 1, 2018. Date of first diagnosis or first anti‐PD medication purchase, the earliest of the two, was defined as the first indication of PD. Additional inclusion criteria were (1) continuous membership in MHS for at least 12 months prior to first PD indication and (2) continuous membership in MHS for at least 12 months thereafter (or until death). Eligible patient records were categorized by PD therapy intensity, with nonintensified therapy (NIT) defined as oral l‐dopa <5 times/day and <1000 mg l‐dopa equivalent dose/day (LEDD). Intensified therapy (IT) was defined as oral l‐dopa ≥5 times/day and/or ≥1000 mg LEDD based on Tomlinson's proposal and our expert's opinion. 14 , 19 , 20 Patients who switched from NIT to IT during the study period were included in the IT cohort only. Index date was set to the first purchase of anti‐PD medication for the NIT group and the first intensified purchase for the IT group.
Study Variables
IT point prevalence on June 1, 2018, was defined as the number of IT patients alive in MHS at that time point, divided by all treated patients with PD (IT + NIT) alive at that point. Data regarding patient demographics, pertinent prescription and medication purchase information, comorbidities, smoking habits, and socioeconomic status (SES) were extracted.
Demographics
SES was derived for commercial purposes by Points Location Intelligence using geographic information systems and financial data, such as expenditures related to retail chains, credit cards, and housing. This score is highly correlated with SES measured by the Central Bureau of Statistics. 21 SES was categorized into low, 1 , 2 , 3 , 4 medium, 5 , 6 and high. 7 , 8 , 9 , 10 Smoking status was defined as the last recorded status in the EMR prior to the index date.
Comorbidities
Cardiovascular disease, 22 diabetes, 23 hypertension, 24 cancer, and chronic kidney disease 25 were identified using previously validated MHS automated chronic disease registries. Registry entry date was considered the comorbidity diagnosis date. Cognitive dysfunction, polyneuropathy, sleep disorders, depression, and chronic upper gastrointestinal disease were defined as at least 1 diagnosis prior to the index date. Identification of these comorbidities was performed using ICD‐9 and internal MHS diagnosis codes.
Disease Burden
Clinical disease burden was assessed by cumulative number of clinical events and healthcare resource use (ie, number of falls, hospitalization days, general physician visits, neurologist visits, emergency room [ER] visits not requiring hospitalization, physical therapy sessions, occupational therapy sessions, speech therapy sessions, and sessions with geriatrician) recorded from the index date (commencement of NIT or IT) until 1 year after the index date unless there was an earlier initiation of DAT, a death, or the end of the study period was reached (June 1, 2018). Clinical burden of disease was also assessed by time to event from index date to death, disability, first hospitalization, and first treatment with DAT. 26 Only falls that required medical attention and were registered by the physician were captured. Disability was defined by the National Insurance Institute in Israel disability status.
Economic disease burden in the first year after the index date was assessed by calculating healthcare resource use costs (ie, hospitalization days, outpatient visits, ER visits, procedures and exams, and medication purchases), which were extracted from the Israeli Ministry of Health Price List for Ambulatory and Hospitalization Services.
Statistical Analysis
Baseline characteristics were analyzed with descriptive statistics. Means and standard deviations and medians and interquartile ranges were used to describe continuous variables, depending on the variable's distribution. Categorical variables are presented by counts and proportions. Comparison between study cohorts was explored using the t test, a parametric test, for continuous variables and the χ2 test for categorical variables. Standard mean difference was calculated to assess the absolute effect size for each comparison. A standard difference <0.1 was taken to indicate a negligible difference in the mean or prevalence of a covariate between treatment groups. 27
Median disease duration at index (defined as time from PD first indication to index date), total disease duration (defined as time from PD first indication to end of follow‐up), and the cumulative incidence of IT were explored using Kaplan–Meier survival tables and curves. Comparisons were performed using a log‐rank test.
The cumulative event rates for burden of disease were compared using generalized linear models (GLMs) with a negative binomial distribution with log link. Time to event was compared between the 2 cohorts using Cox proportional hazard models.
Healthcare costs, measured in New Israeli Shekels, were transformed to cost units (US dollars [USD]) using the purchasing power parities 2018 (end of the study) as a conversion factor (1 USD = 3.752 Israeli shekels). In cases where the year post‐index date was not a full year (as a result of mortality), the data were annualized. 28 Data were analyzed in log scale. The estimated means and 95% confidence intervals (CIs) were extracted from the GLM analyses using the gamma distribution. All multivariate comparisons were adjusted for age at index, sex, index year, and disease duration at index.
All analyses were conducted using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY).
