Abstract
Supporting healthcare decision-making that is patient-centered and evidence-based requires investments in the development of tools and techniques for dissemination of patient-centered outcomes research findings via methods such as clinical decision support (CDS). This article explores the technical landscape for patient-centered CDS (PC CDS) and the gaps in making PC CDS more shareable, standards-based, and publicly available, with the goal of improving patient care and clinical outcomes. This landscape assessment used: (1) a technical expert panel; (2) a literature review; and (3) interviews with 18 CDS stakeholders. We identified 7 salient technical considerations that span 5 phases of PC CDS development. While progress has been made in the technical landscape, the field must advance standards for translating clinical guidelines into PC CDS, the standardization of CDS insertion points into the clinical workflow, and processes to capture, standardize, and integrate patient-generated health data.
Keywords: clinical decision support; patient-centered care, patient-generated health data
INTRODUCTION
Clinical decision support (CDS) enables the timely delivery of evidence-based guidelines at the point of care and supports healthcare decision-making.1 Although CDS was initially presented to clinicians, movement within the policy and research communities toward patient-centered care, as embodied in the Affordable Care Act, has intensified interest in developing patient-centered CDS (PC CDS).2,3 The shift is reflected in the modalities through which CDS is now delivered directly to patients through apps, websites, patient portals, and text messages.
CDS is patient-centered when it incorporates findings from patient-centered outcomes research (PCOR) or patient-specific information and facilitates the participation of patients and their caregivers in health-related decisions and actions.4,5 PC CDS offers novel ways to ensure that patient-specific, highly targeted, evidence-based clinical guidance is delivered to the right recipients, where and when they want to receive it, in a manner that is easy for them to understand and act upon.1,5–7 PC CDS that incorporates patient-generated health data (PGHD) and other patient-centered data (eg, patient preferences, social determinants of health) has tremendous potential to improve patient and clinician decision-making by drawing upon patient-specific data to enhance conversations and support shared decision-making.8,9 PC CDS can be most impactful when these data can be integrated within the electronic health record (EHR) to support patient engagement, produce clinical recommendations, and reduce clinician burden.10,11
There are several factors that will impact the advancement of PC CDS such as policies and regulatory frameworks that influence PC CDS development, use, and implementation,12,13 as well as patients’ and clinicians’ trust in CDS tools and the privacy and security of technologies that collect their health information.13,14 For PC CDS to scale, computable knowledge must be sharable and interoperable and PC CDS tools must interoperate with EHRs, personal health records, and other clinical systems. The development and use of standards for PGHD, for incorporating data into EHRs and other health IT systems, for data exchange, and for developing and sharing CDS (eg, representation of clinical knowledge in a computable form) are key to creating the environment necessary for PC CDS apps to flourish. We explore the current PC CDS technical landscape and assess the challenges in making PC CDS more shareable, standards-based, and publicly available.
OBJECTIVE
We aimed to: (1) assess the current state of PC CDS technical standards; (2) identify challenges; and (3) identify future directions for PC CDS interoperability standards.
MATERIALS AND METHODS
We used a technical expert panel (TEP), a review of peer-reviewed and grey literature, and key informant interviews for our study.
Technical expert panel
The TEP consisted of 22 PC CDS stakeholders with expertise in PC CDS design, implementation, knowledge representation, standards, and measurement. TEP members represented federal agencies (n = 4); academic medical centers (n = 3); health IT developers (n = 4); patient advocacy organizations (n = 2); research organizations (n = 4); health system clinical staff and providers (n = 2); health plans and value-based purchasers (n = 2); and quality standards and measures developers (n = 1). We conducted 2 TEP meetings to gather feedback on and contextualize our findings.
Literature review
We conducted a scoping review to broadly characterize the current state of the technical landscape of PC CDS and identify relevant challenges. We conducted PubMed and Google searches for peer-reviewed and grey literature published between December 2009 and February 2020, supplemented with searches for resources like conference presentations.15 Search terms encompassed key areas of CDS standards, such as: Health Level 7 (HL7®) Fast Healthcare Interoperability Resources (FHIR®), interoperability, decision support, and patient-centeredness (see Supplementary Appendix for additional information on scoping review methods). We reviewed 941 resources, screening titles, and abstracts using inclusion/exclusion criteria and reviewing full text of the articles that remained. We included 22 resources on the PC CDS technology landscape.
