Abstract
Strain VeGlc2, an anaerobic ultramicrobacterium belonging to the Verrucomicrobiales lineage of bacterial descent, fermented glucose to acetate, propionate, succinate, and CO2. The distribution of radiolabel in the fermentation end products produced from position-labelled glucose and in vitro measurements of enzyme activities in crude cell extracts prepared from glucose-grown cells showed that glucose was metabolized via the Embden-Meyerhof-Parnas pathway. The 6-phosphofructokinase (EC 2.7.1.90) activity required pyrophosphate as the phosphoryl donor, and ATP could not replace pyrophosphate. The other enzyme activities were those of a classical Embden-Meyerhof-Parnas pathway. 14CO2 was incorporated into propionate and succinate, suggesting that a carboxylation reaction rather than a transcarboxylation reaction was involved in the reductive pathway leading to succinate and propionate. Difference spectra showed that a type b cytochrome was present, which could be involved in electron transport in the reductive pathway.
The division Verrucomicrobia (14, 39) is a recently recognized division of bacterial descent that is apparently most closely related to the orders Chlamydiales and Planctomycetales (20, 39). This group is presently represented by five species belonging to the order Verrucomicrobiales, Verrucomicrobium spinosum (33, 39) and four species of the genus Prosthecobacter (13, 14, 36). In addition, partial 16S rRNA genes with sequences that indicate that they originated from members of this phylogenetic group have been recovered from a range of soil, water, and sediment samples (17, 23, 39–41). The results of comparative sequence analyses suggest that this group is phlyogenetically very diverse. Recently, three strains of obligately anaerobic bacteria belonging to the order Verrucomicrobiales were isolated from the bulk soil of laboratory-grown rice microcosms (20).
Very little is known about the bacteria belonging to the Verrucomicrobiales lineage of descent. V. spinosum and Prosthecobacter spp. are heterotrophs that are able to grow on sugars but not on amino or organic acids (14, 33, 36). V. spinosum is a facultative anaerobe that is able to grow fermentatively (33) and contains menaquinones (35). The new anaerobic bacteria isolated from the rice paddy soil microcosms have also been characterized phenotypically (20). These three strains grow as oxygen-tolerant fermentative anaerobes on a number of sugars. The pathway of glucose fermentation by one of these isolates, strain VeGlc2, was investigated and was found to be an Embden-Meyerhof-Parnas (EMB) pathway in which a pyrophosphate-dependent 6-phosphofructokinase is used.
MATERIALS AND METHODS
Growth conditions.
Strain VeGlc2 (20) was grown in the bicarbonate-buffered, sulfide-reduced, anoxic mineral medium FM (20). Unless noted otherwise, 4 mM d-glucose was the sole carbon and energy source and was added from filter-sterilized (pore size, 0.2 μm) stock solutions just before the cultures were inoculated. All preparations were incubated at 30°C in the dark.
Analytical methods.
The organic end products of fermentation were determined by high-performance liquid chromatography (22). Hydrogen production was measured with an HgO-to-Hg conversion detector (20). Protein contents were determined by a slight modification of the Bradford (5) method by using bovine serum albumin as a standard. Culture density was routinely monitored by measuring the optical densities at 440 and 650 nm in a 10-mm cuvette. Dry mass growth yields were calculated from culture densities by using a gravimetrically determined conversion factor obtained from cell pellets that were harvested from two 1-liter cultures by centrifugation, washed with 25 mM ammonium acetate, and dried to a constant mass at 105°C. Fermentation balances were calculated as previously described (20).
The protein content of whole cells was estimated by boiling 200 μl of diluted (in 50 mM potassium phosphate buffer [pH 7.0]) cell suspension with 50 μl of 10 M NaOH. Bovine serum albumin standards were treated in the same way. The samples and standards were then centrifuged at 13,000 × g for 5 min, and 200 μl of each resulting supernatant was added to 600 μl of phosphate buffer. The protein contents of these samples and standards were then determined (5).
Preparation of crude cell extracts.