Results
NIT and IT Populations and Baseline Characteristics
In total, 5439 patients with PD registered in the MHS database met the inclusion criteria for this analysis (Fig. 1), 62.6% (n = 3402) of whom were receiving NIT, whereas 37.4% (n = 2037) were receiving IT (Table 1). Of the IT patients, 43.2% (n = 880) fulfilled the oral l‐dopa ≥5 times/day criterion, and 30.1% (n = 614) fulfilled the ≥1000 mg LEDD criterion; 26.6% (n = 543) of the patients met both criteria. Mean age at index in both cohorts was similar (72 years), and more than half (NIT, 52%; IT, 59%) of the patients were men. Median disease duration at index for NIT and IT was 0 years and 3.6 years (95% CI, 3.5–3.8), and median total disease duration was 9.1 years (95% CI, 8.2–9.5) and 12.1 years (95% CI, 11.7–12.5), respectively. A higher prevalence of cognitive dysfunction (7.5% vs. 4.1%) and sleep disorders (30% vs. 23.5%) was noted among IT compared with NIT patients (Table 1). The percentage of smokers in the 2 PD cohorts was similar (NIT, 14.0%; IT, 14.5%). On June 1, 2018, the IT point prevalence was 38%. The 5‐ and 10‐year cumulative incidences of IT were 26.3% and 48%, respectively (Fig. 2).
FIG. 1.
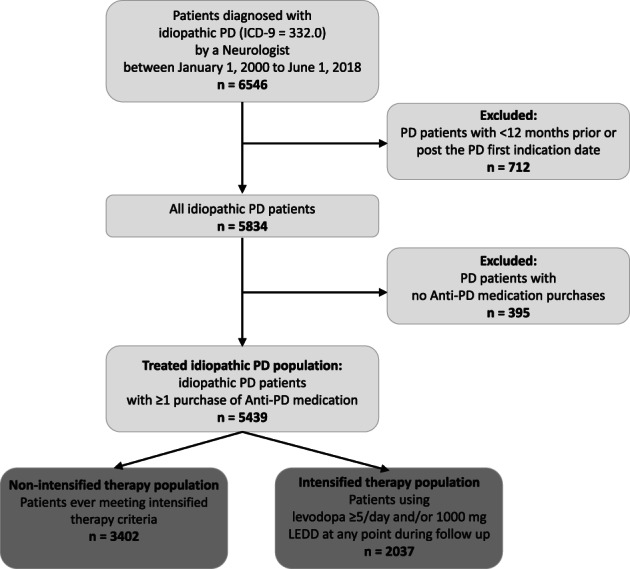
Attrition process. ICD‐9, International Classification of Diseases, Ninth Revision, Clinical Modification; LEDD, levodopa equivalent daily dose; PD, Parkinson's disease.
TABLE 1.
Baseline characteristics of study groups
| Baseline characteristic | Nonintensified, n = 3402 | Intensified, n = 2037 | SMD |
|---|---|---|---|
| Age at index, y, mean (SD) | 72.6 (9.8) | 72.4 (9.7) | 0.014 |
| Time from PD first indication to index, y, median (95% CI) | 0 (NA) | 3.6 (3.5–3.8) | <0.001 a |
| Total disease duration, y, median (95% CI) | 9.2 (8.8–9.5) | 12.1 (11.7–12.5) | <0.001 a |
| Levodopa/LEDD ratio, median (95% CI) | 0.75 (0.714–0.752) | 0.83 (0.826–0.878) | 0.055 |
| Sex, male, n (%) | 1782 (52.4) | 1207 (59.3) | 0.139 b |
| Socioeconomic status, n (%) | 0.087 | ||
| Low | 254 (7.5) | 122 (6.0) | |
| Medium | 2371 (69.7) | 1387 (68.1) | |
| High | 702 (20.6) | 479 (23.5) | |
| Missing | 75 (2.2) | 49 (2.4) | |
| Smoking, ever, n (%) | 218 (14) | 107 (14.5) | 0.016 |
| Cardiovascular disease, n (%) | 746 (21.9) | 501 (24.5) | 0.062 |
| Hypertension, n (%) | 2010 (59.1) | 1308 (64.1) | 0.103 |
| Diabetes, n (%) | 877 (25.8) | 489 (23.9) | 0.043 |
| Cognitive dysfunction, n (%) | 138 (4.1) | 153 (7.5) | 0.148 b |
| Polyneuropathy, n (%) | 355 (10.4) | 272 (13.4) | 0.09 |
| Sleep disorders, n (%) | 800 (23.5) | 612 (30.0) | 0.148 b |
| Depression, n (%) | 47 (1.4) | 40 (2.0) | 0.045 |
| Chronic upper gastrointestinal disease, n (%) | 1295 (38.1) | 871 (42.8) | 0.096 |
Comparisons between nonintensified and intensified patients were made using chi‐square tests for categorical variables and t tests for continuous variables.
For time from PD first indication to index and total disease duration, Kaplan–Meier and log‐rank tests were applied without the ability to calculate SMD. The P value is presented.
SMD > 0.1. SMD was calculated to assess the absolute effect size for each comparison. A standard difference >0.1 was taken to indicate a significant difference between treatment groups.
Abbreviations: SMD, standard mean difference; SD, standard deviation; PD, Parkinson's disease; CI, confidence interval; NA, not applicable; LEDD, levodopa equivalent daily dose.
FIG. 2.
