Abstract
Improving physio-biochemical traits in wheat under drought stress conditions has received more research attention in recent years for better adaptability and higher yield. In this study, we explored the potential bio-physiological mechanisms underlying improved plant growth and water use efficiency in wheat following soil application of potassium (0 and 100 kg ha−1) and seed primed salicylic acid (SA) (150 mg per L) and SA foliar application (100 mg per L) under drought stresses (100%, 60% and 30% FC). Two years' average data revealed that inducing drought stress resulted in a decrease in plant pigments content, growth traits, and plant water status however, the influence was substantially reduced with the combined application of K and SA under drought stress conditions. The SA foliar spray in combination with K had increased chlorophyll a (174% and 83%), chl b (130% and 192%), chl a + b (156% and 120), carotenoid (22% and 11%), proline contents (24% and 29%) leaf relative water content (24% and 29%) while reduced leaf WSD (17% and 20%), WRC (6% and 7%), and WUC (23% and 28%) under mild and severe drought stresses, respectively. The increase in grain yield by 41% and 37% with enhanced water use efficiency was obtained with combined foliar SA and K under mild and severe drought stress, respectively indicating its vital role in overcoming the deleterious effects of drought via regulation of osmotic and metabolic processes and stabilizes cell components. RDA analysis revealed that the studied traits were completely discriminated under severe stress than mild or no drought stress. A positive and significant association was found between plant pigments with seed yield whereas a negative and significant correlation existed between water leaf traits and plant pigments. It was concluded that both foliar SA and seed primed SA with K fertilization combat the adverse effects of drought and improved plant water status as well as growth and bio-physiological traits of wheat under drought stress conditions.
Keywords: Drought stress, Potassium fertilization, Seed priming, Salicylic acid, Foliar spray, Plant pigments
1. Introduction
Drought stress is a global issue, limiting crop productivity and the recent climate change scenarios made it more serious and endanger food security (Abd El–Mageed et al., 2016). Drought is also among the major constraints influencing crop productivity and final yield including major cereals and legumes (Golbashy et al., 2010, Jarzyniak and Jasiński, 2014, Joshi et al., 2016). The severe drought stress condition led to reduced plant growth with retard plants leading to poor dry matter accumulation (Namich and Emara, 2007, Ahmad et al., 2017). Hence, wise and conservative use of water is key for maintaining crop productivity sustainable. Agronomic techniques such as the use of plant growth regulators either through seed priming or foliar application are considered as viable options to cope with abiotic stresses including drought stress for maintaining and increasing crop yield (Karlidag et al., 2009) via lowering abiotic stresses (Elwan and El-Hamahmy, 2009) and improving physiological processes such as plant chlorophyll. Cell membrane stability and leaf area index (Khan et al., 2015). In this regard, Salicylic acid (SA) can be helpful and work in a signaling pathway that improves the tolerance of crop plants to stresses for better crop performance and yield (İsfendiyaroğlu and Oezeker, 2008). SA performs a vital role in improving physiological processes including regulation of stomata, photosynthetic rates, and chlorophyll content under stress conditions (Arfan et al., 2007). Additionally, it also lowers the negative effects of salt and other abiotic stresses as well (Abdel-Wahed et al., 2006, Amin et al., 2007, Eraslan et al., 2007, Hayat et al., 2010, Khan et al., 2015, Malaga et al., 2020). Salicylic acid seed priming lowered the adverse effects of abiotic stresses via improving sugar and proline accumulation (Fahad and Bano, 2012). It was found that SA foliar application may increases plant pigments including chlorophyll a, b and carotenoid content, proline content, and leaf water traits leading to enhance tolerance against drought stress (Brito et al., 2018, Zafar et al., 2021, Saheri et al., 2020). Hence the use of SA is vital for alleviating osmotic stress as short term strategy under drought stress in many plant crops (Brito et al., 2018, Chaves et al., 2002), however further research is needed to clear understanding the mechanism in many crops including wheat (Khan et al., 2015).
Potassium (K) is among the essential macronutrients needed for optimal growth and productivity and have central role in many enzymes activation as well as several important biological processes such as protein formation, stomatal movements, energy transformation including enhances plant tolerance to abiotic stresses (Ul-Allah et al., 2020, Reddy et al., 2004). It has also key role in against formation of reactive oxygen species (ROS) to combat the negative effect of drought stress and maintain crop yield (Aslam et al., 2013; Oerke and Dehne, 2004, Cakmak, 2005). Due to the increased osmotic adjustment, K fertilization can mitigate ROS production under abiotic stresses (Sangakkara et al., 2001). Potassium fertilization with optimal dose may not merely improve tolerance against drought stress but also can improve crop growth and productivity due to proper nutrition (Egilla et al., 2001, Shah et al., 2018). The stomatal regulation under optimal K application is considered important for enhanced photosynthetic rates (Marschner, 2012) as well as transport of photosynthate to sinks as well as to roots (Römheld and Kirkby, 2010) leading to increase dry matter production. The integrity and stability of cell membranes with the application of K under drought stress is important for mitigating drought stress conditions for better crop performance (Ul-Allah et al., 2020, Bajji et al., 2002.
Wheat (Tritium aestivum L.) is among the important crops of the globe and its importance and requirement are increasing due to the increased drought stress condition under the recent changing climate. The conditions even become more serious due to increasing water scarcity. Hence enhancing bio-physiological characteristics of crop plants under drought stress conditions is yet the endeavor of Scientists for food security. There is a lack of proper evidence about SA and K effects on alleviating the environmental stress of field crops. The research on drought stress tolerance in wheat has been laid out, yet the physiological and biochemical traits are seldom studied with the combined application of SA and K under drought stress in field conditions.
We hypothesized that foliar spray of SA combined with soil application of potassium can enhance tolerance against drought stress in wheat from tillering to physiological maturity by improving plant pigments, accumulation of osmolyte content, and enhancing leaf water retention traits. Keeping in view the significance of SA and K for mitigating drought stress, the current study was undertaken to assess the impact of SA and K application on physiological and biochemical parameters of wheat and combat the negative influence of water stress via improving plant pigments accumulation, growth and leaf water deficit, retention and saturation capacity leading to enhance wheat of productivity under field condition.
2. Materials and methods
2.1. Soil physio-chemical properties
The site of the experiment is located at 34°11′52″ latitude to North pole with 72°2′48″ longitudes toward East while the elevation is 282 m above the ocean level. The climate of the site belongs to semi-arid to arid. The soil at the site is clay loam. The soil physio-chemical properties were analyzed before the start of the experiments. Representative soil samples were taken up to the depth of 30 cm and were analyzed. The pH of the soil was 7.9, with EC 0.61dSm−1. The soil available N, P, and extractable K contents were 0.053%, 8.40 mg kg−1, and 116.2 mg kg−1, respectively. The soil field capacity was found on 24%, while the wilting point was 8% soil moisture content. The minimum and maximum temperature ranged between 11℃ to 35℃ during the experiment with total rainfall of 188.2 mm as shown in Table 1. Although the crop was fully protected from rainfall during the drought stress period.
Table 1.
Monthly maximum, minimum and mean temperature, and rainfall data of the experimental site during the experiment.
| Months | Max Temp (°C) | Min Temp (°C) | Average Temp (°C) | Rainfall (mm) |
|---|---|---|---|---|
| Dec-17 | 21.0 | 13.0 | 17.0 | 13.2 |
| Jan-18 | 21.0 | 11.0 | 16.0 | 0.83 |
| Feb-18 | 21.0 | 12.0 | 16.5 | 35.8 |
| Mar-18 | 28.0 | 19.0 | 23.5 | 34.5 |
| Apr-18 | 33.0 | 15.0 | 29.0 | 75.3 |
| May-18 | 35.0 | 24.0 | 30.0 | 28.6 |
2.2. Experimental layout
The experiments were arranged in a Randomized Complete Block Design using split plot procedure with three replications of each treatment. Drought stress levels were kept in the main plot while the salicylic acid (SA) treatments (Seed Priming (SP) and foliar spray (FS)), as well as Potassium (K) fertilization, were arranged in the sub-plots. Muraite of potash was used as a source of K in two levels (0 and 100 kg ha−1). Healthy wheat seeds of cv. PirSabak-2013 were primed in SA solution of 150 mg L−1 for 16 h and then was air-dried for one hour in open shade and were then sowed in the respective subplots and compared with dry seed where no SA seed priming was done. In the case of foliar spray, SA solution of 100 mg L−1 was prepared using distilled water and after calibration was sprayed at tillering stage of the crop and was compared with control. The wheat crop cv. Pir-Sabak-2013 has sown in the second week of December 2017–18. The subplot size was 3.15 m2 having seven rows 1.5 m long and 30 cm apart from each other. The drought stress was imposed at the tillering stage. The sowing was done once the land was properly tilled via Cultivator three times and it was then followed by a rotavator for a smooth seedbed. For optimal soil fertility, recommended amount of nitrogen and phosphorous (120 kg ha−1and 80 kg ha−1, respectively) was also applied to each subplot using Urea and single super phosphate (SSP), respectively. Moreover, P and K fertilization was done during seedbed preparation time whereas Urea was applied in two equal doses both at sowing and at tillering stages. Drought stress in the respective experimental units was maintained on the base of soil moisture content at the root zone and it was determined using as per the gravimetric method. Irrigation was applied via water pipe to the respective plots to ensure the required water content. Drought stress (60% FC) was determined based on full irrigation (100% FC) with 60% management allowable deficit (MAD). Sample for soil moisture was taken from depths of 0–30, 30–60 and 60–100 cm in control plots with frequent intervals as well as before irrigation for estimation of root zone soil moisture depletion. Moreover, the experimental units were fully protected from rainfall using plastic sheath structure and removed in case of no rainfall.