Cells in the late logarithmic phase were harvested by centrifugation under strictly anoxic conditions as previously described (19) and then were washed and resuspended in anoxic 100 mM MOPS (morpholinopropanesulfonic acid) buffer (pH adjusted to 7.0 with NaOH) containing 2.5 mM dithioerythritol and 10 mM MgCl2 for enzyme assays or in 50 mM potassium phosphate buffer (pH 7.0) for cytochrome scans. Crude cell extracts were prepared by French press treatment as previously described (19).
Cytochrome scans.
Reduced-minus-oxidized scans were made with a model U-2000 spectrophotometer (Hitachi, Tokyo, Japan) after one 1-ml aliquot of a crude cell extract was oxidized with a few crystals of sodium persulfate and a second 1-ml aliquot was reduced with a few crystals of sodium dithionite.
Measurement of enzyme activities.
Continuous enzyme activity assays were carried out in 1-ml reaction mixtures in 1.5-ml semimicrocuvettes (under N2 unless noted otherwise) at 30°C by using a model U-2000 spectrophotometer. Linearity with added crude extract was tested, and dependence on key components of the test (e.g., substrate, coenzyme A [CoA], ATP, etc.) was checked. All enzyme assays were carried out under strictly anoxic conditions (6). Enzyme activities were expressed in micromoles of substrate transformed per milligram of cell free crude extract protein, as determined with previously published extinction coefficients (28). Reduction of 2 μmol of methylviologen was defined as oxidation of 1 μmol of substrate. The activities are given below as the means and standard deviations for three to six determinations made with one cell extract. When activities could not be detected, up to four additional attempts were made with cell extracts prepared from independently grown cultures.
Continuous enzyme assays were performed by using the following methods and modifications. Hexokinase, glucose 1-dehydrogenase, glucose-6-phosphate 1-dehydrogenase, phosphogluconate 2-dehydrogenase, glucose-6-phosphate isomerase, 6-phosphofructokinase, and fructose bisphosphate aldolase activities were measured as described previously (21). The assay mixtures used for triose phosphate isomerase, 3-phosphoglycerate kinase, enolase, and phosphoglycerate mutase activity assays (21) contained 0.25 mM NADH. The malate dehydrogenase (6) and glyceraldehyde-3-phosphate dehydrogenase assays (24) were performed with dithioerythritol instead of dithiothreitol. The activities of fumarate hydratase, succinate dehydrogenase (with potassium ferricyanide as an artificial electron acceptor), phosphoenolpyruvate (PEP) carboxylase, and PEP carboxykinase were measured as described by Brune and Schink (6). Pyruvate synthase (11), fumarate reductase (4), pyruvate decarboxylase (3), methylmalonyl-CoA:pyruvate transcarboxylase (37), and methylmalonyl-CoA decarboxylase activities (15) were assayed by using previously described methods. Pyruvate kinase activity was measured as described by Bergmeyer et al. (3). Pyruvate orthophosphate dikinase and pyruvate H2O dikinase activities were measured by using a modification of the pyruvate kinase assay; in the pyruvate orthophosphate dikinase assay 20 mM Na4P2O5 was included and the assay was started with 5 mM AMP instead of ADP, and in the pyruvate H2O dikinase assay 20 mM KH2PO4 was included and the assay was started with 5 mM AMP instead of ADP. Pyruvate carboxylase activity was measured as described by Seubert and Weichler (34); the assay was started with either 1.3 mM ATP or 1.3 mM GTP. Succinate:propionyl-CoA CoA-transferase activity was measured as described by Hilpert et al. (16), and succinate:acetyl-CoA CoA-transferase activity was measured by the same method except that the assay was started with 1 mM acetate instead of propionate. Acetate-CoA ligase activity was measured by using the method of Oberlies et al. (29), while propionate-CoA ligase and succinate-CoA ligase activities were measured by using 1 mM propionate and 1 mM succinate, respectively, instead of acetate in the same assay. Phosphate acetyltransferase activity was measured as described previously (19), and phosphate propionyltransferase activity was measured by the same method except that propionate was used instead of acetate. Acetate kinase activity was measured as described by Bergmeyer et al. (3), while propionate kinase activity was measured by using propionate instead of acetate. Pyrophosphate-dependent acetate kinase and propionate kinase activities were measured by using these assay systems with 5.4 mM Na4P2O5 instead of ATP.