Key informant discussions
In addition to the TEP, we interviewed 18 experts with knowledge in diverse PC CDS disciplines, representing health IT developers (n = 5), healthcare clinical staff and providers (n = 4), clinical content developers (n = 3), research organizations (n = 3), patient advocates (n = 1), federal agencies (n = 1), and payers (n = 1). We used a semi-structured interview guide to fill gaps in the literature and gather perspectives on PC CDS facilitators and future research areas. The guide probed 5 topic areas: (1) critical current standards for PC CDS, (2) gaps in PC CDS standards, (3) experience of using standards in applied projects, (4) identification of barriers, challenges, and other limitations related to using standards, and (5) fundamental limitations in standards that will not carry over to the future.
Analysis and synthesis
We used qualitative thematic synthesis,16 using an inductive approach with simultaneous data collection and analysis.16 After reviewing the literature and interview transcripts, we engaged in iterative discussions to identify primary themes. Two researchers then reviewed the literature and transcripts to refine themes and identify challenges. We reflected on the challenges identified across the literature and interviews to synthesize findings.
RESULTS
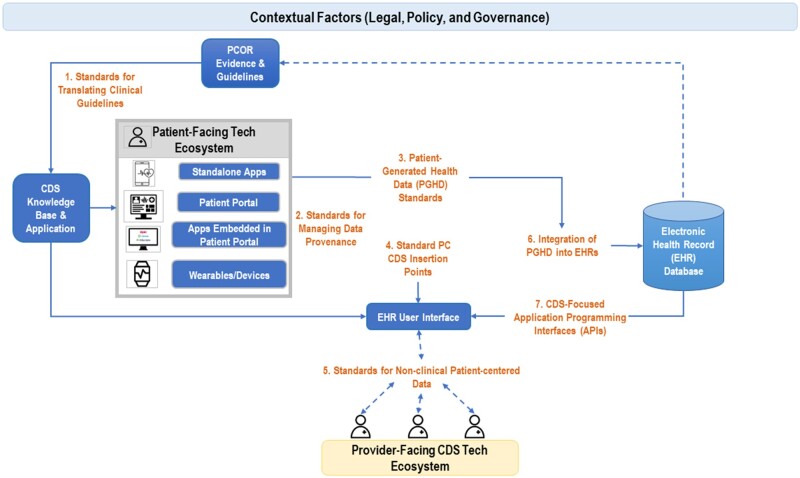
We identified 7 salient challenges for technical processes and standards that span the PC CDS lifecycle.12 Figure 1 displays these challenges and where they apply in the PC CDS landscape.
Figure 1.
Seven salient standards challenges for PC CDS.
Using PCOR evidence/guidance to inform the PC CDS knowledge base requires standards for the translation of clinical guidance (Challenge 1). Evidence-based CDS tools are then made available in both the patient-facing technical ecosystem (eg, apps, patient portals, and wearable devices) and the provider-facing technical ecosystem (ie, the EHR). Leveraging data, such as PGHD, from the patient-facing technical ecosystem, requires a clear understanding of data provenance (Challenge 2) and standards to capture patient-generated data (Challenge 3). To be an effective clinical tool within the provider-facing technical ecosystem, CDS must have standardized insertion points with the EHR (Challenge 4). In addition, we lack a standardized representation of patient-centered data such as patient preferences and social risk factors that can inform clinical decision-making (Challenge 5). Finally, we need a technical infrastructure that supports writing data to the EHR database from the patient-facing technical ecosystem (Challenge 6) and the use of that data to inform PC CDS (Challenge 7). Below, we further describe these 7 challenges.
