Abstract
Background
Flavonoids seem to have hormone-like and anti-hormone properties so that the consumption of flavonoids may have potential effects on hormone-related cancers (HRCs), but the findings have been inconsistent so far. This meta-analysis was aimed to explore the association between flavonoids intake and HRCs risk among observational studies.
Methods
Qualified articles, published on PubMed, EMBASE, and China National Knowledge Infrastructure (CNKI) from January 1999 to March 2022 and focused on relationships between flavonoids (total, subclass of and individual flavonoids) and HRCs (breast, ovarian, endometrial, thyroid, prostate and testicular cancer), were retrieved for pooled analysis. Random effects models were performed to calculate the pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Funnel plots and Begg’s/Egger’s test were used to evaluate the publication bias. Subgroup analyses and sensitivity analyses were conducted to explore the origins of heterogeneity.
Results
All included studies were rated as medium or high quality. Higher consumption of flavonols (OR = 0.85, 95% CI: 0.76–0.94), flavones (OR = 0.85, 95% CI: 0.77–0.95) and isoflavones (OR = 0.87, 95% CI: 0.82–0.92) was associated with a decreased risk of women-specific cancers (breast, ovarian and endometrial cancer), while the higher intake of total flavonoids was linked to a significantly elevated risk of prostate cancer (OR = 1.11, 95% CI: 1.02–1.21). A little evidence implied that thyroid cancer risk was augmented with the higher intake of flavones (OR = 1.24, 95% CI: 1.03–1.50) and flavanones (OR = 1.31, 95% CI: 1.09–1.57).
Conclusions
The present study suggests evidence that intake of total flavonoids, flavonols, flavones, flavanones, flavan-3-ols and isoflavones would be associated with a lower or higher risk of HRCs, which perhaps provides guidance for diet guidelines to a certain extent.
Trial registration
This protocol has been registered on PROSPERO with registration number CRD42020200720.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-022-00778-w.
Keywords: Flavonoids, Flavonoid subclasses, Hormone-related cancers, Observational studies, Meta-analysis
Introduction
Hormone-related cancers (HRCs) are greatly influenced by hormone levels and generally respond to hormone regulation, which plays an indispensable role in tumor growth. Six cancer types, including breast, ovarian, endometrial, thyroid, prostate and testicular cancer, are usually referred to as HRCs since they share the same carcinogenic mechanism [1]. Worldwide, there were about 4.7 million newly diagnosed cases of HRCs in 2018, accounting for more than a quarter of new cancer cases. As the most frequently diagnosed cancer in the great majority of countries, HRCs have become the leading cause of cancer death in over 100 countries [2]. With a cumulative knowledge for the role of hormones in HRCs, hormonal therapy is increasingly important [3, 4], but remains controversial due to more or less inevitable side-effects in the treatment course [5, 6]. Encouragingly, diets rich in vegetables, fruits and tea are found to reduce the cancer risk [7], having the potential to exert chemopreventive effects with the presence of anticarcinogenic phytochemicals [8].
Flavonoids represent one of the largest groups of phytochemicals, as a class of polyphenols with a basic benzo-γ-pyrone structure widely distributed in the plant kingdom, which mainly exist in leaves, flowers, roots, stems, and fruits of plants [9]. The U.S. Department of Agriculture (USDA) database categorizes flavonoids into six subclasses: flavonols, flavones, flavanones, anthocyanidins, flavan-3-ols and isoflavones [10, 11]. Allegedly, flavonoids have multiple functions in different physiological and pathological process of cancer, such as tumor cell proliferation, inflammation, angiogenesis, invasion and metastasis [12–14]. Besides, flavonoids seem to have hormone-like and anti-hormone effects due to their structural similarity to the natural estrogen estradiol, as well as other steroid hormones and steroid hormone antagonists [15]. Further, there is evidence that certain flavonoids may inhibit the activity of aromatase, an enzyme that catalyzes the conversion of androgen to estrogen, and up-regulation of the latter can promote the development of HRCs [16].
Most of these studies on flavonoids and HRCs have been done in vivo or in vitro, but less in humans. The evidence for the relationship between intake of flavonoids and risk of the aforementioned HRCs (except testicular cancer) among observational studies has been summed up by several existing meta-analyses [17–24]. It is noteworthy that these meta-analyses were either only based on individual HRCs type, or just focused on specific flavonoid subclass such as isoflavones, and lack of consistency because of the different methods to assess flavonoids intake. The criteria for evaluating flavonoids intake were not stringent enough, leading to the inclusion of low-quality studies.
As of the last summary of evidence, new research has come out, and an outline of the effects of total, subclass of, and individual flavonoids intake on HRCs has so far not been available. Thus, in this study, a meta-analysis was carried out for a comprehensive evaluation of total/subclass of/individual flavonoids to summarize epidemiological findings and to elucidate the association between flavonoids intake and HRCs risk.
Methods
Search strategy
A systematic search on PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and EMBASE (http://www.embase.com/) databases for English studies and on China National Knowledge Infrastructure (CNKI, https://www.cnki.net/) for Chinese articles published through January 1999 up to March 2022 was performed. The search terms used for retrieval were as follows: ((“flavonoids”[MeSH Terms] OR “flavonoid$”[All Fields]) OR (“flavanones”[MeSH Terms] OR “flavanone$”[All Fields]) OR (“isoflavones”[MeSH Terms] OR “isoflavone$”[All Fields] OR “isoflavonoid$”[all fields]) OR (“flavones”[MeSH Terms] OR “flavone$”[All Fields]) OR (“flavan-3-ol$”[All Fields] OR “flavanol$”[All Fields]) OR (“flavonols”[MeSH Terms] OR “flavonol$”[All Fields]) OR “anthocyanidins”[all fields]) AND (“breast”[all fields] OR (“ovarian”[all fields] OR “ovary”[all fields]) OR “endometrial”[all fields] OR “prostate”[all fields] OR (“testis”[all fields] OR “testicular”[all fields] OR “testicle”[all fields]) OR “thyroid”[all fields]) AND (“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “cancer”[All Fields]) AND (“Epidemiologic Studies”[MeSH Terms]) AND (“1999/1/1”[Date—Publication]: “2022/3/31”[Date—Publication]). Simultaneously, we inspected references cited in retrieved articles to recognize extra studies and undertook to get access to unpublished data. The meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This protocol has been registered on PROSPERO with registration number CRD42020200720 (https://www.crd.york.ac.uk/prospero/).