2.3. Measurement
2.3.1. Physiological traits
Leaf area index (LAI) was noted down at the anthesis stage with a sun scan canopy analyzer (LI-2000, LI-COR, USA). Crop growth rate (CGR) was calculated with an interval of 30 days starting from seedling to physiological maturity. The plants were harvested in each sub-plot in 50 cm row length in three rows. The collected samples were weighed and then oven-dried at 105 °C for 24 h and then were weighed and expressed as g m−2 day−1 as per the method of Radford (1967).
2.3.2. Biochemical traits
The chlorophyll (Chl) content was assessed as per methods prescribed by Arnon, 1949, Davies, 2010. Fresh flag leaves of 0.5 g were kept in 80% Acetone (5 mL) at room temperature overnight and were then centrifuged at 10,000 rpm for 20 min. the absorbance of the supernatant was read using a spectrophotometer at 645, 652, 663, and 480 nm. The final concentrations of total chlorophyll content were calculated according to Arnon, 1949, Davies, 2010. Three leaves were randomly selected in each subplot for proline determination and were measured using the method designated by Bates et al. (1973).
2.3.3. Plant water status traits
To estimate leaf relative water content (RWC), flag leaves were removed from tillers at random in each subplot and their fresh weight was recorded and placed in de-ionized water at room temperature for 16 h to get turgid weight. The samples upon drying in the oven for 24 h at 105 °C were weighed and the RWC was calculated as per the method of Jeon et al. (2006).
RWC (%) = [(FW-DW)/ (TW-DW)] × 100. Moreover, leaf water saturation deficit (WSD), water retention capacity (WRC), and water uptake capacity (WUC) were also determined using Sangakkara et al. (2001) as per the following equations.
Where; FW = Fresh Weight (g), DW = Dry Weight (g), TW = Turgor weight (g).
The water use efficiency (WUE) as expressed in kg m−3) on grain yield basis and calculated by ratio of grain yield (kg ha−1) and applied water via irrigation (m3 ha−1).
2.3.4. Crop yield (kg ha−1)
Wheat seed yield was determined by cutting three middles rows upon maturity in each experimental unit and were sun-dried for several days. The grain collected after manual threshing were measured using electronic balance and expressed in kg per ha.
2.4. Statistical analysis
Data were analyzed using Statistix 10 after checked for following ANOVA assumptions; however, the percent data were first transformed for normality, linearity and independent uni-variance of the data, while the assumptions were met for rest of the data. Statistical analysis was carried out by a generalized linear model (GLM) using the procedure appropriate for a Randomized Complete Block Design with split-plot arrangement (Jan et al., 2009). Drought stress (three levels) and SA (three levels) and K (two levels) were all set as fixed factors and the replications and years as a random factor. Combined analysis of variance was performed to analyze drought stress, SA, K, year, and interactions between SA × K × DS, and K × SA, DS × SA, DS × SA. The simple effect analysis was carried out for interaction between K × SA × DS and the differences between each DS with SA and K were evaluated using Fisher’s least significant difference (LSD) test; at 5% level of probability. Sigma plot 12.0 software was used for graphical representation of the data. The Pearson’s correlation and RDA analysis were performed using Rstudio Team, 3.0.1.
3. Results
3.1. Leaf water status traits
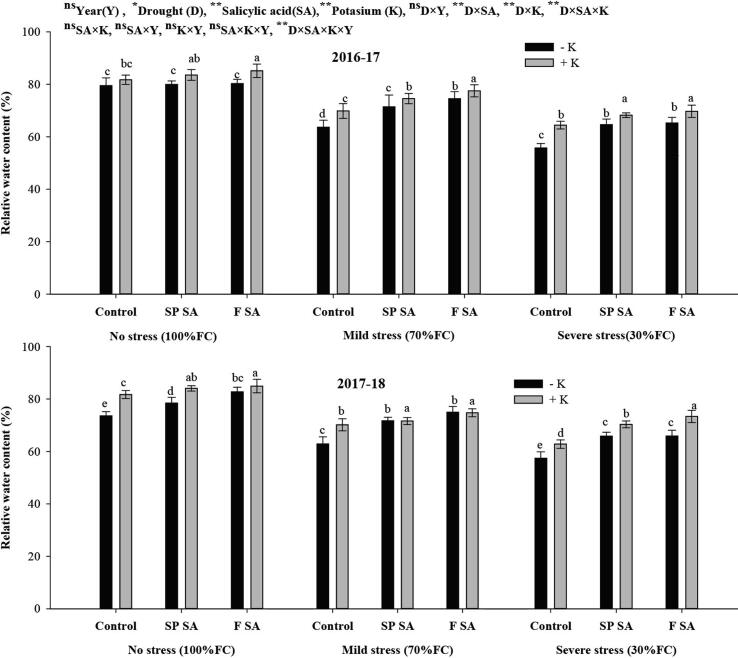
The leaf water status traits were substantially affected by drought stress, SA levels, K application. The RWC substantially reduced in severe and mild drought stresses across SA and K application as compared with no drought stress (Fig. 1). Seed primed with SA and foliar SA significantly enhanced RWC both in no stress and drought stresses, however, a lesser increase was observed under severe stress than no stress plants with SA and K application. Moreover, the plants treated with SA with and without K fertilization showed a 12% and 17%, increase in RWC during 2017–18 while 14% and 12% during the 2nd year of the study under no K with seed priming and foliar spray of SA under moderate stress whereas 17% and 22% increase in RWC in 1st year and 19.3 and 19% in 2nd year was achieved with combine application of K and SA under moderate stress. In case of severe drought stress, RWC was increased by 16 and 17% and 14.7 and 14.8% with sole seed priming and foliar SA in the first and second years, respectively. However, the was 22 and 25% and 23 and 28% in the first year and second year was achieved with seed priming and foliar spray of SA, under K fertilization respectively, as compared to plants without SA under same conditions. Across SA treatments, potassium fertilization had increased RWC by 10 and 16% in 2017–18 and 14 and 13% in 2018–19 under mild and severe drought stresses, respectively, over no K application.
Fig. 1.
Effect of salicylic acid and potassium on relative water content of wheat under drought.
The WSD in flag leaf of wheat increased significantly under mild and severe drought stress and varied due to SA and K application during both years (Table 2). Under no stress condition, the lowest WSD of the wheat leaf was 14.8% and 15.07% when SA was applied on foliage followed by SA seed priming (16.4% and 15.97%). In contrast, the highest WSD of the wheat leaf was 20.5% and 26.45%, respectively, when no SA and K were applied, although it was statistically similar to plots where SA was applied. At 70% FC, the highest water saturation deficit of wheat leaf was 36.4% and 36.54 under mild stress and 44.2% and 42.61% under severe stress when no SA and K was applied during both years, respectively and with SA and K fertilization WSD reduced both in mild and severe drought stress however the reduction was more with foliar SA and K fertilization during both years.
Table 2.
Combine application of SA and K on leaf water status (WSD, WRC and WUC) under drought stress.