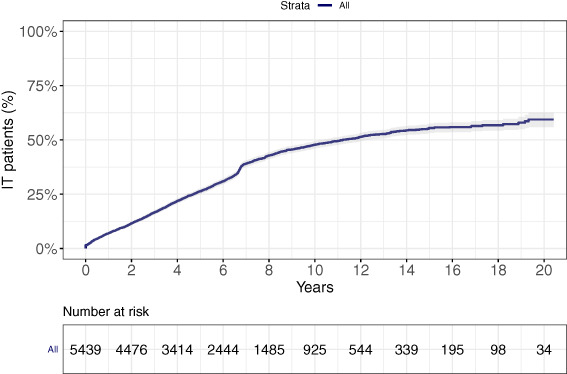
Cumulative incidence of intensified therapy (IT).
Clinical Burden
In the 12 months following the index date, IT patients had 1.5 times (95% CI, 1.2–1.8; P < 0.001) more hospitalization events and 1.9‐fold longer lengths of stay at hospital (95% CI, 1.8–2.1; P < 0.001) compared with NIT patients. Similarly, when compared with NIT patients, IT patients had more visits to a neurologist (incidence rate [IR], 1.2; 95% CI, 1.1–1.3; P < 0.001], geriatrician (IR, 1.7; 95% CI, 1.4–2.0; P < 0.001), physiotherapist (IR, 1.6; 95% CI, 1.4–1.7; P < 0.001), speech therapist (IR, 1.7; 95% CI, 1.2–2.4; P = 0.002), and occupational therapist (IR, 1.8; 95% CI, 1.4–2.2; P < 0.001). Within this same time interval, IT patients had 1.8 times (95% CI, 1.6–2.0; P < 0.001) more documented falls compared with NIT patients. No intercohort differences in the frequencies of general physician and ER visits were noted (Table 2). The risk for mortality (17%; 95% CI, 5%–30%), disability (57%; 95% CI, 41%–75%), and hospitalization (16%; 95% CI, 7%–27%) was increased for the IT patients compared with the NIT patients. As expected, IT patients were 4.7 times more likely to be treated with DAT compared with NIT (hazard ratio, 4.7; 95% CI, 2.9–7.8) (Fig. 3).
TABLE 2.
Cumulative yearly clinical event estimated means and incidence in patients on intensified versus nonintensified anti–Parkinson's disease treatment
| Event | Estimated Mean (95% CI) or n (%) | Incidence Ratio (95% CI) or Odds Ratio (95% CI) | P Value* | |
|---|---|---|---|---|
| Nonintensified | Intensified | |||
| Hospitalization, yes/no | 659 (19.4) | 585 (28.7) | 1.5 (1.2–1.8) | <0.001 |
| Hospital length of stay, days | 2.5 (2.4–2.6) | 4.9 (4.6–5.2) | 2 (1.8–2.1) | <0.001 |
| Visits | ||||
| ER | 0.38 (0.35–0.4) | 0.43 (0.39–0.47) | 1.1 (1–1.3) | 0.063 |
| General physician | 15.0 (14.4–15.6) | 15.2 (14.3–16.0) | 1 (0.9–1.1) | 0.820 |
| Neurologist | 2.9 (2.8–3.0) | 3.5 (3.3–3.74) | 1.2 (1.1–1.3) | <0.001 |
| Geriatric | 0.13(0.11–0.14) | 0.21 (0.19–0.24) | 1.7 (1.4–2) | <0.001 |
| Psychiatrist | 0.36 (0.34–0.39) | 0.29 (0.26–0.33) | 0.8 (0.7–0.9) | 0.004 |
| Sessions | ||||
| Physiotherapist | 2.9 (2.8–3.0) | 4.5 (4.3–4.8) | 1.6 (1.4–1.7) | <0.001 |
| Speech therapist | 0.03 (0.02–0.04) | 0.05 (0.04–0.06) | 1.7 (1.2–2.4) | 0.002 |
| Occupational therapist | 0.09 (0.08–0.11) | 0.17 (0.14–0.19) | 1.8 (1.4–2.2) | <0.001 |
| Falls | 0.35 (0.32–0.37) | 0.6 (0.57–0.7) | 1.8 (1.6–2) | <0.001 |
Estimated means, incidence ratios, and P values for continuous variables were calculated using a generalized linear model with a negative binomial distribution with log link. Odds ratios, 95% CIs, and P values for dichotomous variables were calculated using logistic regression.
Adjusted for age at index, sex, index year, and disease duration at index.
Abbreviations: CI, confidence interval; ER, emergency room.
FIG. 3.
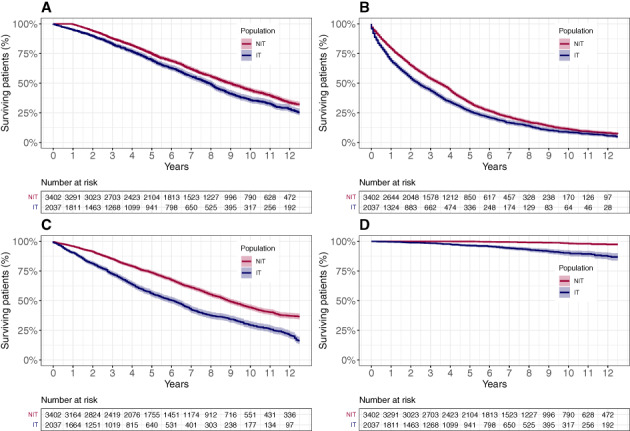
Time to clinical events in patients on intensified (IT) versus nonintensified (NIT) anti‐Parkinson's disease treatment. (A) Mortality (P = 0.004), (B) first hospitalization (P = 0.001), (C) disability (P < 0.001), and (D) device‐aided therapy initiation (P < 0.001). P values were calculated using a Cox proportional hazard model. Analyses were adjusted for age at index, sex, index year, and disease duration at index.
