Abstract
Despite a recent endorsement from official and professional bodies unequivocally recommending COVID-19 vaccination, vaccine hesitancy among pregnant people remains high. The accumulated evidence demonstrates that pregnant people are a special risk group for COVID-19, with an increased risk of intensive care unit admission, extracorporeal membranous oxygenation requirement, preterm birth, and perinatal death. These risks are further increased with some variants of concern, and vaccination of pregnant people reduces the COVID-19–related increase in maternal or fetal morbidity. Data from more than 180,000 vaccinated persons show that immunization against COVID-19 with an mRNA vaccine is safe for pregnant people. Many observational studies comparing perinatal outcomes between vaccinated and unvaccinated pregnant people have had reassuring findings and did not demonstrate harmful effects on pregnancy or the newborn. Immunization with mRNA vaccines does not increase the risk of miscarriage, preterm delivery, low birthweight, maternal or neonatal intensive care unit admission, fetal death, fetal abnormality, or pulmonary embolism. Moreover, observational data corroborate the findings of randomized trials that mRNA vaccination is highly effective at preventing severe SARS-CoV-2 infection in pregnant people, emphasizing that the potential maternal and fetal benefits of vaccination greatly outweigh the potential risks of vaccination. Ensuring pregnant people have unrestricted access to COVID-19 vaccination should be a priority in every country worldwide.
Key words: antibodies, COVID-19, immunogenicity, maternal immunization, pandemic, pregnancy, reactogenicity, SARS-CoV-2, side effects, vaccine
Introduction
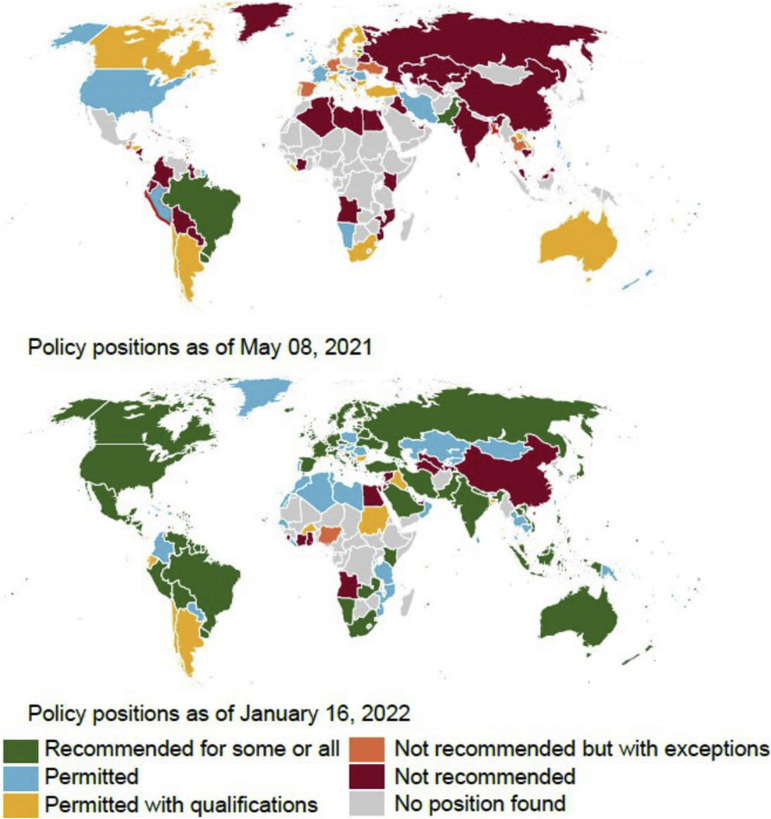
The rapid accumulation of data on COVID-19 and pregnancy during the pandemic resulted in many recommendations from professional bodies, which may, in retrospect, seem contradictory. This is especially true for COVID-19 vaccination during pregnancy. Pregnant people were not prioritized over their age-matched nonpregnant peers in the United States during the initial stages of vaccination rollout, and the Centers for Disease Control and Prevention (CDC) recommended that “getting vaccinated is a personal choice” owing to the lack of substantial safety data. In addition, a cautious approach was employed in other countries, such as the United Kingdom. Initially, pregnant people were recommended vaccination only if they were considered to be at increased risk of acquiring COVID-19 (such as healthcare workers) or having severe diseases if infected (such as those with underlying medical conditions).1 The World Health Organization (WHO) recommended vaccinating pregnant people “where the benefits outweigh the risks” until they changed their position to endorsement in November 2021.2 The accumulation of safety data and increasing evidence of substantial harms of COVID-19 to pregnant people led many professional organizations to fortify their stance, and they now unequivocally “recommend” vaccination of pregnant people.3 The Royal College of Obstetricians and Gynaecologists (RCOG), the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine, and the CDC recommend that all pregnant people should be vaccinated.4 , 5 Many countries underwent substantial policy changes during the pandemic as more permissive policies for vaccination of pregnant people were adopted worldwide. The COVID-19 maternal immunization tracker, a joint initiative by Johns Hopkins University and Berman Institute of Bioethics Center for Immunization Research, investigated and cataloged policy positions worldwide.6 As of April 2022, 162 countries recommend or permit COVID-19 immunization for some or all pregnant people compared with only 21 countries a year ago7 (Figure ). Most recently, the CDC recommended urgent action to increase vaccination uptake among pregnant people, for whom the benefits of vaccination outweigh any potential or unknown risks.8
Figure.
Policy positions of countries around the globe during the pandemic
The figure was created using data and graphics from the COVID-19 maternal immunization tracker website (https://comitglobal.org/explore/public-health-authorities/pregnancy).
Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
Despite a strong endorsement from professional bodies, vaccine hesitancy among pregnant people remains high,9, 10, 11 and strategies that prioritize vaccination of pregnant people are needed. Vaccine hesitancy is particularly high among women who do not feel vulnerable to COVID-19 complications and lack access to accurate information about vaccine safety.12 Caregivers need to stay up to date with available evidence and provide thorough counseling to pregnant people at every stage of pregnancy.
COVID-19 in pregnancy: risk factors and perinatal complications
The susceptibility of pregnant people to severe disease, the mechanism of adverse effects on pregnancy outcomes, and the possibility of vertical transmission are yet to be fully ascertained. However, the accumulated body of evidence confirms that pregnant people are a special risk group for COVID-19. This is due not only to the direct effects of COVID-19 on pregnancy but also to the indirect effects of the pandemic, such as barriers to healthcare access affecting pregnancy outcomes.13 The risk factors for severe disease seem to be similar to those in nonpregnant people: socioeconomic deprivation; increased body mass index; Black and minority ethnicity; preexisting comorbidities, such as pregestational diabetes mellitus, hypertension, or chronic lung disease; and increased maternal age.14, 15, 16, 17, 18 Of note, 1 pregnancy-specific risk factor is timing of infection; women in their third trimester of pregnancy are more susceptible to severe disease, whereas critical COVID-19 rates are very low in the first trimester of pregnancy.18 This increased risk may be partly mediated through the physical burden on the lungs toward the end of pregnancy, as Pineles et al19 have shown that delivery stabilizes the deteriorating pO2-to-FiO2 ratios in pregnant women with respiratory distress.