Challenge 1: standards for translating clinical guidelines into PC CDS
Disseminating evidence-based guidelines for PC CDS requires standards for representing recommended actions and clinical inclusion/exclusion logic in a computable format. To date, guideline developers have not adopted a standard for knowledge representation for implementing PC CDS. Clinical Quality Language (CQL) and HL7 FHIR resources for sharing knowledge artifacts are recently developed standards for this purpose, but they have not been broadly used or implemented. The Centers for Disease Control and Prevention project “Adapting Clinical Guidelines for the Digital Age” aims to improve the consistency with which guidelines are translated and implemented using standards.17 However, additional work is needed to further the development and maturation of consensus-based standards and to promote the adoption of standards for knowledge representation for integrating evidence-based guidelines into PC CDS. The field would benefit from a coordinated federal response that engages stakeholders (eg, standards organizations, developers, health systems, content developers) about consensus-based standards. This response could be organized by the Office of the National Coordinator for Health Information Technology.18
Challenge 2: standards for managing data provenance
Integrating PGHD—health-related data created, recorded, or gathered by or from patients (or caregivers) outside a clinical setting (eg, through a mobile application, device, or patient portal)—into the EHR and PC CDS,19 requires improved data provenance standards. Standards to capture data provenance are in development within FHIR. These solutions could address the lack of validated approaches for assessing the source of PGHD for CDS.20 Consistent use of unique device identifiers to establish device provenance could facilitate linkages between PGHD and data sources.21 Source-specific processes for cleaning, normalizing, and standardizing data must also be defined to meaningfully present PGHD information to healthcare providers.20 However, additional verification methods of PGHD through trusted sources such as healthcare providers or through linkages to complementary information in the EHR may be needed.
Challenge 3: PGHD standards
Standardizing PGHD capture is critical for PC CDS, given that PGHD encompasses a range of data from patients—via assessments, apps, remote monitoring devices, or wearables—providing health information that is not captured in a healthcare setting. Previous efforts have largely focused on electronic patient-reported outcomes (ePROs).22 While some PRO instruments (eg, PROMIS®) are codified in Logical Observation Identifiers Names and Codes (LOINC), a standardized ontology is needed so that measurements can be shared across institutions to enable adoption of quality measurements at a population scale.22 Key informants highlighted the need for ePRO standards for data storage, exchange, and score harmonization (ie, cross-walking ePRO scores across survey instruments on the same topic to compare within similar domains).23 Promising initiatives include HL7® Patient-Reported Outcomes FHIR Implementation Guide,24 the prototype software framework known as SMART Markers,22 tools like Apple’s HealthKit to incorporate PGHD through wearables or a patient portal,25 and the HL7® Personal Health Device Implementation Guide.24 However, PGHD lack standardized, interoperable data definitions and formats (eg, blood pressure), hindering incorporation into care, aggregation from diverse participants and datasets, and analysis and interpretation.21
Challenge 4: standardizing CDS insertion points
To accomplish the 5 Rights,12 there is a need to standardize CDS insertion points into the clinical workflow.6 Key informants noted that creating triggers for PC CDS within existing clinical workflows is difficult and resource intensive. While the CDS Hooks specification was developed to trigger CDS in the workflow,26 there is variation among EHR developers in placing CDS trigger points.27 Additionally, most EHRs limit these integration points, such as only enabling a “hook” in response to a patient’s chart being opened.28 More granular insertion points would accommodate workflows that are patient-centered and directly involve patients.
Challenge 5: standards for non-clinical patient-centered data
Standardized representation of non-clinical patient-centered data (eg, patient-preferences, social risk factor data) that appear in widely used tools, such as the Center for Medicare & Medicaid Innovation’s Health-Related Needs Screening Tool, is critical to propelling PC CDS forward.29 Emerging data collection standards germane to patient-centered care include those that capture patient preferences, social determinants of health (SDOH) data, and other contextual data. While there are some efforts focused on standardizing the collection of patient preference data, the Gravity Project is currently developing a minimum data set for exchanging information related to multiple domains of SDOH such as food insecurity, housing, and transportation. The Gravity Project is developing shared definitions with FHIR-based resources for all domains of its SDOH Core Data for Interoperability.30–32 These standards would enable interoperable exchange of SDOH data, analysis of these data, and their application to PC CDS.