| Treatments | WSD (%) |
WRC |
WUC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Field capacity |
Field capacity |
Field capacity |
|||||||||
| 100% | 70% | 40% | 100% | 70% | 40% | 100% | 70% | 40% | |||
| 2016–17 | 0 K | CK | 20.5a | 36.4a | 44.2a | 5.40a | 6.41a | 8.33a | 0.90a | 1.97a | 3.24a |
| SP | 20.0a | 28.6b | 35.3b | 5.10a | 5.81b | 6.03bc | 0.82a | 1.38b | 1.78c | ||
| FS | 19.7a | 25.5c | 34.7b | 5.33a | 5.84b | 6.07bc | 0.85a | 1.23c | 1.76c | ||
| 100 K | CK | 18.3ab | 30.2b | 35.5b | 5.34a | 5.85b | 6.42b | 0.79a | 1.46b | 1.92b | |
| SP | 16.4bc | 25.4c | 31.8c | 4.24b | 5.45bc | 6.36b | 0.52b | 1.13c | 1.70c | ||
| FS | 14.8c | 22.5d | 30.3c | 5.14a | 5.20c | 5.94c | 0.61b | 0.94d | 1.49d | ||
| Mean | 18.3C | 28.1B | 35.3A | 5.09C | 5.76B | 6.52A | 0.75C | 1.35B | 1.98A | ||
| 2017–18 | 0 K | CK | 26.45a | 36.54a | 42.61a | 5.29ab | 5.77b | 8.04a | 1.14a | 1.74a | 3.00a |
| SP | 21.57b | 27.59b | 34.17c | 5.13b | 5.23c | 6.59bc | 0.89b | 1.17c | 1.91c | ||
| FS | 17.27cd | 24.27c | 34.14c | 5.22ab | 7.34a | 6.32c | 0.73bc | 1.54b | 1.82c | ||
| 100 K | CK | 18.33c | 27.72b | 37.21b | 4.99b | 5.92b | 6.75b | 0.73bc | 1.36b | 2.14b | |
| SP | 15.97de | 29.16b | 29.69c | 5.51a | 5.69b | 6.35c | 0.72bc | 1.37b | 1.59d | ||
| FS | 15.07e | 24.53c | 26.68e | 5.33ab | 5.18c | 5.76d | 0.65c | 1.03c | 1.27e | ||
| Mean | 19.11C | 28.30B | 34.08A | 5.24C | 5.85B | 6.64A | 0.81C | 1.37B | 1.96A | ||
| SOV | WSD | WRC | WUC | ||||||||
| Year(Y) | Ns | Ns | ** | ||||||||
| Drought(D) | ** | ** | ** | ||||||||
| D × Y | * | Ns | Ns | ||||||||
| Salicylic acid(SA) | ** | ** | ** | ||||||||
| Potassium (K) | ** | ** | ** | ||||||||
| SA × Y | Ns | Ns | ** | ||||||||
| D × SA | ** | ** | ** | ||||||||
| K × Y | Ns | Ns | ns | ||||||||
| D × K | ** | ** | ** | ||||||||
| SA × K | ** | ** | ** | ||||||||
| SA × K × Y | Ns | * | * | ||||||||
| D × K × Y | * | Ns | * | ||||||||
| D × SA × K | ** | ** | ** | ||||||||
| D × SA × K × Y | ** | ** | ** | ||||||||
ns non significant, ** significant at 1% level of probability, * significant at 5% level of probability.
The WUC in wheat flag leaf increased significantly under drought conditions, but seed primed SA or foliar SA with soil K fertilization had reduced water uptake capacity during both years (Table 2). Under no drought stress, WUC was the minimum (0.90 and 1.14) with no SA and K applied plots during 1st and 2nd year, respectively, however, it was at par with plots where seed primed and foliar SA with no K fertilization and sole K applied plots. However, WUC was significantly reduced with the combined use of SA and K under no-stress conditions. Under moderate and severe drought stress conditions, WUC has reduced with SA and K fertilization, although the decrease was much higher under severe drought stress conditions during both years.
The WRC was considerably enhanced with inducing drought stress and greater WRC was recorded under severe drought stress (6.52) than moderate stress (5.76) and no drought stress (5.09) across SA and K application. Potassium fertilization with foliar SA had a greater reduction in WRC (5.20 and 5.18) under mild stress where it was higher (5.94, and 5.76) under severe drought stress during 2017–18 and 2018–19, respectively. It was followed by primed SA with K fertilization in the respective drought stress plots. Across SA, K fertilization had reduced WRC (5.85 and 5.18) under moderate stress whereas it was enhanced (6.42 and 6.75) under severe drought stress than no K application during both years, respectively.
3.2. Physiological traits
3.2.1. CGR and LAI
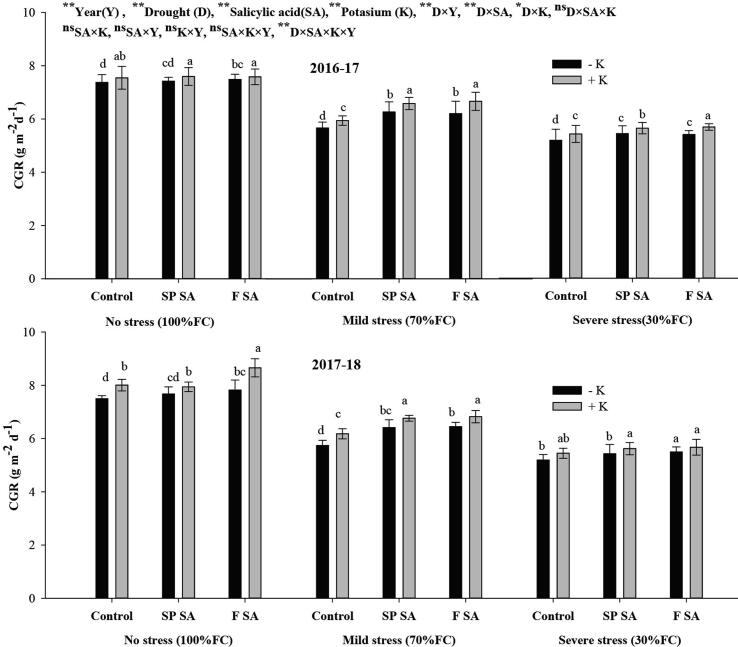
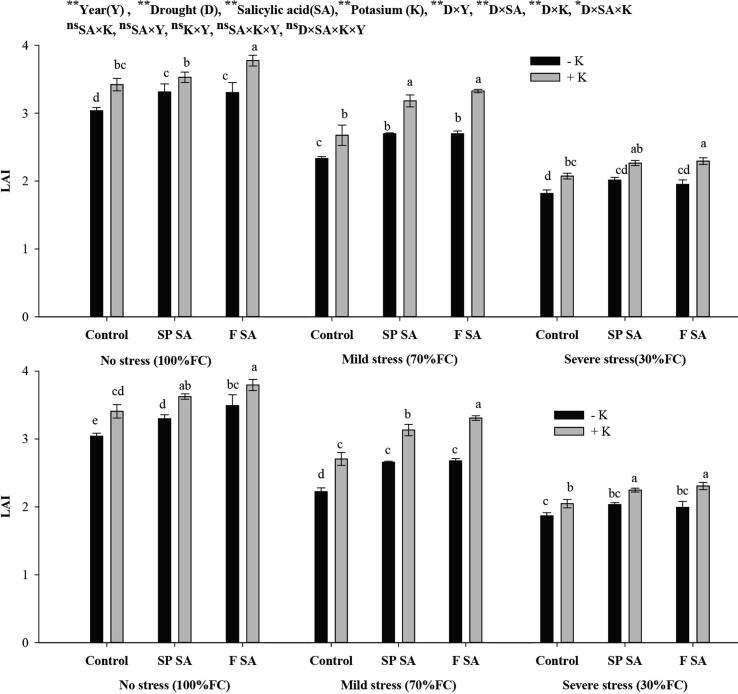
Crop growth rate (CGR) and leaf area index (LAI) were substantially influenced by SA, K, and drought stress. The lower CGR (5.46 and 5.48 g m−2d−1) and LAI (2.07and 2.08) were achieved under severe drought stress followed by moderate stress during the 1st and 2nd year, respectively. Potassium application had resulted in higher CGR and LAI than no K application across drought stress levels. Both foliar SA and seed primed SA in combination with K fertilization had resulted in higher and statistically similar CGR and LAI under moderate stress. In contrast, in case of severe drought stress, the CGR and LAI were greater with foliar SA followed by seed premed SA in the presence of K fertilization during both years. However, no substantial fluctuation in CGR and LAI was recorded with SA and K application under no drought stress (Fig. 2, Fig. 3).
Fig. 2.
Effect of salicylic acid and potassium on CGR of wheat under drought stress during 2016–18.
Fig. 3.
Effect of salicylic acid and potassium on LAI of wheat under drought stress during 2016–18.