SARS-CoV-2 variants of concern are another risk factor with consideration implications on prognosis. Recent studies show that Delta wave infections were associated with increased morbidity and mortality among pregnant people and the risk of advanced respiratory support was increased.20 The finding was consistent among multiple centers worldwide.20, 21, 22 Omicron wave infections seem to be milder, but the data are often confounded by a high vaccination rate in the reported cohorts. Of note, 2 studies reported on the severity of Omicron infection among pregnant people and concluded that Omicron wave infection may be milder than Delta wave infection and not necessarily milder compared with pre-Delta variants.20 , 22 The Omicron variant shows marked evasion of immunity elicited by the current generation of vaccines.23 A recent study showed that breakthrough infections during the Omicron wave were mild without a need for respiratory support for vaccinated individuals and that 5% of unvaccinated individuals needed oxygen supplementation.24 It is important to address the misconceptions about the decreased severity of SARS-CoV-2 with emerging variants and emphasize the need for vaccination despite the apparent decline of protection elicited by the vaccines.
COVID-19 vaccination in pregnant people
Since the start of the vaccination rollout in the United States, and now in the United Kingdom, the recommendation is that COVID-19 vaccination should be offered to all pregnant people in parallel with the rest of the population, according to their age group and comorbidities (Figure). After the initial ambiguous and rapidly changing guidance on COVID-19 vaccination in pregnant people, there is now a renewed effort to prioritize pregnant people as vaccination uptake remains particularly low in pregnancy. All pregnant people need to receive detailed counseling about the benefits of COVID-19 vaccination during pregnancy, including the provision of an up-to-date summary of safety data. Barriers to accessing a consultation with healthcare providers (ie obstetricians, midwives, or general practitioners) should be addressed, and question-and-answer format decision aids should be readily available.25
Available vaccine types and recommendations by the Centers for Disease Control and Prevention
Of note, 3 types of COVID-19 vaccine (mRNA, viral vector, and inactivated) are currently available worldwide, and a fourth type (protein subunit) is awaiting approval by the Food and Drug Administration (FDA) (Table 1 ). Vaccines using mRNA particles (Pfizer or Moderna) are by far the most commonly used type in pregnant people and have the largest accumulated safety data so far. In light of the accumulated safety data and established effectiveness against COVID-19, mRNA vaccines are recommended as the first choice for pregnant people. The development of mRNA vaccines was unprecedented, not only because of their novel mechanism of action but also because of the speed with which they were made available. Traditionally, it takes more than a decade to develop a vaccine and obtain regulatory approval. However, thanks to generous funding by governmental agencies (ie, Operation Warp Speed) and the colossal threat posed by the pandemic, this arduous and lengthy process was cut down to less than a year. These vaccines contain mRNA particles that prompt the muscle cells in the injection area to synthesize and express a portion of the SARS-CoV-2 spike protein. mRNA half-life is very short, so it remains in the tissue for only a few days.25 Antigen-presenting cells show mRNA uptake, which can be observed in regional lymph nodes in the days after the administration of the vaccine.25 For individuals who express vaccine skepticism because of the novel nature of mRNA vaccines, the following points should be made clear. First, mRNA particles cannot interact with hereditary genetic material and cannot change DNA. Second, dissemination in the body is limited mainly to the injection site and the regional lymph nodes, and transplacental passage is unlikely. Once committed, the primary vaccination schedule is considered complete after receiving 2 doses taken 3 to 8 weeks apart.26 There is no need to repeat the first dose if the second dose is delayed more than 2 months. Booster shots are recommended after at least 5 months after the primary vaccination series. Pregnant people without a competent immune system (organ transplant recipients, stem cell transplant, primary or acquired immunodeficiencies, etc.) are required to take 3 vaccines for their primary vaccination series, whereas booster shots are recommended 3 months after the primary vaccination series. Booster shots are required to reverse the decline in the humoral response, which is more pronounced in elderly people, but there are no pregnancy-specific data on the subject. COVID-19 vaccines can be taken with other vaccines in the same visit (flu; tetanus, diphtheria, and pertussis; etc.) without a waiting period. Finally, individuals with SARS-CoV-2 are still recommended to receive their scheduled COVID-19 vaccine after their quarantine period. This recommendation is the same for individuals with SARS-CoV-2 who received a monoclonal antibody treatment; they can be vaccinated as soon as their quarantine period ends.
Table 1.
Vaccine types and recommendations for booster shots
| Vaccine type | Brands | US FDA approved | Recommended for pregnant people in the United States | Booster needed?a | Data on pregnancy outcomes |
|---|---|---|---|---|---|
| mRNA | Pfizer-BioNTech Moderna |
Yes (Pfizer-BioNTech, Moderna) | Yes | At least 5 mo after completing primary COVID-19 vaccination series | Available |
| Viral vector | J&J Janssen AstraZeneca Gamaleya Research Institute |
Yes (J&J Janssen) | Yes with reservationsb | At least 2 mo after receiving J&J Janssen COVID-19 vaccination. mRNA booster is preferred | Limited availability |
| Inactivated | SinoPharm, Sinovac, Bharat Biotech | No | No | No recommendation | Limited availability |
| Protein subunit | Novavax | Under consideration | No | No recommendation | N/A |
FDA, Food and Drug administration; mRNA, messenger RNA; N/A, not applicable.
Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
Centre for Disease Control and Prevention recommendation
Individuals who had a severe reaction after an mRNA vaccine dose or who have a severe allergy to an ingredient of Pfizer-BioNTech or Moderna. Possibility of vaccine-induced thrombosis and thrombocytopenia should be disclosed.
In areas where mRNA vaccines are not widely available, viral vector and inactivated vaccines are also in common use, but safety data for these vaccines in pregnant people are unfortunately relatively lacking. Viral vector vaccines are not recommended for pregnant people in some countries (the United Kingdom27 and Canada), although the CDC states that they can be used in certain situations (Table 1). Booster shots are recommended at least 2 months after a primary vaccination schedule with viral vector vaccines; the booster dose should be an mRNA vaccine.