Inclusion and exclusion criteria
Eligible studies for the pooled analysis were selected as the following criteria: (i) an observational study (an prospective design such as cohort study or nested case–control study, or retrospective design namely case–control study); (ii) the association between intake of total/subclass of/individual flavonoids and the risk of any HRCs was evaluated; (iii) relative risks (RRs), odds ratios (ORs) or hazard ratios (HRs) with the corresponding 95% confidence intervals (CIs) for the highest category of exposure were reported; (iv) evaluating flavonoids consumption by dietary questionnaires and measurement for serum, plasma and urine. Only the research with the longest follow-up period was taken into consideration when multiple articles involving the same cohort have been produced.
The exclusion criteria were as described below: (i) out of adequate statistics; (ii) incomplete evaluation on flavonoids (no total and subclass of flavonoids or less than 3 kinds of flavonoid); (iii) unconfirmed comparison and comparison by per each increase for flavonoids intake; (iv) reported as an abstract, summary, comments, review or editorial; (v) flavonoids intake from sources other than dietary questionnaires, serum, plasma and urine; (vi) amount of soy protein or soy foods or soy supplements consumed as a representative of dietary isoflavone intake. The selection process and the examination for retrieved articles were performed independently by two authors (F. Liu and Y. Peng). Inconsistencies were referred to another author (Yating Qiao) for resolution.
Data extraction
Two authors (F. Liu and Y. Peng) reviewed full-text articles separately, and extracted the following characteristics from the original: first author’s name, publication year, study design (cohort, case–control or nested case–control study), study region (country or area), sex and age range, number of cases and controls or total cohort participants with follow-up period, exposure measurement, the most adjusted ORs in case–control studies or HRs/RRs in cohort studies and the corresponding 95% CIs comparing the highest vs. lowest (or reference) level of flavonoids intake, and the covariates in multivariable models. Dissents on data extraction were settled through argumentum.
Quality assessment
The Newcastle–Ottawa Scale (NOS) quality assessment criteria, consisting of a total of 9 points in three dimensions (4 points in “selection”, 2 points in “comparability” and 3 points in “outcome” for cohort studies or “exposure” for case–control studies), was employed to appraise the quality of selected study [25]. Studies assigned 7–9, 4–6, and 0–3 points were recognized as high-, medium-, and low-quality, respectively (higher score representing higher quality).
Statistical analysis
Statistical analyses were performed by using Stata 15.1 software (StataCorp LP, College Station, TX). ORs with 95% CIs were assessed for ascertaining the association between flavonoids intake and HRCs risk. The merged ORs with 95% CIs calculated by the most adjusted HRs, RRs or ORs with 95% CIs announced in the original were illustrated in forest plots using random effects models depicted by DerSimonian-Laird method [26]. Statistic I2 was applied for the evaluation of heterogeneity among studies [27] and the two-sided p values based on the Q test of heterogeneity were reported (p < 0.05 suggested significant heterogeneity). Publication biases were evaluated by funnel plots, and calculated by Begg’s tests [28] and Egger’s tests [29]. Besides, to explore the source of heterogeneity among the studies, sensitivity analyses were preferred to evaluate the robustness of the results of the combined effects, which were achieved by sequential removal of each study. Further, subgroup analyses were implemented based on study design, menopausal status (in breast cancer) and study region.
Results
Study characteristics
A flowchart of the literature screening process was outlined in Fig. 1, and the details of the selected eligible studies were listed in Supplementary Table S1. Fifty-four published articles contained 51 studies. It consisted of 22 prospective cohort studies, 1 nested case–control study, 18 population-based case–control studies and 10 hospital-based case–control studies. Urine samples were collected in three studies and serum samples were collected in two. The remaining studies assessed flavonoid intake using dietary questionnaires. Most of these studies made adjustment for multiple latent confounding factors, including demographics like age, body mass index (BMI), smoking status, alcohol intake, family history of cancer, energy/caloric intake, as well as female reproduction details such as age at menarche/first birth/menopause and parity, which were confined to women-specific cancers. On assessment of the methodological quality using the NOS, all studies attained the score 5 or above, namely all included studies were of medium- or high-quality (Supplementary Tables S2 and S3).
Fig. 1.
Flowchart for retrieving articles and selecting eligible studies. *CNKI, China National Knowledge Infrastructure; CI, confidence interval
