Abstract
Blood levels of amylase and lipase are the result of a balance between production and clearance of these enzymes. Normal blood level of amylase and lipase is 0–90 U/L and 0–70 U/L, respectively. Rise in blood levels of lipase and amylase was observed in pancreatic injury. This is an observational, cross-sectional study involving 279 patients [176 COVID-positive and 103 COVID-negative] admitted at a tertiary care hospital in Mumbai during COVID pandemic.
The mean age was 45 years with SD of 15 years with men:women ratio of 2.7:1. Serum lipase and amylase were raised in 101 cases (57.4%) and 57 patients (32.4%), respectively. Out of the 101 patients with raised lipase levels, 44 (25%) patients showed a severe rise (> 3 times the normal), and out of 57 patients with raised amylase levels, 12 patients (6.8%) showed a severe rise (> 3 times the normal). From this study, it can be concluded that the prevalence of hyperamylasemia was 32.4% and that of hyperlipasemia was 57.4% in the involved study population with COVID-positive status.
Keywords: COVID-19, Serum amylase, Serum lipase, Biochemistry, Cytology, Surgery
Introduction
Gastrointestinal manifestations of COVID-19 include liver injury, diarrhoea, abdominal pain and vomiting; however, less is known about the viral effect on the pancreas and pancreatic enzymes [1]. A recent report from China described evidence of pancreatic injury, defined as elevated lipase, in up to 17% of active COVID-19 cases [4]. Amylase which is required for starch digestion is mainly secreted by the pancreas and salivary glands, but other organs [2], including the normal and diseased lungs [3], are also potential sources and are cleared by the reticuloendothelial system and the kidneys [2]. Lipase, which is mostly secreted in adults by the pancreas, is a key enzyme for the digestion of triglycerides. Lipase is excreted by the kidneys [2]. In this study, we aimed to assess the impact of COVID-19 on pancreatic enzymes.
Materials and Methods
A retrospective study was performed on the patients admitted in our hospital from May 2020 to October 2020. The COVID was diagnosed with RTPCR (reverse transcriptase polymerase chain reaction) test. In addition to routine blood tests, serum amylase and lipase levels were measured by enzymatic methods of International Federation of Clinical Chemistry (IFCC) on Beckman Coulter analysers. Computed tomography (CT) imaging could not be done due to logistic issues and resource limitation in view of the pandemic. Patients with congenital pancreatic diseases, pancreatic malignancies, solid organ malignancy, haematological cancers, auto immune disorders, other causes of acute pancreatitis such as gall stones, chronic alcoholism, trauma, drugs, hypertriglyceridemia and haemodynamically unstable patients were excluded.
A total of 279 patients (176 COVID-positive cases and 103 COVID-negative control) were studied and their data analysed. The COVID-positive patients were classified into mild, moderate and severe based on symptoms, oxygen saturation by pulse oximetry and ratio of partial pressure of arterial oxygen (PaO2) to a fraction of inspired oxygen (FiO2) i.e. PaO2/FiO2 ratio values and radiological findings, as mentioned in the guidelines for management of COVID-19, Ministry of Health and Family Welfare (MoHFW), Government of India [6].
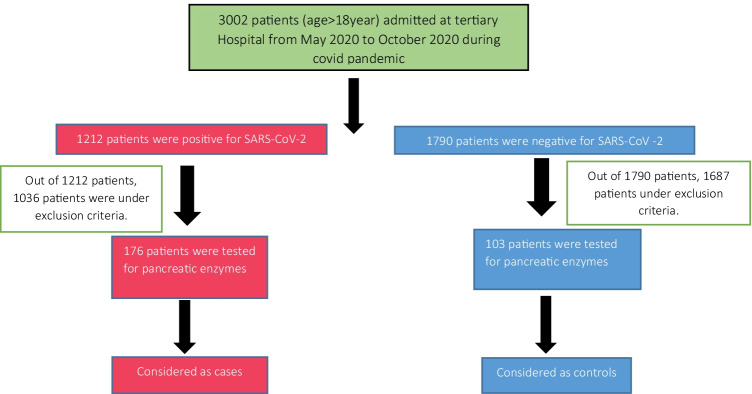
The data was analysed using Microsoft Excel. The variables were compared by chi-square method. The cutoff for statistical significance was taken as a P value < 0.05. Study time: May 2020 to October 2020. Cases, controls and selection criteria are defined in the flow chart (Fig. 1).