3.3. Biochemical traits
3.3.1. Chlorophyll content
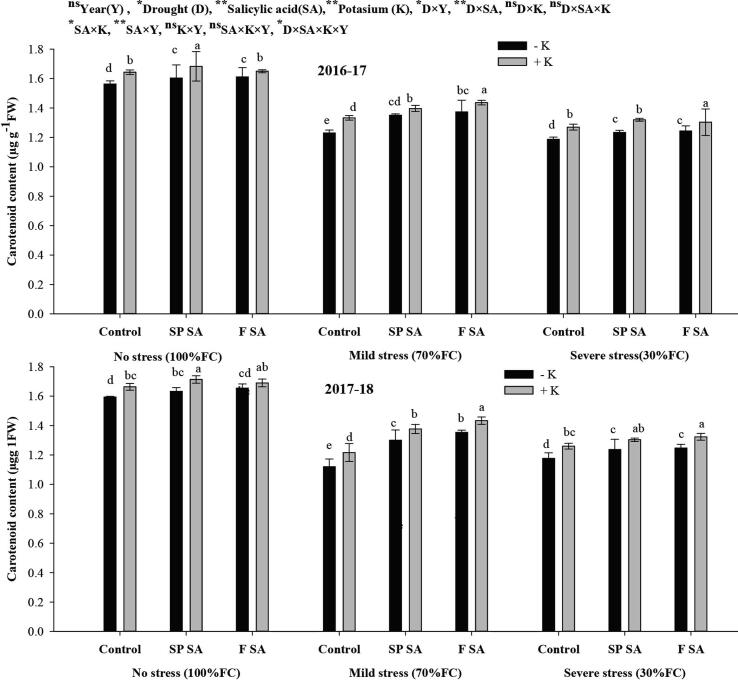
To determine the effect of SA and K on plant pigments in wheat leaves under drought stress, we found a significant increase in Chl a, b, a + b, and carotenoid content with SA seed primed and foliar spray in both with and without K fertilization under mild and severe drought stresses. However, the increase in K fertilization was higher than no K application plots. Moreover, across SA and K fertilization, drought stress reduced chl a, b, total chlorophyll, and carotenoid contents by 50%, 28%, 40%, and 14%, during 1st year and 49%, 25%, 39% and 23% in 2nd year under mild drought stress, respectively and 105%, 47%, 79% and 23% in 1st year while 107%, 51%, 82% and 32% during 2nd year under severe drought stress, respectively as compared with no drought stress. Across SA, the higher contents of Chl a, b, total, and carotenoid content was found in K fertilization as compared with no K. The sole foliar SA had increased Chl a content (19 and 20%) and chl b content (83 and 59) under mild stress while 40% and 30% and 97% and 103% under severe drought stress, respectively. Likewise, the SA seed primed had increased chl a (12.5% and 13.2%) and chl b (62and 53%) under mild stress while chl a (54% and34%) and chl b (125% and 120%) under severe drought stress, during both years, respectively in comparison with no SA. The combined application of foliar SA and soil K fertilization had higher chl a (146 and 117%) and chl b (160 and 151%) under mild stress while 189 and 191% increase in chl a and 124 and 115% increase in chl b under severe drought stress during both years, respectively. Moreover, SA seed primed with K fertilization resulted in higher chl a (113 and 100%) and chl b (139 and 136%) under mild while 167% and 174% increase in chl a and 107% and 106% increase in chl b under severe drought stress during both years, respectively in comparison with control in their respective stress levels. The increase in carotenoid contents was 11 and 21% with foliar SA alone and 4 and 6% under severe stress in both years, respectively followed by SA seed primed with an increase of 10% and 16% under moderate and 3 and 5% under severe drought stress, respectively (Fig. 4). Chl a + b increased by 44 and 38% under mild stress and 60 and 54% under severe stress with sole foliar SA whereas 31.8 and 31.1% under mild and 79 and 63% under severe drought stress with seed priming SA in comparison with no SA during the first and 2nd years. The combined use of foliar SA and K had increased chl a + b by 160 and 157% under mild and 124 and 115% under severe drought stress while that with seed priming SA had increased chl a + b content by 139 and 142% in mild drought stress and 107% and 106% under severe stress during both years, respectively (Table 3).
Fig. 4.
Effect of salicylic acid and potassium on carotenoid content of wheat under drought stress during 2016–18.
Table 3.
Combine application of SA and K on Chl a, Chl b and total chlorophyll of wheat under drought stress.
| Treatments | 0D | 1D | 2D | 0D | 1D | 2D | 0D | 1D | 2D | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016–17 | 0 K | CK | 1.76c | 0.80c | 0.68d | 0.81b | 0.52e | 0.36d | 2.57e | 1.32e | 1.04c |
| SP | 2.02b | 0.90c | 1.05bc | 1.01b | 0.84de | 0.81bc | 3.03d | 1.74d | 1.86b | ||
| FS | 2.26a | 0.95c | 0.95bc | 0.98b | 0.95bc | 0.71c | 3.24cd | 1.90d | 1.66b | ||
| 100 K | CK | 2.02b | 1.45b | 0.92cd | 1.28a | 0.72de | 0.78bc | 3.30bc | 2.17c | 1.70b | |
| SP | 2.19ab | 2.05a | 1.18ab | 1.45a | 1.11ab | 0.96ab | 3.65a | 3.16b | 2.15a | ||
| FS | 2.15ab | 2.15a | 1.29a | 1.34a | 1.28a | 1.04a | 3.50ab | 3.43a | 2.33a | ||
| Mean | 2.07A | 1.38B | 1.01C | 1.15A | 0.90B | 0.78C | 3.21A | 2.29B | 1.79C | ||
| 2017–18 | 0 K | CK | 1.68d | 0.76c | 0.70c | 0.80d | 0.59c | 0.35d | 2.48d | 1.35e | 1.05c |
| SP | 1.95c | 0.86c | 0.94b | 1.06c | 0.90b | 0.77c | 3.01c | 1.77d | 1.71b | ||
| FS | 2.16a | 0.91c | 0.91b | 0.98c | 0.94b | 0.71c | 3.14bc | 1.86d | 1.62b | ||
| 100 K | CK | 1.99bc | 1.41b | 0.83bc | 1.27b | 0.71c | 0.80bc | 3.25b | 2.13c | 1.63b | |
| SP | 2.16ab | 2.01a | 1.20a | 1.45a | 1.18a | 0.96ab | 3.61a | 3.19b | 2.16a | ||
| FS | 2.12abc | 2.11a | 1.24a | 1.38ab | 1.28a | 1.02a | 3.50a | 3.39a | 2.26a | ||
| Mean | 2.01A | 1.35B | 0.97C | 1.16A | 0.93B | 0.77C | 3.17A | 2.28B | 1.74C | ||
| SOV | Chl a | Chlb | Chl a + b | ||||||||
| Year(Y) | Ns | 1 | Ns | Ns | |||||||
| Drought(D) | ** | 2 | ** | ** | |||||||
| D × Y | Ns | 3 | Ns | Ns | |||||||
| Salicylic acid(SA) | ** | 4 | ** | ** | |||||||
| Potassium (K) | ** | 5 | ** | ** | |||||||
| SA × Y | Ns | 6 | ns | ns | |||||||
| D × SA | Ns | 7 | ** | ** | |||||||
| K × Y | Ns | 8 | ns | ns | |||||||
| D × K | * | 9 | ** | ** | |||||||
| SA × K | Ns | 10 | ns | ns | |||||||
| SA × K × Y | Ns | 11 | ns | ** | |||||||
| D × K × Y | Ns | 12 | ns | ns | |||||||
| D × SA × K | ** | 13 | * | ** | |||||||
| D × SA × K × Y | Ns | 14 | ns | ns | |||||||
ns non significant, ** significant at 1% level of probability, * significant at 5% level of probability.
3.3.2. Proline content
Proline content increased in severe and mild drought stress with SA foliar spray followed by SA seed primed with and without K fertilization. SA seed primed at the rate of 150 mL L−1 without K had resulted in a 12% and 8% increase in proline content. In contrast, 11% and 17% with K fertilization under moderate stress, whereas the increase was 19% and 17% without K while 29% and 24% with K fertilization under severe drought stress in 1st and 2nd year, respectively. The foliar SA had increased proline content by 14% and 12% without K, whereas 25% and 22% with K under moderate drought stress while 23% and 26% without K and 33 and 36% with K fertilization under severe drought stress during both years, respectively. Across K and SA, the higher proline content (26% and 20%) was recorded under severe drought stress followed by mild stress (3% and 13%) in comparison with no drought stress (Fig. 5).
Fig. 5.
Effect of salicylic acid and potassium on proline content of wheat under drought stress during 2016–18.
3.3.3. Grain yield and water use efficiency (WUE)
Grain yield of wheat was substantially reduced by 19 and 38% under moderate stress while 62% and 63% were under severe drought stress as compared with no drought stress. The sole foliar SA application had increased grain yield (36 and 34%, respectively) followed by sole SA as seed priming (21 and 20%) under mild drought stress. In comparison, the foliar SA had increased grain yield by 33% and 31% followed by SA seed priming (6.4% and 5.8%) under severe drought stress compared with no SA treated plants in their respective stress levels during the first and 2nd years, respectively (Table 4). The combined application of foliar SA and K at the rate of 100 kg ha−1 had 41 and 40% increase in grain yield under moderate stress, whereas 37.2% and 37.0% under severe drought stress during the 1st and 2nd year, respectively. Likewise, the SA, when applied as seed priming was also effective in combination with K fertilization and had resulted in 37% and 35% increase in grain yield under mild stress while 34% and 31% under severe drought stress during 1st and 2nd year, respectively. Across SA application, K fertilization had resulted in higher grain yield (and 21.4% and 20.8%) than no K fertilization during the 1st and 2nd year, respectively. Drought stress levels increased WUE in wheat crop irrespective of SA and K fertilization. The combined application of both SA applications, either as foliar or seed primed with K, significantly improved the WUE under both stress levels during both years in comparison with their control in corresponding stress levels. The higher WUE achieved with both seed primed and foliar SA in the presence of K under severe and moderate stresses during 1st and 2nd year, although the WUE was also at par in rest of the treatment except control in each stress level. Across SA treatment, K fertilization had resulted in higher WUC compared to no K fertilization under moderate and severe drought stresses during both years (Table 4).
Table 4.