Flavonoids intake and risk of hormone-related cancers
Female breast cancer
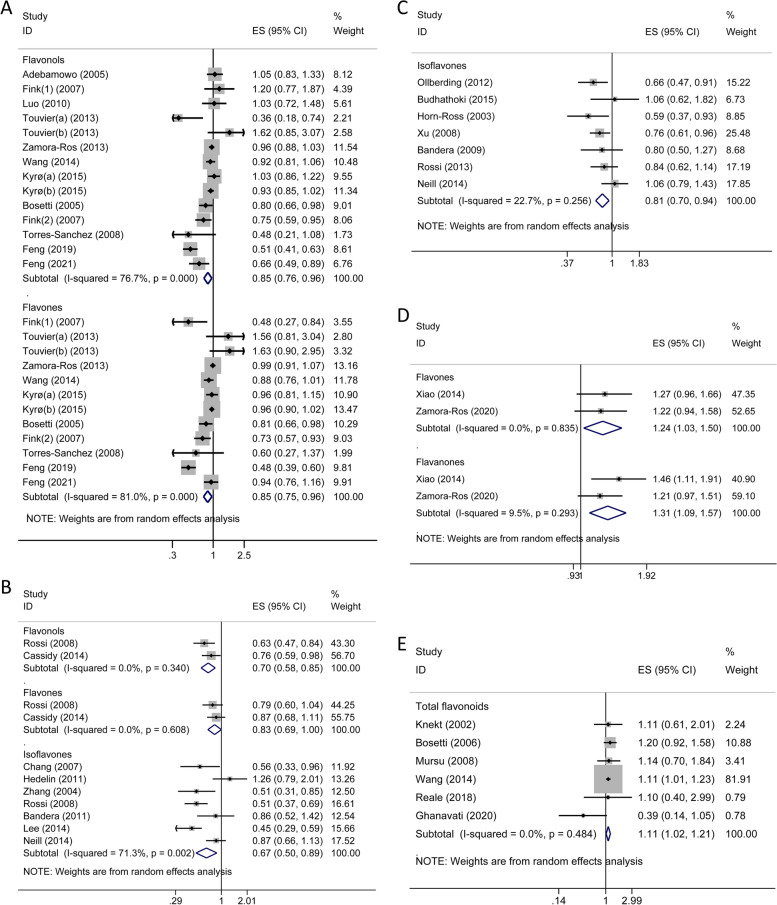
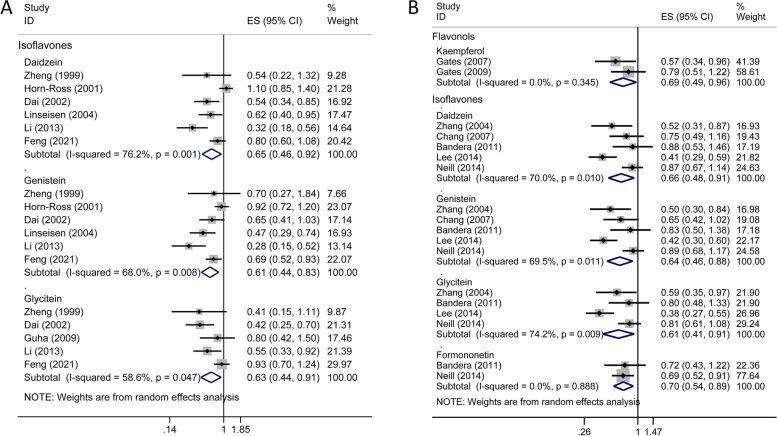
Nineteen studies (9 cohort studies, 10 case–control studies and 1 nested case–control studies) [30–50] were conducted on breast cancer (Supplementary Table S4). Overall, no significant effect of total flavonoids intake on breast cancer risk was observed. The risk of breast cancer significantly decreased in women with higher consumption of flavonols (OR = 0.85; 95% CI, 0.76–0.96; I2 = 76.7%; p < 0.001) and flavones (OR = 0.85; 95% CI, 0.75–0.96; I2 = 81.0%; p < 0.001), compared with that in those with lower consumption (Fig. 2A). These protective effects seemed more pronounced in case–control studies but not in prospective studies. In subgroup analyses, the significant association of flavones with reduced risk of breast cancer was observed in premenopausal women but not in postmenopausal women; Flavonols were the opposite (Supplementary Table S5). Moreover, based on study region, higher consumption of flavones was linked to a decreased risk of breast cancer in non-Asians whereas flavanones and isoflavones had a protective effect only in Asians (Supplementary Table S6). Null results were found for individual compounds of flavonols, flavanones and flavan-3-ols while significant decreased summary risk estimates were retrieved from main individual isoflavones (daidzein, OR = 0.65, 95% CI 0.46–0.92; genistein, OR = 0.61, 95% CI 0.44–0.83; and glycitein, OR = 0.63, 95% CI 0.44–0.91; Fig. 3A and Supplementary Table S4).
Fig. 2.
Forest plots for the association between total/subclass of flavonoids consumption and hormone-related cancer risk. A breast cancer; B ovarian cancer; C endometrial cancer; D thyroid cancer; E prostate cancer. Use (1) and (2) to distinguish two different studies by the same author in the same year; two datasets of the same study were represented by (a) and (b). *ES, effect size; CI, confidence interval
Fig. 3.
Forest plots for the association between individual flavonoid compounds consumption and hormone-related cancer risk. A breast cancer; B ovarian cancer. *ES, effect size; CI, confidence interval
Ovarian cancer
As shown in Fig. 2B and Supplementary Table S4, the combined analyses of the five cohort studies and six case–control studies [7, 40, 51–59] indicated that ovarian cancer risk was significantly reduced in women with the higher intake of flavonols (OR = 0.70; 95% CI, 0.58–0.85; I2 = 0.0%; p = 0.340), and isoflavones (OR = 0.67; 95% CI, 0.50–0.89; I2 = 71.3%; p = 0.002) respectively, but not with higher consumption of total flavonoids and other flavonoid subclasses. And, subgroup analysis suggested isoflavones might be more phylactic against ovarian cancer in case–control studies and in Asian populations (Supplementary Table S6). For individual flavonoids, intake of both kaempferol from the flavonol subclass and all the four individual isoflavones (daidzein, genistein, glycitein and formononetin) contributed to a significantly decreased risk of ovarian cancer (Fig. 3B and Supplementary Table S4).
Endometrial cancer
Three prospective and 5 case–control studies [40, 51, 60–65] of total flavonoids and/or isoflavones subclass were conducted on endometrial cancer. No significant association with endometrial cancer risk was observed for total flavonoids (OR = 0.93; 95% CI, 0.67–1.27; I2 = 32.7%; p = 0.223). Whereas, there was a significant association of isoflavone consumption with reduced risk of endometrial cancer (OR = 0.81; 95% CI, 0.70–0.94; I2 = 22.7%; p = 0.256; Fig. 2C and Supplementary Table S4), which was limited to case–control studies and non-Asian studies by subgroup analyses (Supplementary Table S6). Further, no significant association between individual isoflavone intake and endometrial cancer risk was observed.
Thyroid cancer
Although limited epidemiological studies (2 prospective and 1 case–control studies) [66–68] have been launched to evaluate the flavonoids intake on thyroid cancer risk, we still observed an increment in thyroid cancer risk conferred by the higher intake of flavones (OR = 1.24; 95% CI, 1.03–1.50; I2 = 0%; p = 0.835) and flavanones (OR = 1.31; 95% CI, 1.09–1.57; I2 = 9.5%; p = 0.293) with no evidence of heterogeneity, respectively (Fig. 2D and Supplementary Table S4).
Prostate cancer
Figure 2E and Supplementary Table S4 demonstrated the summarized risk estimates for six cohort and six case–control studies [31, 69–81] on consumption of flavonoids and prostate cancer risk. We observed an increased risk of prostate cancer by higher intake of total flavonoids (OR = 1.11; 95% CI, 1.02–1.21; I2 = 0%; p = 0.484), which was more pronounced in cohort studies. And, this positive relationship was found only in non-Asia populations (Supplementary Table S6). Of note, for the anthocyanidins and flavan-3-ols subclass, the higher intake category presented a markedly elevated prostate cancer risk compared to the lower intake category in prospective studies. For individual compounds, no significant association was attained in our analysis.