Economic Burden
Overall, total healthcare expenditures were 21.5% higher for IT compared with NIT patients (P < 0.001). The higher costs were most significant in medication purchases, hospitalization stays, and ER visits, which were increased by 52.2%, 24.4%, and 12.6%, respectively, in the IT versus NIT groups. Costs associated with outpatient clinic visits and the procedure and exams did not differ between treatment groups (Table 3).
TABLE 3.
Cost analysis a of nonintensified versus intensified anti‐Parkinson's disease treatment
| Healthcare resources | Nonintensified Estimated Mean (95% CI) | Intensified Estimated Mean (95% CI) | P Value |
|---|---|---|---|
| Hospitalization days | 5499 (4898–6182) | 6838 (6013–7789) | 0.03 |
| Outpatient visits | 2132 (2076–2189) | 2241 (2160–2327) | 0.056 |
| ER visits | 330 (317–343) | 371 (352–392) | 0.002 |
| Procedures and exams | 1385 (1298–1478) | 1410 (1286–1547) | 0.782 |
| Medications | 956 (927–985) | 1454 (1391–1520) | <0.001 |
| Total costs | 6075 (5849–6312) | 7381 (6990–7795) | <0.001 |
Estimated means, 95% CIs, and P values were calculated using a generalized linear model with the gamma distribution with log link. Analyses are adjusted for age at index, sex, index year, and disease duration at index.
Costs in US dollars using purchasing power parities.
Abbreviations: CI, confidence interval; ER, emergency room.
Discussion
Intensified drug regimens were associated with multiple indices of PD progression, including deteriorating postural stability, more frequent use of health professionals' services, and a greater likelihood of DAT initiation, disability, hospitalization, and all‐cause mortality. The current observational, EMR‐based cohort study showed that IT, defined by the combination of 2 established criteria (≥5 times daily oral l‐dopa and/or ≥1000 mg LEDD), is a strong proxy for the identification of patients with suspected APD. Despite the power of the “enhanced criteria,” they effectively identified patients with severe disease manifestations, as reflected by higher disease burden. Close monitoring of this highly accessible indicator can facilitate identification of progressing PD and dictate referral to specialized care to improve clinical outcomes and decrease potential health hazards.
The ≥1000 mg LEDD criterion, a long‐standing indicator of APD, has been applied in treatment optimization and healthcare resource cost and use studies. 19 , 29 , 30 Weir et al identified this indicator of advanced disease in 1% of a 7060‐patient study cohort 1 year after PD diagnosis and in 49% of the patients within 10 years of diagnosis. APD was associated with significantly higher annual healthcare use and higher annual healthcare costs compared with patients with milder forms of PD. 30 A recently assessed pharmacy claims‐based (LEDD >1000 mg/day) algorithm 19 found higher odds of deep brain stimulation treatment, falls, hallucinations, and use of specialized equipment or facilities among patients with APD. In parallel, the ≥5 times daily oral l‐dopa criterion, the IT component of the “5‐2‐1” criteria proposed by the Delphi expert consensus panel for flagging patients with suspected APD, has been validated in numerous studies and is included in the recently published MANAGE‐PD (Making Informed Decisions to Aid Timely Management of Parkinson's Disease) comprehensive screening tool. 31 Two studies comparing physician‐diagnosis versus Delphi‐defined PD staging found a significant correlation between the two, 32 , 33 with more than half of the patients with APD meeting the “5” criterion. 33 Studies implementing these criteria confirmed their correlation with established disease burden predictors, including prolonged disease duration, 34 more severe motor and nonmotor burden, and compromised quality of life, compared with patients failing to meet these criteria. 32 , 33 , 34
Risk of PD deterioration, as identified by therapy intensity, increased with duration of follow‐up, with 26.3% and 48% of patients progressing to IT within 5 years and 10 years from first PD indication, respectively. These findings align with the reported steep increase in risk of PD complications noted in a historical cohort analysis of 1232 PD outpatients referred to a single movement disorder clinic. 35 Specifically, evidence from the literature predicted significant intensification of hazard rates for motor fluctuations and dyskinesia and for postural instability and falls 5 to 6 years after diagnosis and of risk of dementia progression 10 years after diagnosis, with differences in risk levels related to sex and time from onset. Similarly, a chart review of 126 incident patients with PD treated with l‐dopa for at least 2 months between the years 1976 and 1990 found 17% and 43% estimated rates of l‐dopa–associated dyskinesia requiring medication adjustments following 5 and 10 years of treatment, respectively. 36 In agreement with the described studies and the current findings, in their meta‐analysis of studies using motor dyskinesia as a measure of PD exacerbation, Ahlskog and Muenter 37 found that the likelihood of motor fluctuations increased from zero to 39% within 4 to 6 years of the start of l‐dopa treatment. A population‐based cohort study involving 279 patients with PD reported a 30% rate of dyskinesia development within a median 4‐year period from l‐dopa initiation. 38