Discontinuous assays of acetate kinase and propionate kinase activities in which ATP was used or in which Na4P2O5 was substituted were carried out by using the method of Rose (31).
Metabolism of radiolabelled glucose and CO2.
Five-milliliter aliquots of FM in vials (total volume each closed vial, 13.2 ml) that had an N2-CO2 (80:20, vol/vol) headspace and contained 4 mM glucose and 30 mM NaHCO3 were each inoculated with 1 ml of a stationary-phase culture of strain VeGlc2 grown under the same conditions. Then 37 kBq of [1-14C]glucose, 37 kBq of [6-14C]glucose, or 300 kBq of NaH14CO3 was added to each vial; these additions changed neither the volumes nor the glucose or CO2 concentrations significantly (i.e., the changes were <1%). One-milliliter samples of the liquid phase were removed and frozen at −20°C. The vials were incubated for 3 days at 30°C, and then additional 1-ml samples were removed and frozen at −20°C. Fifty microliters of 97% H2SO4 was then injected into each vial, and the vials were shaken well by hand for 5 min. Samples were removed from the headspace with a Pressure-Lok series A-2 gas-tight syringe (Precision Sampling Corp., Baton Rouge, La.), and the radiolabel in CO2 was measured with a gas chromatograph and an in-line radioactivity detector (7). The liquid samples were thawed and analyzed to determine their glucose, acetate, propionate, and succinate contents and the amounts of radiolabel in these compounds by using a high-performance liquid chromatograph equipped with an in-line radioactivity detector (22).
RESULTS AND DISCUSSION
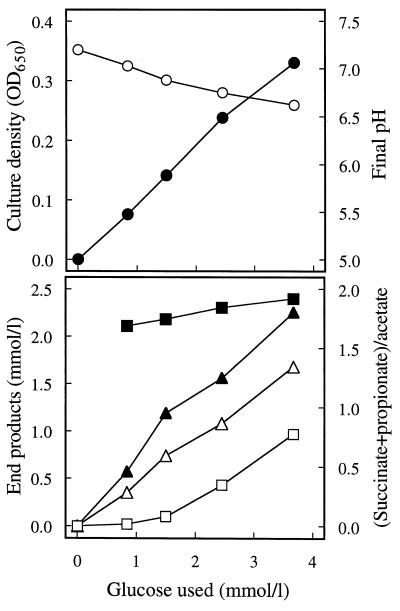
Strain VeGlc2 fermented glucose to acetate, propionate, and succinate (Fig. 1). Carbon dioxide was presumably formed as well but was not measured as it was the buffer system used in the growth medium. The amount of hydrogen produced was always less than 10−4 mol per mol of glucose fermented. The amount of biomass produced, as determined by optical density, was proportional to the amount of glucose utilized (Fig. 1). At low levels of glucose, acetate and propionate were produced, but as the amount of glucose utilized increased, the amount of succinate increased at the expense of the amount of propionate produced. The molar ratio of succinate plus propionate to acetate was always between 1.7 and 2.1 (Fig. 1). The increase in the succinate/propionate ratio may have been triggered by the concomitant decrease in medium pH with increasing glucose utilization (and hence increasing concentrations of acidic end products), but this was not investigated further. More detailed fermentation balance data have been presented elsewhere (20).
FIG. 1.
End products and biomass production from glucose by strain VeGlc2. The fermentation end products from glucose were acetate (▵), propionate (▴), and succinate (□). Also shown are the final pH of the growth medium (○), the ratio of propionate plus succinate to acetate (■), and the amount of biomass produced, as measured by optical density at 650 nm (OD650) (•), after growth on different amounts of glucose. The mean carbon and available H balances of the experiments were 97.3 and 97.6%, respectively.