Vaccine safety
The exclusion of pregnant people from the initial COVID-19 vaccine trials prevented the acquisition of pregnancy-specific safety data and limited the ability of professional organizations to make evidence-based recommendations on COVID-19 vaccination in pregnancy at the initial stage of vaccine implementation. To date, there are ongoing or planned randomized controlled trials of COVID-19 vaccines in pregnancy (Pfizer-BioNTech, Janssen vaccine [BNT16B2b2] Horizon 1 study, and the Preg-CoV pragmatic trial), which will explore the immunogenicity and reactogenicity of different vaccines and different schedules.28, 29, 30 However, the results from these trials are not expected before 2022 to 2023, and it is unclear whether trials will meet their target quota at all, given that vaccines are readily available and recommended to willing pregnant people outside the trial setting.
In the United States, pregnant or lactating women can choose any one of the vaccines licensed by the FDA.31 These include 2 mRNA vaccines (Pfizer-BioNTech [New York] and Moderna [mRNA1273; Cambridge, Massachusetts]) and 1 viral vector vaccine (Johnson & Johnson [J&J] Janssen [Titusville, New Jersey]). A large cohort of pregnant people is being followed up as part of the CDC V-safe and V-safe COVID-19 Vaccine Pregnancy Registry (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html).32 A preliminary report of more than 35,000 women,33 most vaccinated with an mRNA vaccine, identified no safety concern, and no “red flag signal” has been raised from the entire cohort. Several other vaccine safety surveillance systems are in place, including the Vaccine Adverse Event Reporting System (VAERS) implemented by the CDC in the United States, the Public Health England Inadvertent Vaccination in Pregnancy system, and the UK Medicines and Healthcare Products Regulatory Agency “Yellow Card” monitoring system. Public Health England and Public Health Scotland have reported that more than 81,000 pregnancies in England and more than 19,000 pregnancies in Scotland have received a COVID-19 vaccine.34 Reassuringly, reactogenicity rates seem to be similar in pregnant and nonpregnant individuals receiving the vaccine.35 Although pregnant people reported slightly higher rates of injection site pain, they reported lower rates of headache, myalgia, chills, and fever. A recent US study suggested that the incidence of adverse reactions, including fever, was lower in pregnant people compared with those who were neither pregnant nor lactating.35
VAERS is an invaluable source for monitoring vaccine safety, but healthcare providers should be aware that the data have been misappropriated by some groups on social media, claiming vaccines are not safe for pregnant people. It is important to understand that social media is an important information source for some patients and knowledge level along with its sources may need to be queried. Clarifying that both the CDC and ACOG unequivocally recommend COVID-19 vaccination without reservations about its safety can be important for reducing vaccine hesitancy in some individuals.
Vaccine-associated myocarditis
Myocarditis following COVID-19 vaccination seems to be specific to mRNA vaccines. According to the US VAERS reports, there is an apparent increase in observed compared with expected rates of myocarditis following mRNA vaccination among young individuals.36 This increased risk is observed predominantly in males below 40 years of age. Published studies corroborate the VAERS reports, with cases occurring at a median age below 30 years, approximately 90% after the second dose, and overwhelmingly (98%) in males.36 In women of reproductive age, the potential risk of mRNA vaccine-associated myocarditis is approximately 2 and approximately 4 to 5 cases per million vaccinations (after the second dose) for women aged 25 to 40 years and 18 to 25 years, respectively. The excess risk of myocarditis that could be attributed to mRNA vaccination is <4 per million vaccination doses. Most cases of vaccine-associated myocarditis are mild and resolve spontaneously or with nonsteroidal antiinflammatory drugs.36 Vaccine-associated myocarditis should be suspected in pregnant people with chest pain, pressure, discomfort, shortness of breath, or palpitations occurring within approximately 5 days after vaccination. Elevated cardiac enzymes, abnormal cardiac rhythm, echocardiogram, or magnetic resonance imaging in the absence of other obvious causes should prompt consideration of vaccine-related myocarditis. Documentation of possible cases and outcomes is important for continued safety surveillance of mRNA vaccines. Reassuringly, in the hitherto published literature, there is no report of vaccine-associated myocarditis in the pregnant people.
Vaccine-induced thrombosis and thrombocytopenia
The rare syndrome of vaccine-induced thrombosis and thrombocytopenia (VITT), also known as thrombosis with thrombocytopenia syndrome (TTS), has been reported in rare instances with the viral vector vaccines (AstraZeneca and J&J Janssen) at a rate of 7 cases per million doses.37 The condition more commonly affects adults under the age of 50 years. It is an idiosyncratic reaction often occurring after the first dose of a vaccine but also possible after the second dose, although rarely. The condition does not seem to be more likely in those at prothrombotic risk, including pregnant people. Patients with VITT typically present within a few weeks of vaccination (approximately 5 to 30 days).38 , 39 The diagnosis is more likely in persons presenting with thrombosis in atypical locations, such as splanchnic and cerebral veins, thrombocytopenia (<150×109/L), and high D-dimer levels (>2000 μg/L).39 The exact pathogenesis of VITT is not clear, but antibodies against platelet factor 4 polyanion complexes were detected in most cases.40 In cases with clinical suspicion, the diagnosis can be confirmed by demonstrating platelet factor 4 antibodies using an enzyme-linked immunosorbent assay.38 The management of VITT is complex as patients are prone to both coagulopathy and hemorrhage. Intravenous immunoglobulin (IVIg) infusion is recommended to alleviate thrombocytopenia. Cases refractory to IVIg infusion have been successfully treated with plasma exchange.40 , 41 Anticoagulation should be achieved with direct oral anticoagulants (fondaparinux, danaparoid, or argatroban), although concurrent bleeding is not a contraindication to anticoagulation, and decisions should be made on a case-by-case basis. The use of unfractionated heparin is not recommended as it may aggravate the condition. There is a single reported case of VITT in a pregnant people,42 who presented with headache, bilateral leg pain, and malaise 7 days after the first dose of AstraZeneca viral vector vaccine. She had mild thrombocytopenia (121×109/L), but unfortunately, a diagnosis of VITT was not made at the time. The woman was readmitted with severe headache and thrombocytopenia (33×109/L) 4 days after her initial discharge. Computed tomography revealed cerebral venous sinus thrombosis and parenchymal hemorrhage in the temporal lobe. Despite medical and surgical management of complications, both the mother and fetus died. This tragic case emphasized the importance of awareness of the condition.