IT patients had greater risks for mortality, disability, and hospitalization compared with NIT patients, as was shown in previous publications evaluating the change in risk with increased disease severity. Forsaa et al showed that each 10‐point increase in Unified PD Rating Scale (UPDRS; a measure of disease severity with increasing scores indicating increasing severity) 39 , 40 was associated with a 1.25‐fold increase in risk for mortality. In addition, every 100‐mg increase in LEDD was associated with a 1.07‐fold increased risk for mortality. 40 Levy et al reported that every 1‐point increase in the motor UPDRS score was associated with a 1.04‐fold increased risk for mortality. 41 In a 13‐year follow‐up of the DATATOP (Deprenyl and tocopherol antioxidative therapy of parkinsonism) cohort, 42 a cohort of 800 patients with early PD without severe motor symptoms at baseline, an increased risk of mortality was associated with a Hoehn and Yahr (H&Y) 43 stage increase and with a 10‐point increase in total UPDRS score. 44 Kaltenboeck et al found that 24%, 55%, 80%, and 63% of patients with an ambulatory assistance device, PD‐related claims for skilled nursing, early disease, or newly diagnosed disease, respectively, were alive at year 5 post index. 45 Disability, measured as loss of independence, was associated with the interaction between increased total UPDRS score and H&Y stage. 46 Disease severity, manifested as higher H&Y stage, more motor fluctuations, and prolonged timed “up and go” test, 47 was associated with an increased risk for 1 or more hospital encounters. 48
The present analysis also showed that IT patients had higher healthcare resource use and, as a result, higher economic burden compared with NIT patients. Disease severity has previously been associated with higher medical costs. Kaltenboeck et al showed that patients with an ambulatory assistance device or patients with PD‐related claims for skilled nursing had higher medical costs compared with newly diagnosed patients and patients with early disease. 45 It has also been shown that medical costs increased with the progression in H&Y disease stage (in patients with motor fluctuations), with increase in medication off time during the day and with longer time of dyskinesia. 49 Another work reported on higher healthcare resource use, such as hospitalizations (number of admissions and length of stay), medical specialist services, imaging, and allied health services (physiotherapy, podiatry, occupational therapy, speech therapy, and psychology), in patients with moderate–severe PD compared with those with mild disease. As a result, patients with moderate–severe disease had higher medical costs compared with patients with mild disease. 50
Compared with NIT patients, IT patients were more likely to have underlying sleep disorders and cognitive dysfunction at baseline. Indeed, sleep disorders are among the most frequently reported nonmotor symptoms in patients with PD, and they generally exacerbate with disease progression, 51 affecting as many as 78% of patients in advanced stages of the disease. 52 The trajectory of chronic PD pathology can be directly linked to dementia‐inducing processes. However, studies reporting on the practical inevitability of cognitive decline with advancing age 53 , 54 , 55 , 56 raise questions regarding the contribution of age and accumulating comorbidities to these events. In the present analysis, the IT cohort reported on higher rates of cognitive impairment, yet mean age was the same as the mean age of NIT patients, suggesting that the cognitive dysfunction might be correlated to disease progression.
Study strengths included the large sample size in this analysis of real‐world, systematic, longitudinal, prospectively collected care data, suggesting the generalizability of the presented findings to PD populations in Israel. Moreover, in efforts to ensure a validated and highly specific definition of PD, only patients diagnosed by a neurologist as having idiopathic PD (ICD‐9: 332.0) and who were treated with antiparkinsonian medications were included in the analysis. In addition, visit as opposed to referral measures were collected for this analysis, as they are more likely to reflect actual healthcare use statistics. Identification of patients with suspected APD solely based on LEDD may exclude elderly or comorbid patients with limited tolerability to high‐dose therapies. 57 Yet, expansion of the treatment intensification definition through integration of the 2 intensified treatment criteria, as was done in the current analysis, may partially overcome these limitations and consequently identify a larger set of patients with PD who could potentially benefit from adjusted or customized treatment and more targeted surveillance.
Limitations of the analysis included failure to capture services provided beyond the framework of the MHS basic or supplementary insurance packages. However, given the low copayment rates in Israel, it can be assumed that the majority of patients received MHS‐subsidized treatments only. In addition, although there was no validation that purchased medications were actually administered, our earlier study demonstrated that patients routinely purchasing medications are likely to consume them. 58 Miscoding of atypical parkinsonian syndromes (eg, multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, Lewy body dementia) may have led to the inclusion of non‐PD patients in the collected data set but was assumed to have had a negligible skewing effect. Furthermore, as a retrospective analysis of a healthcare system database, the diagnostic accuracy of common disease symptoms may have been overrated, whereas others less recognized as features of PD may have been underreported. Furthermore, the burden of disease was only determined for the first year post index, which is likely to underestimate the actual burden over time. In addition, the current analysis was not able to detect major complications of advanced disease that are nondopaminergic in nature, such as psychosis, autonomic disturbances, cognitive deterioration, and balance impairment. All of these complications can elevate disease burden and most likely are not reflected in our IT cohort. Lastly, several studies reported that men are treated with higher LEDD dosages compared with women, thus creating a potential selection bias in the current cohort. 59 However, combining 2 criteria for the definition of IT may reduce this bias.
