Highlights
-
•
SEEG stimulation of the hippocampus produced the auditory aura by itself and prior to the seizure.
-
•
Heshcl’s gyri stimulation produced another distinctive sound like the “buzz of a loudspeaker”.
-
•
Resection of mesial temporal structures resulted in seizure freedom since the last 3 years.
-
•
This is an initial description of auditory aura arising from the hippocampus using Stereo EEG.
Keywords: Temporal plus epilepsy, Auditory aura, Stereotactic EEG, Heschl’s gyrus, Cortical stimulation
Abbreviations: Stereotactic Electroencephalogram, SEEG
Abstract
Epileptic auras provide the initial clues to identify the probable region of ictal onset. In temporal lobe epilepsies, auras are most commonly experiential or viscerosensory, when they arise from mesial structures. Whereas sensations such as vertigo and auditory hallucinations are more likely to occur from the lateral temporal lobe structures. Auditory auras have been described from the lateral temporal neocortex, Heschl’s gyri, frontal operculum and posterior insula. We herein describe a patient with temporal lobe epilepsy with an auditory aura who was localized to have the onset of seizures from the hippocampus using stereotactic EEG (SEEG). Stimulation of the hippocampal contacts also reproduced the habitual auditory aura. Anterior temporal lobectomy with amygdalohippocampectomy resulted in complete seizure freedom for 3 years.
This is an initial description of auditory aura elicited from the hippocampus using SEEG.
Introduction
Epileptic auras provide important clinical clues to help localize the ictal onset zone. They usually arise close to, but not always necessarily from the ictal onset zone, as there can be functionally silent areas nearby. Auras must hence be interpreted with caution. With respect to the temporal lobe, auras are most commonly viscero-sensory, when they arise from the mesial structures such as the hippocampus and amygdala. They are described as epigastric discomfort, experiential auras (déjà vu/jamais vu), fearfulness and/or olfactory disturbances. Whereas, sensations such as vertigo and auditory hallucinations are more likely to occur from the lateral temporal lobe structures. Auditory auras have been described from Heschl’s gyri, lateral temporal neocortex, posterior insula, and frontal operculum, but not usually from mesial temporal structures [1], [2]. We herein describe a patient with drug resistant temporal lobe epilepsy with an auditory aura, which was proven by stereotactic-EEG (SEEG) to arise from the hippocampus.
Case report
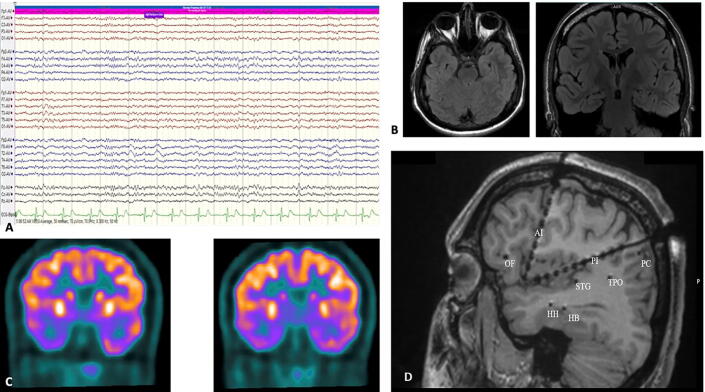
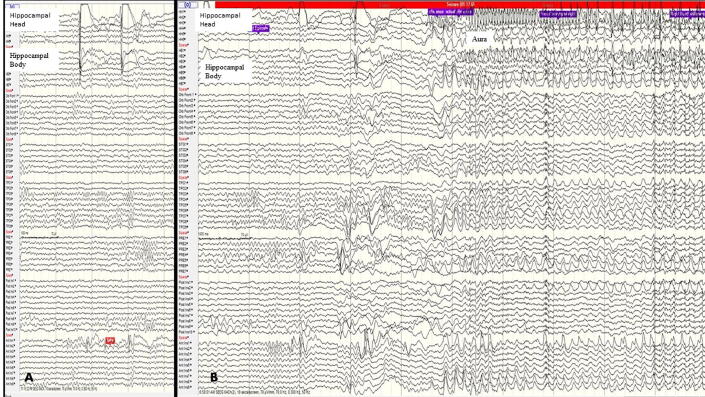
An Indian male, nineteen years of age, presented with history of seizures since the age of three years. He had an unremarkable birth and developmental history. He had no history of febrile seizures or any other early insult. A detailed family history taken, revealed no history of epilepsy in 3 generations. His first seizure at the age of three years, was a left focal to bilateral tonic clonic seizure. Subsequently, he was seizure-free for about a year and his habitual events have occurred since the age of five years. They were characterized by an auditory aura – ringing sensation in both ears similar to the ringing of a telephone, followed by behavioral arrest and staring. The aura lasted for a few seconds, about 3 to 4. All seizures were preceded by the same aura. He had no automatisms or irrelevant speech during the seizure. These events tended to occur about twice a month. There were no focal seizures with awareness. Our clinical hypothesis alluded to a seizure onset from the lateral temporal lobe structures (probably Heschl’s gyrus). Onset from the posterior insula in such instances is not uncommon either. There were no features in the semiology of the habitual seizure which could aid in the lateralization of the ictus. However, the first seizure lateralized to the right hemisphere. Interictal EEG showed right temporal polymorphic delta slowing (Fig. 1a.). He underwent imaging with a 3.0T MRI scanner under an epilepsy protocol, which was normal (Fig. 1b.). He was admitted to the epilepsy monitoring unit for long-term EEG monitoring (LTM) and no habitual event was recorded over five days. LTM recorded at another center captured one seizure from the right temporal region, however this record could not be reviewed. Interictal FDG-PET showed hypometabolism in the right mesial temporal structures and temporal pole. No abnormal areas of hypometabolism were detected in the insula or lateral temporal region (Fig. 1c.). Overall, the patient had an MR negative, PET positive right temporal/temporal plus epilepsy. As auditory auras have been described from the insula, a possibility of tempo- insular form of Temporal plus epilepsy was considered. The option of repeat LTM vs. SEEG implantation and extra-operative monitoring was discussed. 