The specific growth yield on glucose was 45.5 ± 2.8 g per mol (mean ± standard deviation; n = 4). In growth medium FM at pH 7.2 and 30°C, the specific growth rate was 0.117 ± 0.0027 h−1 (mean ± standard deviation; n = 3), corresponding to a doubling time of 5.92 ± 0.13 h. The protein content of the dry mass was 45%. From specific growth yields of 42.1 to 48.0 g (dry weight) per mol of glucose and specific growth rates of 0.115 to 0.120 h−1 maximum specific substrate turnover rates of 2.40 to 2.85 mmol of glucose per g (dry weight) per h were calculated, which corresponded to 0.088 to 0.106 μmol of glucose per mg of protein per min.
The in vitro assays of enzyme activities in crude cell extracts of glucose-grown strain VeGlc2 cells showed that all of the activities for a functional EMP pathway were present (Table 1). The EMP pathway enzyme activities detected were mainly just a little lower than the calculated maximum specific substrate turnover rate, or they were much higher. Glucose was apparently phosphorylated by an ATP-dependent hexokinase and was further metabolized to pyruvate by enzymes of the classical EMP pathway. The 6-phosphofructokinase reaction apparently required inorganic pyrophosphate as the phosphoryl donor instead of ATP. Pyrophosphate-dependent 6-phosphofructokinase activities have been found in a number of different bacteria, including Propionibacterium shermanii (30), Spirochaeta thermophila (21), and Amycolatopsis methanolica (2). No other pyrophosphate-dependent enzyme activities were detected in strain VeGlc2, but since the acetate, propionate, and succinate formation pathways have not been completely elucidated, the presence of these activities cannot be ruled out yet.
TABLE 1.
Mean specific activities of enzymes in crude extracts of glucose-grown strain VeGlc2 cellsa
| Enzyme activity | EC no. | Sp act (μmol per mg of protein per min) |
|---|---|---|
| Hexokinase (ATP) | 2.7.1.1/2 | 0.036 (0.004)b |
| Hexokinase (PEP) | <0.001 | |
| Hexokinase (P2O54−) | <0.001 | |
| Glucose-6-phosphate 1-dehydrogenase (NADP) | 1.1.1.49 | 0.019 (0.003) |
| Phosphogluconate 2-dehydrogenase (NAD) | 1.1.1.43 | 0.012 (0.001) |
| Phosphogluconate 2-dehydrogenase (NADP) | 1.1.1.43 | 0.014 (0.001) |
| Glucose-6-phosphate isomerase | 5.3.1.9 | 0.188 (0.009) |
| 6-Phosphofructokinase (ATP) | 2.7.1.11 | <0.001 |
| 6-Phosphofructokinase (P2O54−) | 2.7.1.90 | 1.93 (0.21) |
| Fructose bisphosphate aldolase | 4.1.2.13 | 0.048 (0.018) |
| Triose phosphate isomerase | 5.3.1.1 | 47.9 (9.5) |
| Glyceraldehyde-3-phosphate dehydrogenase | 1.2.1.12 | 1.48 (0.24) |
| 3-Phosphoglycerate kinase | 2.7.2.3 | 0.778 (0.065) |
| Enolase | 4.2.1.11 | 2.46 (0.04) |
| Phosphoglycerate mutase | 5.4.2.1 | 0.071 (0.005) |
| Pyruvate kinase | 2.7.1.40 | 0.037 (0.004) |
| Pyruvate synthase | 1.2.7.1 | 0.026 (0.003) |
| Malate dehydrogenase (NAD) | 1.1.1.37 | 28.5 (2.3) |
| Fumarate hydratase | 4.2.1.2 | 5.56 (0.82) |
| Succinate dehydrogenase | 1.3.99.1 | 0.045 (0.024) |
| Fumarate reductase | 1.3.99.1 | 0.019 (0.007) |
| Succinate-CoA ligase | 6.2.1.4/5 | 0.112 (0.013) |
The specific activities of the following enzymes were less than 0.001 μmol per mg of protein per min: glucose 1-dehydrogenase (NAD) (EC 1.1.1.118), glucose 1-dehydrogenase (NADP) (EC 1.1.1.119), glucose-6-phosphate 1-dehydrogenase (NAD) (EC 1.1.1.49), malate dehydrogenase (NADP) (EC 1.1.1.82), pyruvate orthophosphate dikinase (EC 2.7.9.1), pyruvate H2O dikinase (EC 2.7.9.2), acetate kinase (ATP) (EC 2.7.2.1), acetate kinase (P2O54−) (EC 2.7.2.12), propionate kinase (ATP) (EC 2.7.2.-), propionate kinase (P2O54−) (EC 2.7.2.-), pyruvate decarboxylase (EC 4.1.1.1), methylmalonyl-CoA:pyruvate transcarboxylase (EC 2.1.3.1), PEP carboxylase (EC 4.1.1.31), PEP carboxykinase (EC 4.1.1.32), pyruvate carboxylase (ATP) (EC 6.4.1.1), pyruvate carboxylase (GTP) (EC 6.4.1.-), methylmalonyl-CoA decarboxylase (EC 4.1.1.41), succinate:propionyl-CoA CoA-transferase (EC 2.8.3.-), succinate:acetyl-CoA CoA-transferase (EC 2.8.3.-), acetate-CoA ligase (EC 6.2.1.1/13), propionate-CoA ligase (EC 6.2.1.17), phosphate acetyltransferase (EC 2.3.1.8), and phosphate propionyltransferase (EC 2.3.1.-).