Taken together, treatment intensity can serve as an objective and robust indicator of deterioration of PD symptom control. This readily extractable data can be easily integrated into EMR alerts to actively target more advanced patients likely to be inadequately controlled on oral medications to guide risk‐appropriate care and to streamline specialty referrals. Early identification of failing effectiveness of current treatments using a simple and easily accessible indicator will assist in optimizing PD treatment strategies and standardizing clinical practice while reducing healthcare expenditures. Furthermore, such a proxy model can potentially serve as a meaningful end point for assessing the impact of investigational products on disease progression in future disease‐modifying trials.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
Y.B.: 1B, 1C, 2A, 2B, 3A
T.G.: 1A, 2C, 3B
G.C.: 1A, 1B, 2A, 2C, 3B
N.G.: 1A, 2C, 3B
R.G.: 1A, 1B, 2C, 3B
R.C.: 1A, 2C, 3B
L.B.: 1A, 2C, 3B
Y.J.J.: 1A, 2C, 3B
V.S.: 1A, 2C, 3B
M.G.: 2B, 3B
A.T.: 1A, 1B, 2C, 3B
Disclosures
Ethical Compliance Statement
This study was approved by the Bait Ba'lev Hospital Ethics Board and was granted a waiver of consent. Informed patient consent was not necessary for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The study was funded by AbbVie. The design and study conduct were performed in collaboration of AbbVie with Maccabi healthcare services as well as participation of the interpretation of data, review, and approval of the publication. No honoraria or payments were made for authorship. Y.B., G.C., and V.S. have no conflict of interest.
Financial Disclosures for the Previous 12 Months
T.G. has served as an advisor for Cytora, Synnerva, AbbVie, Teva, Medison, and Allergan and received honoraria from AbbVie and Neuroderm; research support from the Parkinson's Foundation, University Tel‐Aviv, Phonetica Ltd, and Israel innovation authority; and travel support from AbbVie, Medison, Medtronic, and Allergan; and is an investigator in clinical trials in advanced PD, including trials supported by AbbVie. N.G. serves as a member of the editorial board for the Journal of Parkinson's Disease. He serves as a consultant to Sionara, Teva, NeuroDerm, Intec Pharma, Pharma2B, Denali, and AbbVie. He receives royalties from Lysosomal Therapeutics (LTI) and payment for lectures at Teva, UCB, AbbVie, Sanofi‐Genzyme, Bial, and the Movement Disorder Society. He received research support from The Michael J. Fox Foundation, the National Parkinson Foundation, the European Union 7th Framework Program, and the Israel Science Foundation as well as from the Teva NNE program, Biogen, LTI, and Pfizer. R.G. is a former employee of AbbVie, currently employed by BOL Pharma Ltd and may hold AbbVie stock. R.C., L.B., Y.J.J., and M.G.J. are employees of AbbVie and own AbbVie stock. A.T. received honoraria from AbbVie.
Acknowledgments
We thank Yehudit Posen and Sharon Tal of Posen Scientific Writing Ltd. for their medical editing of this manuscript.
References
- 1. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2014;29(13):1583–1590. 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2. Voon V, Napier TC, Frank MJ, et al. Impulse control disorders and levodopa‐induced dyskinesias in Parkinson's disease: an update. Lancet Neurol 2017;16(3):238–250. 10.1016/S1474-4422(17)30004-2. [DOI] [PubMed] [Google Scholar]
- 3. Clarke CE, Worth P, Grosset D, Stewart D. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson's disease. Parkinsonism Relat Disord 2009;15(10):728–741. 10.1016/j.parkreldis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 4. Jann MW. Advanced strategies for treatment of Parkinson's disease: the role of early treatment. Am J Manag Care 2011;17(Suppl 12):S315–S321. [PubMed] [Google Scholar]
- 5. Kulisevsky J, Oliveira L, Fox SH. Update in therapeutic strategies for Parkinson's disease. Curr Opin Neurol 2018;31(4):439–447. 10.1097/WCO.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 6. Wirdefeldt K, Odin P, Nyholm D. Levodopa‐carbidopa intestinal gel in patients with Parkinson's disease: a systematic review. CNS Drugs 2016;30(5):381–404. 10.1007/s40263-016-0336-5. [DOI] [PubMed] [Google Scholar]
- 7. Antonini A, Yegin A, Preda C, Bergmann L, Poewe W, Global study investigators and coordinators . Global long‐term study on motor and non‐motor symptoms and safety of levodopa‐carbidopa intestinal gel in routine care of advanced Parkinson's disease patients; 12‐month interim outcomes. Parkinsonism Relat Disord 2015;21(3):231–235. 10.1016/j.parkreldis.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8. Murman DL. Early treatment of Parkinson's disease: Opportunities for managed care. Am J Manag Care 2012;18(7 Suppl):S183–S188. [PubMed] [Google Scholar]
- 9. Moro E, Allert N, Eleopra R, Houeto JL, Phan TM, Stoevelaar H, International Study Group on Referral Criteria for DBS . A decision tool to support appropriate referral for deep brain stimulation in Parkinson's disease. J Neurol 2009;256(1):83–88. 10.1007/s00415-009-0069-1. [DOI] [PubMed] [Google Scholar]