Vaccination against SARS-CoV-2 using viral vector vaccines seems to be safe for pregnant people notwithstanding the extremely rare risk of VITT. Viral vector vaccines do not require stringent transfer and storage conditions and are more widely available in low- and middle-income countries (LMICs), where they may be of importance for preventing COVID-19–associated mortality and morbidity in pregnant people. As of December 14, 2021, the WHO Strategic Advisory Group of Experts has classified the Bharat Biotech Covaxin (BBV152 A, BBV152 B, BBV152 C; Hyderabad, India), Sinopharm BIBP-CorV (Hayat-Vax; Beijing, China), and Sinovac (CoronaVac; Beijing, China) (SARS-CoV-2–inactivated vaccine) vaccines to be “permitted” for pregnant people, which implies that all pregnant people can receive or choose to receive these vaccines.43
In the United States, the advice is that pregnant people can be offered the viral vector vaccine with some reservations. They should be informed of the possible risk of VITT, particularly in individuals under the age of 50 years, and the fact that alternatives (ie, mRNA vaccines) are available.37
Adverse perinatal outcomes following vaccination
Although regulatory bodies and public health agencies collect postmarketing surveillance data and monitor for safety signals, observational studies investigating perinatal outcomes in vaccinated women are equally important. Several studies compared perinatal outcomes between vaccinated and unvaccinated mothers, and the findings were reassuring. Table 2 lists the observational studies published before December 2021, reporting the perinatal outcomes of pregnant people who received COVID-19 vaccination during pregnancy and those who did not.44, 45, 46, 47, 48, 49, 50
Table 2.
Published observational studies before December 2021 reporting on the perinatal outcomes of pregnant people who received COVID-19 vaccine during pregnancy and those who did not
| Study | Sample size (of whom outcomes are reported) | Outcomes reported | Rate in vaccinated women, % (n/N) | Rate in unvaccinated women, % (n/N) | Effect estimate |
|---|---|---|---|---|---|
| Goldshtein et al,44 2021 | 7530 vaccinated vs 7530 unvaccinated | SARS-CoV-2 infection (≥28 d after the first dose) | 0.2 (10/4788) | 1.0 (46/4788) | OR: 0.21 (95% CI, 0.09–0.43) |
| Abortion | 1.7 (128/7530) | 1.6 (118/7530) | OR: 1.08 (95% CI, 0.83–1.41) | ||
| Fetal growth restriction | 0.5 (36/7530) | 0.5 (38/7530) | OR: 0.94 (95% CI, 0.58–1.53) | ||
| Preeclampsia | 0.3 (20/7530) | 0.3 (21/7530) | OR: 0.95 (95% CI, 0.48–1.84) | ||
| Stillbirth | <0.1 (1/7530) | <0.1 (2/7530) | OR: 0.49 (95% CI, 0.01–9.60) | ||
| Maternal death | 0.0 (0/7530) | 0.0 (0/7530) | Not estimable | ||
| Pulmonary embolism | 0.0 (0/7530) | 0.0 (0/7530) | Not estimable | ||
| Preterm birth at <37 wk | 5.6 (77/1387) | 5.9 (85/1427) | OR: 0.92 (95% CI, 0.66–1.29) | ||
| Blakeway et al,45 2022 | 133 vaccinated vs 399 unvaccinated | SGA | 12.0 (16/133) | 12.0 (48/399) | OR: 1.00 (95% CI, 0.55–1.82) |
| Fetal abnormalities | 2.3 (3/133) | 2.5 (10/399) | OR: 0.89 (95% CI, 0.24–3.31) | ||
| Stillbirth | 0.0 (0/133) | 0.2 (1/399) | Not estimable | ||
| Cesarean delivery | 30.8 (41/133) | 34.1 (136/399) | OR: 0.86 (95% CI, 0.56–1.31) | ||
| Postpartum hemorrhage | 9.8 (13/133) | 9.0 (36/399) | OR: 1.09 (95% CI, 0.56–2.12) | ||
| Intrapartum fever | 3.7 (5/133) | 1.0 (4/399) | OR: 3.85 (95% CI, 1.01–14.60) | ||
| Placental abruption | 0.0 (0/133) | 0.0 (0/133) | Not estimable | ||
| NICU admission | 5.3 (7/133) | 5.0 (20/399) | OR: 1.05 (95% CI, 0.43–2.54) | ||
| Maternal ICU admission | 6.0 (8/133) | 4.0 (16/399) | OR: 1.53 (95% CI, 0.64–3.66) | ||
| Theiler et al,46 2021 | 140 vaccinated vs 1862 unvaccinated | SARS-CoV-2 infection (any time) | 1.4 (2/140) | 11.3 (210/1862) | OR: 0.11 (95% CI, 0.01–0.42) |
| Maternal death | 0.0 (0/140) | 0.0 (0/1862) | Not estimable | ||
| Pulmonary embolism | 0.0 (0/129) | 0.1 (2/1580) | Not estimable | ||
| Early neonatal death | 0.0 (0/140) | 0.0 (0/1862) | Not estimable | ||
| NICU admission | 0.7 (1/140) | 0.5 (11/1862) | OR: 1.31 (95% CI, 0.03–9.17) | ||
| Maternal ICU admission | 0.7 (1/140) | 0.1 (2/1862) | OR: 7.24 (95% CI, 0.12–14.02) | ||
| Postpartum hemorrhage | 4.3 (6/140) | 3.1 (57/1862) | OR: 1.54 (95% CI, 0.53–3.67) | ||
| Transfusion | 17.8 (25/140) | 12.9 (241/1862) | OR: 1.61 (95% CI, 0.97–2.58) | ||
| Cesarean delivery | 31.4 (44/140) | 29.8 (555/1862) | OR: 1.21 (95% CI, 0.81–0.80) | ||
| Preeclampsia or eclampsia | 0.7 (1/140) | 1.2 (23/1862) | OR: 0.62 (95% CI, 0.01–3.91) | ||
| Stillbirth | 0.0 (0/140) | 0.4 (6/1862) | Not estimable | ||
| Low birthweight (<2500 g) | 2.1 (3/140) | 6.5 (121/1862) | OR: 0.34 (95% CI, 0.07–1.05) | ||
| Beharier et al,47 2021 | 92 vaccinated vs 66 unvaccinated | Preterm birth at <37 wk | 4.3 (4/92) | 7.6 (5/66) | OR: 0.55 (95% CI, 0.10–2.70) |
| NICU admission | 4.3 (4/92) | 1.5 (1/66) | OR: 2.93 (95% CI, 0.28–14.76) | ||
| Dagan et al,48 2021 | 10,861 vaccinated vs 10,861 unvaccinated | SARS-CoV-2 infection (≥28 d after the first dose) | 0.04 (3/7577) | 0.80 (64/7519) | RR: 0.04 (95% CI, 0.00–0.11) |
| Kharbanda et al,49 2021 | 15,079 vaccinated vs 90,367 unvaccinated | Miscarriage | Vaccination rate in miscarriages is 8.6 (1128/13,160) Vaccination rate in ongoing pregnancies is 8.0(20,139/250,944) |