Combine application of SA and K on water use efficiency (WUE) and grain yield (GY) of wheat under drought stress.
| WUE (g m−3) |
Grain yield (t ha−1) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | 0D | 1D | 2D | 0D | 1D | 2D | ||
| 2016–17 | 0 K | CK | 0.62b | 0.67c | 0.84d | 3.20b | 2.24c | 1.72c |
| SP | 0.64ab | 0.75b | 0.96c | 3.31b | 2.72b | 1.83bc | ||
| FS | 0.65ab | 0.80b | 1.09b | 3.26b | 3.05a | 2.29a | ||
| 100 K | CK | 0.64ab | 0.81b | 1.06b | 3.22b | 2.72b | 1.94b | |
| SP | 0.71a | 0.90a | 1.16a | 3.56a | 3.07a | 2.31a | ||
| FS | 0.70a | 0.93a | 1.17a | 3.57a | 3.16a | 2.36a | ||
| Mean | 0.66C | 0.81B | 1.05B | 3.35A | 2.83B | 2.07C | ||
| 2017–18 | 0 K | CK | 0.63b | 0.73c | 0.85c | 3.27b | 2.26d | 1.73c |
| SP | 0.66ab | 0.78bc | 0.93b | 3.28b | 2.71c | 1.83b | ||
| FS | 0.68ab | 0.81b | 0.97b | 3.30b | 3.03b | 2.27a | ||
| 100 K | CK | 0.67ab | 0.79b | 0.96b | 3.23b | 2.73c | 1.93b | |
| SP | 0.69a | 0.87a | 1.10a | 3.55a | 3.06ab | 2.27a | ||
| FS | 0.70a | 0.91a | 1.15a | 3.57a | 3.16a | 2.37a | ||
| Mean | 0.67C | 0.81B | 0.99A | 3.37A | 2.82B | 2.07C | ||
| SOV | WUE | GY | ||||||
| Year(Y) | Ns | Ns | ||||||
| Drought(D) | ** | ** | ||||||
| D × Y | * | Ns | ||||||
| Salicylic acid(SA) | ** | ** | ||||||
| Potassium (K) | ** | ** | ||||||
| SA × Y | ns | ns | ||||||
| D × SA | ** | ** | ||||||
| K × Y | Ns | Ns | ||||||
| D × K | ** | ** | ||||||
| SA × K | ns | * | ||||||
| SA × K × Y | Ns | Ns | ||||||
| D × K × Y | Ns | Ns | ||||||
| D × SA × K | ns | ** | ||||||
| D × SA × K × Y | ns | ns | ||||||
Ns non significant, ** significant at 1% level of probability, * significant at 5% level of probability.
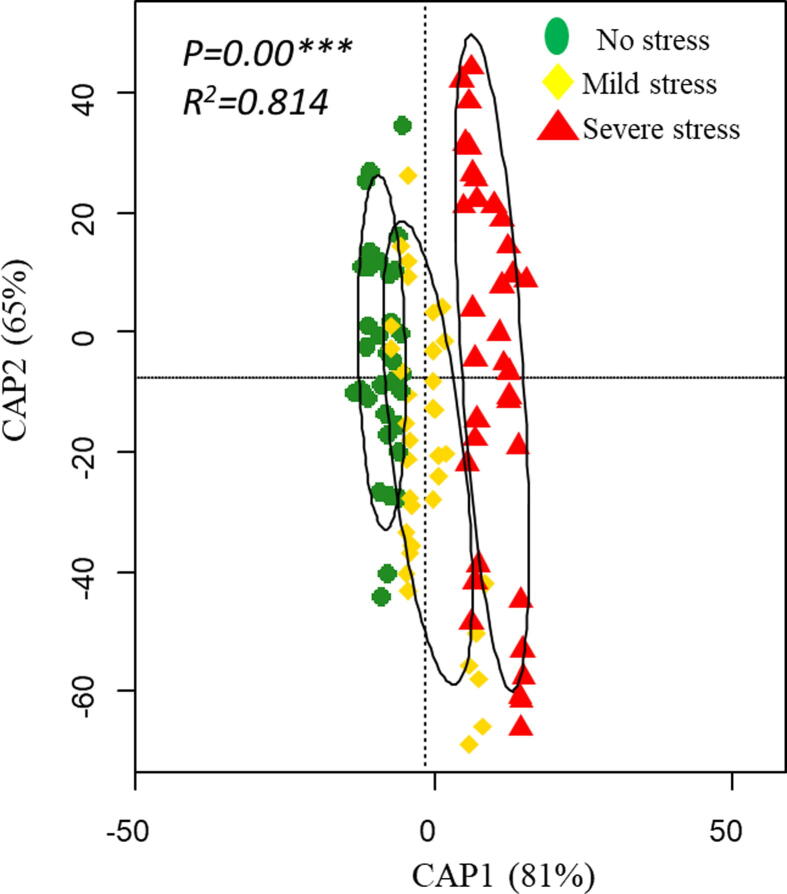
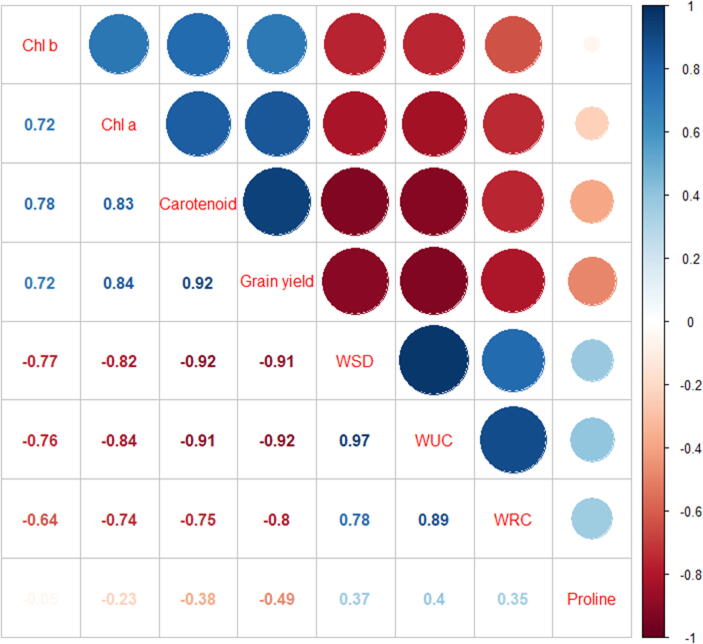
4. RDA and correlation matrix
The first two RDA axis showed high eigen values as compared to others and explained 81% and 65% variation in different traits under drought stress. All the traits were completely discriminated under severe stress as compared to other treatments (Fig. 6). Correlation matrix showed that positive correlation was found between chl a with chl b, carotenoid and grain yield while the WSD, WUC, WRC and proline have negative correlation with chl a. Chl b have same trend as for chl b. we also found strong positive between carotenoid content and seed yield whereas there was negative correlation with other traits. Grain yield have a negative correlation with WSD, WUC, WRC and proline. WSD have positive correlation with WUC, WRC and proline and same trend was observed for WUC and WRC (Fig. 7).
Fig. 6.
Ordination plots of the results from the redundancy analysis (RDA) to identify the relationships among the drought levels for different traits. Samples were analyzed in triplicates. No stress (100 %FC, Mild stress (70 %FC) and Severe stress (30 %FC).
Fig. 7.
Correlation coefficient matrix for different traits under drought stress. Light and dark red represent the degree of negative correlation. Light and dark blue represent the degree of positive correlation.
5. Discussion
Drought stress is the most harmful factor affecting crop productivity across the globe with the bigger challenge for food security in the future (Ahmed et al., 2017, Fang et al., 2017, Maswada et al., 2018). The decrease in crop productivity might differ from 50% to 73% under drought stress conditions (Berry et al., 2013). The adoption of a specific strategy cannot stop drought conditions, yet it helps decrease its deleterious effects (Fahad et al., 2017). In the present study, we attempted to reduce the deleterious effect of drought stress on wheat with seed priming and foliar SA and K fertilization to the soil. Our results revealed that drought stress significantly reduced CGR and LAI compared with no stress due to less photosynthetic pigments needed for assimilates production, ultimately decreasing dry matter production (Marschner, 2012). We also found that both foliar and seed priming SA in the presence of K ameliorated the harmful effect of drought and enhanced CGR and LAI of wheat in comparison with control in their respective drought stresses. The role of SA in drought might be due to the accumulation of bio-synthesis of tryptophan, a precursor of auxin hormone, and mainly responsible for rapid growth rate lead to enhance plant growth. Moreover, Somayyeh and Sepehri (2012) also reported that the SA resulted in enhanced CGR in maize.