- 10. Regnault A, Boroojerdi B, Meunier J, Bani M, Morel T, Cano S. Does the MDS‐UPDRS provide the precision to assess progression in early Parkinson's disease? Learnings from the Parkinson's progression marker initiative cohort. J Neurol 2019;266(8):1927–1936. 10.1007/s00415-019-09348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puschmann A, Brighina L, Markopoulou K, et al. Clinically meaningful parameters of progression and long‐term outcome of Parkinson disease: an international consensus statement. Parkinsonism Relat Disord 2015;21(7):675–682. 10.1016/j.parkreldis.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22(13):1901–1911. 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 13. Luquin MR, Kulisevsky J, Martinez‐Martin P, Mir P, Tolosa ES. Consensus on the definition of advanced Parkinson's disease: a neurologists‐based Delphi study (CEPA study). Parkinsons Dis 2017;2017:4047392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson's disease: a multi‐country Delphi‐panel approach. Curr Med Res Opin 2018;34(12):2063–2073. 10.1080/03007995.2018.1502165. [DOI] [PubMed] [Google Scholar]
- 15. Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77(9):851–857. 10.1212/WNL.0b013e31822c9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson's disease. Mov Disord 2007;22(4):515–522. 10.1002/mds.21311. [DOI] [PubMed] [Google Scholar]
- 17. Yilmaz R, Hopfner F, van Eimeren T, Berg D. Biomarkers of Parkinson's disease: 20 years later. J Neural Transm (Vienna) 2019;126(7):803–813. 10.1007/s00702-019-02001-3. [DOI] [PubMed] [Google Scholar]
- 18. Gwinn K, David KK, Swanson‐Fischer C, Albin R, Hillaire‐Clarke CS, Sieber BA, et al. Parkinson's disease biomarkers: perspective from the NINDS Parkinson's disease biomarkers program. Biomark Med 2017;11(6):451–473. 10.2217/bmm-2016-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahodwala N, Pettit AR, Jahnke J, et al. Use of a medication‐based algorithm to identify advanced Parkinson's disease in administrative claims data: associations with claims‐based indicators of disease severity. Clin Park Relat Disord 2020;3:100046. 10.1016/j.prdoa.2020.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 21. Israel Central Bureau of Statistics . Characterization and classification of geographic units by the soci‐economic level of the population 2008. Jerusalem, Israel: 2013. Publication No. 1530
- 22. Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol 2011;152(3):345–349. 10.1016/j.ijcard.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 23. Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 2003;18(12):1143–1146. 10.1023/b:ejep.0000006635.36802.c8. [DOI] [PubMed] [Google Scholar]
- 24. Weitzman D, Chodick G, Shalev V, Grossman C, Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension 2014;64(3):501–507. 10.1161/HYPERTENSIONAHA.114.03718. [DOI] [PubMed] [Google Scholar]
- 25. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA 2014;311(24):2518–2531. 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dodel RC, Berger K, Oertel WH. Health‐related quality of life and healthcare utilisation in patients with Parkinson's disease: impact of motor fluctuations and dyskinesias. Pharmacoeconomics 2001;19(10):1013–1038. 10.2165/00019053-200119100-00004. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46(3):399–424. 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999;20:125–144. 10.1146/annurev.publhealth.20.1.125. [DOI] [PubMed] [Google Scholar]
- 29. Dahodwala N, Jahnke J, Pettit AR, et al. Low sustainment of high‐dose oral medication regimens for advanced Parkinson's disease in medicare beneficiaries. J Parkinsons Dis 2021;11:675–684. 10.3233/JPD-202147. [DOI] [PubMed] [Google Scholar]
- 30. Weir S, Samnaliev M, Kuo TC, Tierney TS, Walleser Autiero S, Taylor RS, Schrag A. Short‐ and long‐term cost and utilization of health care resources in Parkinson's disease in the UK. Mov Disord 2018;33(6):974–981. 10.1002/mds.27302. [DOI] [PubMed] [Google Scholar]
- 31. Antonini A, Odin P, Schmidt P, et al. Validation and clinical value of the MANAGE‐PD tool: a clinician‐reported tool to identify Parkinson's disease patients inadequately controlled on oral medications. Parkinsonism Relat Disord 2021;92:59–66. 10.1016/j.parkreldis.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 32. Fasano A, Fung VSC, Lopiano L, Elibol B, Smolentseva IG, Seppi K, et al. Characterizing advanced Parkinson's disease: OBSERVE‐PD observational study results of 2615 patients. BMC Neurol 2019;19(1):50. 10.1186/s12883-019-1276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aldred J, Anca‐Herschkovitsch M, Antonini A, et al. Application of the '5‐2‐1′ screening criteria in advanced Parkinson's disease: interim analysis of DUOGLOBE. Neurodegener Dis Manag 2020;10(5):309–323. 10.2217/nmt-2020-0021. [DOI] [PubMed] [Google Scholar]