Testicular cancer
Only one case–control study [82] investigated the consumption of total flavonoids and isoflavones subclass and risk of testicular cancer but showed no significant results (data not shown).
Women-specific cancers
A total of 16 aforementioned prospective (15 cohort and 1 nested case–control studies) and 20 case–control studies on breast, endometrial and ovarian cancer were selected for the analysis on the flavonoid intake with the risk of women-specific cancers. No evidence on association between total flavonoids intake and women-specific cancer risk was found in our analysis. For flavonoid subclasses, higher consumption of flavonols (OR = 0.85; 95% CI, 0.76–0.94; I2 = 75.5%; p < 0.001), flavones (OR = 0.85; 95% CI, 0.77–0.95; I2 = 76.3%; p < 0.001) and isoflavones (OR = 0.87; 95% CI, 0.82–0.92; I2 = 73.8%; p < 0.001) was associated with a decreased risk of women-specific cancers, especially among the case–control studies (Supplementary Table S4). Similar results were also observed for the three main individual compounds of isoflavones (daidzein, OR = 0.69, 95% CI 0.58–0.83; genistein, OR = 0.67, 95% CI 0.57–0.80; and glycitein, OR = 0.68, 95% CI 0.57–0.83). In subgroup analyses based on region, the lower women-specific cancer risk was more reported for flavonols (OR = 0.90; 95% CI, 0.82–0.98) and flavones (OR = 0.90; 95% CI, 0.84–0.97) in non-Asian studies, but for flavanones (OR = 0.74; 95% CI, 0.60 to 0.91)and isoflavones (OR = 0.64; 95% CI, 0.55–0.75) in Asians (Supplementary Table S6).
Men-specific cancers
Out of the 6 cohort and 7 case–control studies, only the higher consumption of total flavonoids (OR = 1.11; 95% CI, 1.02–1.22; I2 = 0%; p = 0.537) was associated with significant increased risk of men-specific cancers including prostate and testicular cancer, which was restricted to prospective studies (Supplementary Table S4).
Publication bias
As shown in Fig. 4 and Supplementary Table S7, results from both Begg's tests and Egger’s tests indicated little evidence of asymmetry of the funnel plots among total flavonoids and flavonoid subclasses except isoflavones in breast cancer (Begg’s test: Z = 2.85, pZ = 0.004; and Egger’s test: t = -2.90, pt = 0.013) and women-specific cancers (Begg’s test: Z = 1.92, pZ = 0.055; and Egger’s test: t = -4.70, pt < 0.001).
Fig. 4.
Funnel plots for the association between flavonoid consumption and hormone-related cancer risk. A breast cancer; B ovarian cancer; C endometrial cancer; D prostate cancer
Sensitivity analysis
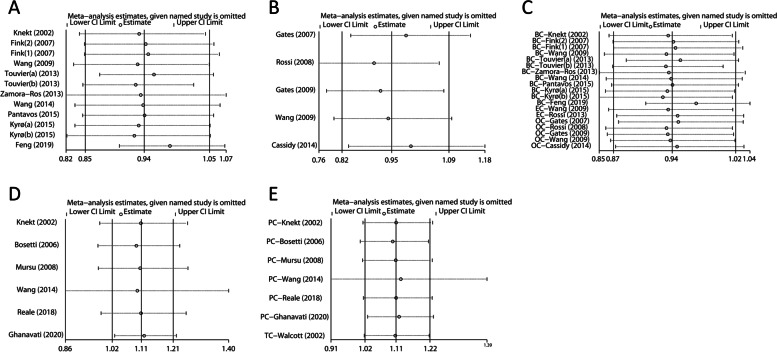
Sensitivity analyses for total flavonoids and HRCs were performed (Fig. 5 and Supplementary Table S8). When any study except Ghanavati’s [81] was eliminated, there was no evidence that total flavonoids had a detrimental effect on prostate cancer. When Wang’ study [77] was eliminated, the dangerous effect of total flavonoids on men-specific cancers disappeared. For other cancers, no statistically significant change in combined effect sizes was detected after excluding each study separately.
Fig. 5.
Plots of sensitivity analyses by sequential removal of each study. A breast cancer; B ovarian cancer; C women-specific cancers; D prostate cancer; E men-specific cancers. *BC, breast cancer; EC, endometrial cancer; OC, ovarian cancer; PC, prostate cancer; TC, testicular cancer
Discussion
In the present study, a comprehensive meta-analysis of existing observational studies on the consumption of flavonoids and risk of HRCs were conducted. The current results showed that higher consumption of total flavonoids was only associated with an increased risk of men-specific cancers, mainly prostate cancer. For the subclasses, flavonols, flavones, and isoflavones, as well as the three main individual compounds of isoflavones (daidzein, genistein and glycitein) may have protective effects on women-specific cancers, whereas potentially dangerous effects of flavones and flavanones have been found in thyroid cancer. There was no evidence in support of any role for anthocyanidins in HRCs.
Flavonoids can interfere with the initiation, promotion and development of cancer through directly inhibiting the process of oxidative stress and oxidative damage [14] and indirectly affecting hormone activity [83]. Aromatase, a rate-limiting enzyme responsible for the biotransformation of estrogen in vivo, can catalyze the transition from androgen to estrogen. Thus, the regulation of estrogen synthesis in vivo by inhibiting aromatase activity has become a way to treat HRCs. Flavanones, flavones and isoflavones, considered to be strong antioxidants due to the phenyl hydroxyl groups on the nucleus of flavonoids, have long been supposed to have the capacity to inhibit the activity of aromatase [84–86]. Some phytoestrogens, such as genistein, apigenin, naringenin, and kaempferol, can bind to estrogen receptors (ER) and compete fiercely with 17β-estradiol in binding to ER [87]. This may be due to the similarity of the flavonoid structure to the polyphenol ring in the steroidal core of 17β-estradiol. Scherbakov et al. found that high dose of phytoestrogens apigenin, genistein, quercetin and naringenin had inhibitory effect on ER ( +) or ER (-) breast cancer cells [88]. Daidzein, genistein, quercetin and luteolin possess the ability of inhibiting the proliferative activity induced by environmental estrogen, which suggests anti-estrogen and anti-cancer functions by such individual compounds of flavonoids [89].