Challenge 6: integration of PGHD into EHRs
Using PGHD in health settings is challenging because: (1) most organizations lack infrastructure to receive and store PGHD; and (2) the standards needed to effectively integrate PGHD within the EHR are lacking, immature, or not widely adopted.20 Several key informants, as well as the literature, noted that data collected through PC CDS apps are siloed and often maintained and analyzed outside of the EHR. As CDS moves outside the clinical setting and directly to patients through apps and devices, the risk increases that PGHD will remain inaccessible to clinicians via the EHR without mature, widely adopted, data exchange standards.
Challenge 7: CDS-focused application programming interfaces
While FHIR standards have improved access to data for use in CDS, several issues remain—including variability of implementation, inconsistent availability of FHIR standards across developers, and limited functionality.33 Interpretation and implementation of FHIR profiles can differ within the same health system.32 The definitions of FHIR profiles (eg, Patient Reported Outcomes FHIR Implementation Guide) must be more specific to support interoperability—so that there is a use of standard terminology rather than developer- or institution-specific terms.24,34 Efforts are underway to extend standard terminologies to include concepts for PGHD and PC CDS.35,36
Key informants noted the application programming interfaces (APIs) to access many data types within EHRs remain proprietary.33 Furthermore, even when EHRs have APIs for FHIR resources, they do not usually support writing data from apps to the EHR.28 To promote interoperability, EHR developers should use FHIR APIs that allow data to be passed from an external application to the EHR (ie, write APIs), such as those required for real-time interactions during order entry and documentation.37
DISCUSSION
The development, use, and wide scale implementation of PC CDS is challenging because the patient-centered focus accentuates the complex, adaptive, sociotechnical systems involved. These challenges increase exponentially when you combine the complexity of the healthcare delivery system with the systems required to design, develop, implement, and deliver evidence-based PC CDS that meets patients’ and clinicians’ needs, and fits into their respective routines and workflows. Addressing these challenges will also require patient and clinician trust in PC CDS and mechanisms to assess trustworthiness of CDS.
Technical limitations of PC CDS include immaturity and limited adoption of standards that promote interoperability in representing and sharing this knowledge; lack of PGHD standards and ‘true’ integration of PGHD into EHRs; and immature and widely variable adoption and use of FHIR APIs, and CDS standards (ie, CQL and CDS hooks). The lack of industry-wide standards for PGHD collection, transfer, and tracking using different technologies, and for interoperability across devices, limits its clinical utility. Additional research should focus on developing and piloting standards for PGHD and curating and controlling its flow to providers, so it is meaningful and actionable.38
Finally, within the healthcare ecosystem, the level of integration of a PC CDS intervention in the EHR has implications for adoption. Clinician-facing PC CDS needs to be incorporated into the traditional EHR-based workflow. In addition, PC CDS must surpass traditional clinical workflows and office-based settings into modalities that reach patients.
CONCLUSION
We identified technical processes and standards needed to support the implementation of evidence-based clinical guidelines in patient-centered care delivery. Future PC CDS efforts should focus on expanding standards development to address gaps, piloting new standards where there are deficiencies, accelerating standards adoption by EHR developers, and promoting research into scalability through cooperation with standards development organizations, EHR developers, and other healthcare stakeholders. A robust technical infrastructure will ensure that PC CDS is more easily deployed for patients and incorporated in clinical workflows.
FUNDING
This work is based on research conducted by NORC at the University of Chicago under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSP233201500023I).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design of this research. PD, SFS, KH-H, LSH, DFL, and DFS led data acquisition and the analysis and interpretation of data. All authors were involved in drafting the manuscript or revising it critically for important intellectual content and gave final approval of the version published.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Andrew Chiao for his assistance in manuscript preparation.
CONFLICT OF INTEREST STATEMENT
AB owns stock in Elimu Informatics. Other authors do not have any competing interests to declare.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Prashila Dullabh, NORC at the University of Chicago, Bethesda, Maryland, USA.
Krysta Heaney-Huls, NORC at the University of Chicago, Bethesda, Maryland, USA.
David F Lobach, Elimu Informatics, El Cerrito, California, USA.
Lauren S Hovey, NORC at the University of Chicago, Bethesda, Maryland, USA.
Shana F Sandberg, NORC at the University of Chicago, Bethesda, Maryland, USA.