The values in parentheses are standard deviations, based on three to six determinations.
Pyruvate was cleaved by pyruvate synthase. Methylviologen was used as an electron acceptor in the in vitro pyruvate synthase assays, but the physiological electron acceptor is not known. The pyruvate synthase reaction was CoA dependent. The roles of the glucose-6-phosphate 1-dehydrogenase and phosphogluconate 2-dehydrogenase activities are not known. Some of the enzyme activities leading to acetate, propionate, and succinate formation could not be detected in crude cell extracts or in cells permeabilized in a cuvette with cetyltrimethylammonium bromide or with toluene-ethanol, despite attempts in which different substrate concentrations and different buffers were used (18). Enzyme activities catalyzing the steps leading to acetate production from acetyl-CoA could not be detected. Most of the enzymes of the reductive pathway leading to succinate and propionate formation could be detected; the exceptions were the enzymes of the first (oxaloacetate-forming) step and the last steps catalyzing the conversion of succinyl-CoA to propionate. The succinate dehydrogenase activity was measured by using ferricyanide as an artificial electron acceptor. The physiological acceptor is not known. Whether the enzymes that have not been detected yet are not present or the assay conditions have not been suitable cannot be determined at present.
Strain VeGlc2 fermented [1-14C]glucose and [6-14C]glucose to labelled acetate, succinate, and propionate (with approximately equal specific activities in each product), but no label was detected in CO2 (Table 2). The labelling pattern obtained with 14C-labelled glucose also suggests that the EMP pathway is the major pathway of glucose catabolism in this strain. Operation of the Entner-Doudoroff pathway or of a hexose monophosphate pathway would result in the formation of radiolabelled CO2 from [1-14C]glucose (12, 27). Although low activities of some hexose monophosphate pathway enzymes were detected (Table 1), the [14C]glucose-labelling patterns and generally higher specific activities of EMP pathway enzymes strongly suggest that these reactions are of minor importance, and they probably serve to supply pentose precursors for anabolic pathways. A minor role for other pathways in glucose metabolism cannot be ruled out.
TABLE 2.
Amounts of products formed in 5-ml mixtures and distribution of radiolabel from glucose and bicarbonate in the products of glucose fermentation by strain VeGlc2
| Addition | Products
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Succinate
|
Acetate
|
Propionate
|
CO2
|
|||||
| Amt (μmol) | Amt of radiolabel (Bq/μmol) | Amt (μmol) | Amt of radiolabel (Bq/μmol) | Amt (μmol) | Amt of radiolabel (Bq/μmol) | Amt (μmol)a | Amt of radiolabel (Bq/μmol) | |
| [1-14C]Glucose | 4.10 | 886 | 8.68 | 951 | 11.42 | 1,013 | 4.58 | <20 |
| [6-14C]Glucose | 4.21 | 879 | 8.67 | 940 | 11.27 | 930 | 4.46 | <20 |
| NaH14CO3 | 4.10 | 1,269 | 8.43 | <20 | 11.22 | 767 | 4.33 | NAb |
Calculated as follows: moles of CO2 = moles of acetate − moles of succinate.