Consistent with the findings herein, Chang et al. [18] implies significant association of flavonols and flavones intake with a reduced risk of breast cancer and a protective effect of flavan-3-ols only in postmenopausal women. Higher intake of kaempferol from the flavonol subclass and isoflavones including daidzein, genistein, glycitein and formononetin was observed to be linked to a decreased risk of ovarian cancer in this analysis, as reported by the two meta-analyses for ovarian cancer [20, 24]. Hua et al. [20] confused isoflavones and quercetin with total flavonoids when evaluating flavonoids intake, which potentially lead to bias. Given that the approach to evaluate flavonoids intake was different from the current research, there was actually no evidence supporting the effect of total flavonoids against ovarian cancer. No striking discordance was found in all pooled analyses for endometrial cancer and prostate cancer among observational studies including this study, demonstrating higher consumption of isoflavones with a decreased risk of endometrial cancer among case–control studies and the higher intake category of total flavonoids with an elevated risk of prostate cancer confined to prospective studies. Nevertheless, a meta-analysis of randomized controlled trials (RCTs) revealed an effect by higher isoflavone intake for a lower risk of prostate cancer [90]. For thyroid cancer, a cohort study evaluating flavonoid subclasses and a case–control study assessing isoflavones only have been reported by Grosso’s meta-analysis [24]. The current analysis added another prospective cohort study evaluating flavonoid subclasses. For flavonoid subclasses except isoflavones, the results of the aforementioned two cohort studies were pooled and uncovered potentially pernicious effects of flavones and flavanones, the latter of which was consistent with Grosso's findings [24].
Menopausal status as an effect modifier may have an influence on the association between flavonoids intake and women-specific cancer risk. Since most studies reported menopausal status limited to breast cancer, the present study conducted subgroup analyses just for breast cancer. A significant association of higher flavones intake in premenopausal women and of higher flavan-3-ols intake in postmenopausal women with reduced risk of breast cancer was found in this study. Isoflavones intake has been reported to be negatively associated with the risk of breast cancer in postmenopausal women [17, 19], but no association was observed in this study. The content of isoflavones in soy products decreases with the processing of raw soybeans. Second-generation foods derived from soy, such as soy bread and biscuits, have much lower levels of isoflavones (2.4 to 18.1 mg/100 g) compared to raw soy flour (exceeds 100 mg/100 g) [21]. Therefore, significant differences in isoflavone component levels in various soy products may lead to different findings. Such inconsistent findings may be attributed to the fact that the study included not soy foods but pure isoflavones when evaluating consumption of isoflavones, which to a certain extent avoided some heterogeneity. Subgroup analyses based on study region were also performed to explore the heterogeneity among studies in different areas. There were great differences between Asian and non-Asian regions in the association between isoflavones intake and the risk of women-specific cancers as well as the flavan-3-ols intake and prostate cancer risk; especially, the results of region-based subgroup analyses limited to breast cancer in up-to-date meta-analyses were consistent with this study [17, 19, 24]. These suggested the existence of an ethnic disparity necessitated for consideration in evaluating the role of flavonoids intake on cancer risks.
Dietary flavonoids are widely present in plant-based foods. Flavonols, the most abundant flavonoids in foods, are mainly found in onion, red wine, olive oil, berries, and grapefruit. The primary sources of flavones, flavanones, flavan-3-ols and anthocyanidins are vegetables, citrus fruits, green tea and colored berries, respectively. Isoflavones are present in significant quantities in legumes mainly soybeans, green beans, mung beans. The bean of the soy plant has been used in both foods and phytotherapeutic supplements. Evidence from pre-clinical studies supports a role for soy/soy isoflavone in reducing the risk of developing prostate cancer and inhibiting its progression [90]. Therefore, as an eminent clinical intervention agent or chemoprevention agent, flavonoids will have great potential for future. In addition, plant-based food consumption and flavonoids intake vary among populations and ethnicities due to regional differences in dietary habits, especially for isoflavones. Higher intake (over 35 ~ 63 mg/d) of isoflavones in Asians tend to be more than ten times of the higher intake (over 0.03 ~ 4 mg/d) in Westerners, due to the quite low soy consumption in the West. Thus a validated and reliable dietary questionnaire for soy isoflavones taking regional variations into account is often required to assess isoflavone intake [19], when setting dietary standards in the future. Moreover, the findings of the present meta-analysis suggested that an intake of approximately more than 14.5 ~ 60 mg/d of flavonols and over 0.2 ~ 31 mg/d of flavones may have a preventive effect on breast cancer.
Due to the wide varieties of available flavonoids, it is tough to assess the consumption of total flavonoids or each kind of flavonoid. The difficulty of measuring flavonoids intake and the limited epidemiological studies made our research to a certain degree of heterogeneity. Most studies used a food frequency questionnaire (FFQ) or a dietary history questionnaire (DHQ) to evaluate flavonoid consumption, which is likely to lead to measurement bias; while other studies assessed exposure on urine or serum levels, but just reflected the short-term exposure of subjects, in consequence, randomness cannot be ignored. Besides, in addition to studies on morbidity, we also included some cohort studies with follow-up outcomes of recurrence or death to jointly probe the association between flavonoids and HRCs, which is presumably one of the origins of heterogeneity.
The research has some advantages that deserve attention. First, this meta-analysis systematically summarized the effective evidence between flavonoids and HRCs. Second, we objectively launched a comprehensive search for studies from 1999 to 2022. Compared with previous meta-analyses, this study included more cohort studies and case–control studies, which undoubtedly provides more tangible and favorable evidence for guiding flavonoids in the prevention of HRCs. Third, most categories of flavonoids were evaluated, including total flavonoids, flavonoid subclasses, and individual compounds in each subclass. Moreover, inclusion and exclusion criteria established were relatively strict. All individual studies included were rated as medium or high quality, which somewhat avoided the insufficiency and unreliability of the results conferred by low-quality studies.
On the contrary, there are some limitations and challenges to be settled in the research. As only observational studies were included, evidence power is not very strong yet. The sample size of individual studies is small and there may still be other unknown confounding factors affecting the results. This limits the opportunities for extrapolation. Further intervention studies are needed to confirm our conclusion. The number of eligible studies is still limited, especially for certain cancers, thus flavonoid subclasses cannot be synthetically analyzed. Further, the criteria for the included studies allowed no subgroup analyses based on dietary and other sources (urine/serum/plasma) of flavonoids to be performed. For the sake of avoiding unnecessary heterogeneity, RCTs were not included in our meta-analysis. Limited clinical trials have prevented our results from providing more conclusive evidence.