8 SEEG electrodes (Ad-Tech, 10 contact with 7 mm spacing) were implanted in the right hippocampus (HC) (head and body), posterior superior temporal gyrus (STG) (covering the Heschl’s gyrus), temporo-parieto-occipital junction (TPO), precuneus, orbitofrontal region and the anterior and posterior insula (Fig. 1d.). Interictal abnormalities were seen predominantly in the head and body of the hippocampus and anterior insula (Fig. 2a.). Six habitual events were recorded which began in the head of the right HC (Fig. 2b.). At onset and during the aura, rhythmic gamma activity was observed from the head of the hippocampus. There was no spread to the Heschl’s gyrus, after perception of aura/seizure onset.
Fig. 1.
a. Interictal right temporal polymorphic delta slowing. b. MRI brain revealed no abnormalities. c. Interictal PET depicting right mesial temporal hypometabolism. d. Implanted SEEG electrodes: HH: Hippocampal head, HB: Hippocampal body, STG: Superior temporal gyrus, OF: Orbitofrontal lobe, AI: Anterior insula, PI: Posterior insula, TPO: Temperoparietooccipital Junction, PC: Precuneus.
Fig. 2.
a. Interictal abnormalities in the head and body of the hippocampus and anterior insula. b. Ictal SEEG of habitual event: onset from the head of the right HC.
Cortical stimulation was carried out using the Nikolet cortical stimulator with paired electrodes. The stimulation parameters were set at a frequency of 50 Hz, stimulus train of 500 ms, stimulus duration of 2 to 3 s, and the current was raised in steps of 0.5 to 1 m amp, up to 5 m Amp, to look for a response such as seizure, or till after discharges occurred.
The auditory aura alone without a seizure, was elicited from HH1 and HH2 contacts when stimulated at 1 m amp, with a lag of 1 s, after ictal onset of EEG changes. The seizure was elicited with auditory aura, when stimulated at 2 m amp. Stimulation of the Heschl’s gyrus revealed an auditory phenomenon which the patient described as similar to the “buzzing of a loud speaker”. He could clearly distinguish the epileptic aura produced by stimulating the hippocampal head (HH1 and HH2), from this.
The patient underwent a right anterior temporal lobectomy with amygdalohippocampectomy (ATL + AH). Post- surgery, the patient had no motor or cognitive deficits and recovered well. He has been followed up after surgery for 3 years and has remained seizure free (Engel 1a).
Histopathology of the resected specimen showed reactive gliosis in the temporal pole and amygdala. No focal cortical dysplasia or hippocampal sclerosis was detected.
Discussion
Aura has been described in the 1981 International Classification of Epileptic seizures, as “that portion of the seizure which occurs before consciousness is lost and for which memory is retained afterwards”. Although the seizure can arise from functionally silent areas and then propagate to a symptomatogenic area, producing an aura, it is generally considered close to and functionally connected to the ictal onset zone. Studies using SEEG for localizing auditory auras are few. Auditory auras have been localized to lateral temporal cortex, posterior insula and frontal operculum using Intracranial EEG [3], [4]. They have not been described from the hippocampus.
Studies in rats have identified that hippocampal neurons are involved in auditory processing, particularly auditory fear conditioning. Voxel based fMRI studies in humans have identified that the HC, in addition to the planum temporale, plays a role in identifying learned acoustic patterns [5], [6].
Mesial temporal auras have usually psychic/ viscero-sensory components. However, studies have shown that up to 6–7% of patients with hippocampal sclerosis (HS) may have auditory auras [7], [8].
Auditory auras such as elementary sounds have been traditionally described from the Heschls’ gyri. Ictal deafness has also been described from the dominant temporal lobe [9]. More complex auditory auras such as illusions have been described from the auditory association cortex, posterior insula and frontal operculum [2], [3]. More complex auras such as hallucinations, auditory and verbal may arise from the lateral neocortex and posterior language areas in the dominant hemisphere [10] (Table 1).
Table 1.
Localization of Auditory auras.