NA, not applicable.
When unlabelled glucose was fermented in the presence of NaH14CO3, propionate and succinate were labelled, but no label was incorporated into acetate (Table 2). The specific activity of radiolabel incorporated into the succinate produced was almost twice the specific activity of radiolabel incorporated into propionate. This suggests that there was a carboxylation reaction producing oxaloacetate from pyruvate or PEP rather than a transcarboxylase activity from methylmalonyl-CoA to pyruvate. Also, a transcarboxylase would not allow the variability in succinate-to-propionate ratios observed. The enzyme activities that can be measured suggest that there is reductive production of succinate, with some methylmalonyl-CoA decarboxylase activity producing propionate. The enzymes of this pathway are presumably similar to the enzymes found in other succinate- and propionate-producing bacteria (1, 25, 26, 32, 37).
Reduced-minus-oxidized spectra of crude extracts of strain VeGlc2 cells grown on glucose contained peaks with maxima at 427, 525, and 557.5 nm, indicative of a type b cytochrome. By using a previously published extinction coefficient (9), a cytochrome b content of 158 nmol per g of cell protein was estimated. The function of type b cytochromes in succinate- and propionate-producing bacteria appears to be in the transfer of electrons to the fumarate reductase reaction (9, 10, 26), and in strain VeGlc2 type b cytochromes may transfer electrons arising from oxidations in the EMP pathway to the fumarate reductase reaction.
The specific growth yield of strain VeGlc2 on glucose was 45.5 g (dry weight) of cells per mol of glucose, which corresponds to an ATP yield of about 4 mol of ATP per mol of glucose, assuming a biomass yield of 10.5 g (dry weight) of cells per mol of ATP (38). The enzyme activities measured indicate that there was a net ATP yield of 1 mol per mol of glucose in the EMP pathway to pyruvate (one ATP was used in the hexokinase reaction, two ATP equivalents were used in the 6-phosphofructokinase reaction, and four ATP were produced in the 3-phosphoglycerate kinase and pyruvate kinase reactions). There must, therefore, be additional ATP-forming steps in the pathways leading to acetate, propionate, and succinate. The possibilities include an as-yet-undetected acetate kinase reaction, a sodium-pumping methylmalonyl-CoA decarboxylase reaction, and/or electron transport-mediated generation of a proton motive force during the reduction of fumarate to succinate.
The Verrucomicrobiales represent a lineage of descent that is well-separated from the well-studied lineages of the Proteobacteria and the gram-positive bacteria (20, 39). The central metabolic pathway of one representative, strain VeGlc2, appears to be slightly different from the classical EMP pathway found in many other bacteria.
ACKNOWLEDGMENT
I thank Alexandra Schuhmann-Pidun for excellent technical assistance.