Conclusions
In conclusion, there is a small amount of evidence that total flavonoids, flavonols, flavones, flavanones, flavan-3-ols and isoflavones may be associated with a lower or higher risk of certain HRCs, which maybe provide guidance for diet guidelines to a certain extent in the future. Nonetheless, due to limited data, further prospective studies and a larger number of subjects are warranted to be verified and provide stronger evidence.
Supplementary Information
Acknowledgements
We would like to thank the Cancer Biobank of Tianjin Medical University Cancer Institute and Hospital, founded by National Human Genetic Resources Sharing Service Platform (2005DKA21300), and The National Key Research and Development program of China: The Net construction of human genetic resource Bio-bank in North China (2016YFC1201703).
Abbreviations
- BMI
Body mass index
- Cis
Confidence intervals
- CNKI
China National Knowledge Infrastructure
- DHQ
Dietary history questionnaire
- ER
Estrogen receptors
- FFQ
Food frequency questionnaire
- HRCs
Hormone-related cancers
- HRs
Hazard ratios
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS
Newcastle–Ottawa Scale
- ORs
Odds ratios
- RCTs
Randomized controlled trials
- RRs
Relative risks
- USDA
The U.S. Department of Agriculture
Authors’ contributions
YH, MZ and FfS designed research; FL, YP and YQ conducted research; FL and YP analyzed data or performed statistical analysis; FL and YQ wrote paper – original draft; FjS and FfS wrote paper – review & editing; MZ and FfS had primary responsibility for final content. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFC1302703), the National Natural Science Foundation of China (81974488), Tianjin Science and Technology Committee Foundation (17JCYBJC25300) and Chinese National Key Research and Development Project (2018YFC1315600).
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no potential competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Zhang, Email: mingle1981@163.com.
Fangfang Song, Email: double1980@163.com.
References
- 1.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Student S, Hejmo T, Poterala-Hejmo A, Lesniak A, Buldak R. Anti-androgen hormonal therapy for cancer and other diseases. Eur J Pharmacol. 2020;866:172783. doi: 10.1016/j.ejphar.2019.172783. [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2015;418(Pt 3):240–244. doi: 10.1016/j.mce.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Cagnacci A, Venier M. The Controversial History of Hormone Replacement Therapy. Medicina (Kaunas) 2019;55:602. doi: 10.3390/medicina55090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Weelden WJ, Massuger L. Enitec, Pijnenborg JMA, Romano A: Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front Oncol. 2019;9:359. doi: 10.3389/fonc.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr. 2014;100:1344–1351. doi: 10.3945/ajcn.114.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Chen YM, Huang J, Fang YJ, Huang WQ, Yan B, Lu MS, Pan ZZ, Zhang CX. Flavonoid intake from vegetables and fruits is inversely associated with colorectal cancer risk: a case-control study in China. Br J Nutr. 2016;116:1275–1287. doi: 10.1017/S0007114516003196. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran MP, McKay DL, Blumberg JB. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J Nutr Gerontol Geriatr. 2012;31:176–189. doi: 10.1080/21551197.2012.698219. [DOI] [PubMed] [Google Scholar]
- 10.USDA Database for the Flavonoid Content of Selected Foods, Release 3.3 [https://www.ars.usda.gov/]
- 11.USDA Database for the Isoflavone Content of Selected Foods, Release 2.1 [https://www.ars.usda.gov/]
- 12.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Fang H, Xu W. Recent advance in the research of flavonoids as anticancer agents. Mini Rev Med Chem. 2007;7:663–678. doi: 10.2174/138955707781024463. [DOI] [PubMed] [Google Scholar]
- 14.Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res Treat. 2000;62:35–49. doi: 10.1023/A:1006422302173. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen AJ, McNulty J. Polyphenolic natural products and natural product-inspired steroidal mimics as aromatase inhibitors. Med Res Rev. 2019;39:1274–1293. doi: 10.1002/med.21536. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS ONE. 2014;9:e89288. doi: 10.1371/journal.pone.0089288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang H, Xie Q, Zhang QY, Peng XL, Zhu JD, Mi MT. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS ONE. 2013;8:e54318. doi: 10.1371/journal.pone.0054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315–323. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 20.Hua X, Yu L, You R, Yang Y, Liao J, Chen D, Yu L. Association among Dietary Flavonoids, Flavonoid Subclasses and Ovarian Cancer Risk: A Meta-Analysis. PLoS ONE. 2016;11:e0151134. doi: 10.1371/journal.pone.0151134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong XS, Ge J, Chen SW, Xiong YQ, Ma SJ, Chen Q. Association between Dietary Isoflavones in Soy and Legumes and Endometrial Cancer: A Systematic Review and Meta-Analysis. J Acad Nutr Diet. 2018;118:637–651. doi: 10.1016/j.jand.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Guo K, Liang Z, Liu L, Li F, Wang H. Flavonoids intake and risk of prostate cancer: a meta-analysis of observational studies. Andrologia. 2016;48:1175–1182. doi: 10.1111/and.12556. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Cornago A, Appleby PN, Boeing H, Gil L, Kyro C, Ricceri F, Murphy N, Trichopoulou A, Tsilidis KK, Khaw KT, et al. Circulating isoflavone and lignan concentrations and prostate cancer risk: a meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int J Cancer. 2018;143:2677–2686. doi: 10.1002/ijc.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosso G, Godos J, Lamuela-Raventos R, Ray S, Micek A, Pajak A, Sciacca S, D'Orazio N, Del Rio D, Galvano F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol Nutr Food Res. 2017;61:1600930. [DOI] [PubMed]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol. 2001;154:434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 31.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 32.Linseisen J, Piller R, Hermann S, Chang-Claude J, German Case-Control S. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2004;110:284–290. doi: 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- 33.Bosetti C, Spertini L, Parpinel M, Gnagnarella P, Lagiou P, Negri E, Franceschi S, Montella M, Peterson J, Dwyer J, et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomarkers Prev. 2005;14:805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 34.Adebamowo CA, Cho E, Sampson L, Katan MB, Spiegelman D, Willett WC, Holmes MD. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- 35.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol. 2007;165:514–523. doi: 10.1093/aje/kwk033. [DOI] [PubMed] [Google Scholar]