Drought stress induces proline accumulation in plants (Kuznetsov and Shevyakova, 1999) which contribute to the reduction of deleterious effects of stress factors (Morsy et al., 2007, Hayat et al., 2012). In the current study, the higher proline accumulation was found under drought stress conditions compared with control. Proline accumulated under stress might serve as a source for energy and ammonia, directly involves in plant metabolism after stress removal (Ramanjulu et al., 1997, Hussain et al., 2019). In our case, the foliar and seed primed SA with and without K fertilization had given more proline accumulation as compared with control, although the accumulation was comparatively less when no K was applied along with SA. The accumulation of proline by osmoregulation helps protect the plants in stabilizing enzymes, membranes, and detoxification of ROS (Ashraf and Foolad, 2007, Basu et al., 2016, Hussain et al., 2019). Likewise, SA application resulted in higher proline content (27%) as compared with no SA application (Kordi et al. (2013).
Higher leaf water status including RWC has been reported as an important indicator of drought stress in leaves, which is directly related to soil water content (Soltys-Kalina et al., 2016, Ahmad et al., 2017). We found reduced leaf water uptake, water retention, water saturation, and relative water content under severe and mild drought stresses in comparison with no stress. Cell membranes are exposed to variation under stress conditions; most often associated with an increase in permeability of the cell (Iqbal, 2009). However, In the present study, the foliar and seed primed SA with K fertilization had resulted in lesser reduction in WRC, WUC, and WSC under mild and severe drought stress express lower damage in cell structure due to drought than no SA and K application. Likewise, the reduction in the leaf turgid weight/dry weight could be due to an increased amount of cellulose and hemicellulose content in the cell well (Chowdhury et al., 2017). SA helped in saving the plants from leaf damage under stressed conditions thus giving very prominent results and improved leaf water status (Rao et al., 2012, Aslam et al., 2013, Zamaninejad et al., 2013). Likewise, Keyvan (2010) also reported that high osmotic regulation is a mechanism to withstand drought stress. It is well understood that, when transpiration goes beyond water intake, cell turgidity falls as RWC and cell volume decrease (Lawlor and Cornic, 2002) thus resultantly low turgidity and leaf water staus slow down plant growth and decrease stomatal conductance. The results of this study agreed with the conclusion of Rao et al. (2012) who observed that SA application enhanced RWC under mild stress. The application of SA also changed the quantity and amount of ions under water stress conditions (Zahra et al., 2011). The present study proposes that SA application may have lessened the adverse effects of drought stress in numerous crops exposed to water stress.
Chlorophyll and carotenoid are important for different vital processes inside plants including photosynthesis, light energy reactions, cell membranes stabilization and energy transduction (Huang et al., 2015, Cannella et al., 2016, Shah et al., 2017). Chlorophyll a and b regulate the process of photosynthesis (Kalaji et al., 2011). In the present study, we found a substantial reduction in plant pigments (chl a, b, a + b, and carotenoid content) under both severe and mild drought stresses as compared to no drought stress. Reduction in leaf chlorophyll content might be due to hamper of photosynthetic activity and reduced plant growth (Décima Oneto et al., 2016). Moreover, it could be due to the damage of the thylakoid membrane and metabolic impairment (Fahad et al., 2017). The same was also reported by Zhang et al. (2004) in soybean and Farooq et al. (2009) in maize. The foliar SA followed by seed primed SA with and without K fertilization had substantially improved plant pigments under drought stress conditions. Although the improvement under K fertilization was more. SA increases antioxidant activity under abiotic stresses that might help to protect the leaf from damage (Manzoor et al., 2015).
There was a considerable reduction in crop yield under drought stress conditions in the present study. This could be due to severe influence on the meristematic activity leads to retard cell growth and elongation, and abscission of leaves and roots, thus diminishing photosynthesis and dry matter production (Zamaninejad et al., 2013). The photosynthetic capacity reduction under drought stress resulted in decreased crop yield (Chaves et al., 2011, Ashraf et al., 2012). In the present study, the foliar SA and seed primed SA with and without K fertilization improved wheat yield under drought stress, however sole use of K fertilization fetch less increase in yield than combined use of SA and K. SA primed plants enhanced drought tolerance and resulted in higher plant dry matter under drought stress (Sayyari et al., 2013). The underlying mechanisms of SA in reducing the drought effects mediated osmolytes accumulation and enhance plant pigment content for maintaining osmotic homeostasis and regulating plant nutrient uptake as well as other vital plant growth pathways (Horváth et al., 2007, Khan et al., 2015). The limited water present in the root zone might be utilized more efficiently under drought-stressed led to maintaining plant growth even under stress conditions. Higher soil moisture conservation was noted by some plants under water stress conditions due to greater accumulation of osmolyte content (El Hadi and Khadr, 2003). SA application also improved WUE under stressful conditions and this may be attributed to the increased leaf diffusive resistance and lower transpiration rates (Bakry et al., 2012). Likewise, Subramanian et al. (2006) also reported improved WUE under drought stress than normal conditions due to lower transpiration.
In the current study, we found the tolerance effect of potassium fertilization under drought stress and enhanced CGR, LAI and proline content. The improved growth of crop plants may be due to the optimal photosynthetic processes with K fertilization. Synergistic effects were found with K fertilization between dry matter accumulation and CGR in the present study. Potassium fertilization showed a positive and significant linear relationship with CGR, NAR and LAI (Szewczuk et al., 2009, Nadim et al., 2012). The application of K had increased proline content in order to lessen the damaging effect of severe stress thus worked as stress mitigator (Oddo et al., 2011). The deleterious effects of drought stress were reduced due to higher proline accumulation under K fertilization (Aslam et al., 2013, Ahmad et al., 2018). According to the present results, K application at 100 kg ha−1 had improved RWC, WSD, WRC and WUC under severe and drought stress as compare to control in respective stresses. The substantial influence of K on leaf water status was also reported (Umar and Moinuddin, 2002). Potassium might have a greater role in the maintenance of the water economy of plant by increasing relative water contents under water stress (Zörb et al., 2014).
We found that K fertilization had enhanced photosynthetic pigments. This might be the availability of K+ ion to the plant that might help in inducing stressed conditions and enhance tolerance to abiotic stress. This might be due to the vital role of K in many enzymes activation, protein synthesis, photosynthesis, water relation, and stomatal movement in plants (Egilla et al., 2001, Amanullah, 2016). Likewise, (Kalaji et al., 2011) also found that well-watered plots had higher chlorophyll contents in the presence of potassium fertilization. Various studies have shown that K fertilization ameliorates the harmful effects of drought stress on growth and yield (Ahmad et al., 2014, Jatav et al., 2014, Erel et al., 2015). In the present study, K application had improved grain yield and was found very helpful in reducing stressful conditions in improving wheat yield along SA. Similarly, Shekhawat et al. (2013) also reported an increase in wheat yield with the application of K fertilizer. Enhanced maize yield was also achieved with potassium application (Shahzad et al., 2017). In like manner, Maleki et al. (2014) also found higher maize yield and yield components with K application under drought stress. We found an increase in WUE with K fertilization which might be due to morphological and biochemical alterations such as improved leaf water traits, triggering the accumulation of proline and plant pigments with enhancing crop growth under drought stress (Abid et al., 2018, Maswada et al., 2020). The same was also reported by Hattori et al. (2005) who found that K application increased drought tolerance of maize via the enhancement of water uptake ability.
6. Conclusion
It was concluded that both foliar SA and seed primed SA with K fertilization combat the adverse effects of drought by inducing tolerance by enhancing leaf water status as well as growth and bio-physiological traits of wheat under drought stress conditions. Exposure to both mild and severe drought stress has shown a decrease in the growth and physiology traits of wheat. However, the SA and K application under mild and severe drought stresses have shown better tolerance and enhanced wheat yield by 41% and 37% under mild and severe drought stress over no SA and K application, respectively.