REFERENCES
- 1.Allen S H G, Kellermeyer R W, Sternholm R L, Wood H G. Purification and properties of enzymes involved in the propionic acid fermentation. J Bacteriol. 1964;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves A M C R, Euverink G J W, Hektor H J, Hessels G I, van der Vlag J, Vrijbloed J W, Hondmann D, Visser J, Dijkhuizen L. Enzymes of glucose and methanol metabolism in the actinomycete Amycolatopsis methanolica. J Bacteriol. 1994;176:6827–6835. doi: 10.1128/jb.176.22.6827-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmeyer H U, Gawehn K, Grassl M. Enzymes as biochemical reagents. In: Bergmeyer H U, editor. Methods of enzymatic analysis, 2nd English ed. Vol. 1. Weinheim, Germany: Verlag Chemie; 1974. pp. 425–522. [Google Scholar]
- 4.Boonstra J, Huttunen M T, Konings W N, Kaback H R. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975;250:6792–6798. [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brune A, Schink B. A complete citric acid cycle in assimilatory metabolism of Pelobacter acidigallici, a strictly anaerobic, fermenting bacterium. Arch Microbiol. 1990;154:394–399. [Google Scholar]
- 7.Conrad R, Mayer H-P, Wüst M. Temporal changes of gas metabolism by hydrogen-syntrophic methanogenic bacterial associations in anoxic paddy soil. FEMS Microbiol Ecol. 1989;62:265–274. [Google Scholar]
- 8.Deeb S S, Hager L P. Crystalline cytochrome b1 from Escherichia coli. J Biol Chem. 1964;239:1024–1031. [PubMed] [Google Scholar]
- 9.de Vries W, Aleem M I H, Hemrika-Wagner A, Stouthamer A H. The functioning of cytochrome b in the electron transport to fumarate in Propionibacterium freudenreichii and Propionibacterium pentosaceum. Arch Microbiol. 1977;112:271–276. doi: 10.1007/BF00413091. [DOI] [PubMed] [Google Scholar]
- 10.de Vries W, van Wijck-Kapteyn W M C, Oosterhuis S K H. The presence and function of cytochromes in Selenomonas ruminantium, Anaerovibrio lipolytica and Veillonella alcalescens. J Gen Microbiol. 1974;81:69–78. doi: 10.1099/00221287-81-1-69. [DOI] [PubMed] [Google Scholar]
- 11.Diekert G, Thauer R K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978;136:597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottschalk G. Bacterial metabolism. 2nd ed. New York, N.Y: Springer Publishing Co., Inc.; 1986. [Google Scholar]
- 13.Hedlund B P, Gosink J J, Staley J T. Phylogeny of Prosthecobacter, the fusiform caulobacters: members of a recently discovered division of the Bacteria. Int J Syst Bacteriol. 1996;46:960–966. doi: 10.1099/00207713-46-4-960. [DOI] [PubMed] [Google Scholar]
- 14.Hedlund B P, Gosink J J, Staley J T. Verrucomicrobia div. nov., a new division of the Bacteria containing three new species of Prosthecobacter. Antonie Leeuwenhoek. 1997;72:29–38. doi: 10.1023/a:1000348616863. [DOI] [PubMed] [Google Scholar]
- 15.Hilpert W, Dimroth P. Purification and characterization of a new sodium-transport decarboxylase. Methylmalonyl-CoA decarboxylase from Veillonella alcalescens. Eur J Biochem. 1983;132:579–587. doi: 10.1111/j.1432-1033.1983.tb07403.x. [DOI] [PubMed] [Google Scholar]
- 16.Hilpert W, Schink B, Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J. 1984;3:1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiorns W D, Methé B A, Nierzwicki-Bauer S A, Zehr J P. Bacterial diversity in Adirondack mountain lakes as revealed by 16S rRNA gene sequences. Appl Environ Microbiol. 1997;63:2957–2960. doi: 10.1128/aem.63.7.2957-2960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen, P. H. Unpublished data.