Priyanka J Desai, NORC at the University of Chicago, Bethesda, Maryland, USA.
Edwin Lomotan, Center for Evidence and Practice Improvement, Agency for Healthcare Research and Quality, Rockville, Maryland, USA.
James Swiger, Center for Evidence and Practice Improvement, Agency for Healthcare Research and Quality, Rockville, Maryland, USA.
Michael I Harrison, Center for Evidence and Practice Improvement, Agency for Healthcare Research and Quality, Rockville, Maryland, USA.
Chris Dymek, Center for Evidence and Practice Improvement, Agency for Healthcare Research and Quality, Rockville, Maryland, USA.
Dean F Sittig, School of Biomedical Informatics, University of Texas Health Science Center, Houston, Texas, USA.
Aziz Boxwala, Elimu Informatics, El Cerrito, California, USA.
REFERENCES
- 1. Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patient Protection and Affordable Care Act, Pub. L. No. 111–148, 124 Stat. 119. 2010.
- 3. Smith M, Saunders R, Stuckhardt L, et al. , eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. doi: 10.17226/13444. [DOI] [PubMed] [Google Scholar]
- 4.Frequently Asked Questions (FAQ). Patient-Centered Clinical Decision Support Learning Network. 2020. https://pccds-ln.org/FAQ. Accessed May 16, 2021.
- 5. Marcial LH, Richardson JE, Lasater B, et al. The imperative for patient-centered clinical decision support. EGEMS (Wash DC) 2018; 6 (1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Middleton B, Sittig DF, Wright A. Clinical decision support: a 25 year retrospective and a 25 year vision. Yearb Med Inform 2016; Suppl 1: S103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berman L, Curry L, Goldberg C, et al. Pilot testing of a decision support tool for patients with abdominal aortic aneurysms. J Vasc Surg 2011; 53 (2): 285–92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gimbel RW, Rennert LM, Crawford P, et al. Enhancing patient activation and self-management activities in patients with type 2 diabetes using the US Department of Defense Mobile Health Care Environment: Feasibility Study. J Med Internet Res 2020; 22 (5): e17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavallee DC, Lee JR, Austin E, et al. mHealth and patient generated health data: stakeholder perspectives on opportunities and barriers for transforming healthcare. mHealth 2020; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen DJ, Keller SR, Hayes GR, et al. Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project health design. JMIR Hum Factors 2016; 3 (2): e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye J. The impact of electronic health record–integrated patient-generated health data on clinician burnout. J Am Med Inform Assoc 2021; 28 (5): 1051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Analytic Framework for Action. Patient-Centered Clinical Decision Support Learning Network. 2020. https://pccds-ln.org/analytic-framework Accessed November 2, 2021.
- 13. Petersen C, DeMuro P. Legal and regulatory considerations associated with use of patient-generated health data from social media and mobile health (mHealth) devices. Appl Clin Inform 2015; 6 (1): 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson JE, Middleton B, Platt JE, et al. Building and maintaining trust in clinical decision support: recommendations from the Patient‐Centered CDS Learning Network. Learn Health Sys 2019; 4 (2): e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8 (1): 19–32. [Google Scholar]
- 16. Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Adapting Clinical Guidelines for the Digital Age. Public Health Information Office (PHIO). 2021. https://www.cdc.gov/csels/phio/clinical-guidelines/index.html Accessed May 16, 2021.
- 18.Final Report of the Health Information Technology Advisory Committee’s Interoperability Standards Priorities (ISP). 2021. https://www.healthit.gov/sites/default/files/page/2021-07/2021-06-09_ISP_TF_2021_HITAC%20Recommendations_Report_Signed_508.pdf. Accessed January 13, 2022.
- 19.The Office of the National Coordinator for Health Information Technology. Patient-Generated Health Data. HealthIT.gov. 2018. https://www.healthit.gov/topic/scientific-initiatives/patient-generated-health-data. Accessed January 13, 2022.