Fig. 1.
Flow chart
The mean age was 45 years with SD of 15 years with men:women ratio of 2.7:1.
Table 1 shows that 100 COVID-negative (97.1%) and 75 COVID-positive (42.6%) patients had normal lipase levels. Three COVID-negative (2.9%) and 57 COVID-positive (32.4%) patients had lipase level 1–3 times the normal. Forty-four COVID-positive (25%) patients had lipase level > 3 times the normal, whereas no COVID-negative patient had shown lipase level > 3 times the normal.
Table 1.
Comparison of serum lipase levels between cases and controls
| Lipase value (U/L) | Controls | Cases |
|---|---|---|
| Normal lipase | 100 (97.1%) | 75 (42.6%) |
| 1–3 Times upper level of normal lipase | 3 (2.9%) | 57 (32.4%) |
| > 3 Times upper level of normal lipase | 0 (0%) | 44 (25%) |
U/L = units per litre
Table 2 shows that 100 COVID-negative (97.1%) and 119 COVID-positive (67.6%) patients had normal amylase levels. Three COVID-negative (2.9%) and 45 COVID-positive (25.6%) patients had amylase level 1–3 times the normal. Twelve COVID-positive (6.8%) patients had lipase level > 3 times the normal, whereas no COVID-negative patient had shown amylase level > 3 times the normal.
Table 2.
Comparison of serum amylase levels between cases and controls
| Amylase value (U/L) | Control | Case |
|---|---|---|
| Normal amylase | 100 (97.1) | 119 (67.6) |
| 1–3 Times upper level of normal amylase | 3 (2.9) | 45 (25.6) |
| > 3 Times upper level of normal amylase | 0 (0) | 12 (6.8) |
U/L = units per litre
Tables 3 and 4 show the correlation of serum lipase and amylase values, respectively, in cases of mild, moderate and severe COVID infection. Out of 176 patients, 128 had a mild disease, while 5 patients and 43 patients had moderate and severe diseases, respectively.
Table 3.
Lipase levels in COVID-19 patients according to severity
| Lipase value (U/L) | Mild | Moderate | Severe | Total | P value |
|---|---|---|---|---|---|
| Normal lipase | 75 (58.6) | 0 (0) | 0 (0) | 75 (42.6) | 0.001 |
| 1–3 Times upper level of normal lipase | 52 (40.6) | 5 (100) | 0 (0) | 57 (32.4) | |
| > 3 Times upper level of normal lipase | 1 (0.8) | 0 (0) | 43 (100) | 44 (25) | |
| Total | 128 (100) | 5 (100) | 43 (100) | 176 (100) |
U/L = units per litre
Table 4.
Amylase levels in COVID-19 patients according to severity
| Amylase value (U/L) | Mild | Moderate | Severe | Total | P value |
|---|---|---|---|---|---|
| Normal amylase | 119 (93) | 0 (0) | 0 (0) | 118 (67.6) | 0.001 |
| 1–3 Times upper level of normal amylase | 9 (7) | 5 (100) | 31 (72.1) | 45 (25.6) | |
| > 3 Times upper level of normal amylase | 0 (0) | 0 (0) | 12 (27.9) | 12 (6.9) | |
| Total | 128 (100) | 5 (100) | 43 (100) | 176 (100) |
U/L = units per litre
Table 3 shows out of the 128 patients with mild disease, 52 (40.6%) patients had borderline-elevated lipase levels, while only 1 patient had severely elevated lipase levels more than three times the upper limit of normal. All the 5 patients with moderate disease had borderline-elevated lipase levels. All the 43 patients with severe disease had grossly deranged lipase levels with levels exceeding more than three times the upper limit of normal values.
Table 4 shows out of the 128 patients with mild disease, 119 had normal amylase levels, while only 9 patients had borderline-elevated amylase levels. All 5 with moderate disease had borderline-elevated amylase levels. A total of 43 had severe disease of which 31 (72.1%) had mild elevations in amylase levels, while 12 (27.9%) patients had gross elevations with amylase values exceeding three times the upper limit of normal.
Table 5 shows the prevalence of increased amylase and lipase values in the control group.
Table 5.