OR: 1.02 (95% CI, 0.96–1.08) | |
| Rottenstreich et al,50 2022 | 712 vaccinated vs 1063 unvaccinated | Preterm birth at <37 wk | 1.0 (7/712) | 0.9 (10/1063) | OR: 1.04 (95% CI, 0.33–3.05) |
| Cesarean delivery | 15.6 (111/712) | 10.8 (115/1063) | OR: 1.04 (95% CI, 0.33–3.05) | ||
| Postpartum hemorrhage | 7.3 (52/712) | 10.0 (106/1063) | OR: 0.71 (95% CI, 0.49–1.01) | ||
| Placental abruption | 1.1 (8/712) | 2.3 (25/1063) | OR: 0.47 (95% CI, 0.18–1.08) | ||
| Maternal ICU admission | 0.0 (0/712) | 0.0 (0/1063) | Not estimable | ||
| Transfusion | 0.5 (4/712) | 0.6 (7/1063) | OR: 0.85 (95% CI, 0.18–3.36) | ||
| Puerperal fever | 3.2 (23/712) | 3.4 (36/1063) | OR: 0.95 (95% CI, 0.53–1.66) | ||
| Magnus et al,51 2021 | 1003 vaccinated vs 17,474 unvaccinated | Miscarriage within the 5-wk exposure window | 23.0 (231/1003) | 24.5 (4290/17474) | OR: 0.91 (95% CI, 0.75–1.10) |
| Wainstock et al52 | 913 vaccinated vs 3486 unvaccinated | Hypertensive disorders of pregnancy | 5.5 (50/913) | 4.7 (165/3486) | OR: 1.17 (95% CI, 0.84–1.61) |
| 5-min Apgar score of <7 | 0.4 (2/913) | 1.1 (30/3486) | OR: 0.33 (95% CI, 0.08–1.40) | ||
| Cesarean delivery | 19.9 (182/913) | 17.2 (601/3486) | OR: 1.19 (95% CI, 0.99–1.44) | ||
| Placental abruption | 0.3 (3/913) | 0.3 (11/3486) | OR: 1.04 (95% CI, 0.29–3.74) | ||
| Postpartum hemorrhage | 1.1 (10/913) | 0.9 (30/3486) | OR: 1.28 (95% CI, 0.62–2.62) | ||
| Postpartum fever | 0.2 (2/913) | 0.3 (12/3486) | OR: 0.64 (95% CI, 0.14–2.85) | ||
| SGA | 2.8 (26/913) | 3.8 (131/3486) | OR: 0.75 (95% CI, 0.49–1.15) | ||
| Butt et al53 | 407 vaccinated vs 407 unvaccinated | SARS-CoV-2 infection | 10.5 per 10,000 person-weeks | 82.5 per 10,000 person-weeks | HR: 0.12 (95% CI, 0.03–0.56) |
CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; NICU, neonatal intensive care unit; OR, odds ratio; RR, relative risk; SGA, small for gestational age.
Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
A common concern for women is pregnancy loss after vaccination. A preliminary report by the CDC showed the observed spontaneous miscarriage rate after vaccination (12.6%) is not increased over the known background rate.32 However, a subsequent correction acknowledged that, because follow-up data were not yet available for most vaccinated pregnant people before 20 weeks of gestation, no proportion could be determined for their risk of spontaneous abortion. Moreover, the miscarriage rate after vaccination was recently reported with reassuring findings.49 , 54 None demonstrated an increased risk of miscarriage after vaccination. The study by Kharbanda et al49 included a control group and demonstrated that the miscarriage rate was not significantly different within 1 month of vaccination compared with controls. The finding was consistent in subgroups of women who received vaccination at 6 to 8, 9 to 13, and 14 to 19 weeks of gestation. Similarly, another larger case-control study based on data from Norwegian registries did not find any evidence supporting an increased risk of early pregnancy loss within either 3 or 5 weeks after COVID-19 vaccination.51 A study from Israel, including 7530 women vaccinated with the Pfizer-BioNTech vaccine during pregnancy, found no evidence of an increased risk of adverse pregnancy outcomes, including abortions (both spontaneous and induced), intrauterine growth restriction, preeclampsia, stillbirth, maternal death, pulmonary embolism, birthweight, and gestational age at birth.44 Other studies comparing adverse outcomes between vaccinated and unvaccinated women did not demonstrate an increased risk of almost all reported perinatal outcomes.45 , 46 , 50 Intrapartum fever was reported to be slightly higher in vaccinated women compared with unvaccinated women (3.7% vs 1.0%, respectively), but the association was not replicated in a study by Rottenstreich et al50 (3.2% vs 3.4%, respectively). The increased risk in the study by Blakeway et al45 was not because of an excessively increased prevalence in the vaccinated cohort but rather a lower-than-expected rate in the matched unexposed cohort. Rottenstreich et al50 demonstrated that the composite neonatal adverse outcomes were reduced by 48% in vaccinated women but also reported a significantly increased cesarean delivery rate (15.6% vs 10.8%). To date, the safety data are driven almost entirely by studies reporting on mRNA vaccines.
No long-term follow-up study of the infants of mothers vaccinated during pregnancy is available yet. Such follow-up studies are essential to provide the reassurance that many pregnant people require; the V-safe COVID-19 Vaccine Pregnancy Registry will follow up women and their babies until 3 months after birth, and the Preg-CoV trial will follow up mothers and infants until 1 year after the birth.
All available COVID-19 vaccines in the United States are considered safe for lactating mothers; there is no theoretical risk, and there is no vaccine that has been demonstrated in practice to date. It should be borne in mind that even live vaccines, such as measles, mumps, and rubella, can be safely given to lactating women without any adverse effects on the neonate.