Florindo et al studied auditory aura by dividing their patients into four groups, viz, simple/ complex hallucinations and positive / negative illusions. Those with unilateral auditory aura, did not show any difference in lateralization, ipsilaterally or contralaterally. However, those patients who underwent presurgical evaluation, showed a contralateral epileptogenic zone. Both complex hallucinations and negative illusions lateralized to the dominant hemisphere [11].
Our patient, had an elementary auditory aura of ringing in both ears. As both lateral temporal neocortex and posterior insula have been described to produce auditory auras, we initially considered our patient with MRI negative temporal lobe epilepsy to be a part of possibly temperoinsular type of temporal plus syndrome. Such patients tend to do poorly if only conventional ATL + AH is done [12]. This situation is more likely to occur in MRI negative cases, wherein the epileptogenic substrate is most likely to be a subtle focal cortical dysplasia (FCD).
Hence the epileptogenic network was studied with SEEG, and both the spontaneous and stimulated seizures localized to the hippocampus. Also, the typical aura was produced without a seizure at lower currents of stimulation, thereby confirming the hippocampal origin of the aura. Stimulation of the Heschl’s gyri also produced a different sound. Post ATL + AH, the patient continues to be seizure-free for 3 years. We believe this to be an initial description of auditory aura elicited from the hippocampus using SEEG.
Interestingly, in our case, the substrate remains unknown as the resected specimen failed to show abnormalities suggestive of an FCD.
Conclusions
In our patient, auditory auras arose from the hippocampus confirmed by SEEG recording. Localization of an auditory aura was reproduced by direct electrical stimulation of the hippocampal SEEG electrodes, to further support a mesial temporal origin.
Conflict of interest
This is to declare that the authors do not have any declaration of interest. Also, no funding was used for this study. This work has not been submitted to any other journal or published elsewhere. Informed consent of the patient was taken before writing this manuscript.
Ethical statement
This is to certify that, this work is the authors’ original work and has not been published elsewhere.
This paper is also not being considered for publication elsewhere.
All authors were involved in the care of the patient and also in preparation and revision of the manuscript.
No funding was used in this study.
There was no conflict of interest of any of the authors in the conduct of this study.
Informed consent was taken from the patient for this study.
References
- 1.Perven G., So K. Epileptic auras: phenomenology and neurophysiology. Epileptic Disord. 2015;17(4):349–362. doi: 10.1684/epd.2015.0786. [DOI] [PubMed] [Google Scholar]
- 2.Isnard J., Guenot M., Sindou M., Mauguiere F. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004;45(9):1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson S., Alexopoulos A., Bingaman W., et al. Auditory aura in frontal opercular epilepsy: sounds from afar. Epileptic Disord. 2015;17(2):150–155. doi: 10.1684/epd.2015.0742. [DOI] [PubMed] [Google Scholar]
- 4.Penfield W., Perot P. The brain's record of auditory and visual experience: a final summary and discussion. Brain. 1963;86(4):595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C., Liu Y., Xu J., Gan X., Xiao Z. Septal and hippocampal neurons contribute to auditory relay and fear conditioning. Front Cell Neurosci. 2018;12:102. doi: 10.3389/fncel.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Bonnici H.M., Teki S., Agus T.R., Pressnitzer D., Maguire E.A., et al. Representations of specific acoustic patterns in the auditory cortex and hippocampus. Proc R Soc B. 2014;281(1791):20141000. doi: 10.1098/rspb.2014.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari-Marinho T., Caboclo L.O.S.F., Marinho M.M., Centeno R.S., Neves R.S.C., Santana M.T.C.G., et al. Auras in temporal lobe epilepsy with hippocampal sclerosis: relation to seizure focus laterality and post surgical outcome. Epilepsy Behav. 2012;24(1):120–125. doi: 10.1016/j.yebeh.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Asadi Pooya A.A., Nei M., Sharan A., Sperling M.R. Auras in patients with temporal lobe epilepsy and mesial temporal sclerosis. J Neurol Sci. 2016;364:24–26. doi: 10.1016/j.jns.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh D., Mohanty G., Prabhakar S. Ictal deafness—a report of three cases. Seizure. 2001;10:130–133. doi: 10.1053/seiz.2000.0476. [DOI] [PubMed] [Google Scholar]
- 10.Serino A., Heydrich L., Kurian M., Spinelli L., Seeck M., Blanke O. Auditory, verbal hallucinations of epilepstic origin. Epilepsy Behav. 2014;31:181–186. doi: 10.1016/j.yebeh.2013.12.014. Epub 2014 Jan 14. [DOI] [PubMed] [Google Scholar]
- 11.Florindo I., Bisulli F., Pittau F., Naldi I., Striano P., Striano S., et al. Lateralizing value of the auditory aura in partial seizures. Epilepsia. 2006;47(Suppl. 5):68–72. doi: 10.1111/j.1528-1167.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 12.Kahane P., Barba C., Rheims S., Job-Chapron A.S., Minotti L., Ryvlin P. The concept of temporal ‘plus’ epilepsy. Revue Neurologique. 2015;171(3):267–272. doi: 10.1016/j.neurol.2015.01.562. [DOI] [PubMed] [Google Scholar]