- 36.Torres-Sanchez L, Galvan-Portillo M, Wolff MS, Lopez-Carrillo L. Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Public Health Nutr. 2009;12:825–831. doi: 10.1017/S136898000800325X. [DOI] [PubMed] [Google Scholar]
- 37.Touvier M, Druesne-Pecollo N, Kesse-Guyot E, Andreeva VA, Fezeu L, Galan P, Hercberg S, Latino-Martel P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: a prospective cohort study. Breast Cancer Res Treat. 2013;137:225–236. doi: 10.1007/s10549-012-2323-y. [DOI] [PubMed] [Google Scholar]
- 38.Zamora-Ros R, Ferrari P, Gonzalez CA, Tjonneland A, Olsen A, Bredsdorff L, Overvad K, Touillaud M, Perquier F, Fagherazzi G, et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res Treat. 2013;139:163–176. doi: 10.1007/s10549-013-2483-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Gapstur SM, Gaudet MM, Peterson JJ, Dwyer JT, McCullough ML. Evidence for an association of dietary flavonoid intake with breast cancer risk by estrogen receptor status is limited. J Nutr. 2014;144:1603–1611. doi: 10.3945/jn.114.196964. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009;89:905–912. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Zhang M, Holman CD. Population versus hospital controls in the assessment of dietary intake of isoflavone for case-control studies on cancers in China. Nutr Cancer. 2013;65:390–397. doi: 10.1080/01635581.2013.767915. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Gao YT, Chow WH, Shu XO, Li H, Yang G, Cai Q, Rothman N, Cai H, Shrubsole MJ, et al. Urinary polyphenols and breast cancer risk: results from the Shanghai Women's Health Study. Breast Cancer Res Treat. 2010;120:693–702. doi: 10.1007/s10549-009-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W. Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev. 2002;11:815–821. [PubMed] [Google Scholar]
- 44.Kyro C, Zamora-Ros R, Scalbert A, Tjonneland A, Dossus L, Johansen C, Bidstrup PE, Weiderpass E, Christensen J, Ward H, et al. Pre-diagnostic polyphenol intake and breast cancer survival: the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Breast Cancer Res Treat. 2015;154:389–401. doi: 10.1007/s10549-015-3595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantavos A, Ruiter R, Feskens EF, de Keyser CE, Hofman A, Stricker BH, Franco OH, Kiefte-de Jong JC. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: the Rotterdam Study. Int J Cancer. 2015;136:2178–2186. doi: 10.1002/ijc.29249. [DOI] [PubMed] [Google Scholar]
- 46.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Gaudet MM, Abrahamson PE, Bell P, Schroeder JC, Teitelbaum SL, et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol Biomarkers Prev. 2007;16:2285–2292. doi: 10.1158/1055-9965.EPI-07-0245. [DOI] [PubMed] [Google Scholar]
- 47.Zheng W, Dai Q, Custer LJ, Shu XO, Wen WQ, Jin F, Franke AA. Urinary excretion of isoflavonoids and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:35–40. [PubMed] [Google Scholar]
- 48.Feng XL, Ho SC, Mo XF, Lin FY, Zhang NQ, Luo H, Zhang X, Zhang CX. Association between flavonoids, flavonoid subclasses intake and breast cancer risk: a case-control study in China. Eur J Cancer Prev. 2020;29:493–500. [DOI] [PubMed]
- 49.Feng XL, Zhan XX, Zuo LS, Mo XF, Zhang X, Liu KY, Li L, Zhang CX. Associations between serum concentration of flavonoids and breast cancer risk among Chinese women. Eur J Nutr. 2021;60:1347–1362. doi: 10.1007/s00394-020-02331-z. [DOI] [PubMed] [Google Scholar]
- 50.Feng XL, Ho SC, Zhan XX, Zuo LS, Mo XF, Zhang X, Abulimiti A, Huang CY, Zhang CX. Serum isoflavones and lignans and odds of breast cancer in pre- and postmenopausal Chinese women. Menopause. 2021;28:413–422. doi: 10.1097/GME.0000000000001715. [DOI] [PubMed] [Google Scholar]
- 51.Neill AS, Ibiebele TI, Lahmann PH, Hughes MC, Nagle CM, Webb PM. Australian Ovarian Cancer Study G, Australian National Endometrial Cancer Study G: Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. Br J Nutr. 2014;111:1430–1440. doi: 10.1017/S0007114513003899. [DOI] [PubMed] [Google Scholar]
- 52.Chang ET, Lee VS, Canchola AJ, Clarke CA, Purdie DM, Reynolds P, Anton-Culver H, Bernstein L, Deapen D, Peel D, et al. Diet and risk of ovarian cancer in the California Teachers Study cohort. Am J Epidemiol. 2007;165:802–813. doi: 10.1093/aje/kwk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 54.Rossi M, Negri E, Lagiou P, Talamini R, Dal Maso L, Montella M, Franceschi S, La Vecchia C. Flavonoids and ovarian cancer risk: A case-control study in Italy. Int J Cancer. 2008;123:895–898. doi: 10.1002/ijc.23549. [DOI] [PubMed] [Google Scholar]
- 55.Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124:1918–1925. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hedelin M, Lof M, Andersson TM, Adlercreutz H, Weiderpass E. Dietary phytoestrogens and the risk of ovarian cancer in the women's lifestyle and health cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:308–317. doi: 10.1158/1055-9965.EPI-10-0752. [DOI] [PubMed] [Google Scholar]
- 58.Lee AH, Su D, Pasalich M, Tang L, Binns CW, Qiu L. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr Res. 2014;34:302–307. doi: 10.1016/j.nutres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Zhang M, Xie X, Lee AH, Binns CW. Soy and isoflavone intake are associated with reduced risk of ovarian cancer in southeast china. Nutr Cancer. 2004;49:125–130. doi: 10.1207/s15327914nc4902_2. [DOI] [PubMed] [Google Scholar]