Vaccine effectiveness
In addition, the exclusion of pregnant people from the initial COVID-19 vaccine trials hindered the assessment of vaccine efficacy in pregnancy. Modi et al55 reviewed the ramifications of this initial exclusion of pregnant people from clinical trials, which led, understandably, to inconsistent advice being given by different public health, governmental, and professional authorities worldwide. Despite widespread calls for inclusion, the decision to exclude pregnant people was based on generic grounds rather than specific grounds. This made counseling challenging for physicians and likely reduced vaccine uptake among pregnant people because of inconsistent messaging, an effect that persists.56
Despite the dearth of randomized pregnancy-specific data, there is little historic precedence to assume that vaccine efficacy would be reduced during pregnancy.57 Immunogenicity studies show that mRNA vaccines produce a similar humoral immune response in pregnant people to that in nonpregnant women.58, 59, 60, 61 Therefore, the mRNA vaccines are likely to confer a similar level of protection against SARS-CoV-2 infection and serious infection, like that seen in nonpregnant individuals. Goldshtein et al44 performed a 1:1 matched analysis of vaccinated and unvaccinated pregnant people and demonstrated a considerable reduction in new SARS-CoV-2 infections in the vaccinated group (0.33%) compared with the unvaccinated group (1.64%) at 70 days follow-up. Most cases occurring in the vaccinated group were within 1 month after vaccination. More recently, Dagan et al48 reported a very high vaccine effectiveness rate of 96% against proven SARS-CoV-2 infection among pregnant people.46 IgG antibodies have been shown to efficiently cross the placenta and are present in umbilical cord blood at birth.59 , 60 , 62 Antibodies elicited by the vaccine were greater in quantity and more durable compared with those elicited by SARS-CoV-2 infection. Edlow et al63 found the transplacental transfer of antibodies elicited by SARS-CoV-2 infection was not efficient and occurred at a lower rate than influenza hemagglutinin A antibodies. The finding was corroborated by Atyeo et al64 who showed that SARS-CoV-2–specific antibodies had lower transplacental transfer than influenza or pertussis-specific antibodies. This was not the case with vaccination, which elicited efficient transfer of antibodies, which were also more durable.65 Moreover, higher levels of IgA antibodies were detected in the breastmilk of vaccinated individuals.60 , 62 However, the degree of protection afforded to the infant by neonatal IgG and breastmilk IgA antibodies is not yet clear.
Timing of vaccination
Advice about the timing of vaccination is based on expert opinion rather than on experimental data. The current evidence suggested that these vaccines are safe at any point in pregnancy, including the first trimester of pregnancy, although some women, who conscious of the fact that the first trimester of pregnancy is the gestational age most associated with the development of fetal structural anomalies, may choose to delay vaccination until after 12 weeks of gestation. More pregnancy outcome data are needed for those vaccinated in the first or second trimesters of pregnancy, but preliminary findings from both preclinical and observational studies regarding fetal development are reassuring.39 , 57 , 58 However, if there is an increased risk of acquiring the infection or severe COVID-19 because of preexisting risk factors, vaccination should be advised, even early in pregnancy. Ideally, the second dose should be administered before the third trimester of pregnancy at the latest, the time of greatest risk to pregnant people, allowing 2 weeks after the second dose to develop the maximal immune response. Those planning pregnancy should be reassured that there is no need to delay vaccination because they are trying to conceive or planning fertility treatment and there is no need to delay trying to conceive after vaccination. Both animal and clinical studies do not suggest a detrimental effect of mRNA vaccines.66 , 67 Current advice in the United Kingdom is that if a woman has had a viral vector vaccine before pregnancy, the second dose should preferably also be a viral vector vaccine; however, it is also permissible to use an mRNA vaccine. Mixing vaccine types may be beneficial in the light of recent evidence showing that heterologous combinations generate a better antibody response. However, this potential benefit should be balanced against the apparent higher reactogenicity of the heterologous combination.
The UK Preg-CoV trial will examine the effect of different intervals between doses of COVID-19 vaccines, currently of the Pfizer-BioNTech or Moderna vaccines.30 This study plans to randomize 600 pregnant people at an interval of 4 to 6 weeks or 8 to 12 weeks between the first and second doses. The immune response and reactogenicity of different dose intervals will be investigated. The main aim of this study was to devise the most effective schedule for protecting pregnant people against the virus.
Another issue is the need for booster doses for pregnant people. The efficacy of vaccination decreases with time, especially in elderly populations.68 The need for boosters would be affected by the incidence of breakthrough infections in the target population, the emergence of newer variants of the SARS-CoV-2 virus with potential for increased transmissibility and immune evasion, and the rate of decline in protection against symptomatic and severe disease. There are scant data about breakthrough infections and the decline in vaccine effectiveness in pregnant people, and more research is needed. In the meantime, a booster shot may be beneficial for women who have previously been vaccinated with less effective formulations, such as inactivated or viral vector vaccines. Currently, the advice in the United Kingdom is that pregnant people are eligible for a booster vaccine dose if 12 weeks have passed since their second dose. This booster should be with an mRNA vaccine, regardless of the type of vaccine received for the first 2 doses, because of their established higher efficacy and lesser risk of TTS, compared with the viral vector vaccines. In addition, the CDC recommends a booster dose for pregnant people parallel to the general population.69 Booster shots seem to be the most effective option for protection against infection with the Omicron variant, which has substantial immune escape from humoral immunity elicited by mRNA vaccines.70
Vaccine hesitancy
Vaccine hesitancy seems to be greater in pregnant people compared with their nonpregnant peers.9 , 68 , 71 In the United States, a study, including a large cohort of 135,968 pregnant people, found that only 16.3% received at least 1 dose of the COVID-19 vaccine and that only 11.1% had both doses during pregnancy.9 The rates of vaccination uptake during pregnancy were the lowest among those of Black (6.0%) and Hispanic (11.1%) ethnicity and younger women (18 to 24 years) (5.5%). Pregnant people were less likely to be vaccinated than nonpregnant women of reproductive age (fully vaccinated: 11.1% vs 24.9%). In addition, similar findings were reported in the United Kingdom, with lower vaccine uptake in ethnic minorities and deprived (social or economic) populations.45 Moreover, a national level study from Scotland showed that vaccine uptake was the lowest in the most deprived population.72 In Scotland, the rate of vaccination was twice as high in the least deprived quintile than the most deprived quintile throughout the vaccination rollout.72 In the United Kingdom, an RCOG survey71 found that only 42% of those who had been offered a vaccine had accepted it; and of those not yet offered, approximately the same proportion (40%) said that they would accept; another 41% said they would not accept, whereas 18% were undecided. Many of those refusing or undecided said it was because they were waiting for further safety data. As mentioned above, in both the United States and the United Kingdom, COVID-19 vaccination has been unequivocally recommended for pregnant people since July 2021.
The reason for hesitancy should be explored and any erroneous beliefs addressed. Social media is awash with false information about COVID-19 vaccination. Women should be reassured that there is no plausible scientific mechanism by which the vaccine could affect fertility and that there is no evidence showing any effect on fertility. It is important to emphasize that vaccines cannot cause COVID-19 and that they cannot alter a person’s DNA (mRNA does not enter the nucleus of the host cell, and so it remains separate from the host DNA). The detailed explanation may provide the reassurance that some women need to accept vaccination. Emphasizing that the potential maternal and fetal benefits of vaccination greatly outweigh the potential risks of vaccination by citing specific figures may be especially useful (Table 3 ).74 However, an informed choice to refuse should be respected. Pregnant people refusing vaccination should be encouraged to follow other infection prevention and control measures, such as wearing facemasks or following social distancing.