CRediT authorship contribution statement
Fazal Munsif: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Tariq Shah: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Muhammad Arif: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Muhammad Jehangir: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Muhammad Zahir Afridi: . Ijaz Ahmad: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Basit Latief Jan: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration. Saleh Alansi: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/168), King Saud University, Riyadh, Saudi Arabia.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Availability of data and material
All data and materials as well as software application or custom code support our claims and comply with field standards. All data generated or analyzed during this study are included in this published article.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El–Mageed T.A., El-Sherif A.M.A., Ali M.M., Abd El-Wahed M.H. Combined effect of deficit irrigation and potassium fertilizer on physiological response, plant water status and yield of soybean in calcareous soil. Arch. Agron. Soil Sci. 2016;63:827–840. doi: 10.1080/03650340.2016.1240363. [DOI] [Google Scholar]
- Abdel-Wahed M., Amin A., El-Rashad S. Physiological effect of some bioregulators on vegetative growth, yield and chemical constituents of yellow maize plants. World J. Agric. Sci. 2006;2:149–155. [Google Scholar]
- Abid M., Ali S., Qi L.K., Zahoor R., Tian Z., Jiang D., Snider J.L., Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci. Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P., Ashraf M., Hakeem K.R., Azooz M.M., Rasool S., Chandna R., Akram N.A. Potassium starvation-induced oxidative stress and antioxidant defense responses in Brassica juncea. J. Plant Interact. 2014;9(1):1–9. [Google Scholar]
- Ahmad Z., Anjum S., Waraich E.A., Ayub M.A., Ahmad T., Tariq R.M.S., Ahmad R., Iqbal M.A. Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress – a review. J. Plant Nutr. 2018;41(13):1734–1743. doi: 10.1080/01904167.2018.1459688. [DOI] [Google Scholar]
- Ahmad Z., Waraich E.A., Ahmad R., Shahbaz M. Modulation in water relations, chlorophyll contents and antioxidants activity of maize by foliar phosphorus application under drought stress. Pak. J. Bot. 2017;49:11–19. [Google Scholar]
- Ahmed M., Haq M.E., Hossain M.M., Maruf M., Hasan M.M. Performance of four different rice cultivars under drought stress in the North-Western part of Bangladesh. Int. J. Agric. For. 2017;7:134–139. [Google Scholar]
- Amanullah M.H.K. Difference in dry matter accumulation with variable rates of sulphur and potassium application under calcareous soils in Brassica napus vs. B. juncea. J. Oilseed Brassica. 2016;1:241–248. [Google Scholar]
- Amin A., Rashad M., El-Abagy H. Physiological effect of indole-3-butyric acid and salicylic acid on growth, yield and chemical constituents of onion plants. J. Appl. Sci. Res. 2007;3:1554–1563. [Google Scholar]
- Arfan M., Athar H.R., Ashraf M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007;164(6):685–694. doi: 10.1016/j.jplph.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Ahmad M.S.A., Öztürk M., Aksoy A. In: Crop Production for Agricultural Improvement. Ashraf M., Öztürk M., Ahmad M.S.A., Aksoy A., editors. Springer Netherlands; Dordrecht: 2012. Crop Improvement Through Different Means: Challenges and Prospects; pp. 1–15. [DOI] [Google Scholar]
- Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Aslam M., Zamir M., Afzal I., Yaseen M., Mubeen M., Shoaib A. Drought stress, its effect on maize production and development of drought tolerance through potassium application. Cercetări Agronomice în Moldova. 2013;46:99–114. [Google Scholar]
- Bajji M., Kinet J.-M., Lutts S. Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae) Can. J. Bot. 2002;80(3):297–304. doi: 10.1139/b02-008. [DOI] [Google Scholar]
- Bakry B., El-Hariri D., Sadak M., El-Bassiouny H. Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soil. J. Appl. Sci. Res. 2012;8:3503–3514. [Google Scholar]
- Basu S., Ramegowda V., Kumar A., Pereira A. Plant adaptation to drought stress. F1000Res. 2016;5 doi: 10.12688/f1000research.7678.1. F1000 Faculty Rev-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Berry P., Ramirez-Villegas J., Bramley H., Mgonja M.A., Samarendu M. Regional impacts of climate change on agriculture and the role of adaptation. Plant Genetic Resources Climate Change. 2013;4:78–97. doi: 10.1079/9781780641973.0078. [DOI] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005;168(4):521–530. doi: 10.1002/jpln.200420485. [DOI] [Google Scholar]
- Cannella D., Möllers K.B., Frigaard N.U., Jensen P.E., Bjerrum M.J., Johansen K.S., Felby C. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 2016;7:11134. doi: 10.1038/ncomms11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M.M., Miguel Costa J., Madeira Saibo N.J. In: Advances in Botanical Research. Bourgougnon N., editor. Elsevier; 2011. Recent Advances in Photosynthesis Under Drought and Salinity; pp. 49–104. [Google Scholar]
- Chowdhury J.A., Karim M.A., Khaliq Q.A., Ahmed A.U., Mondol A.M. Effect of drought stress on water relation traits of four soybean genotypes. SAARC J. Agric. 2017;15:163–175. doi: 10.3329/sja.v15i2.35146. [DOI] [Google Scholar]
- Davies P.J. In: Plant Hormones. Davies P.J., editor. Springer; Netherlands: 2010. The Plant Hormones: Their Nature, Occurrence, and Functions; pp. 1–15. [Google Scholar]
- Décima Oneto C., Otegui M.E., Baroli I., Beznec A., Faccio P., Bossio E., Blumwald E., Lewi D. Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress- and maturation-induced promoter. J. Biotechnol. 2016;220:66–77. doi: 10.1016/j.jbiotec.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Egilla J.N., Davies F.T., Drew M.C. Effect of potassium on drought resistance of Hibiscus rosa-sinensis cv. Leprechaun: Plant growth, leaf macro-and micronutrient content and root longevity. Plant Soil. 2001;229:213–224. [Google Scholar]
- El Hadi, A., Khadr, M., 2003. Effect of potassium fertilization under saline and drought condition in Egyptian soils. Potassium and water management in West Asia and North Africa Proceedings of the Regional. Work-shop of the International Potash Institute. Amman. Jordan, from, 5-5.
- Elwan M.W.M., El-Hamahmy M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009;122(4):521–526. doi: 10.1016/j.scienta.2009.07.001. [DOI] [Google Scholar]
- Eraslan F., Inal A., Gunes A., Alpaslan M. Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hortic. 2007;113(2):120–128. doi: 10.1016/j.scienta.2007.03.012. [DOI] [Google Scholar]
- Erel R., Yermiyahu U., Ben-Gal A., Dag A., Shapira O.r., Schwartz A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant Physiol. 2015;177:1–10. doi: 10.1016/j.jplph.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., Ihsan M.Z., Alharby H., Wu C., Wang D., Huang J. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahad S., Bano A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot. 2012;44:1433–1438. [Google Scholar]
- Fang Y., Du Y., Wang J., Wu A., Qiao S., Xu B., Zhang S., Siddique K.H.M., Chen Y. Moderate Drought Stress Affected Root Growth and Grain Yield in Old, Modern and Newly Released Cultivars of Winter Wheat. Front. Plant Sci. 2017;8:672. doi: 10.3389/fpls.2017.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: effects, mechanisms and management. Agron. Sustainable Dev. 2009;29(1):185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Golbashy M., Ebrahimi M., Khorasani S.K., Choukan R. Evaluation of drought tolerance of some corn (Zea mays L.) hybrids in Iran. Afr. J. Agric. Res. 2010;5:2714–2719. [Google Scholar]
- Hattori T., Inanaga S., Araki H., An P., Morita S., Luxova M., Lux A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005;123(4):459–466. doi: 10.1111/j.1399-3054.2005.00481.x. [DOI] [Google Scholar]
- Hayat Q., Hayat S., Irfan M., Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ. Exp. Bot. 2010;68(1):14–25. [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth E., Szalai G., Janda T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007;26(3):290–300. doi: 10.1007/s00344-007-9017-4. [DOI] [Google Scholar]
- Huang C.J., Wei G., Jie Y.C., Xu J.J., Zhao S.Y., Wang L.C., Anjum S.A. Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie (Boehmeria nivea L.) cultivars. Photosynthetica. 2015;53(3):455–463. doi: 10.1007/s11099-015-0127-0. [DOI] [Google Scholar]
- Hussain H.A., Men S., Hussain S., Chen Y., Ali S., Zhang S., Zhang K., Li Y., Xu Q., Liao C., Wang L. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019;9:3890. doi: 10.1038/s41598-019-40362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, S., 2009. Physiology of wheat (Triticum aestivum L.) accessions and the role of phytohormones under water stress. Quaid-I-Azam University Islamabad.