- 20. Abdolkhani R, Gray K, Borda A, DeSouza R. Patient-generated health data management and quality challenges in remote patient monitoring. JAMIA Open 2019; 2 (4): 471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarver M. Executive Summary for the Patient Engagement Advisory Committee Meeting. Connected and Empowered Patients. ePlatforms Potentially Expanding the Definition of Scientific Evidence. 2018. https://www.fda.go10.1093/jamiaopen/ooz036v/media/122887/download. Accessed May 16, 2021.
- 22. Sayeed R, Gottlieb D, Mandl KD. SMART Markers: collecting patient-generated health data as a standardized property of health information technology. NPJ Digit Med 2020; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin E, LeRouge C, Hartzler AL, Chung AE, Segal C, Lavallee DC. Opportunities and challenges to advance the use of electronic patient-reported outcomes in clinical care: a report from AMIA workshop proceedings. JAMIA Open 2019; 2 (4): 407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patient Reported Outcomes FHIR Implementation Guide. HL7 FHIR Implementation Guide. 2019. http://hl7.org/fhir/us/patient-reported-outcomes/2019May/. Accessed August 23, 2020.
- 25.EHRIntelligence. Top Challenges to Leveraging Patient-Generated Health Data. EHRIntelligence. 2016. https://ehrintelligence.com/news/top-challenges-to-leveraging-patient-generated-health-data. Accessed August 23, 2020.
- 26. Narus SP, Rahman N, Mann DK, He S, Haug PJ. Enhancing a commercial EMR with an open, standards-based publish-subscribe infrastructure. AMIA Annu Symp Proc 2018; 2018: 799–806. [PMC free article] [PubMed] [Google Scholar]
- 27. Tcheng JE, Bakken S, Bates DW, eds. Optimizing Strategies for Clinical Decision Support: Summary of a Meeting Series. Washington, DC: National Academy of Medicine; 2017. [PubMed] [Google Scholar]
- 28. Bresnick J. FHIR is Blazing a Path to Patient-Centered, Data-Driven Healthcare. HealthITAnalytics. 2018. https://healthitanalytics.com/features/fhir-is-blazing-a-path-to-patient-centered-data-driven-healthcare. Accessed May 16, 2021.
- 29.Billioux A, Verlander K, Anthony S, Alley D. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives. Washington, DC: National Academy of Medicine; 2017.
- 30. Nelson LR. Gravity Project FHIR IG—Patient Care—Confluence. Confluence. 2019. https://confluence.hl7.org/display/PC/Gravity+Project+FHIR+IG. Accessed January 13, 2022.
- 31. Melnick ER, Holland WC, Ahmed OM, et al. An integrated web application for decision support and automation of EHR workflow: a case study of current challenges to standards-based messaging and scalability from the EMBED trial. JAMIA Open 2019; 2 (4): 434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kukhareva P, Warner P, Rodriguez S, et al. Balancing functionality versus portability for SMART on FHIR applications: case study for a neonatal bilirubin management application. AMIA Annu Symp Proc 2019; 2019: 562–71. [PMC free article] [PubMed] [Google Scholar]
- 33. Wright A, Sittig DF, Ash JS, et al. Lessons learned from implementing service-oriented clinical decision support at four sites: a qualitative study. Int J Med Inform 2015; 84 (11): 901–11. [DOI] [PubMed] [Google Scholar]
- 34. Horvath K, Sengstack P, Opelka F, et al. ; Association of American Medical Colleges. A vision for a person-centered health information system. NAM Perspectives 2018; 8 (10): 13. [Google Scholar]
- 35.Health Level Seven® International. The Gravity Project Completes Food Insecurity and Housing Data Identification. The Standard. 2020. http://blog.hl7.org/gravity-project-completes-food-insecurity-and-housing-data-identification. Accessed January 13, 2022.
- 36.Regenstrief Institute. Survey Instruments. LOINC. https://loinc.org/panels/category/survey-instruments/. Accessed August 23, 2020.
- 37. Payne TH, Corley S, Cullen TA, et al. Report of the AMIA EHR-2020 Task Force on the status and future direction of EHRs. J Am Med Inform Assoc 2015; 22 (5): 1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bradway M, Giordanengo A, Joakimsen R, et al. Measuring the effects of sharing mobile health data during diabetes consultations: protocol for a mixed method study. JMIR Res Protoc 2020; 9 (2): e16657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.