Table 3.
Maternal and perinatal adverse outcomes associated with SARS-CoV-2 infection and messenger RNA COVID-19 vaccination
| Maternal adverse outcomes | SARS-CoV-2 infection | mRNA COVID-19 vaccination | Adverse outcome more common with |
|---|---|---|---|
| Fever | 4:10 | ∼1:10 | SARS-CoV-2 infection |
| Injection site pain | NA | ∼9:10 | Vaccination |
| Shortness of breath | 2:10 | NA | SARS-CoV-2 infection |
| Muscle pain | 2:10 | ∼1–2:10 | SARS-CoV-2 infection |
| Cough | 4:10 | NA | SARS-CoV-2 infection |
| Anaphylaxis | NA | 2–5:1,000,000 | Vaccination |
| Myocarditis | ∼5:100 | ∼1:1,000,000 | SARS-CoV-2 infection |
| Oxygen support | 2:10 | NA | SARS-CoV-2 infection |
| Intensive care unit admission | 4:100 | NA | SARS-CoV-2 infection |
| Death | 1–2:100 | NA | SARS-CoV-2 infection |
| Perinatal adverse outcomes | |||
| Preterm delivery | 2.2-fold increase (baseline 1:10) | Not increased over baseline | SARS-CoV-2 infection |
| Fetal death | 2.2-fold increase (baseline ∼1:200) | Not increased over baseline | SARS-CoV-2 infection |
| Preeclampsia | 1.5-fold increase (baseline ∼3:100) | Not increased over baseline | SARS-CoV-2 infection |
| Cesarean delivery | 1.63-fold increase (baseline rates variable) | Not increased over baseline | SARS-CoV-2 infection |
A survey of vaccine uptake among pregnant people in 16 countries12 found that vaccine hesitancy is less in countries where the effects of the pandemic have been the greatest. Unfortunately, in many of those countries, the main barrier pregnant people face is a lack of vaccine availability. The lower rate of vaccine hesitancy creates an opportunity for equity, as far fewer (10-fold) vaccinations are needed to prevent 1 maternal death in some LMICs compared with high-income countries.75 The strongest predictors of vaccine acceptance are confidence in vaccine safety and effectiveness, the level of worry about COVID-19, belief in the importance of vaccines to their country, compliance with mask guidelines, trust in public health agencies and health science, and attitudes toward routine vaccination.12
Conclusion
Pregnant people should be prioritized for vaccination ahead of their nonpregnant peers of similar age in every country worldwide. Compared with their nonpregnant peers, pregnant people are at increased risk of severe disease, and COVID-19 is also associated with preeclampsia, preterm birth, stillbirth, and birth by cesarean delivery. A large body of evidence suggests that COVID-19 vaccination during pregnancy is effective and safe. Waiting for the evidence of no harm is no longer acceptable when the evidence of harm from the virus is already present.
Footnotes
P.O.B. is cochair of the Royal College of Obstetricians and Gynaecologists (RCOG) Vaccine Committee. P.O.B. is the RCOG vice president. P.H. is a member of the RCOG Vaccine Committee. P.H. is chief investigator of the Preg-CoV trial (UK multicenter COVID-19 vaccination in pregnancy). A.K. is the obstetrical principal investigator (PI) of the Preg-CoV trial (UK multicenter COVID-19 vaccination in pregnancy). A.K. is a member of the COVAX Working Group on COVID-19 vaccination in pregnancy. A.K. is the PI of the Pfizer COVID-19 vaccination in pregnancy trial. E.K. and S.P. report no conflict of interest.
This study received no funding.
Supplementary Data
COVID-19 vaccination in pregnancy: Expert Review
<VIDSE>Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
COVID-19 vaccination in pregnancy: Expert Review
<VIDSE>Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
References
- 1.Public Health England JCVI issues new advice on COVID-19 vaccination for pregnant women. gov.uk. 2021 https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women Available at: [Google Scholar]
- 2.World Health Organization The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: what you need to know. 2022. https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine Available at:
- 3.Martins I., Louwen F., Ayres-de-Campos D., Mahmood T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256–258. doi: 10.1016/j.ejogrb.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The American College of Obstetricians and Gynecologists ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. 2021. https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals Available at:
- 5.Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at:
- 6.Comitglobal.org COMIT: Covid-19 maternal immunization tracker. 2021. https://www.comitglobal.org/vaccines/matrix/pregnancy Available at:
- 7.Public health authority pregnancy policy explorer. Available at: https://www.comitglobal.org/explore/public-health-authorities/pregnancy. Accessed January 3, 2022
- 8.Centers for Disease Control and Prevention COVID-19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID-19. 2021. https://emergency.cdc.gov/han/2021/han00453.asp Available at: cdc.gov. Available at:
- 9.Razzaghi H., Meghani M., Pingali C., et al. COVID-19 vaccination coverage among pregnant women during pregnancy - eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health England COVID-19 vaccine surveillance report – week 13. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1008919/Vaccine_surveillance_report_-_week_31.pdf Available at:
- 11.Cui Y., Binger K., Tsaih S., Palatnik A. Understanding COVID-19 vaccine hesitancy in pregnant patients. Am J Obstet Gynecol. 2021;226:S658. [Google Scholar]
- 12.Skjefte M., Ngirbabul M., Akeju O., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielewska B., Barratt I., Townsend R., et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badr D.A., Mattern J., Carlin A., et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764–768. doi: 10.1016/j.ajog.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokken E.M., Huebner E.M., Taylor G.G., et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225:77.e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight M., Bunch K., Vousden N., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galang R.R., Newton S.M., Woodworth K.R., et al. Risk factors for illness severity among pregnant women with confirmed severe acute respiratory syndrome coronavirus 2 infection-surveillance for emerging threats to mothers and babies network, 22 state, local, and territorial health departments, 29 March 2020-5 March 2021. Clin Infect Dis. 2021;73(Suppl3):S17–S23. doi: 10.1093/cid/ciab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalafat E., Prasad S., Birol P., et al. An internally validated prediction model for critical COVID-19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol. 2022;226:403.e1–403.e13. doi: 10.1016/j.ajog.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineles B.L., Stephens A., Narendran L.M., et al. The relationship between delivery and the PaO2/FiO2 ratio in COVID-19: a cohort study. BJOG. 2022;129:493–499. doi: 10.1111/1471-0528.16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birol Ilter P., Prasad S., Mutlu M.A., et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obstet Gynecol. 2022 doi: 10.1002/uog.24916. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSisto C.L., Wallace B., Simeone R.M., et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engjom H.M., Ramakrishnan R., Vousden N., et al. Severity of maternal SARS-CoV-2 infection and perinatal outcomes during the Omicron variant dominant period: UK Obstetric Surveillance System national cohort study. medRxiv. 2022 doi: 10.1136/bmjmed-2022-000190. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J., Peng P., Cao X., et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19:293–295. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birol Ilter P., Prasad S., Berkkan M., et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet Gynecol. 2022;59:560–562. doi: 10.1002/uog.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsay K.E., Bhosle S.M., Zurla C., et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nat Biomed Eng. 2019;3:371–380. doi: 10.1038/s41551-019-0378-3. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#primary-series Available at:
- 27.Royal College of Obstetricians & Gynaecologists. COVID-19 vaccines, pregnancy and breastfeeding FAQs 2021. https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/ Available at:
- 28.Clinicaltrials.gov Study to evaluate the safety, tolerability, and immunogenicity of SARS CoV-2 RNA vaccine candidate (BNT162b2) against COVID-19 in healthy pregnant women 18 years of age and older. 2021. https://clinicaltrials.gov/ct2/show/NCT04754594 Available at:
- 29.Clinicaltrials.gov A study of Ad26.COV2.S in healthy pregnant participants (COVID-19) (HORIZON 1) 2021. https://clinicaltrials.gov/ct2/show/NCT04765384 Available at:
- 30.St George’s Vaccine Institute Preg-CoV trial. 2021. https://vaccine.ac.uk/research/preg-cov-trial/ Available at:
- 31.U.S. Food and Drug Administration FDA approves first COVID-19 vaccine: approval signifies key achievement for public health. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine Available at:
- 32.Centers for Disease Control and Prevention V-safe COVID-19 vaccine pregnancy registry. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html Available at:
- 33.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Health Service. NHS encourages pregnant women to get COVID-19 vaccine 2021. https://www.england.nhs.uk/2021/10/nhs-encourages-pregnant-women-to-get-covid-19-vaccine/# Available at: England.nhs.uk. Available at:
- 35.Kachikis A., Englund J.A., Singleton M., Covelli I., Drake A.L., Eckert L.O. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacNeil J.R., Su J.R., Broder K.R., et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institute for Health and Care Excellence COVID-19 rapid guideline: vaccine-induced immune thrombocytopenia and thrombosis (VITT) 2022. https://www.nice.org.uk/guidance/ng200/resources/covid19-rapid-guideline-vaccineinduced-immune-thrombocytopenia-and-thrombosis-vitt-pdf-51036811744 Available at: [PubMed]
- 39.Arepally G.M., Ortel T.L. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138:293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavord S., Scully M., Hunt B.J., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendes-de-Almeida D.P., Martins-Gonçalves R., Morato-Santos R., et al. Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia following ChAdOx1 nCOVID-19 vaccine in a pregnant woman. Haematologica. 2021;106:3025–3028. doi: 10.3324/haematol.2021.279407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization; 2022. Covid-19 Maternal Immunization Tracker.https://www.comitglobal.org/authority/who Available at: [Google Scholar]
- 44.Goldshtein I., Nevo D., Steinberg D.M., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blakeway H., Prasad S., Kalafat E., et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226:236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theiler R.N., Wick M., Mehta R., Weaver A.L., Virk A., Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3:100467. doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beharier O., Plitman Mayo R., Raz T., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131 doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagan N., Barda N., Biron-Shental T., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 49.Kharbanda E.O., Haapala J., DeSilva M., et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rottenstreich M., Sela H.Y., Rotem R., Kadish E., Wiener-Well Y., Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129:248–255. doi: 10.1111/1471-0528.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnus M.C., Gjessing H.K., Eide H.N., Wilcox A.J., Fell D.B., Håberg S.E. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385:2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wainstock T., Yoles I., Sergienko R., Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butt A.A., Chemaitelly H., Khal A.A., Coyle P.V., Saleh H., Kaleeckale A.H., et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest [Internet] 2021;131(23) doi: 10.1172/JCI153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zauche L.H., Wallace B., Smoots A.N., et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modi N., Ayres-de-Campos D., Bancalari E., et al. Equity in coronavirus disease 2019 vaccine development and deployment. Am J Obstet Gynecol. 2021;224:423–427. doi: 10.1016/j.ajog.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chervenak F.A., McCullough L.B., Bornstein E., et al. Professionally responsible coronavirus disease 2019 vaccination counseling of obstetrical and gynecologic patients. Am J Obstet Gynecol. 2021;224:470–478. doi: 10.1016/j.ajog.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlaudecker E.P., McNeal M.M., Dodd C.N., Ranz J.B., Steinhoff M.C. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis. 2012;206:1670–1673. doi: 10.1093/infdis/jis592. [DOI] [PubMed] [Google Scholar]
- 58.Prabhu M., Murphy E.A., Sukhu A.C., et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient maternofetal transplacental transfer of anti- severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies after antenatal SARS-CoV-2 BNT162b2 messenger RNA vaccination. Clin Infect Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray K.J., Bordt E.A., Atyeo C., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collier A.Y., McMahan K., Yu J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baird J.K., Jensen S.M., Urba W.J., Fox B.A., Baird J.R. SARS-CoV-2 antibodies detected in mother’s milk post-vaccination. J Hum Lact. 2021;37:492–498. doi: 10.1177/08903344211030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edlow A.G., Li J.Z., Collier A.Y., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atyeo C., Pullen K.M., Bordt E.A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shook L.L., Atyeo C.G., Yonker L.M., et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowman C.J., Bouressam M., Campion S.N., et al. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol. 2021;103:28–35. doi: 10.1016/j.reprotox.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wesselink A.K., Hatch E.E., Rothman K.J., et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022 doi: 10.1093/aje/kwac011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at:
- 70.Schmidt F., Muecksch F., Weisblum Y., et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Royal College of Obstetricians & Gynaecologists Maternity colleges express concern over vaccine hesitancy in pregnant women. 2021. https://www.rcog.org.uk/en/news/maternity-colleges-express-concern-over-vaccine-hesitancy-in-pregnant-women/ Available at:
- 72.Stock S.J., Carruthers J., Calvert C., et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28:504–512. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckley B.J.R., Harrison S.L., Fazio-Eynullayeva E., Underhill P., Lane D.A., Lip G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Investig. 2021;51 doi: 10.1111/eci.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalafat E., Magee L.A., von Dadelszen P., O’Brien P., Khalil A. SARS-CoV-2 vaccination in pregnancy: a unique opportunity for equity. Lancet. 2021;398:951. doi: 10.1016/S0140-6736(21)01756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
COVID-19 vaccination in pregnancy: Expert Review
<VIDSE>Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.
COVID-19 vaccination in pregnancy: Expert Review
<VIDSE>Kalafat. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol 2022.