- İsfendiyaroğlu M., Oezeker E. Rooting of Olea europaea “Domat” cuttings by auxin and salicylic acid treatments. Pak. J. Bot. 2008;40:1135–1141. [Google Scholar]
- Jarzyniak K.M., Jasiński M. Membrane transporters and drought resistance–a complex issue. Front. Plant Sci. 2014;5:687. doi: 10.3389/fpls.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatav K.S., Agarwal R., Tomar N.S., Tyagi S. Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J. Indian Bot. Soc. 2014;93:177–189. [Google Scholar]
- Jeon M.-W., Ali M.B., Hahn E.-J., Paek K.-Y. Photosynthetic pigments, morphology and leaf gas exchange during ex vitro acclimatization of micropropagated CAM Doritaenopsis plantlets under relative humidity and air temperature. Environ. Exp. Bot. 2006;55(1-2):183–194. [Google Scholar]
- Joshi R., Wani S.H., Singh B., Bohra A., Dar Z.A., Lone A.A., Pareek A., Singla-Pareek S.L. Transcription factors and plants response to drought stress: current understanding and future directions. Front. Plant Sci. 2016;7:1029. doi: 10.3389/fpls.2016.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaji H.M., Govindjee, Bosa K., Kościelniak J., Żuk-Gołaszewska K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011;73:64–72. doi: 10.1016/j.envexpbot.2010.10.009. [DOI] [Google Scholar]
- Karlidag H., Yildirim E., Turan M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agricola. 2009;66(2):180–187. [Google Scholar]
- Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010;8:1051–1060. [Google Scholar]
- Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordi S., Saidi M., Ghanbari F. Induction of Drought Tolerance in Sweet Basil (Ocimum basilicum L) by Salicylic Acid. Int. J. Agric. Food Res. 2013;2 doi: 10.24102/ijafr.v2i2.149. [DOI] [Google Scholar]
- Kuznetsov V.V., Shevyakova N. Proline under stress: biological role, metabolism, and regulation. Russ. J. Plant Physiol. 1999;46:274–287. [Google Scholar]
- Lawlor D.W., Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Malaga S., Janeczko A., Janowiak F., Waligórski P., Oklestkova J., Dubas E., Krzewska M., Nowicka A., Surówka E., Rapacz M., Wójcik-Jagła M., Kopeć P., Hura T., Ostrowska A., Kaczanowska K., Żur I. Involvement of homocastasterone, salicylic and abscisic acids in the regulation of drought and freezing tolerance in doubled haploid lines of winter barley. Plant Growth Regul. 2020;90(1):173–188. [Google Scholar]
- Maleki A., Fazel S., Naseri R., Rezaei K., Heydari M. The effect of potassium and zinc sulfate application on grain yield of maize under drought stress conditions. Adv. Environ. Biol. 2014:890–894. [Google Scholar]
- Manzoor K., Ilyas N., Batool N., Ahmad B., Arshad M. Effect of Salicylic Acid on the Growth and Physiological Characteristics of Maize under Stress Conditions. J. Chem. Soc. Pak. 2015;37 [Google Scholar]
- Marschner H. Academic press; Cambridge, MA: 2012. Marschner’s mineral nutrition of higher plants. [Google Scholar]
- Maswada H.F., Abd El-Razek U.A., El-Sheshtawy A.-N., Elzaawely A.A. Morpho-physiological and yield responses to exogenous moringa leaf extract and salicylic acid in maize (Zea mays L.) under water stress. Arch. Agron. Soil Sci. 2018;64(7):994–1010. doi: 10.1080/03650340.2017.1406079. [DOI] [Google Scholar]
- Maswada H.F., Sunoj V.S.J., Prasad P.V.V. A comparative Study on the Effect of Seed Pre-sowing Treatments with Microwave Radiation and Salicylic Acid in Alleviating the Drought-Induced Damage in Wheat. J. Plant Growth Regul. 2020;40(1):48–66. doi: 10.1007/s00344-020-10079-3. [DOI] [Google Scholar]
- Morsy M.R., Jouve L., Hausman J.-F., Hoffmann L., Stewart J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007;164(2):157–167. doi: 10.1016/j.jplph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Nadim M., Awan I., Baloch M., Khan E., Naveed K., Khan M. Response of wheat (Triticum aestivum L.) to different micronutrients and their application methods. J. Anim. Plant Sci. 2012;22:113–119. [Google Scholar]
- Namich, M., Emara, M., 2007. Response of cotton cultivar Giza 86 to application of glycine betaine under drought conditions. In: Plant Nutrient Management under stress conditions-17th International Symposium of CIEC, pp. 429-440.
- Oddo E., Inzerillo S., La Bella F., Grisafi F., Salleo S., Nardini A. Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol. 2011;31(2):131–138. doi: 10.1093/treephys/tpq115. [DOI] [PubMed] [Google Scholar]
- Oerke E.-C., Dehne H.-W. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 2004;23(4):275–285. doi: 10.1016/j.cropro.2003.10.001. [DOI] [Google Scholar]
- Radford P. Growth analysis formulae-Their use and abuse. Crop Sci. 1967;7777:171–175. [Google Scholar]
- Ramanjulu S., Sreenivasulu N., Sudhakar C. Effect of Water Stress on Photosynthesis in Two Mulberry Genotypes with Different drought Tolerance. Photosynthetica. 1997;35(2):279–283. [Google Scholar]
- Rao S., Qayyum A., Razzaq A., Ahmad M., Mahmood I., Sher A. Role of foliar application of salicylic acid and l-tryptophan in drought tolerance of maize. J. Animal Plant Sci. 2012;22:768–772. [Google Scholar]
- Reddy A.R., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161(11):1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Römheld V., Kirkby E.A. Research on potassium in agriculture: needs and prospects. Plant Soil. 2010;335(1-2):155–180. doi: 10.1007/s11104-010-0520-1. [DOI] [Google Scholar]
- Sangakkara U.R., Frehner M., Nosberger J. Influence of Soil Moisture and Fertilizer Potassium on the Vegetative Growth of Mungbean (Vigna radiata L. Wilczek) and Cowpea (Vigna unguiculata L. Walp) J. Agron. Crop Sci. 2001;186(2):73–81. [Google Scholar]
- Sayyari M., Ghavami M., Ghanbari F., Kordi S. Assessment of salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. Int. J. Agric. Crop Sci. (IJACS) 2013;5:1951–1957. [Google Scholar]
- Shah S., Houborg R., McCabe M. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.) Agronomy. 2017;7(3):61. doi: 10.3390/agronomy7030061. [DOI] [Google Scholar]
- Shah T., Khan H., Noor M.A., Ghoneim A., Wang X., Sher A., Nasir M., Basahi M.A. Effects of potassium on phenological, physiological and agronomic traits of maize (zea mays l.) under high nitrogen nutrition with optimum and reduced irrigation. Appl. Ecol. Environ. Res. 2018;16(5):7079–7097. [Google Scholar]
- Shahzad A.N., Fatima A., Sarwar N., Bashir S., Rizwan M., Qayyum M.F., Qureshi M.K., Javaid M.H., Ahmad S. Foliar Application of Potassium Sulfate Partially Alleviates Pre-anthesis Drought-induced Kernel Abortion in Maize. Int. J. Agric. Biol. 2017;19(03):495–501. [Google Scholar]
- Shekhawat P., Shaktawat R., Rathore D. Effect of nitrogen and potassium levels on growth and yield of barley (Hordeum vulgare L.) in loamy sand soil of Rajasthan. Environ. Ecol. 2013;31:1303–1306. [Google Scholar]
- Soltys-Kalina D., Plich J., Strzelczyk-Żyta D., Śliwka J., Marczewski W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of 'Katahdin'-derived potato cultivars. Breed Sci. 2016;66(2):328–331. doi: 10.1270/jsbbs.66.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayyeh B., Sepehri A. Paclobutrazol and salicylic acid application ameliorates the negative effect of water stress on growth and yield of maize plants. J. Res. Agric. Sci. 2012;8:127–139. [Google Scholar]
- Subramanian K.S., Santhanakrishnan P., Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006;107(3):245–253. [Google Scholar]
- Szewczuk A., Komosa A., Gudarowska E. Effect of different potassium soil levels and forms of potassium fertilizers on micro-elemental nutrition status of apple trees in early fruition period. J. Elementol. 2009;14:553–562. [Google Scholar]
- Umar, S., Moinuddin, 2002. Genotypic differences in yield and quality of groundnut as affected by potassium nutrition under erratic rainfall conditions. J. Plant Nutrit. 25, 1549-1562.
- Zahra S., Amin B., Ali V.S.M., Ali Y., Mehdi Y. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl) J. Biophys. Struct. Biol. 2011;2:35–41. [Google Scholar]
- Zamaninejad M., Khorasani S.K., Moeini M.J., Heidarian A.R. Effect of salicylic acid on morphological characteristics, yield and yield components of corn (Zea mays L.) under drought condition. Europ. J. Exp. Biol. 2013;3:153–161. [Google Scholar]
- Zhang M., Duan L., Zhai Z., Li J., Tian X., Wang B., He Z., Li Z. In: Proceedings of the 4th international crop science congress, Brisbane, Australia. 2004. Effects of plant growth regulators on water deficit-induced yield loss in soybean; pp. 252–256. [Google Scholar]
- Zörb C., Senbayram M., Peiter E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014;171(9):656–669. doi: 10.1016/j.jplph.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Zafar, Rasheed, Z., Atif, F., Javed, R.M., Maqsood, M.A., Gailing, M., Foliar, O., 2021. Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants 10, 1242. https://doi.org/10.3390/plants10061242. [DOI] [PMC free article] [PubMed]
- Chaves M.M., Pereira J.S., Maroco J., Rodrigues M.L., Ricardo C.C.P., Osório M.L., Carvalho I., Faria T., Pinheiro T. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002;89:907–916. doi: 10.1093/aob/mcf105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheri F., Barzin G., Pishkar L., Boojar M.M.A., Babaeekhou L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia. 2020;75(12):2189–2200. [Google Scholar]
- Brito C., Dinis L., Ferreira H., Moutinho-Pereira J., Correia C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018;226:56–63. doi: 10.1016/j.jplph.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Ul-Allah S., Ijaz M., Nawaz A., Sattar A., Sher A., Naeem M., Shahzad U., Farooq U., Nawaz F., Mahmood K. Potassium Application Improves Grain Yield and Alleviates Drought Susceptibility in Diverse Maize hybrid. Plants. 2020;9:75. doi: 10.3390/plants9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, M.T., Shah, P., Hollington, P.A., Khan, M.J., Sohail, Q., 2009. Agriculture Research: Design and Analysis, A Monograph. Agriculture University Peshawar Pakistan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials as well as software application or custom code support our claims and comply with field standards. All data generated or analyzed during this study are included in this published article.