Amylase and lipase levels in COVID-19 patients according to severity
| Amylase and lipase value | Mild | Moderate | Severe | Total | P value |
|---|---|---|---|---|---|
| Both normal | 75 (90.4) | 0 (0) | 0 (0) | 75 (75) | 0.001 |
| Both raised 1–3 times upper level of normal | 8 (9.6) | 5 (100) | 0 (0) | 13 (13) | |
| Both raised > 3 times upper level of normal | 0 (0) | 0 (0) | 12 (100) | 12 (12) | |
| Total | 83 (100) | 5 (100) | 12 (100) | 100 (100) | |
All the corroborations have a statistical P value of less than 0.05 which brings out the findings to be statistically significant.
Discussion
COVID-19 has been associated with gastrointestinal symptoms including abdominal pain, and viral RNA has been identified in the gastrointestinal tract of patients with COVID-19. Pancreatic inflammation is reportedly known to occur with illnesses caused by several viruses viz. mumps, cytomegalovirus, coxsackie B virus and HIV. In the novel coronavirus (nCoV-SARS-2019) disease, the primary organ of damage has been the respiratory system. Effect on other systems has been variable, and several associations have been reported based on clinical observations. The pancreatic injury could be due to direct viral invasion, or secondary to hypoxic effects or cytokine-mediated injury, the exact mechanism is difficult to surmise at this juncture. Further studies on extra-pulmonary effects of COVID-19 will help us understand its pathophysiology.
Acute pancreatitis was diagnosed employing the revised Atlanta classification and was reported from Copenhagen in two out of three family members with COVID-19 infection. Gadiparthi et al. 5 have described a case of acute pancreatitis in a COVID-19 patient on a background of hypertriglyceridemia and newly-diagnosed type-2 DM. Wang et al. 4 from Wuhan, China defined pancreatic injury as any abnormality in amylase (normal range, 0–90 U/L) or lipase (normal range, 0–70 U/L), and their study reported that at admission 17% of 52 patients with COVID-19 had slightly abnormal amylase or lipase. It was suggested that pancreatic injury in COVID-19 might be caused directly by the cytopathic effect mediated by local SARS-CoV-2 replication or caused indirectly by systemic responses to respiratory failure or the harmful immune response induced by SARS-CoV-2 infection, which also leads to damage in multiple organs.
This study also highlights hyperamylasemia and hyperlipasemia in approximately one-third and half of the patients, respectively. In this study, elevated amylase and lipase levels did not correlate with the severity of COVID-19 disease or mortality. However, all the five patients with severe disease had grossly deranged amylase and lipase values with the serum values of both the enzyme exceeding more than three times the upper normal limit (refer to tabulated data).
In this study, elevated amylase and lipase levels did not correlate with the severity of COVID-19 disease or mortality. Although, all the five patients with elevated lipase levels had severe COVID pneumonia, and two patients with over 3 times elevation of lipase levels expired, there was no defined association observed of serum lipase levels with mortality, which could probably be due to the small sample size of the study.
Conclusion
Though pancreatic injury may be seen in one-third of patients with COVID-19, acute pancreatitis is uncommon. One should be careful if there is simultaneous elevation of amylase and lipase, as it may be associated with a worse prognosis. One cannot determine the pathophysiological mechanism behind elevation of amylase and lipase levels in COVID-positive patients, as it primarily appears to have a multifactorial pathogenesis. Further extensive studies on the extra pulmonary manifestations of COVID 19 are required to better understand the pathophysiology behind the rise of pancreatic enzymes in COVID-positive patients.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 1990;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieper-Bigelow C, Strocchi A, Levitt MD. Where does serum amylase come from and where does it go? Gastroenterol Clin North Am. 1990;19:793–810. doi: 10.1053/j.gastro.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Berk JE, Shimamura J, Fridhandler L. Amylase changes in disorders of the lung. Gastroenterology. 1978;74:1313–1317. doi: 10.1053/j.gastro.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159(1):367–370. doi: 10.1053/j.gastro.2020.03.05510.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadiparthi C, Bassi M, Yegneswaran B, Ho S, Pitchumoni CS. Hyperglycemia, hypertriglyceridemia, and acute pancreatitis in COVID-19 infection: clinical implications. Pancreas. 2020;49:e62–e63. doi: 10.1097/mpa.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health and Family Welfare, Government of India 2020 Guidance document on appropriate management of suspected/confirmed cases of COVID-19 – types of COVID-19 dedicated facilities dated 7th April, 2020