- 19.Janssen P H, Schink B. Pathway of anaerobic poly-β-hydroxybutyrate degradation by Ilyobacter delafieldii. Biodegradation. 1993;4:179–185. [Google Scholar]
- 20.Janssen P H, Schuhmann A, Mörschel E, Rainey F A. Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol. 1997;63:1382–1388. doi: 10.1128/aem.63.4.1382-1388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen P H, Morgan H W. Glucose catabolism by Spirochaeta thermophila RI 19.B1. J Bacteriol. 1992;174:2449–2453. doi: 10.1128/jb.174.8.2449-2453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumböck M, Conrad R. Metabolism of position-labelled glucose in anoxic methanogenic paddy soil and lake sediment. FEMS Microbiol Ecol. 1991;85:247–256. [Google Scholar]
- 23.Kuske C R, Barns S M, Busch J D. Diverse uncultivated bacterial groups from soils of the arid southwestern United States that are present in many geographic regions. Appl Environ Microbiol. 1997;63:3614–3621. doi: 10.1128/aem.63.9.3614-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamed R, Zeikus J G. Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol. 1980;141:1251–1257. doi: 10.1128/jb.141.3.1251-1257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melville S B, Michel T A, Macy J A. Pathway and sites for energy conservation in the metabolism of glucose by Selenomonas ruminantium. J Bacteriol. 1988;170:5298–5304. doi: 10.1128/jb.170.11.5298-5304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller T L. The pathway of formation of acetate and succinate from pyruvate by Bacteroides succinogenes. Arch Microbiol. 1978;117:145–152. doi: 10.1007/BF00402302. [DOI] [PubMed] [Google Scholar]
- 27.Moat A G, Foster J W. Microbial physiology. 3rd ed. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 28.Möller D, Schauder R, Fuchs G, Thauer R K. Acetate oxidation to CO2 via a citric acid cycle involving an ATP-citrate lyase: a mechanism for the synthesis of ATP via substrate level phosphorylation in Desulfobacter postgatei growing on acetate and sulfate. Arch Microbiol. 1987;148:202–207. [Google Scholar]
- 29.Oberlies G, Fuchs G, Thauer R K. Acetate thiokinase and the assimilation of acetate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1980;128:248–252. doi: 10.1007/BF00406167. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien W E, Bowien S, Wood H G. Isolation and characterization of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J Biol Chem. 1975;250:8690–8695. [PubMed] [Google Scholar]
- 31.Rose I R. Acetate kinase of bacteria (acetokinase) Methods Enzymol. 1955;1:591–595. [Google Scholar]
- 32.Schink B, Kremer D R, Hansen T A. Pathway of propionate formation from ethanol in Pelobacter propionicus. Arch Microbiol. 1987;147:321–327. [Google Scholar]
- 33.Schlesner H. Verrucomicrobium spinosum gen. nov., sp. nov.: a fimbriated prosthecate bacterium. Syst Appl Microbiol. 1987;10:54–56. [Google Scholar]
- 34.Seubert W, Weichler H. Pyruvate decarboxylase from Pseudomonas. Methods Enzymol. 1969;13:258–262. [Google Scholar]
- 35.Sittig M, Schlesner H. Chemotaxonomic investigation of various prosthecate and/or budding bacteria. Syst Appl Bacteriol. 1993;16:92–103. [Google Scholar]
- 36.Staley J T, de Bont J A M, de Jonge K. Prosthecobacter fusiformis nov. gen. et sp., the fusiform caulobacter. Antonie Leeuwenhoek. 1976;42:333–342. doi: 10.1007/BF00394132. [DOI] [PubMed] [Google Scholar]
- 37.Stams A J M, Kremer D R, Nicolay K, Weenk G H, Hansen T A. Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol. 1984;139:167–173. [Google Scholar]
- 38.Stouthamer A H. The search for correlation between theoretical and experimental growth yields. In: Quayle J R, editor. International review of biochemistry. 21. Microbial biochemistry. Baltimore, Md: University Park Press; 1979. pp. 1–47. [Google Scholar]
- 39.Ward-Rainey N, Rainey F A, Schlesner H, Stackebrandt E. Assignment of hitherto unidentified 16S rDNA species to a main line of descent within the domain Bacteria. Microbiology. 1995;141:3247–3250. [Google Scholar]
- 40.Wise M G, McArthur J V, Shimkets L J. Bacterial diversity of a Carolina bay as determined by 16S rRNA gene analysis: confirmation of novel taxa. Appl Environ Microbiol. 1997;63:1505–1514. doi: 10.1128/aem.63.4.1505-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwart G, Huismans R, van Agterveld M P, Van de Peer Y, De Rijk P, Eenhorn H, Muyzer G, van Hannen E J, Gons H J, Laanbroek H J. Divergent members of the bacterial division Verrucomicrobiales in a temperate freshwater lake. FEMS Microbiol Ecol. 1998;25:159–169. [Google Scholar]