- 60.Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95:1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 61.Xu WH, Zheng W, Cai Q, Cheng JR, Cai H, Xiang YB, Shu XO. The Asp(327)Asn polymorphism in the sex hormone-binding globulin gene modifies the association of soy food and tea intake with endometrial cancer risk. Nutr Cancer. 2008;60:736–743. doi: 10.1080/01635580802192833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandera EV, Williams MG, Sima C, Bayuga S, Pulick K, Wilcox H, Soslow R, Zauber AG, Olson SH. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009;20:1117–1127. doi: 10.1007/s10552-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ollberding NJ, Lim U, Wilkens LR, Setiawan VW, Shvetsov YB, Henderson BE, Kolonel LN, Goodman MT. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst. 2012;104:67–76. doi: 10.1093/jnci/djr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi M, Edefonti V, Parpinel M, Lagiou P, Franchi M, Ferraroni M, Decarli A, Zucchetto A, Serraino D, Dal Maso L, et al. Proanthocyanidins and other flavonoids in relation to endometrial cancer risk: a case-control study in Italy. Br J Cancer. 2013;109:1914–1920. doi: 10.1038/bjc.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Budhathoki S, Iwasaki M, Sawada N, Yamaji T, Shimazu T, Sasazuki S, Inoue M, Tsugane S, Group JS Soy food and isoflavone intake and endometrial cancer risk: the Japan Public Health Center-based prospective study. BJOG. 2015;122:304–311. doi: 10.1111/1471-0528.12853. [DOI] [PubMed] [Google Scholar]
- 66.Xiao Q, Park Y, Hollenbeck AR, Kitahara CM. Dietary flavonoid intake and thyroid cancer risk in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2014;23:1102–1108. doi: 10.1158/1055-9965.EPI-13-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horn-Ross PL, Hoggatt KJ, Lee MM. Phytoestrogens and thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2002;11:43–49. [PubMed] [Google Scholar]
- 68.Zamora-Ros R, Cayssials V, Franceschi S, Kyro C, Weiderpass E, Hennings J, Sandstrom M, Tjonneland A, Olsen A, Overvad K, et al. Polyphenol intake and differentiated thyroid cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2020;146:1841–1850. doi: 10.1002/ijc.32589. [DOI] [PubMed] [Google Scholar]
- 69.Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, Negri E, Montella M, Lagiou P, Franceschi S, La Vecchia C. Flavonoids and prostate cancer risk: a study in Italy. Nutr Cancer. 2006;56:123–127. doi: 10.1207/s15327914nc5602_1. [DOI] [PubMed] [Google Scholar]
- 70.Hedelin M, Balter KA, Chang ET, Bellocco R, Klint A, Johansson JE, Wiklund F, Thellenberg-Karlsson C, Adami HO, Gronberg H. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate. 2006;66:1512–1520. doi: 10.1002/pros.20487. [DOI] [PubMed] [Google Scholar]
- 71.Hedelin M, Klint A, Chang ET, Bellocco R, Johansson JE, Andersson SO, Heinonen SM, Adlercreutz H, Adami HO, Gronberg H, Balter KA. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden) Cancer Causes Control. 2006;17:169–180. doi: 10.1007/s10552-005-0342-2. [DOI] [PubMed] [Google Scholar]
- 72.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007;98:388–396. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 73.Nagata Y, Sonoda T, Mori M, Miyanaga N, Okumura K, Goto K, Naito S, Fujimoto K, Hirao Y, Takahashi A, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr. 2007;137:1974–1979. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 74.Mursu J, Nurmi T, Tuomainen TP, Salonen JT, Pukkala E, Voutilainen S. Intake of flavonoids and risk of cancer in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int J Cancer. 2008;123:660–663. doi: 10.1002/ijc.23421. [DOI] [PubMed] [Google Scholar]
- 75.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN, Multiethnic Cohort S. Legume and isoflavone intake and prostate cancer risk: The Multiethnic Cohort Study. Int J Cancer. 2008;123:927–932. doi: 10.1002/ijc.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geybels MS, Verhage BA, Arts IC, van Schooten FJ, Goldbohm RA, van den Brandt PA. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol. 2013;177:1388–1398. doi: 10.1093/aje/kws419. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Stevens VL, Shah R, Peterson JJ, Dwyer JT, Gapstur SM, McCullough ML. Dietary flavonoid and proanthocyanidin intakes and prostate cancer risk in a prospective cohort of US men. Am J Epidemiol. 2014;179:974–986. doi: 10.1093/aje/kwu006. [DOI] [PubMed] [Google Scholar]
- 78.Reale G, Russo GI, Di Mauro M, Regis F, Campisi D, Giudice AL, Marranzano M, Ragusa R, Castelli T, Cimino S, Morgia G. Association between dietary flavonoids intake and prostate cancer risk: A case-control study in Sicily. Complement Ther Med. 2018;39:14–18. doi: 10.1016/j.ctim.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Russo GI, Di Mauro M, Regis F, Reale G, Campisi D, Marranzano M, Lo Giudice A, Solinas T, Madonia M, Cimino S, Morgia G. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male. 2018;21:48–54. doi: 10.1080/13685538.2017.1365834. [DOI] [PubMed] [Google Scholar]
- 80.Reger MK, Zollinger TW, Liu Z, Jones JF, Zhang J. Dietary intake of isoflavones and coumestrol and the risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2018;142:719–728. doi: 10.1002/ijc.31095. [DOI] [PubMed] [Google Scholar]
- 81.Ghanavati M, Clark CCT, Bahrami A, Teymoori F, Movahed M, Sohrab G, Hejazi E. Dietary intake of polyphenols and total antioxidant capacity and risk of prostate cancer: A case-control study in Iranian men. Eur J Cancer Care (Engl) 2021;30:e13364. doi: 10.1111/ecc.13364. [DOI] [PubMed] [Google Scholar]
- 82.Walcott FL, Hauptmann M, Duphorne CM, Pillow PC, Strom SS, Sigurdson AJ. A case-control study of dietary phytoestrogens and testicular cancer risk. Nutr Cancer. 2002;44:44–51. doi: 10.1207/S15327914NC441_6. [DOI] [PubMed] [Google Scholar]
- 83.Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 84.Kellis JT, Jr, Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225:1032–1034. doi: 10.1126/science.6474163. [DOI] [PubMed] [Google Scholar]
- 85.Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT, Jr, Vickery LE. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;44:147–153. doi: 10.1016/0960-0760(93)90022-O. [DOI] [PubMed] [Google Scholar]
- 86.Hodek P, Trefil P, Stiborova M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139:1–21. doi: 10.1016/S0009-2797(01)00285-X. [DOI] [PubMed] [Google Scholar]
- 87.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 88.Scherbakov AM, Andreeva OE. Apigenin Inhibits Growth of Breast Cancer Cells: The Role of ERalpha and HER2/neu. Acta Naturae. 2015;7:133–139. doi: 10.32607/20758251-2015-7-3-133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem. 2002;66:1479–1487. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]
- 90.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–130. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].