- 34. Santos‐Garcia D, de Deus Fonticoba T, Suarez Castro E, Aneiros Diaz A, McAfee D. 5‐2‐1 criteria: a simple screening tool for identifying advanced PD patients who need an optimization of Parkinson's treatment. Parkinsons Dis 2020;2020:7537924. 10.1155/2020/7537924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prange S, Danaila T, Laurencin C, et al. Age and time course of long‐term motor and nonmotor complications in Parkinson disease. Neurology 2019;92(2):e148–e160. 10.1212/WNL.0000000000006737. [DOI] [PubMed] [Google Scholar]
- 36. Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE. Levodopa‐associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976‐1990. Arch Neurol 2006;63(2):205–209. 10.1001/archneur.63.2.205. [DOI] [PubMed] [Google Scholar]
- 37. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16(3):448–458. 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 38. Turcano P, Mielke MM, Bower JH, Parisi JE, Cutsforth‐Gregory JK, Ahlskog JE, et al. Levodopa‐induced dyskinesia in Parkinson disease: a population‐based cohort study. Neurology 2018;91(24):e2238–e2243. 10.1212/WNL.0000000000006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fahn S, Elton RL. Unified parkinsons disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease, Vol 2. Florham Park, NJ: MacMillan Healthcare Information; 1987:153–163. [Google Scholar]
- 40. Forsaa EB, Larsen JP, Wentzel‐Larsen T, Alves G. What predicts mortality in Parkinson disease?: A prospective population‐based long‐term study. Neurology 2010;75(14):1270–1276. 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- 41. Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 2002;59(11):1708–1713. 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 42. Steering D. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Arch Neurol 1989;46(10):1052–1060. [DOI] [PubMed] [Google Scholar]
- 43. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1998;50(2):318. [DOI] [PubMed] [Google Scholar]
- 44. Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Rudolph A, et al. Survival in Parkinson disease: thirteen‐year follow‐up of the DATATOP cohort. Neurology 2005;64(1):87–93. 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 45. Kaltenboeck A, Johnson SJ, Davis MR, Birnbaum HG, Carroll CA, Tarrants ML, Siderowf AD. Direct costs and survival of medicare beneficiaries with early and advanced Parkinson's disease. Parkinsonism Relat Disord 2012;18(4):321–326. 10.1016/j.parkreldis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 46. Shulman LM, Gruber‐Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, Weiner WJ. The evolution of disability in Parkinson disease. Mov Disord 2008;23(6):790–796. 10.1002/mds.21879. [DOI] [PubMed] [Google Scholar]
- 47. Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed "Up & Go" test in people with Parkinson disease. Phys Ther 2001;81(2):810–818. 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 48. Hassan A, Wu SS, Schmidt P, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson's disease. Parkinsonism Relat Disord 2013;19(11):949–954. 10.1016/j.parkreldis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 49. Dodel RC, Berger K, Oertel WH. Health‐related quality of life and healthcare utilisation in patients with Parkinson's disease. Pharmacoeconomics 2001;19(10):1013–1038. 10.2165/00019053-200119100-00004. [DOI] [PubMed] [Google Scholar]
- 50. Bohingamu Mudiyanselage S, Watts JJ, Abimanyi‐Ochom J, Lane L, Murphy AT, Morris ME, et al. Cost of living with Parkinson's disease over 12 months in Australia: a prospective cohort study. Parkinsons Dis 2017;2017:5932675. 10.1155/2017/5932675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zoccolella S, Savarese M, Lamberti P, Manni R, Pacchetti C, Logroscino G. Sleep disorders and the natural history of Parkinson's disease: the contribution of epidemiological studies. Sleep Med Rev 2011;15(1):41–50. Epub 2010/07/17. 10.1016/j.smrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 52. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24(11):1641–1649. 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 53. Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol 2012;69(5):601–607. 10.1001/archneurol.2011.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23(6):837–844. 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 55. Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson's disease: a 20‐year neuropsychological study (Sydney multicentre study). J Neurol Neurosurg Psychiatry 2011;82(9):1033–1037. 10.1136/jnnp.2010.232678. [DOI] [PubMed] [Google Scholar]
- 56. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12‐year population study. Neurology 2008;70(13):1017–1022. 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 57. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311(16):1670–1683. 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 58. Shalev V, Goldshtein I, Halpern Y, Chodick G. Association between persistence with statin therapy and reduction in low‐density lipoprotein cholesterol level: analysis of real‐life data from community settings. Pharmacotherapy 2014;34(1):1–8. 10.1002/phar.1326. [DOI] [PubMed] [Google Scholar]
- 59. Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: a review. J Neurol 2017;264(8):1583–1607. [DOI] [PubMed] [Google Scholar]


